Abstract
Agriculture plays a major role on society, especially in developing countries which rely on commodity exportation markets. To maintain high crop productivity, the use of agrochemicals was once employed as the main strategy, which in turn affected soil, water, and human health. In order to aid this issue, identifying some alternatives, such as the implementation of biofertilizers and inoculants as bioinputs in modern agriculture, are imperative to improve ecosystem quality. Among these bioinputs, a few bioproducts have shown good performances, such as phytohormones (e.g., auxins and giberellins), biosurfactants, and other enzymes; thus, it is extremely important to assure the quality and feasibility of their production in biorefinery scenarios. These bioproducts can be synthesized through fermentation processes through utilizing plant biomasses and agricultural byproducts as carbon sources. In this sense, to increase the tecno-economical availability of these processes, the implementation of solid-state fermentation (SSF) has shown great potential due to its ease of operation and cost-attractiveness. Therefore, this study aims to describe the main substrates used in SSF systems for the production of potential bioinputs; their associated operation hurdles, parameters, and conditions selection; the most suitable microorganisms; and the underlying mechanisms of these molecules in soil dynamics. Within this context, this study is expected to contribute to the development of new processes in modern biorefineries and to the mitigation of environmental impacts.
1. Introduction
Since the Upper Paleolithic, agriculture has evolved from primitive, almost “artisanal”, methods focused on individual or small-group feeding to mechanized methods involving the intensive use of synthetic inputs and even the adoption of genetic engineering in cultivars [1,2]. Currently, agriculture is the economic basis of several countries, especially developing ones, which comprise the main exporters of agricultural products. On the other hand, agriculture in the Contemporary Era, especially after the Green Revolution that began in the 1960s in the USA and Europe, has started to focus on productivity, income, and profit rather than subsistence, as was the case in the past [3].
Current agriculture has become more harmful than symbiotically helpful, establishing a destructive relationship with land in an attempt to increase productivity and profit [4]. In recent years, the adoption of synthetic agrochemical processes and products has been observed, and these synthetic processes and products contaminate soil, water sources, and the air, causing environmental imbalances ranging from pest invasions due to insects and microorganisms to climate change [5].
In recent years, sustainable agriculture has been presented as an alternative to avoid the problems previously reported, making the agricultural techniques and products used less harmful to the planet. The use of agricultural bioinputs has been well evaluated and increasingly explored among small- and medium-sized rural producers in several developed countries [6]. The global market size for bioinputs was estimated to be worth USD 8.8 billion in 2019 and is expected to grow at a CAGR of 13.6% to reach a value of USD 18.9 billion by 2025 [7].
Bioinputs are products based on microbial cells and metabolites, plant materials, organic or natural materials, and they are used in agriculture to combat pests and diseases, improving soil fertility and nutrient availability for plants. Furthermore, the foods produced from agricultural practices that use bioinputs are free from the agrochemical residues that are associated with the risk of neurodegenerative diseases and cancers. When compared to synthetic products, bioinputs have low toxicity and high biodegradability, which reduces environmental and human health impacts. Geopolitical and economic factors are also preponderant for the adoption of bioinputs in agriculture, since several commodity-producing countries import synthetic fertilizers that are essential for agricultural activity and food security from Canada and Russia, thus impacting trade balances. The most common examples of bioinputs that can be found on the market today are agricultural inoculants, biofertilizers, biosurfactants, phytohormones, and biological control agents. These bioproducts are obtained mainly by fermentative processes, generally by solid-state fermentation (SSF) [7,8].
SSF is an ancient technique that was first used in 2600 BC in Egypt before passing through Asia, Africa, and Europe. The technique was used in the production of some traditional foods and drinks, and from the mid-19th century onwards, it became popular in the Western world, being used in the production of enzymes, medicines, organic acids, foods, and products for agricultural use [9]. In this type of bioprocess, solid substrates, generally of natural origin, are used as a support or nutritional substrate in an environment with minimal water activity, allowing the microorganisms to grow and driving its metabolism to the target of interest [10]. As previously reported, several bioinputs used in agriculture are currently produced through SSF. The present literature review aims to describe the ways in which some bioinputs of agricultural interest are produced via SSF, the advantages and disadvantages of these bioprocesses, and the main challenges to improving these processes and increasing yields in order to reach a larger market and promote the development of sustainable agriculture.
