Bio-Inspired Eco-Composite Materials Seaweed Waste Integration for Sustainable Structural Applications
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Sample Development
2.3. Aging Test Conditions
2.4. Characterization Techniques
2.4.1. Weight Loss
2.4.2. Morphology Evaluation—Scanning Electron Microscopy (SEM)
2.4.3. Fourier Transform Infrared Spectroscopy (FTIR)
2.4.4. Mechanical Characterization—Flexural Test
3. Results and Discussion
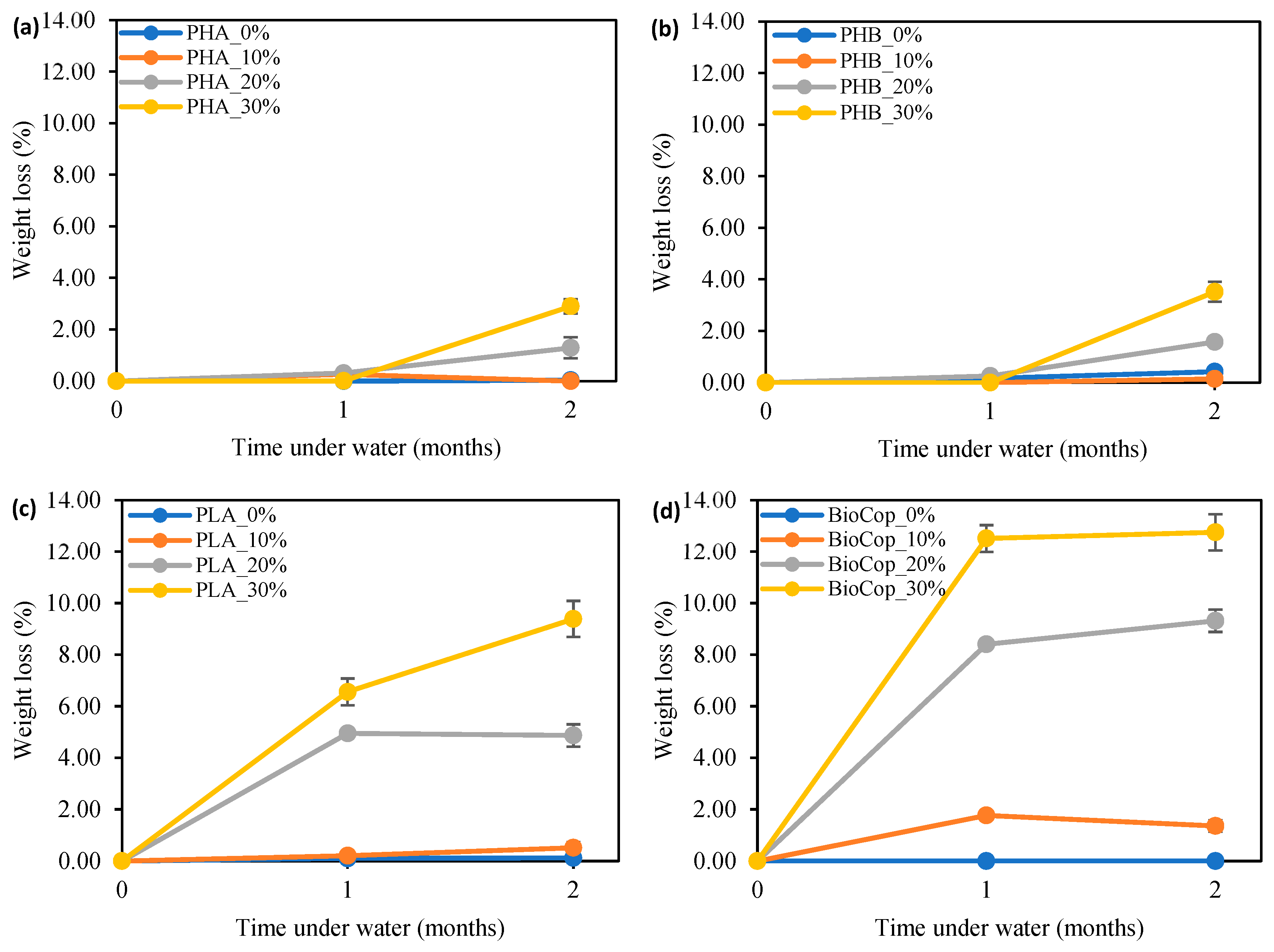
