Ecological Assessment of Phytoplankton Diversity and Water Quality to Ensure the Sustainability of the Ecosystem in Lake Maybalyk, Astana, Kazakhstan
Abstract
:1. Introduction
2. Materials and Methods
2.1. Case Study Description
2.2. Sample Collection and Treatment
2.2.1. Hydrobiological Analysis
2.2.2. Hydrochemical Analysis
2.2.3. Data Analysis
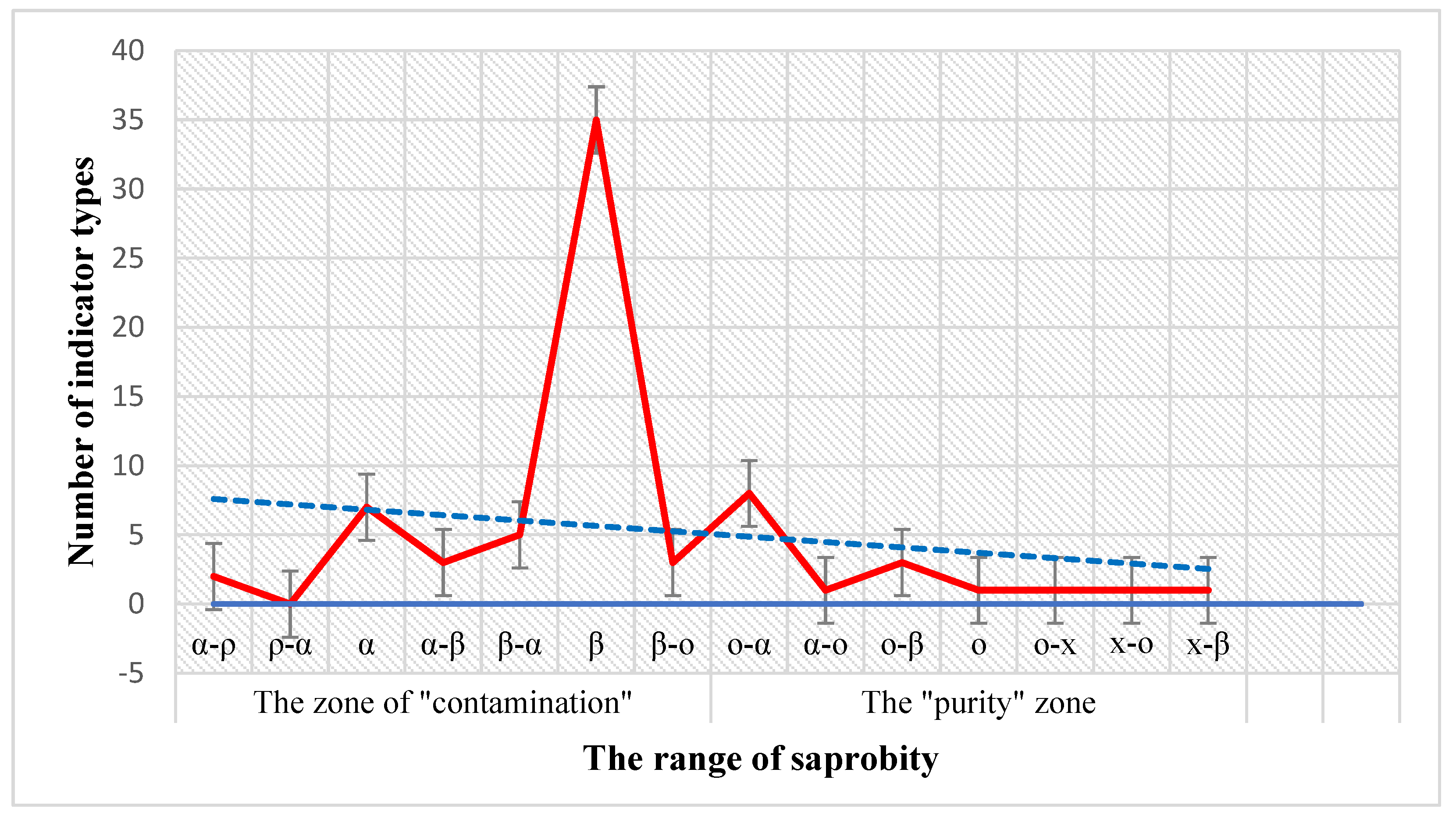
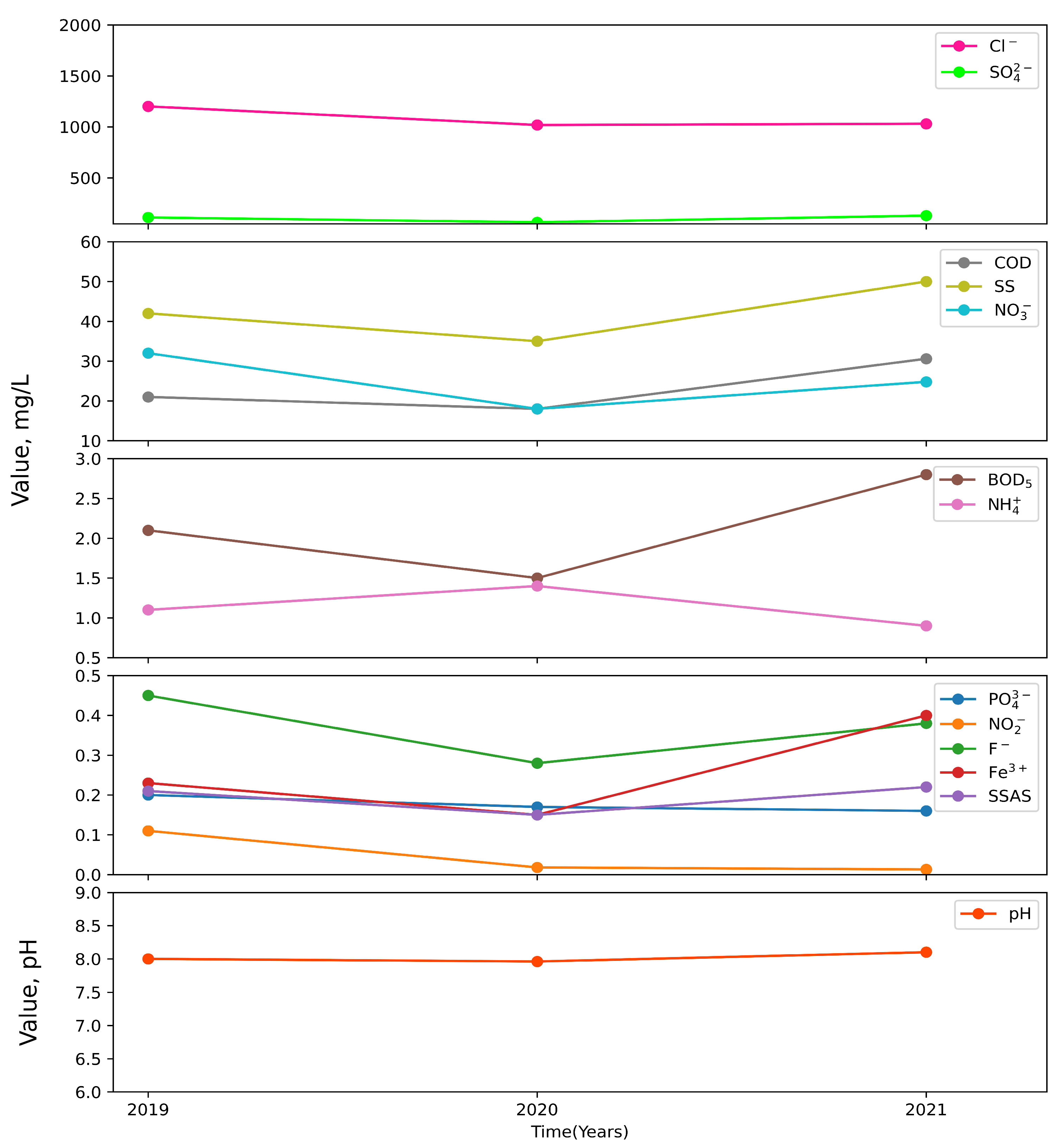
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, F.; Zhan, X.; Xu, H.; Zhu, G.; Zou, W.; Zhu, M.; Kang, L.; Guo, Y.; Zhao, X.; Wang, Z.; et al. New insights into eutrophication management: Importance of temperature and water residence time. J. Environ. Sci. 2022, 111, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.S.; Bali, R.; Khan, H.; Mohamed, H.I.; Sharma, S.K. Improved water resource management framework for water sustainability and security. Environ. Res. 2021, 201, 111527. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.; Larson, A.; Goodwill, J.; Wang, Y.; Cardace, D.; Akanda, A.S. Water Quality Observations from Space: A Review of Critical Issues and Challenges. Environments 2022, 9, 125. [Google Scholar] [CrossRef]
- Bhat, R.A.; Singh, D.V.; Qadri, H.; Dar, G.H.; Dervash, M.A.; Bhat, S.A.; Unal, B.T.; Ozturk, M.; Hakeem, K.R.; Yousaf, B. Vulnerability of municipal solid waste: An emerging threat to aquatic ecosystems. Chemosphere 2022, 287, 132223. [Google Scholar] [CrossRef]
- Chowdhury, S.; Mazumder, M.A.J.; Al-Attas, O.; Husain, T. Heavy metals in drinking water: Occurrences, implications, and future needs in developing countries. Sci. Total Environ. 2016, 569–570, 476–488. [Google Scholar] [CrossRef]
- Alexander, T.J.; Vonlanthen, P.; Seehausen, O. Does eutrophication-driven evolution change aquatic ecosystems? Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160041. [Google Scholar] [CrossRef]
- Scholz, S.N.; Esterhuizen-Londt, M.; Pflugmacher, S. Rise of toxic cyanobacterial blooms in temperate freshwater lakes: Causes, correlations and possible countermeasures. Toxicol. Environ. Chem. 2017, 99, 543–577. [Google Scholar] [CrossRef]
- Laplace-Treyture, C.; Feret, T. Performance of the Phytoplankton Index for Lakes (IPLAC): A multimetric phytoplankton index to assess the ecological status of water bodies in France. Ecol. Indic. 2016, 69, 686–698. [Google Scholar] [CrossRef]
- Cartuche, A.; Guan, Z.; Ibelings, B.W.; Venail, P. Phytoplankton Diversity Relates Negatively with Productivity in Tropical High-Altitude Lakes from Southern Ecuador. Sustainability 2019, 11, 5235. [Google Scholar] [CrossRef]
- Abzhalelov, A.B.; Tekebayeva, Z.B.; Aituganov, K.A. The Role of Algoflora in the Purification of Reservoirs Contaminated with Various Pollutants; Print House LLP “Master Po”: Astana, Kazakhstan, 2017; pp. 1–84. [Google Scholar]
- Beauger, A.; Voldoire, O.; Allain, E.; Gosseaume, P.; Blavignac, C.; Baker, L.A.; Wetzel, C.E. Biodiversity and Environmental Factors Structuring Diatom Assemblages of Mineral Saline Springs in the French Massif Central. Diversity 2023, 15, 283. [Google Scholar] [CrossRef]