2. Production of Agricultural Bioinputs by Solid-State Fermentation: General Concepts
Agricultural bioinputs are products based on microbial cells and metabolites, plant materials, or even animal materials that are used in agricultural production to combat insects and phytopathogenic microorganisms, improving soil fertility and maintaining plant fitness. Among the existing bioinputs, biofertilizers, inoculants, and biocontrol agents stand out. These biological compounds present numerous environmental advantages for farmers and consumers. The main advantages include greater sustainability with respect to the production process; reduced application costs; the greater biological fixation of nitrogen, phosphorus, and potassium micronutrients; and an increased production of substances that act on plant growth, immune modulators, and antimicrobials [11].
Different microorganisms can be used in the production of bioinputs, such as bacteria, filamentous fungi, and yeasts. Generally, the microorganisms are isolated from the plants themselves and can be classified as rhizospheric, epiphytic, or endophytic. Knowledge of the part of the plant from which the microorganism is isolated is important. Indeed, a microorganisms’ application in an inoculant formulation will highly depend on its origin, and inoculant formulations can improve the lifespan and proliferation of plants. The methods of application range from seed coating application, root application, and foliar application, as well as incorporation directly into the soil to improve nutrient availability. Examples of microorganisms that promote plant growth include bacteria of the genera Rhizobium, Azospirillum, Bacillus, Streptomyces, Gluconoacetobacter, and Pseudomonas and filamentous mycorrhizal fungi such as Claroideoglomus, Glomus, and Rhizophagus, which are used in the production of bioinoculants [12,13,14]. Recently, there has also been an increase in studies on yeasts such as those of the genera Candida spp., Rhodotorula spp., Cryptococcus spp., and Saccharomyces sp., which have been reported to promote plant growth [15].
With the aim of outlining lower-emission bioprocesses, combined with the sustainable and eco-friendlier nature of bioinputs compared to synthetic agrochemicals, the current focus of several studies is to realize the production of different plant growth promoters by utilizing renewable and low-cost feedstock in fermentation processes [16]. In this sense, solid-state fermentation (SSF) stands out for its versatility in terms of the use of raw materials. In fact, agricultural byproducts such as lignocellulosic biomasses, brans, and others can be used in SSF to produce a variety of bio-based products, including bioinputs [17,18,19]. SSF is commonly recognized as a process in which microorganisms are cultivated on insoluble solid substrates or in a solid matrix embedded with a limited availability of moisture (low water activity), allowing for microbial growth [20,21].
Despite the fact that a few drawbacks must still be overcome, in comparison to other techniques such as submerged fermentation (SmF), the conduction of fermentation processes under SSF exhibits attractive advantages, as summarized in Table 1. As highlighted by Manan and Webb [22], SSF offers several advantages in terms of techno-economic, environmental, and biological characteristics. However, when comparing SSF with submerged fermentation (SmF), it can be observed that SmF provides better control of environmental parameters, a broader spectrum of applications, and system homogeneity. Furthermore, these features ensure predictable and reliable product uniformity through different kinetic models and operation mode strategies [23,24,25,26]. Despite these disadvantages, SSF is still preferable for the production of agricultural bioinputs in on-farm mode due to its ease of installation, ease of maintenance for the farmer, and the lower costs associated with it.

Table 1.
Advantages and disadvantages of using SSF as a fermentation strategy for the production of bioproducts.
In SSF, the use of agricultural byproducts serves as a solid matrix (inert support or support) and/or substrate (nutrient source) for microbial fermentation, allowing for the generation of various biological-based products. The choice of which substrate to use is extremely dependent on microbial metabolic requirements such as the carbon and nitrogen balance and the balance of other micronutrients. The structure, chemical composition, and porosity of a substrate also influence microbial development; thus, an appropriate choice of material is fundamental to facilitate its growth. Due to these aforementioned properties, a few byproducts must be pretreated (e.g., lignocellulosic biomasses) in order to promote the fragmentation of recalcitrant components, the disruption of the fibers, and detoxification, hence facilitating enzymatic access to the substrate. Table 2 displays a variety of pretreatment techniques that are employed in current agricultural practice, including chemical, physical, physical–chemical, and biological techniques, and mainly used to treat lignocellulosic materials.

Table 2.
Types of pretreatments employed to treat lignocellulosic biomasses for use in fermentation processes.