3.1. Weight Loss
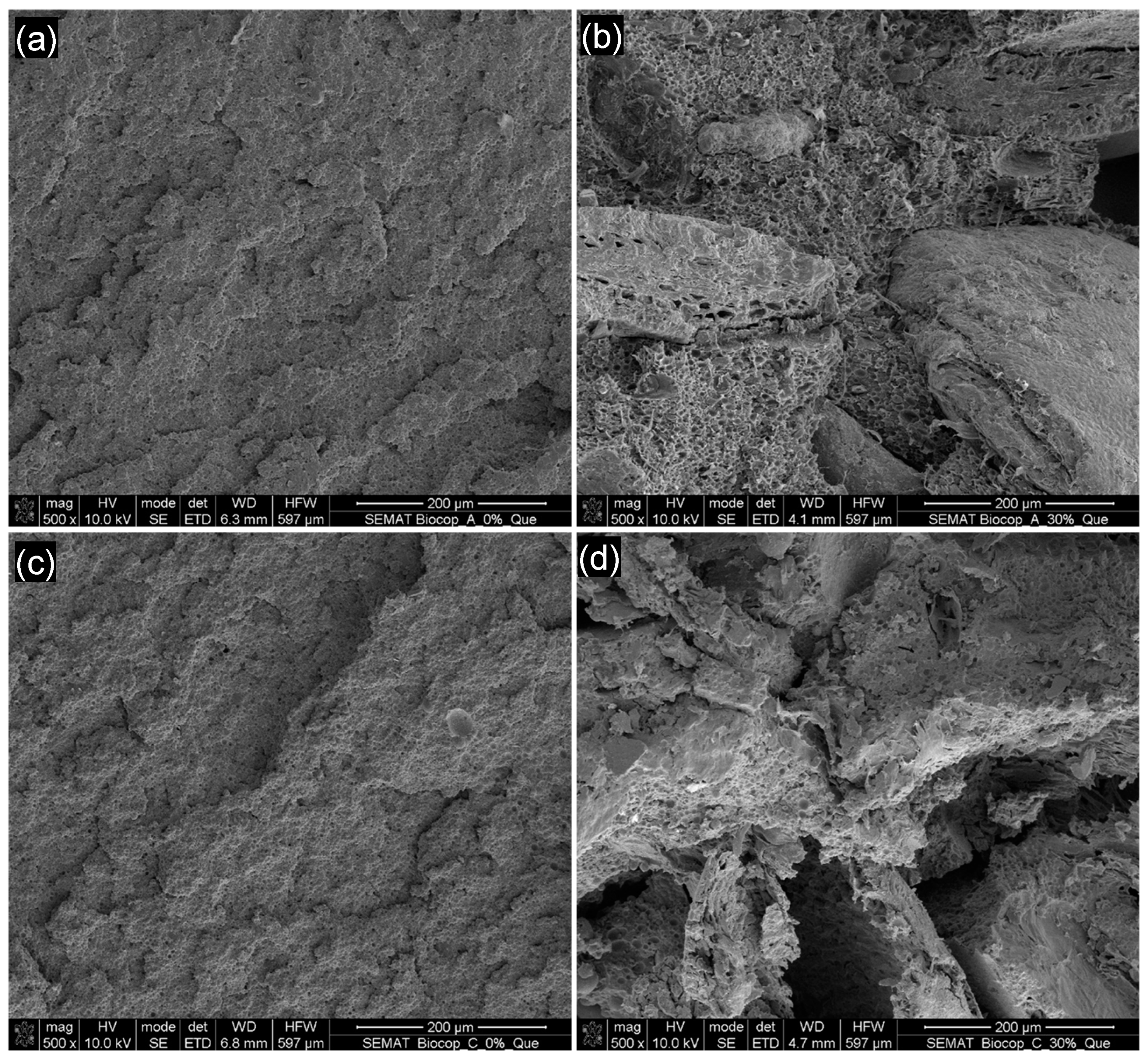
3.2. Morphology Evaluation
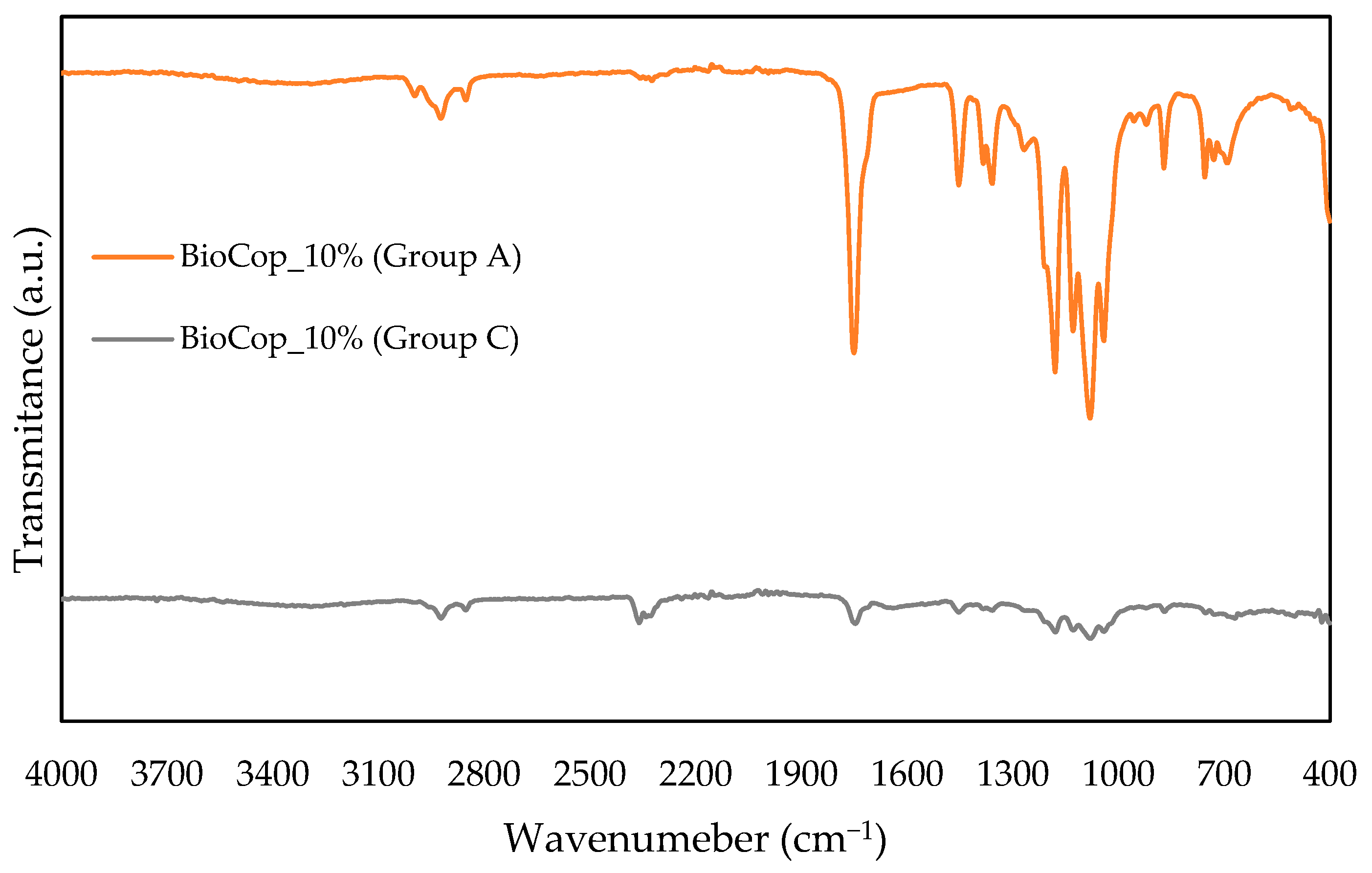
3.3. Chemical Evaluation—FTIR
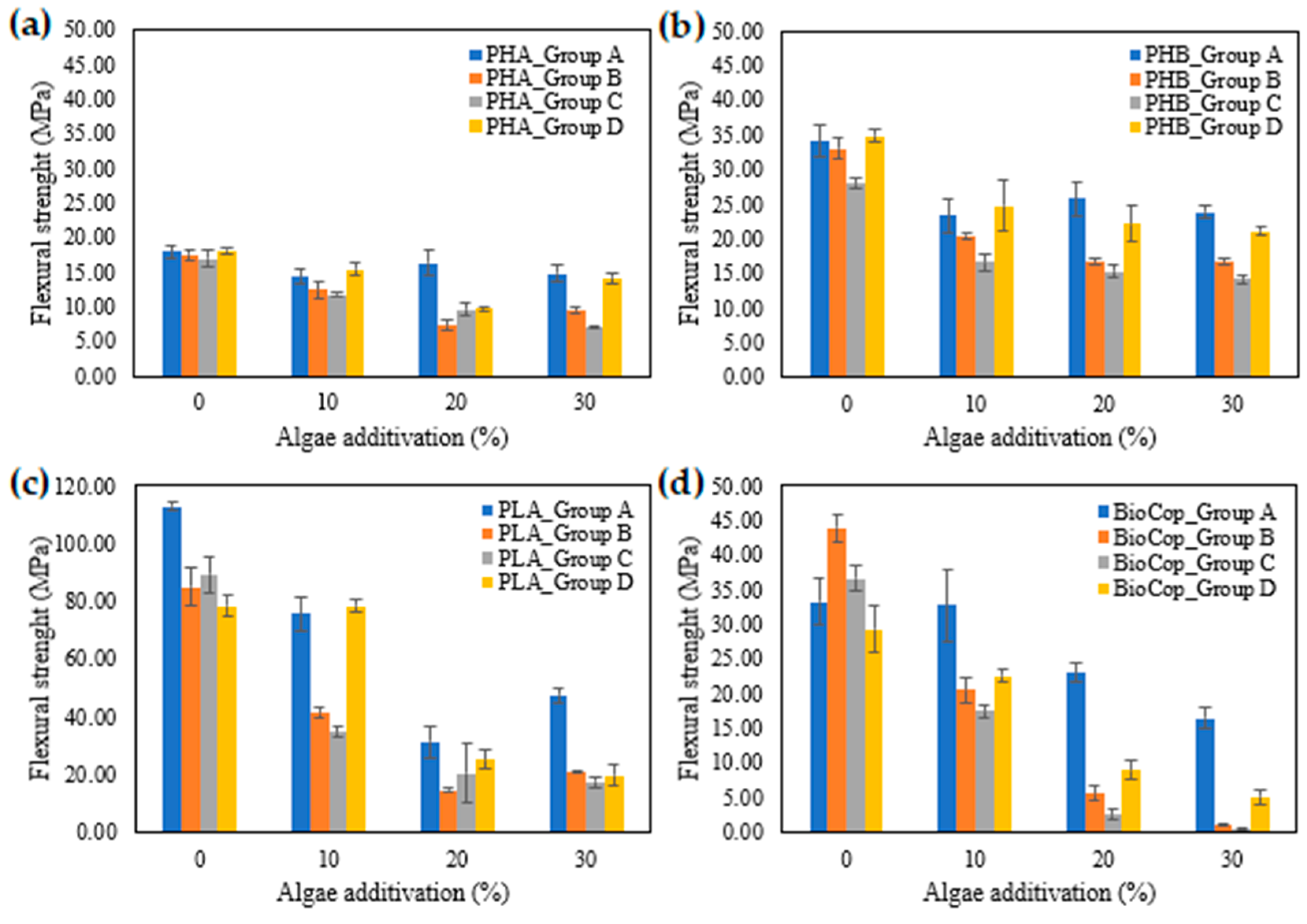
3.4. Flexural Test
4. Conclusions
- -
- Algae-infused materials, particularly those with higher algae content, showed greater mass loss. Notably, PLA- and BioCop-based composites experienced the most substantial weight loss after two months of seawater exposure.