- Chiaramonti, D.; Prussi, M.; Casini, D.; Tredici, M.R.; Rodolfi, L.; Bassi, N.; Zittelli, G.C.; Bondioli, P. Review of energy balance in raceway ponds for microalgae cultivation: Re-thinking a traditional system is possible. Appl. Energy 2013, 102, 101–111. [Google Scholar] [CrossRef]
- Cai, Y.; Qi, L.; Shan, T.; Liu, Y.; Zhang, N.; Lu, X.; Fan, Y. Application of Phytoplankton Taxonomic α-Diversity Indices to Assess Trophic States in Barrier Lake: A Case of Jingpo Lake. Diversity 2022, 14, 1003. [Google Scholar] [CrossRef]
- Liu, W.C.; Liu, H.M.; Yam, R.S.W. A three-dimensional coupled hydrodynamic-ecological modeling to assess the planktonic biomass in a subalpine lake. Sustainability 2021, 13, 12377. [Google Scholar] [CrossRef]
- Barinova, S.; Krupa, E.; Khitrova, E. Spatial Distribution of the Taxonomic Diversity of Phytoplankton and Bioindication of the Shallow Protected Lake Borovoe in the Burabay National Natural Park, Northern Kazakhstan. Diversity 2022, 14, 1071. [Google Scholar] [CrossRef]
- Duan, T.; Feng, J.; Chang, X.; Li, Y. Evaluation of the effectiveness and effects of long-term ecological restoration on watershed water quality dynamics in two eutrophic river catchments in Lake Chaohu Basin, China. Ecol. Indic. 2022, 145, 109592. [Google Scholar] [CrossRef]
- Smith, J.E.; Wolny, J.L.; Hill, R.L.; Stocker, M.D.; Pachepsky, Y. Examining the Relationship between Phytoplankton Community Structure and Water Quality Measurements in Agricultural Waters: A Machine Learning Application. Environments 2022, 9, 142. [Google Scholar] [CrossRef]
- Baek, S.H.; Lee, M.; Park, B.S.; Lim, Y.K. Variation in Phytoplankton Community Due to an Autumn Typhoon and Winter Water Turbulence in Southern Korean Coastal Waters. Sustainability 2020, 12, 2781. [Google Scholar] [CrossRef]
- Ratté-Fortin, C.; Chokmani, K.; Laurion, I. Spatiotemporal Variability in Phytoplankton Bloom Phenology in Eastern Canadian Lakes Related to Physiographic, Morphologic, and Climatic Drivers. Environments 2020, 7, 77. [Google Scholar] [CrossRef]
- Ampofo, J.; Abbey, L. Microalgae: Bioactive Composition, Health Benefits, Safety and Prospects as Potential High-Value Ingredients for the Functional Food Industry. Foods 2022, 11, 1744. [Google Scholar] [CrossRef]
- Sim, B.R.; Kim, H.C.; Kim, C.S.; Kim, J.H.; Park, K.W.; Lim, W.A.; Lee, W.C. Seasonal Distributions of Phytoplankton and Environmental Factors Generate Algal Blooms in the Taehwa River, South Korea. Water 2020, 12, 3329. [Google Scholar] [CrossRef]
- Madeira, M.S.; Cardoso, C.; Lopes, P.A.; Coelho, D.; Afonso, C.; Bandarra, N.M.; Prates, J.A.M. Microalgae as feed ingredients for livestock production and meat quality: A review. Livest. Sci. 2017, 205, 111–121. [Google Scholar] [CrossRef]
- Niccolai, A.; Chini Zittelli, G.; Rodolfi, L.; Biondi, N.; Tredici, M.R. Microalgae of interest as food source: Biochemical composition and digestibility. Algal Res. 2019, 42, 101617. [Google Scholar] [CrossRef]