The pretreatments used in lignocellulosic biomass aim to promote its fragmentation, increase its surface area and solubility, lower the cellulose crystallinity and lignin content, and increase the porosity of the cellulose fiber to facilitate microbial growth [28]. Note that the choice of the pretreatment process must take into account parameters such as efficiency, waste generation, energy requirements, and cost-attractiveness.
In SSF, in addition to the selection of supports/substrates, variables such as the standardization of particle size (granulometry), quantity and age of inoculum, specific additives, pH, and process temperature must also be considered [22]. Another parameter of fundamental importance for the performance of the SSF is the selection of the bioreactor, including its geometry and configuration, which influence the mass and energy transfer of the system. The main types of bioreactors for SSF are tray bioreactors, packed beds, air-solid fluidized bed stirred bioreactors, and rotating drum bioreactors. Tray fermenters are the most commonly used bioreactors for SSF due to their simple design [29].
Given the world’s current socioeconomic and environmental scenario, there is an urgent need to adopt sustainable processes and products aimed at developing bioeconomy. Therefore, several bioproducts can be synthesized using lignocellulosic biomasses in biorefineries, meeting the requirements for more ecological practices. In the following sections, some important agricultural bioinputs, as well as their characteristics and production parameters in the context of SSF, are presented.
3. Inoculants and Biological Controlling Agents as Bioinputs
The advancement of green and circular economy concepts worldwide has intensified the search for sustainable and highly efficient products that can replace synthetic products derived from petroleum and mineral extraction. Recently, in the agricultural sector, the excessive use of NPK fertilizers (nitrogen, phosphate, and potassium compounds) and synthetic pesticides has shown the harmful effects of these toxic compounds in the reduction of the biodiversity present in soils, as well as the contamination of water sources, groundwater, and food, causing several health problems for organisms living on the planet [30,31]. An alternative to replace these synthetic products and restore the balance of soil biodiversity is the use of inoculants and biological control agents [32].
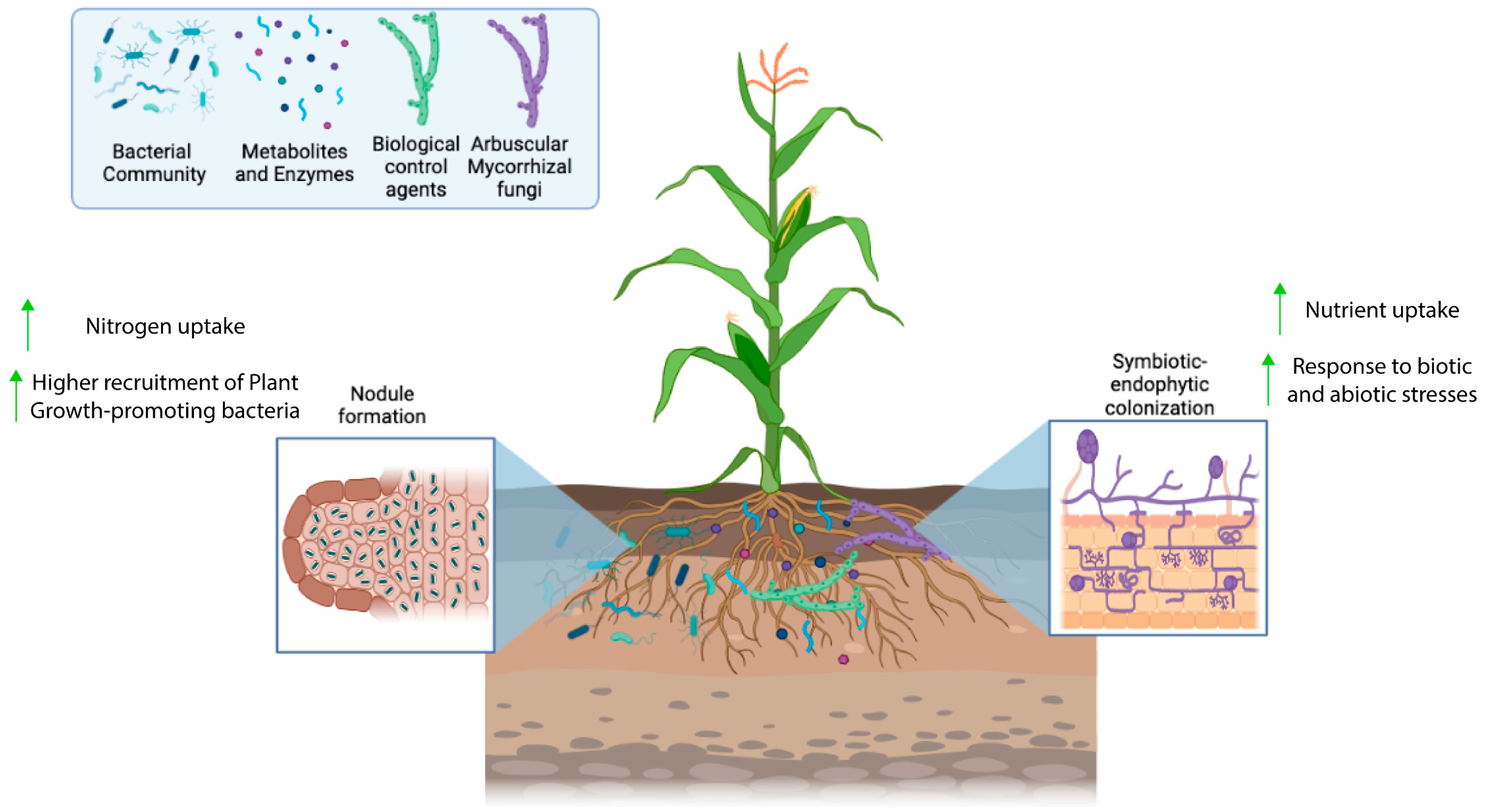