- -
- Scanning Electron Microscopy (SEM) analysis indicated that an increase in algae content within BioCop compositions led to both surface and internal breakdown when immersed in seawater, especially for BioCop_30.
- -
- Chemical analysis through FTIR of the BioCop compositions revealed that while algae addition did not alter chemical bonds, it weakened the peak intensity, indicating bond weakening. Comparison of FTIR spectra before and after seawater exposure showed bond weakening or disappearance due to material degradation.
- -
- Flexural tests further demonstrated that PLA compositions exhibited higher mechanical resistance than other combinations, while increased algae content reduced flexural strength across all samples. Two months of seawater immersion contributed to a further reduction in flexural strength due to structural degradation.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, Z.; Ren, X. Impact of natural resources, resilient economic growth, and energy consumption on CO2 emissions. Resour. Policy 2024, 90, 104714. [Google Scholar] [CrossRef]
- Xiao, Q.; Zhao, Y.; Shen, S. The impact of environmental tournament on CO2 emissions from the perspective of synergizing the reduction of pollution and carbon emissions. Sci. Total Environ. 2024, 907, 168181. [Google Scholar] [CrossRef]
- Naguib, H.M.; Hou, G. Exploitation of Natural and Recycled Biomass Resources to Get Eco-friendly Polymer. J. Polym. Environ. 2023, 31, 533–540. [Google Scholar] [CrossRef]
- Liu, S.-Y.; Ren, B.; Li, H.-Y.; Yang, Y.-Z.; Wang, Z.-Q.; Wang, B.; Xu, J.-C. CO2 storage with enhanced gas recovery (CSEGR): A review of experimental and numerical studies. Pet. Sci. 2022, 19, 594–607. [Google Scholar] [CrossRef]
- Ashley, M.; Magiera, C.; Ramidi, P.; Blackburn, G.; Scott, T.; Gupta, R.; Wilson, K.; Ghosh, A.; Biswas, A. Nanomaterials and processes for carbon capture and conversion into useful by-products for a sustainable energy future. Greenh. Gases Sci. Technol. 2012, 2, 419–444. [Google Scholar] [CrossRef]
- Xue, W.; Wang, Y.; Chen, Z.; Liu, H. An integrated model with stable numerical methods for fractured underground gas storage. J. Clean. Prod. 2023, 393, 136268. [Google Scholar] [CrossRef]
- Geographic, N. Photosynthesis. National Geographic. Available online: https://education.nationalgeographic.org/resource/photosynthesis/ (accessed on 22 February 2024).
- Zhao, Y.; Tao, S.; Hu, T. Research advances on impacts micro/nanoplastics and their carried pollutants on algae in aquatic ecosystems: A review. Aquat. Toxicol. 2023, 264, 106725. [Google Scholar] [CrossRef] [PubMed]
- Environmental, F. lgae, Phytoplankton and Chlorophyll. Fundamentals of Environmental Measurements. 22 October 2014. Available online: https://www.fondriest.com/environmental-measurements/parameters/water-quality/algae-phytoplankton-chlorophyll/ (accessed on 22 February 2023).