- Guedes, A.C.; Sousa-Pinto, I.; Malcata, F.X. Application of Microalgae Protein to Aquafeed. Handb. Mar. Microalgae Biotechnol. Adv. 2015, 8, 93–125. [Google Scholar] [CrossRef]
- Hemaiswarya, S.; Raja, R.; Kumar, R.R.; Ganesan, V.; Anbazhagan, C. Microalgae: A sustainable feed source for aquaculture. World J. Microbiol. Biotechnol. 2011, 27, 1737–1746. [Google Scholar] [CrossRef]
- Wan, A.H.L.; Davies, S.J.; Soler-Vila, A.; Fitzgerald, R.; Johnson, M.P. Macroalgae as a sustainable aquafeed ingredient. Rev. Aquac. 2019, 11, 458–492. [Google Scholar] [CrossRef]
- Michalak, I.; Mahrose, K. Seaweeds, Intact and Processed, as a Valuable Component of Poultry Feeds. J. Mar. Sci. Eng. 2020, 8, 620. [Google Scholar] [CrossRef]
- Fredriksson, S.; Elwinger, K.; Pickova, J. Fatty acid and carotenoid composition of egg yolk as an effect of microalgae addition to feed formula for laying hens. Food Chem. 2006, 99, 530–537. [Google Scholar] [CrossRef]
- Oostlander, P.C.; van Houcke, J.; Wijffels, R.H.; Barbosa, M.J. Microalgae production cost in aquaculture hatcheries. Aquaculture 2020, 525, 735310. [Google Scholar] [CrossRef]
- Sfez, S.; Van Den Hende, S.; Taelman, S.E.; De Meester, S.; Dewulf, J. Environmental sustainability assessment of a microalgae raceway pond treating aquaculture wastewater: From up-scaling to system integration. Bioresour. Technol. 2015, 190, 321–331. [Google Scholar] [CrossRef]
- Shah, M.R.; Lutzu, G.A.; Alam, A.; Sarker, P.; Kabir Chowdhury, M.A.; Parsaeimehr, A.; Liang, Y.; Daroch, M. Microalgae in aquafeeds for a sustainable aquaculture industry. J. Appl. Phycol. 2018, 30, 197–213. [Google Scholar] [CrossRef]
- Sui, Y.; Jiang, Y.; Moretti, M.; Vlaeminck, S.E. Harvesting time and biomass composition affect the economics of microalgae production. J. Clean. Prod. 2020, 259, 120782. [Google Scholar] [CrossRef]
- Dineshbabu, G.; Goswami, G.; Kumar, R.; Sinha, A.; Das, D. Microalgae–nutritious, sustainable aqua- and animal feed source. J. Funct. Foods 2019, 62, 103545. [Google Scholar] [CrossRef]
- Nagappan, S.; Das, P.; AbdulQuadir, M.; Thaher, M.; Khan, S.; Mahata, C.; Al-Jabri, H.; Vatland, A.K.; Kumar, G. Potential of microalgae as a sustainable feed ingredient for aquaculture. J. Biotechnol. 2021, 341, 1–20. [Google Scholar] [CrossRef]
- Poikane, S.; Zohary, T.; Cantonati, M. Assessing the ecological effects of hydromorphological pressures on European lakes. Inland Waters 2019, 10, 241–255. [Google Scholar] [CrossRef]
- Parikhani, F.; Atazadeh, E.; Razeghi, J.; Mosaferi, M.; Kulikovskiy, M. Using Algal Indices to Assess the Ecological Condition of the Aras River, Northwestern Iran. J. Mar. Sci. Eng. 2023, 11, 1867. [Google Scholar] [CrossRef]
- Barinova, S.; Gabyshev, V.; Gabysheva, O. Phytoplankton in the Ecological Assessment of the Mining Facilities Influence on the Anabar River in the Permafrost Zone of the Arctic, Eastern Siberia, Russia. Land 2023, 12, 1775. [Google Scholar] [CrossRef]