Inoculants are biological products composed of living microorganisms that benefit the development of different plant species, increasing the availability of nutrients for plants and improving the absorption of nutrients by plants. They also induce plant immunity and favor soil quality [33,34]. The microorganisms used in the preparation of inoculant formulations are known as plant growth promoters, examples of which include bacteria and fungi which can be found adhered to the roots (rhizospheric), inside plant tissues (endophytic), or in plant leaves. These microorganisms stand out for being nitrogen fixers (diazotrophs); phosphate solubilizers; and producers of siderophores, phytohormones, antimicrobial compounds, and enzymes. Examples of microorganisms used in inoculant formulations include bacteria of the genera Burkholderia, Pantoea, Enterobacter, Pseudomonas, Massilia, Sphingobium, Sphingomonas, Agrobacterium, Rhizobium, Bradyrhizobium, and Ochrobactrum and fungi of the genera Penicillium and Mycorrhiza [12,35,36]. A schematic representation of the interactions among those microorganisms occurring in the rhizosphere is exhibited in Figure 1.
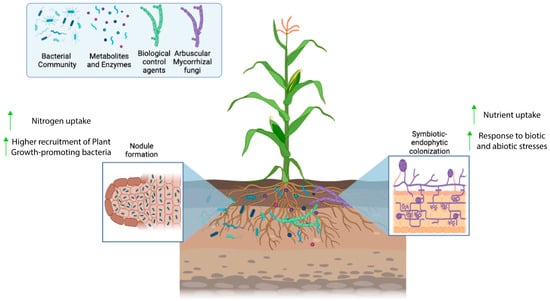
Figure 1.
Biological processes occurring in the rhizosphere, including the growth and colonization of the plant roots by different types of microorganisms.
Biological control agents are bioproducts composed of micro or macroorganisms that act to reduce the biotic and abiotic factors that may negatively interfere in agricultural production [37]. They are used in important methods for protecting plants against attacks by arthropod pests and phytopathogenic microorganisms, proving to be effective without inflicting ecological damage [38]. This study will focus on the production of microbial biological control agents or bioinsecticides, more specifically those composed of entomopathogenic bacteria and fungi, which infect insect pests and have antagonistic activity against phytopathogenic microorganisms.
Among the most common formulations of these biological control agents on the market, those containing bacteria from the genera Agrobacterium, Bacillus, Pseudomonas, Streptomyces, and Paenibacillus and also fungi from the genera Trichoderma, Metarhizium, and Beauveria are some examples of effective and well-studied agents that act against several plant diseases [12,16]. Biological control agents may act under hyperparasitism, competition, the secretion of lytic enzymes, and plant growth promotion, inducing systemic host resistance and antibiosis [39].