- Tsai, D.; Chen, P.; Ramaraj, R. The potential of carbon dioxide capture and sequestration with algae. Ecol. Eng. 2017, 98, 17–23. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, X.; Chen, J. Carbon sequestration performance, enzyme and photosynthetic activity, and transcriptome analysis of algae-bacteria symbiotic system after antibiotic exposure. Sci. Total Environ. 2023, 902, 166486. [Google Scholar] [CrossRef]
- Goswami, R.K.; Mehariya, S.; Verma, P. Advances in microalgae-based carbon sequestration: Current status and future perspectives. Environ. Res. 2024, 249, 118397. [Google Scholar]
- Gad, N. Evaluation for biosorption of rare earth elements from gebel serbal granite using different types of algae. Acta Ecol. Sin. 2022, 42, 572–582. [Google Scholar] [CrossRef]
- Yang, Y.; Tang, S.; Chen, J.P. Carbon capture and utilization by algae with high concentration CO2 or bicarbonate as carbon source. Sci. Total Environ. 2024, 918, 170325. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, P.; Gururani, P.; Parveen, A. Algae: A promising and sustainable protein-rich food ingredient for bakery and dairy products. Food Chem. 2024, 441, 138322. [Google Scholar] [CrossRef]
- Gómez, I.; Huovinen, P. Lack of Physiological Depth Patterns in Conspecifics of Endemic Antarctic Brown Algae: A Trade-Off between UV Stress Tolerance and Shade Adaptation? PLoS ONE 2015, 10, e0134440. [Google Scholar] [CrossRef]
- Li, K.; Liu, Q.; Luo, R.; Lu, Q. Microalgae-based wastewater treatment for nutrients recovery: A review. Bioresour. Technol. 2019, 291, 121934. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhou, M.; Luo, J. Carbon dioxide sequestration accompanied by bioenergy generation using a bubbling-type photosynthetic algae microbial fuel cell. Bioresour. Technol. 2019, 280, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Hu, C.; Wang, J. A precise microalgae farming for CO2 sequestration: A critical review and perspectives. Sci. Total Environ. 2023, 2023, 166013. [Google Scholar] [CrossRef] [PubMed]
- Priyadharsini, P.; Nirmala, N.; Dawn, S. Genetic improvement of microalgae for enhanced carbon dioxide sequestration and enriched biomass productivity: Review on CO2 bio-fixation pathways modifications. Algal Res. 2022, 66, 102810. [Google Scholar] [CrossRef]
- Jiang, X.J.; Gao, Z.; Zhang, Q. Remote sensing methods for biomass estimation of green algae attached to nursery-nets and raft rope. Mar. Pollut. Bull. 2020, 150, 110678. [Google Scholar] [CrossRef]
- Kim, Y.-D.; Hong, J.-P.; Song, H.-I. Studies on technology for seaweed forest construction and transplanted Ecklonia cava growth for an artificial seaweed reef. J. Environ. Biol. 2012, 33, 969. [Google Scholar]
- Hariz, H.; Lawton, R.; Craggs, R. Effects of seeding method and single versus mixed species assemblages on the performance of Filamentous Algae Nutrient Scrubbers (FANS) for the treatment of agricultural drainage. Agric. Water Manag. 2023, 280, 108238. [Google Scholar] [CrossRef]
- Harnon, V.; Wolfrum, E.; Knoshaug, E.; Davis, R. Reliability metrics and their management implications for open pond algae cultivation. Algal Res. 2021, 55, 102249. [Google Scholar] [CrossRef]