- Stoyneva-Gärtner, M.P.; Descy, J.P.; Uzunov, B.A.; Miladinov, P.; Stefanova, K.; Radkova, M.; Gärtner, G. Diversity of the Summer Phytoplankton of 43 Waterbodies in Bulgaria and Its Potential for Water Quality Assessment. Diversity 2023, 15, 472. [Google Scholar] [CrossRef]
- Ramón, J.; Sierra, A.; Newman, R.; Barinova, S.; Krupa, E.; Khitrova, Y. Phytoplankton Diversity and Bioindication of the Lakes in the Burabay National Natural Park, Northern Kazakhstan. Ecologies 2023, 4, 242–268. [Google Scholar] [CrossRef]
- Huo, S.; Li, X.; Xi, B.; Zhang, H.; Ma, C.; He, Z. Combining morphological and metabarcoding approaches reveals the freshwater eukaryotic phytoplankton community. Environ. Sci. Eur. 2020, 32, 37. [Google Scholar] [CrossRef]
- Unified Methods of Water Quality Research. Part 3. Methods of Biological Analysis of Waters. Appendix 1. Saprobity Indicators; Unoin for Mutual Economic Assistance, CMEA: Moscow, Russia, 1977; pp. 11–42.
- Tekebayeva, Z.; Temirbekova, A.; Bazarkhankyzy, A.; Bissenova, G.; Abzhalelov, A.; Tynybayeva, I.; Temirkhanov, A.; Askarova, N.; Mkilima, T.; Sarmurzina, Z. Selection of Active Microorganism Strains Isolated from a Naturally Salty Lake for the Investigation of Different Microbial Potentials. Sustainability 2023, 15, 51. [Google Scholar] [CrossRef]
- Kozlov, A.V.; Markova, D.S.; Bodyakshina, M.A.; Klochkov, E.A.; Zakharova, A.A.; Savinov, M.I. Ecology-Geochemical Characteristics of Lake Water of the Zavolzhye of the Nizhny Novgorod Region with the Status of Specially Protected Natural Areas. Adv. Curr. Nat. Sci. 2020, 11, 69–75. [Google Scholar] [CrossRef]
- Baturina, M.A.; Makarevich, O.A.; Kaygorodova, I.A.; Zhukova, T.V.; Adamovich, B. The role of annelida in the lakes of the naroch system (BELARUS). Int. J. Appl. Fundam. Res. 2018, 12-1, 56–59. [Google Scholar]
- Imant, E.N.; Novoselov, A.P. Qualitative and quantitative indicators of zooplankton in lakes lacha (arkhangelsk region) and golodnaya guba (nenets autonomous district). Int. J. Appl. Fundam. Res. 2018, 12-2, 226–271. [Google Scholar]
- Hanžek, N.; Gligora Udovič, M.; Kajan, K.; Borics, G.; Várbíró, G.; Stoeck, T.; Žutinić, P.; Orlić, S.; Stanković, I. Assessing ecological status in karstic lakes through the integration of phytoplankton functional groups, morphological approach and environmental DNA metabarcoding. Ecol. Indic. 2021, 131, 108166. [Google Scholar] [CrossRef]
- Gabyshev, V.A.; Gabysheva, O.I. To the study of the influence of concentration of organic and biogenic substances on the cenotic and floristic structure of mixotrophic phytoflagellate communities of large subarctic rivers of eastern siberia. Int. J. Appl. Fundam. Res. 2018, 6, 93–97. [Google Scholar]
- Matafonov, P.V. The spatial distribution of lithorial zoobentos in the lake arakhley in the low-water period. Int. J. Appl. Fundam. Res. 2018, 5, 180–184. [Google Scholar]
- The Sixth National Report on Biological Diversity in the Republic of Kazakhstan; Convention on Biological Diversity: Astana, Kazakhstan, 2018; Available online: https://www.cbd.int/doc/nr/nr-06/kz-nr-06-en.pdf (accessed on 4 November 2022).