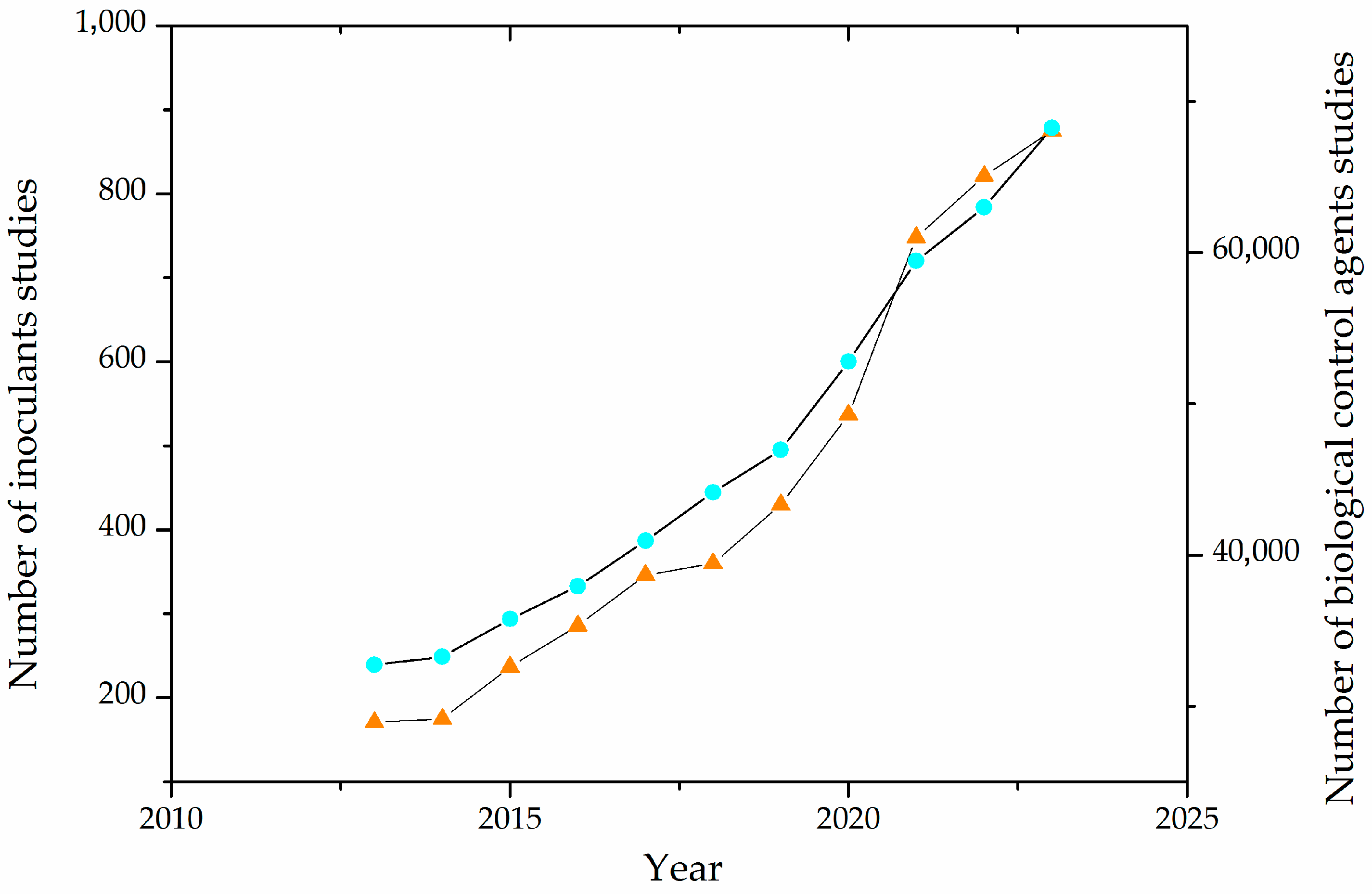
In recent years, there has been an increasing number of works regarding the production and effects of inoculants and biological control agents, as depicted in Figure 2.
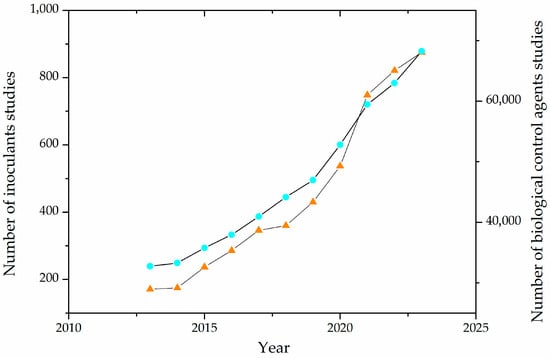
Figure 2.