- Kwon, G.; Cho, D.-W.; Park, J. A review of plastic pollution and their treatment technology: A circular economy platform by thermochemical pathway. Chem. Eng. J. 2023, 464, 142771. [Google Scholar] [CrossRef]
- Harris, P.; Maes, T.; Raubenheimer, K. A marine plastic cloud-Global mass balance assessment of oceanic plastic pollution. Cont. Shelf Res. 2023, 255, 104947. [Google Scholar] [CrossRef]
- Deville, A.; Ian, V.-R.; Ita-Nagy, D. Ocean-based sources of plastic pollution: An overview of the main marine activities in the Peruvian EEZ. Mar. Pollut. Bull. 2023, 189, 114785. [Google Scholar] [CrossRef] [PubMed]
- Mathis, J.; Gillet, M.; Disselkoen, H. Reducing ocean plastic pollution: Locally led initiatives catalyzing change in South and Southeast Asia. Mar. Policy 2022, 143, 105127. [Google Scholar] [CrossRef]
- Liotta, I.; Avolio, R.; Castaldo, R. Mitigation approach of plastic and microplastic pollution through recycling of fishing nets at the end of life. Process Saf. Environ. Prot. 2024, 182, 1143–1152. [Google Scholar] [CrossRef]
- Citterich, F.; Giudice, A.; Azzaro, M. A plastic world: A review of microplastic pollution in the freshwaters of the Earth’s poles. Sci. Total Environ. 2023, 869, 161847. [Google Scholar] [CrossRef] [PubMed]
- Cheah, W.; Er, A.C.; Aiyub, K. Current status and perspectives of algae-based bioplastics: A reviewed potential for sustainability. Algal Res. 2023, 71, 103078. [Google Scholar] [CrossRef]
- Cerbule, K.; Herrmann, B.; Petric, M. Use of biodegradable materials to reduce marine plastic pollution in small scale coastal longline fisheries. J. Nat. Conserv. 2023, 74, 126438. [Google Scholar] [CrossRef]
- Qiang, T.; Ren, W.; Chen, L. Biodegradable, high mechanical strength, and eco-friendly pectin-based plastic film. Food Hydrocoll. 2024, 149, 109539. [Google Scholar] [CrossRef]
- Chen, G.; Li, J.; Sun, Y. Replacing Traditional Plastics with Biodegradable Plastics: Impact on Carbon Emissions. Engineering 2023, 32, 152–162. [Google Scholar] [CrossRef]
- Younus, M.; Naguib, H.M.; Fekry, M.; Elsawy, M. Pushing the limits of PLA by exploring the power of MWCNTs in enhancing thermal, mechanical properties, and weathering resistance. Sci. Rep. 2023, 13, 16588. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Hao, X.; An, D.; Tillotson, M. Unveiling the potential for artificial upwelling in algae derived carbon sink and nutrient mitigation. Sci. Total Environ. 2023, 905, 167150. [Google Scholar] [CrossRef]
- Le, C.-Y.; Feng, J.-C.; Sun, L. Co-benefits of carbon sink and low carbon food supply via shellfish and algae farming in China from 2003 to 2020. J. Clean. Prod. 2023, 414, 137436. [Google Scholar] [CrossRef]
- Zhang, X.; An, L.; Tian, J. Microalgal capture of carbon dioxide: A carbon sink or source? Bioresour. Technol. 2023, 390, 129824. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Yang, T.; Dindoruk, B. Investigation the impact of methane leakage on the marine carbon sink. Appl. Energy 2024, 360, 122880. [Google Scholar] [CrossRef]
- Scherer, M.; Pinto, F.; Santos, P.; Costa, C. Integrated Coastal Zone Management: Preservation, adaptation, and monitoring. J. Integr. Coast. Zone Manag. 2021, 21, 5–9. [Google Scholar]
- ISO 178:2001; Plastic—Determination of Flexural Properties. European Standard: Brussels, Belgium, 2005.