- Krupa, E.G.; Barinova, S.S.; Romanova, S.M. Ecological Mapping in Assessing the Impact of Environmental Factors on the Aquatic Ecosystem of the Arys River Basin, South Kazakhstan. Diversity 2019, 11, 239. [Google Scholar] [CrossRef]
- Jiyenbekov, A.; Barinova, S.; Bigaliev, A.; Nurashov, S.; Sametova, E.; Fahima, T. Ecological diversity of algae in the alakol lake natural reserve, Kazakhstan. Bot. Pacifica 2019, 8, 63–74. [Google Scholar] [CrossRef]
- Krupa, E.; Barinova, S.; Aubakirova, M. Tracking pollution and its sources in the catchment-lake system of major waterbodies in Kazakhstan. Lakes Reserv. Res. Manag. 2020, 25, 18–30. [Google Scholar] [CrossRef]
- Krupa, E.G.; Barinova, S.S.; Romanova, S.M. Zooplankton Size Structure in the Kolsay Mountain Lakes (Kungei Alatau, Southeastern Kazakhstan) and Its Relationships with Environmental Factors. Water Resour. 2019, 46, 403–414. [Google Scholar] [CrossRef]
- Recreation Areas Are Organized Near the Lake Maybalyk, Taldykol and Maly Taldykol. Available online: https://profi.travel/news/30150/details (accessed on 4 November 2020).
- Sirenko, L.A.; Sakevich, A.I.; Osipov, L.F.; Lukina, L.F.; Kuzmenko, M.I.; Kozitskaya, V.N.; Velichko, I.M.; Myslovich, V.O.; Gavrilenko, M.Y.; Arendarchuk, V.V.; et al. Methods of Physiological and Biochemical Research of Algae in Hydrobiological Practice; Naukova Dumka: Kiev, Ukraine, 1975. [Google Scholar]
- Abakumov, V.A.; Sushchenya, L.M. Hydrobiological Monitoring of Freshwater Ecosystems and Ways to Its Improvement. Ekol. Modif. Kriter. Ekol. Normirovaniya 1991, 41–52. [Google Scholar]
- Wasser, S.P.; Kondratieva, N.V.; Masyuk, N.P.; Palamar-Mordvintseva, G.M.; Vetrova, Z.I.; Kordyum, E.I.; Moshkova, N.A.; Prikhodkova, L.P.; Kovalenko, O.V.; Stupina, V.V.; et al. Algae; Naukova Dumka: Kiev, Ukraine, 1989. [Google Scholar]
- AlgaeBase: Listing the World’s Algae. Available online: https://www.algaebase.org/ (accessed on 10 November 2023).
- Genkal, S.I.; Chekryzheva, T.A.; Komulaynen, S.F. Diatom Algae in Waterbodies and Watercourses of Karelia; Scientific World: Moscow, Russia, 2015. [Google Scholar]
- Melkumov, G.M. Algology: A Textbook for Higher Education Institutions; VSU: Voronezh, Russia, 2015. [Google Scholar]
- Sládeček, V. System of Water Quality from the Biological Point of View. Achieves Hydrobiol. Beih. Ergeb. Limnol. 1973, 7, 1–218. [Google Scholar]
- Adhiambo, R.; Mensah, P.K.; Acheampong, E. Widespread Geographical Disparities in Phytoplankton Ecology Research in the Face of Climate Change: A Review. Water 2023, 15, 4288. [Google Scholar] [CrossRef]