Evolution of the number of publications on agricultural inoculants and biological control agents in the last decade. The light blue circles (○) represent studies of biological control agents, and the orange triangles (Δ) represent studies of inoculants.
From a technological point of view, the increasing study of inoculants and biological control agents is due to the advancements in fermentative processes and molecular biology techniques, the development of biorefinery concepts, and the imperative need for more sustainable agricultural inputs.
The main steps involved in the bioprocess to obtain inoculant formulations and biological control agents consist of the selection of suitable microbial strains; the characterization of their morphological, physiological, and biochemical properties; the evaluation of fermentation conditions (optimization of nutritional and physical parameters); and the development of biopinputs using cheap reagents, adjuvants, and vehicles which will stabilize the bioactive agents in the formulation.
Aiming to reduce production costs, combined with the sustainable concepts of biorefineries and green and circular economies, lignocellulosic biomass has started to be used as a raw material in the development of inoculant formulations and agricultural biological control agents, being considered a viable alternative.
Production of Inoculants and Biological Control Agents by SSF
Inoculants had a global market share of approximately USD 1.0 to USD 2.3 billion in 2020, and their market share is expected to reach USD 3.9 billion by 2025, with a CAGR ranging between 11 and 12.8%. Biological control agents currently have a market worth approximately USD 6.6 billion, and this market worth is expected to grow at a CAGR of 15.8% to reach USD 13.7 billion by 2027. The leading global inoculant and biological control agent manufacturers are Novozymes (Frederiksberg, Denmark), BASF SE (Ludwigshafen am Rhein, Germany), Premier Tech (Boizenburg, Germany), Bioceres Crop Solutions (Rosario, Argentine), Marrone Bio Innovations Inc. (Davis, CA, USA), Bayer CropScience AG (Monheim am Rhein, Germany), and Valent Biosciences (Libertyville, IL, USA) [40,41].
With the advancements in the use of inoculants and biological control agents, the productivity of these compounds is one of the main issues that need to be evaluated for their large-scale production and global implementation. The steps that need to be taken to choose the proper microbial strain in the bioinput strikingly rely on the cultivar species, the type of disease, and the desired effect upon the system, ranging from the induction of innate plant immunity and the direct antagonism of the pathogen to the production of enzymes or improvement of soil quality. Subsequently, process conditions and substrate composition alter the dynamics of microbial metabolism; thus, the microbial strain must be cautiously selected in order to drive the production to either biomass or metabolite excretion. Both strategies contribute to the suppression of pathogens; however, the best option must be examined in situ and will depend on the prevalence of their positive effects over time or over the cultivation period.
Regarding substrate type, medium composition influences microbial growth and metabolite production. The main carbon sources assimilated by microorganisms used in inoculant and biological control formulations are sugars and polyols such as glucose, sucrose, xylose, lactose, glycerol, mannitol, and others. These nutrients can be found in different substrates. Considering the cost and availability of raw materials and substrates, agro-industrial byproducts such as lignocellulosic biomasses mainly composed of a carbohydrate portion rich in cellulose and hemicellulose stand out. As previously reported, lignocellulosic materials are promising nutritional sources that can be used in SSF for the production of bioinputs such as inoculants and control agents. In addition to supports/substrates, lignocellulosic materials can also be used as vehicles for inoculant formulations and biological control agents [42].
Zhao et al. [43] reported the production of Rhizobium leguminosarum biomass by SSF using fresh wheat straw and charcoal as supports. In SSF with wheat straw, it was observed that after 72 h of cultivation, there was approximately a thousand-fold increase in the number of viable cells, starting from an initial concentration of 7.3 to approximately 10.0 log cfu/g substrate. When charcoal was used, after 72 h of cultivation, a 10-fold increase in the number of viable cells was observed. The results obtained in this study showed that although charcoal is known as an excellent inert support for biofertilizer production, wheat straw presented a better performance.