- Deroiné, M.; Duigou, A.; Corre, Y.M.; Gac, P. Seawater accelerated ageing of poly(3-hydroxybutyrate-co-3-hydroxyvalerate). Polym. Degrad. Stab. 2014, 105, 237–247. [Google Scholar] [CrossRef]
- Shah, A.A.; Hasan, F.; Hameed, A.; Ahmed, S. Biological degradation of plastics: A comprehensive review. Biotechnol. Adv. 2008, 26, 246–265. [Google Scholar] [CrossRef] [PubMed]
- Sivan, A. New perspectives in plastic biodegradation. Curr. Opin. Biotechnol. 2011, 22, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Hadiyanto, H.; Adian, K.; Pratiwi, W. The effect of salinity on the interaction between microplastic polyethylene terephthalate (PET) and microalgae Spirulina sp. Environ. Sci. Pollut. Res. 2022, 29, 7877–7887. [Google Scholar] [CrossRef]
- Simões, A.V.B. Redução da Quantidade e do Tipo de Plástico Colocado no Mercado. Master’s Thesis, Universidade de Aveiro, Aveiro, Portugal, 2022. [Google Scholar]
- Palai, B.; Mohanty, S.; Nayak, S. A Comparison on Biodegradation Behaviour of Polylactic Acid (PLA) Based Blown Films by Incorporating Thermoplasticized Starch (TPS) and Poly (Butylene Succinate co Adipate) (PBSA) Biopolymer in Soil. J. Polym. Environ. 2021, 29, 2772–2788. [Google Scholar] [CrossRef]
- Bachchan, A.; Das, P.; Chaudhary, V. Effects of Moisture on the Structure and Properties of Natural Fiber Reinforced Polymer Composites. Mater. Today Proc. 2021, 49, 3403–3408. [Google Scholar] [CrossRef]
- Moll, V.; Beć, K.B.; Grabska, J.; Huck, C.W. Investigation of Water Interaction with Polymer Matrices by Near-Infrared (NIR) Spectroscopy. Polymers 2022, 27, 5882. [Google Scholar] [CrossRef]





| Polymer | Melting (°C) | Polymer:Algae Ratio (%) | Reference | Polymer | Melting (°C) | Polymer:Algae Ratio (%) | Reference |
|---|---|---|---|---|---|---|---|
| PHA | 175 | 0 | PHA_0 | PLA | 185 | 0 | PLA_0 |
| 10 | PHA_10 | 10 | PLA_10 | ||||
| 20 | PHA_20 | 20 | PLA_20 | ||||
| 30 | PHA_30 | 30 | PLA_30 | ||||
| PHB | 180 | 0 | PHB_0 | BioCop | 200 | 0 | BioCop_0 |
| 10 | PHB_10 | 10 | BioCop_10 | ||||
| 20 | PHB_20 | 20 | BioCop_20 | ||||
| 30 | PHB_30 | 30 | BioCop_30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barros, D.; Nobre, L.; Bessa, J.; Leite, L.; Mota, C.; Cunha, F.; Fangueiro, R. Bio-Inspired Eco-Composite Materials Seaweed Waste Integration for Sustainable Structural Applications. Sustainability 2024, 16, 11051. https://doi.org/10.3390/su162411051
Barros D, Nobre L, Bessa J, Leite L, Mota C, Cunha F, Fangueiro R. Bio-Inspired Eco-Composite Materials Seaweed Waste Integration for Sustainable Structural Applications. Sustainability. 2024; 16(24):11051. https://doi.org/10.3390/su162411051
Chicago/Turabian StyleBarros, Daniel, Luís Nobre, João Bessa, Liliana Leite, Carlos Mota, Fernando Cunha, and Raúl Fangueiro. 2024. "Bio-Inspired Eco-Composite Materials Seaweed Waste Integration for Sustainable Structural Applications" Sustainability 16, no. 24: 11051. https://doi.org/10.3390/su162411051
APA StyleBarros, D., Nobre, L., Bessa, J., Leite, L., Mota, C., Cunha, F., & Fangueiro, R. (2024). Bio-Inspired Eco-Composite Materials Seaweed Waste Integration for Sustainable Structural Applications. Sustainability, 16(24), 11051. https://doi.org/10.3390/su162411051