- Filiz, N.; Işkın, U.; Beklioğlu, M.; Öğlü, B.; Cao, Y.; Davidson, T.A.; Søndergaard, M.; Lauridsen, T.L.; Jeppesen, E. Phytoplankton Community Response to Nutrients, Temperatures, and a Heat Wave in Shallow Lakes: An Experimental Approach. Water 2020, 12, 3394. [Google Scholar] [CrossRef]
- Zhu, H.; Liu, X.G.; Cheng, S.P. Phytoplankton community structure and water quality assessment in an ecological restoration area of Baiyangdian Lake, China. Int. J. Environ. Sci. Technol. 2021, 18, 1529–1536. [Google Scholar] [CrossRef]
- Barinova, S.; Gabyshev, V.; Genkal, S.; Gabysheva, O. Diatoms of Small Water Bodies as Bioindicators in the Assessment of Climatic and Anthropogenic Impacts on the Coast of Tiksi Bay, Russian Arctic. Water 2023, 15, 1533. [Google Scholar] [CrossRef]
- Jiang, L.; Yao, Y.; Zhang, S.; Wan, L.; Zhou, Z. Effects of Stream Connectivity on Phytoplankton Diversity and Community Structure in Sunken Lakes: A Case Study from an August Survey. Diversity 2023, 15, 291. [Google Scholar] [CrossRef]
- Arsad, S.; Sihombing, R.S.P.; Mahmudi, M.; Luthfi, O.M.; Safitri, I.; Pratiwi, F.D. Benthic and Planktonic Microalgae Community in Probolinggo Beach. J. Aquac. Fish Health 2024, 13, 1–11. [Google Scholar] [CrossRef]
- Mesquita, M.C.B.; Graco-Roza, C.; de Magalhães, L.; Ger, K.A.; Marinho, M.M. Environmental Variables Outpace Biotic Interactions in Shaping a Phytoplankton Community. Diversity 2024, 16, 438. [Google Scholar] [CrossRef]
- de-los-Ríos-Mérida, J.; Guerrero, F.; Arijo, S.; Muñoz, M.; Gilbert, J.D.; Álvarez-Manzaneda, I.; Reul, A. Implications of Anthropic Activities in the Catchment Area of a Temporary Mediterranean Wetland Complex in the South of Spain. Sustainability 2024, 16, 1685. [Google Scholar] [CrossRef]
- MohanapriyaK, R.; Geetharamani, D. Fresh water Micro algal Diversity of Noyyal River at Tamil Nadu State, India. J. Algal Biomass Utln 2014, 5, 12–20. [Google Scholar]
- Severes, A.; Nivas, S.; D’Souza, L.; Hegde, S. Diversity study of freshwater microalgae of some unexplored water bodies of a rapidly developing industrial region in India. J. Algal Biomass Utln. 2018, 9, 31–40. [Google Scholar]

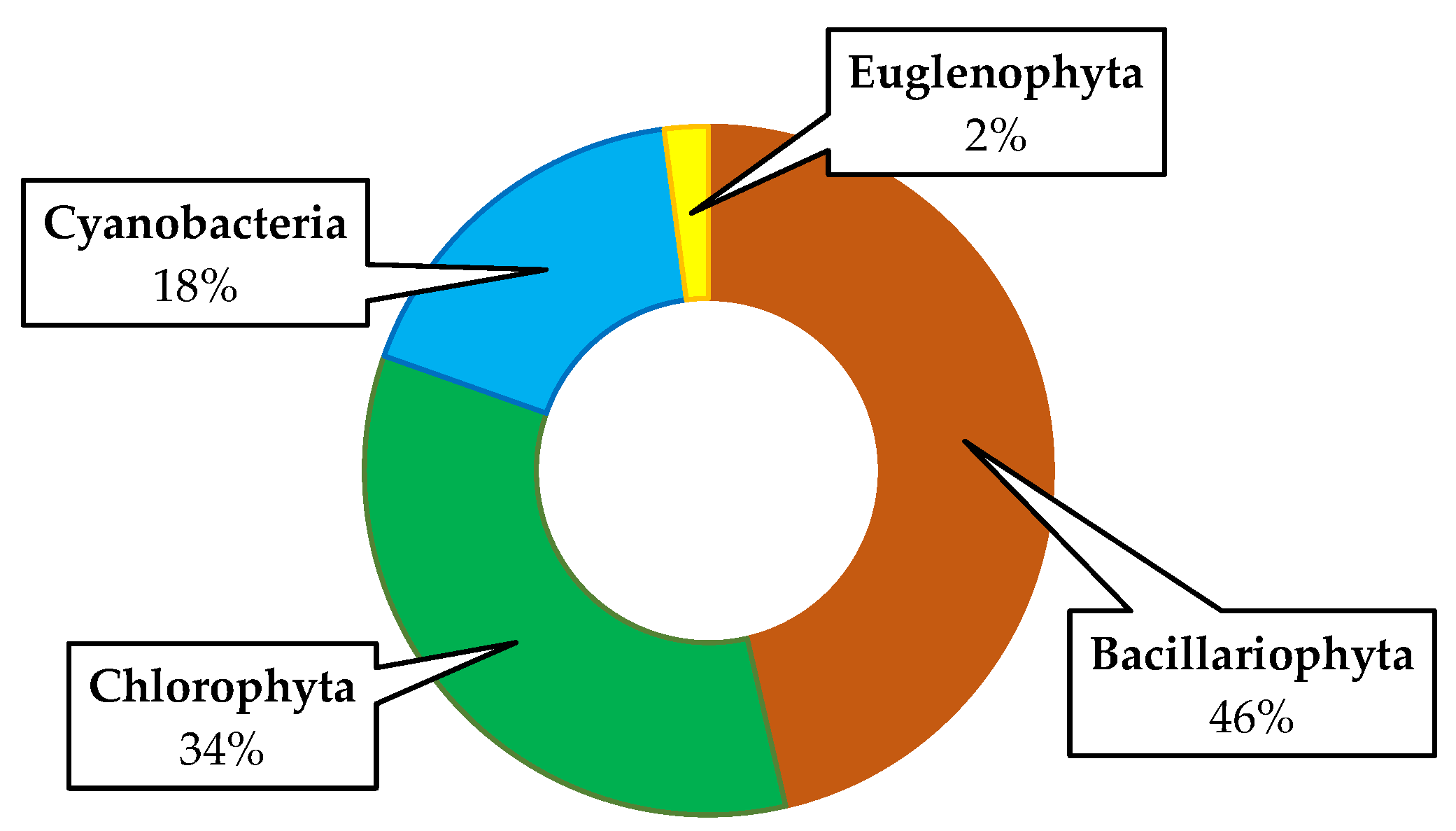
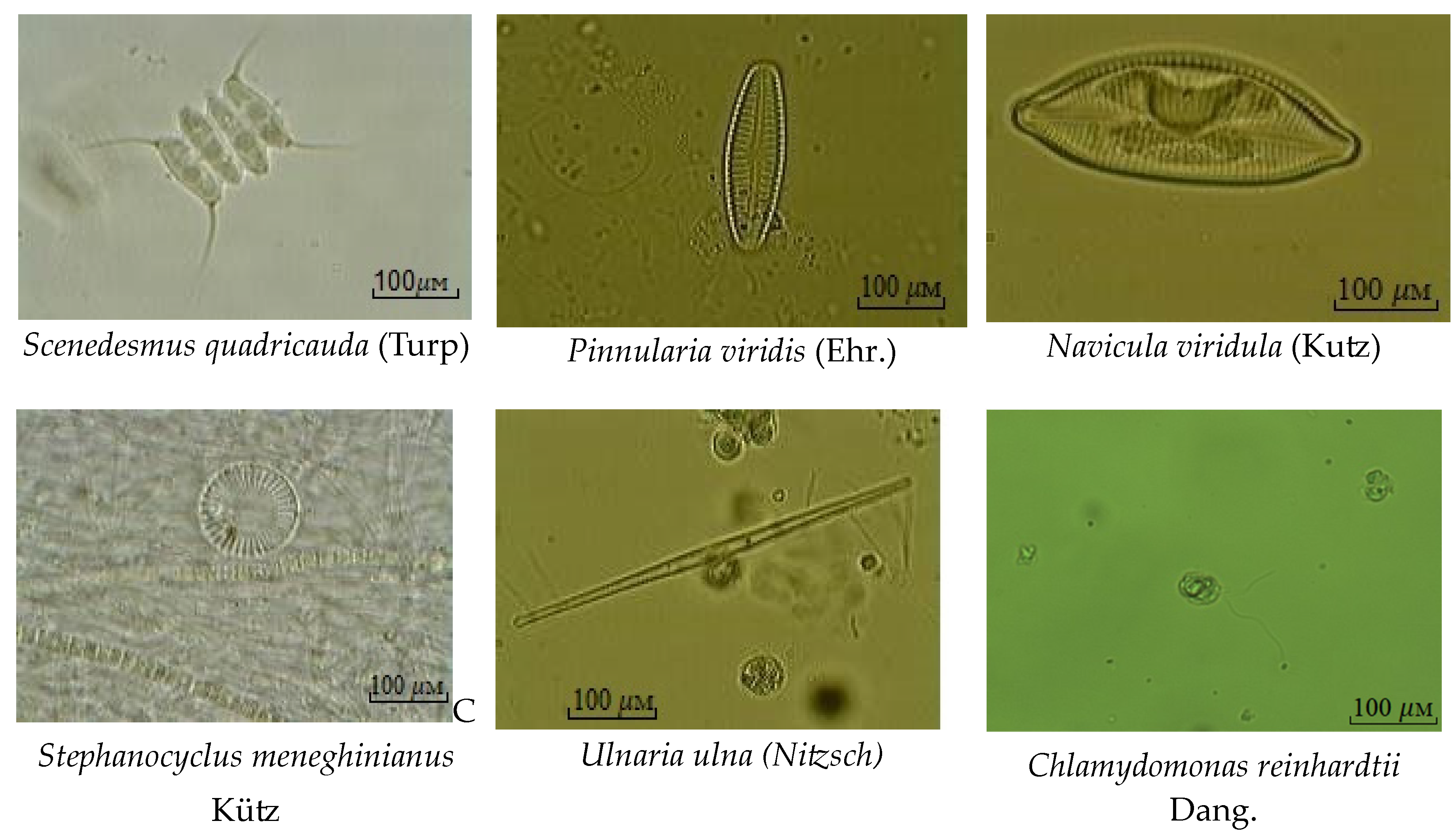
| №. | Name of the Algae | s | S |
|---|---|---|---|
| 1 | 2 | 3 | 4 |
| Bacillariophyta | |||
| 1 | Aulacoseira granulata Ehr. | β | 2.0 |
| 2 | Bacillaria paxillifera (Müll) | β | 2.8 |
| 3 | Caloneis amphisbaena Bory | β–α | 2.3 |