Bacillus thuringensis is a type of bacteria present in the soil that produces certain types of proteins, known as Cry, with an insecticidal effect on Lepidoptera, Coleoptera, and Diptera; thus, it is normally used as a potential bioinsecticide. Within this context, Molina-Peñate et al. [44] reported on the use of solid waste treated with lytic enzymes (e.g., cellulases, hemicellulases, pectinases, and amylases) as raw material for the mass growth of Bacillus thuringiensis in SSF. After 100 h of cultivation, approximately 108 viable cells/g of substrate and 108 spores/g of substrate were obtained. The presence of Cry protein crystals, essential for insecticidal action, was also observed. Accordingly, this strategy proved to be viable on the scale at which it was carried out, being an alternative process with a value-added bioinput for future biorefineries.
There are also several studies that have exploited the use of corncobs, food waste, rice husk, and coconut husks as substrates/supports for the production of cellular biomass aiming towards biological pest control [16]. Table 3 shows more examples of renewable and lignocellulosic substrates/supports used in the production of inoculants and biological control agents by SSF.

Table 3.
Renewable substrates used in SSF for the production of sustainable inoculants and biological control agents as agricultural bioinputs.
Some authors also consider inoculants and biological control agents as biofertilizers, as in addition to microorganisms, these formulations can also contain substances such as organic acids, amino acids, vitamins, enzymes, micronutrients, and phytohormones, which promote plant growth [53]. Thus, in Table 4 these mentioned substances are classified according to their function upon the stimulation of growth.

Table 4.
Classification of bioinputs according to their major functions as exhibited in inoculants and biological control agents.
In addition to the studies cited in Table 3, there are also works that describe the production of biofertilizers using lignocellulosic residues under anaerobic digestion (AD) to generate biogas [60]. In AD, organic matter is degraded and stabilized by microbial action in an anoxic environment, generating biogas as the main product [61]. At the end of AD, organic acids (acetic acid, propionic acid, butyric acid, caproic acid, and valeric acid), solvents (acetone), and digestate (solid material resulting from the process) are also observed as byproducts [62]. The digestate can be used as a biofertilizer for agricultural crops, replacing mineral fertilizers. The application of digestate as a biofertilizer depends on its physicochemical composition. Its composition is highly variable depending upon the feedstock, pretreatment process, inoculums used, and AD operating conditions like pH and temperature. Commonly, it is characterized by an alkaline pH; N–NH4+ (46.2–79%); macronutrients such as potassium (28–95 g/kgDM), phosphorus (8–42 g/kgDM), sulfur (2.9–14.7 g/kgDM); and trace elements such as cobalt, iron, and selenium [63].
Regarding the production of biofertilizers, Vassilev et al. [64] demonstrated the feasibility of using solid beet residues for the growth of microbial biomass of Aspergillus niger, which, by producing organic acids during the fermentation process, solubilized the natural phosphate, making it available for the plant.
Ajala et al. [65] showed that A. niger, when cultivated on cassava peel by SSF, produces a concentration of citric acid three-fold higher in comparison to SmF. Devi and Sumathy [66] evaluated the production of biofertilizers in SSF using different fruit residues (papaya, watermelon, pineapple, custard apple, and guava). Microorganisms isolated from soil samples were evaluated, and the growth of Pennisetum glaucum was observed upon their application. After microbial growth, the plants were inoculated with fermented custard apple, guava, and watermelon. Greater seed germination and more pronounced elongation regarding the roots and shoots were observed in the plants treated with fermented solutions compared to the control plants.
Solid biofertilizers obtained by SSF from lignocellulosic biomass when applied to the soil are also important for the formation of humic substances. These substances have long been recognized as the most widely distributed organic component on the planet. Humic substances are formed mainly from the biological degradation of plant and animal residues. Their structures have an abundance of carbonyl and phenolic groups that contribute to their complexation and have amphipathic characteristics that can bind to mineral soil surfaces [67,68,69,70]. The main types of humic substances found in soil are fulvic acids, humins, and humic acids, which act at different pH. Humic substances contribute to heat retention in the soil, which stimulates seed germination and plant root development; they act against erosion, preventing runoff due to the fact that they contain aggregates from combining with clays, enabling a better water retention capacity [71]. When the solid biofertilizer obtained by SSF, still containing a significant lignin content, comes into contact with soil microorganisms such as rhizospheric bacteria, it will be degraded by the action of phenoloxidases and enzymes that produce hydrogen peroxide. The resulting phenolic compounds will be precursors of humic substances. Some studies have demonstrated the efficient production of humic acids by SSF using lignocellulosic agro-industrial byproducts such as raw empty fruit bunch and rice straw fibers [72,73].