| 4 | Cocconeis pediculus Her | o-α | 1.8 |
| 5 | Cymbella aspera Ehr. | o-α | 1.8 |
| 6 | Diatoma vulgaris Bory | β | 2.2 |
| 7 | Diploneis elliptica (Kutz.) Cl. | o-χ | 0.6 |
| 8 | Fragilaria vaucheriae (Kutz.) Boye P | β–α | 2.2 |
| 9 | Gomphonema trigonocephalum Ehr. | o-β | 1.4 |
| 10 | Gomphonema constrictum Ehr. | β | 2.2 |
| 11 | Gyrosigma acuminatum (Kutz.) | o-α | 1.9 |
| 12 | Gyrosigma attenuatum (Kutz.) Raben. | o-α | 1.8 |
| 13 | Hippodonta capitata Ehr. | β–α | 2.4 |
| 14 | Melosira varians Ag | β | 2.1 |
| 15 | Neidiomorpha binodis Ehr. | β-o | 1.6 |
| 16 | Navicula cincta (Ehr.) Kutz. | χ-o | 0.5 |
| 17 | Navicula cryptocephala Kutz. | β | 2.1 |
| 18 | Navicula cuspidate Kutz. | α-β | 2.7 |
| 19 | Navicula salinarum Kolbe | β | 2.1 |
| 20 | Navicula viridula (Kutz) | β | 2.2 |
| 21 | Nitzschia acicularis W. Sm. | α | 2.4 |
| 22 | Nitzschia obtusa W. Sm. | β–α | 2.4 |
| 23 | Nitzschia palea (Kutz.) W. Sm. | α-o | 2.8 |
| 24 | Nitzschia tryblionella Hantz. | α | 2.7 |
| 25 | Pantocsekiella kuetzingiana (Thw) | β | 2.0 |
| 26 | Pinnularia major Kutz. var. major | β | 2.1 |
| 27 | Pinnularia viridis (Ehr) var. viridis | β | 2.1 |
| 28 | Placoneis gastrum Ehr. | β | 2.0 |
| 29 | Pleurosigma elongatum W. Sm. | β | 2.0 |
| 30 | Stauroneis legumen Ehr. | ο | 1.0 |
| 31 | Stephanocyclus meneghinianus Kütz | α-β | 2.8 |
| 32 | Surirella librile Ehr. | β | 2.2 |
| 33 | Synedra pulchella (Ralfs) Kutz. | β | 2.2 |
| 34 | Tabularia tabulata (Ag.) | β-α | 2.5 |
| 35 | Ulnaria acus (Kütz) | o-α | 1.85 |
| 36 | Ulnaria ulna (Nitzsch) | β | 2.0 |
| Chlorophyta | |||
| 37 | Ankistrodesmus acicularis (A.Br.) | β | 2.2 |
| 38 | Ankistrodesmus falcatus (Corda) Ralfs | β | 2.3 |
| 39 | Chlamydomonas proboscigera Korsch. | α | 3.1 |
| 40 | Chlamydomonas reinhardtii Dang. | α | 3.1 |
| 41 | Chlorella vulgaris Beijer. | α | 3.1 |
| 42 | Closterium gracile Breb. | o-β | 1.5 |
| 43 | Crucigenia quadrata Morren. | o-α | 1.9 |
| 44 | Crucigenia tetrapedia (Kirchn.) | β | 2.0 |
| 45 | Dictyosphaerium pulchellum Woodvar. pulchella | β | 2.3 |
| 46 | Hindakia tetrachotoma Printz. | β | 2.5 |
| 47 | Gonatozygon monotaenium de Bary | χ-β | 0.8 |
| 48 | Lagerheimia marssonii Lemm. | β | 2.1 |
| 49 | Neglectella solitaria (Wittr) | β-o | 1.7 |
| 50 | Pediastrum boryanum (Turp.) Menegh. | o-α | 1.9 |
| 51 | Tetradesmus lagerheimii M.J. Wynne | β | 2.2 |
| 52 | Acutodesmus acutiformis (Schröder) | o-α | 1.8 |
| 53 | Scenedesmus bijugatus (Turp) | β | 2.0 |
| 54 | Scenedesmus quadricauda (Turp) | β | 2.1 |
| 55 | Tetradesmus obliquus (Turpin) | α-β | 2.8 |
| 56 | Scenedesmus obtusus Meyen | β | 2.0 |
| 57 | Selenastrum bibraianum Reinsch. | β-o | 2.25 |
| 58 | Tetraedron minimum A. Br. Hansg. | β | 2.1 |
| 59 | Tetrastrum triacanthum Korsch. | β | 2.2 |
| 60 | Ulothrix subtilis Kütz. | β | 2.0 |
| Cyanobacteria | |||
| 61 | Dolichospermum flosaquae (Bornet and Flahault) | β | 2.0 |
| 62 | Aphanizomenon flos-aquae Ralf. | β | 2.2 |
| 63 | Aphanothece clathrata | β | 2.1 |
| 64 | Microcystis aeruginosa Kutz. | β | 2.1 |
| 65 | Nostoc carneum Ag. | β | 2.0 |
| 66 | Phormidium chalybeum (Mert.) Gom. | α | 3.0 |
| 67 | Spirulina jenneri Elenk. | α | 3.0 |
| 68 | Oscillatoria princeps Vauch. | α-ρ | 2.8 |
| 69 | Phormidesmis mollis (Gomont) | β | 2.0 |
| 70 | Spirulina subsalsa Oersted | o-β | 1.4 |
| Euglenophyta | |||
| 71 | Euglena viridis Ehr. var. viridis | α-ρ | 3.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tekebayeva, Z.; Bazarkhankyzy, A.; Temirbekova, A.; Rakhymzhan, Z.; Kulzhanova, K.; Beisenova, R.; Kulagin, A.; Askarova, N.; Yevneyeva, D.; Temirkhanov, A.; et al. Ecological Assessment of Phytoplankton Diversity and Water Quality to Ensure the Sustainability of the Ecosystem in Lake Maybalyk, Astana, Kazakhstan. Sustainability 2024, 16, 9628. https://doi.org/10.3390/su16229628
Tekebayeva Z, Bazarkhankyzy A, Temirbekova A, Rakhymzhan Z, Kulzhanova K, Beisenova R, Kulagin A, Askarova N, Yevneyeva D, Temirkhanov A, et al. Ecological Assessment of Phytoplankton Diversity and Water Quality to Ensure the Sustainability of the Ecosystem in Lake Maybalyk, Astana, Kazakhstan. Sustainability. 2024; 16(22):9628. https://doi.org/10.3390/su16229628
Chicago/Turabian StyleTekebayeva, Zhanar, Aidana Bazarkhankyzy, Aliya Temirbekova, Zhanar Rakhymzhan, Kamshat Kulzhanova, Raikhan Beisenova, Andrey Kulagin, Nurgul Askarova, Dinara Yevneyeva, Aslan Temirkhanov, and et al. 2024. "Ecological Assessment of Phytoplankton Diversity and Water Quality to Ensure the Sustainability of the Ecosystem in Lake Maybalyk, Astana, Kazakhstan" Sustainability 16, no. 22: 9628. https://doi.org/10.3390/su16229628
APA StyleTekebayeva, Z., Bazarkhankyzy, A., Temirbekova, A., Rakhymzhan, Z., Kulzhanova, K., Beisenova, R., Kulagin, A., Askarova, N., Yevneyeva, D., Temirkhanov, A., & Abzhalelov, A. (2024). Ecological Assessment of Phytoplankton Diversity and Water Quality to Ensure the Sustainability of the Ecosystem in Lake Maybalyk, Astana, Kazakhstan. Sustainability, 16(22), 9628. https://doi.org/10.3390/su16229628