In addition to lignocellulosic substrates, other materials can be used in the production of inoculants, biological control agents, and biofertilizers. Examples include cheese whey, beetroot and sugarcane molasses, glycerol, solid residues from olive oil mills, and vinasse, which can be used in SSF as a substrate/support or moistening solution [21,64,74,75].
In addition to inoculants and biological control agents, other bioinputs such as biosurfactants and phytohormones can also be produced by SSF, as reported in the next sections.
4. Biosurfactants: Promising and Emerging Agricultural Bioinputs
Biosurfactants (or biological surfactants) are microbial metabolites with an amphipathic structure that have outstanding physicochemical and biological properties such as the ability to reduce surface tension, emulsifying capacity, and antimicrobial potential. According to the biochemical groups present in their structures, biosurfactants can be classified as glycolipids, lipopeptides, lipoproteins, phospholipids, and similar molecules [76]. Glycolipids and lipopeptides are the most common microbial biosurfactants.
Glycolipids are formed by the union of sugars (monosaccharides and polyols) and lipids, and they have molar masses between 476 and 848 Da and critical micellar concentrations (CMCs) between 20 and 366 mg/L [77,78]. Among the most common biosurfactant glycolipids are rhamnolipids, produced by bacteria, and sophorolipids, produced by yeast.
Lipopeptides are biosurfactants formed by the union between peptides and lipids and have molar masses greater than 1000 Da; due to this, they are called high-molar-mass biosurfactants, and they have CMCs around 10μmol/L or 23 mg/L. Surfactin is the lipopeptide produced by some bacteria best described in [77].
In agriculture, microbial biosurfactants are commonly used as emulsifiers in agrochemical formulations. However, in recent years, with the search for “clean” methodologies for more sustainable agricultural production, it has been observed that biosurfactants can also be used as antimicrobials, germinating agents, and growth stimulants.
Due to their antimicrobial properties, in recent years biosurfactants have been the target of agronomic studies aiming to use them as biopesticides to control pathogens in crops. The antimicrobial activity of biosurfactants is due to their membranotropic effects. Upon contact with pathogen cells, biosurfactants cause the permeabilization of the target cell through interacting with lipids and membrane proteins, causing cell disruption and death. The interaction mechanisms of the interactions between biosurfactants and membrane molecules involve three processes: (I) a change in membrane hydrophobicity through the intermolecular interactions between different functional groups present in biosurfactants, lipids, and proteins [79]; (II) the fluidization of the cell membrane, which alters the balance between anchored saturated and unsaturated fatty acids [80,81]; (III) increasing the exchange of charge between the anchored molecules and thus opening pores and channels that, in turn, favor greater permeabilization of the cell membrane [82,83]. In turn, these aforementioned mechanisms cause the efflux of bioactive intracellular compounds such as enzymes, lipopolysaccharides (LPS), polypeptides (PP), and functional effectors, namely Microbial Associated Molecular Patterns (MAMPs), which could stimulate or “sensitize” plants, leading to a faster response of systemic resistance to pathogens [84].
Still, regarding their agricultural applications, there are also reports that biosurfactants produced by some endophytic microorganisms act as protective molecules, acting significantly on growth and against some biotic and abiotic factors [85,86].
Production of Biosurfactants in SSF
Despite being molecules of fundamental importance for the industrial and agricultural sectors, the production costs of biosurfactants are still high. This fact is due to the raw materials used and the purification processes adopted [86]. In recent years, the use of agro-industrial byproducts for the production of biosurfactants has increased. Various lignocellulosic materials can be used in the production of biosurfactants via SSF, as can be seen in Table 5.

Table 5.
Different approaches for the production of different types of biosurfactants via SSF using renewable feedstocks.
As previously reported, obtaining biosurfactants by SSF is still carried out on small scales, with only 2% of studies reporting a yield of between 5 and 10 kg of bioproduct. Furthermore, 62% of the studies on obtaining biosurfactants by SSF reported the production of glycolipids (sophorolipids, rhamnolipids, and others) and lipopeptides (surfactin, iturin, and others), and 40% of the studies reported the use of packed beds or porcelain fully aerated and agitated bioreactors [98].
When analyzing the advantages of obtaining biosurfactants by SSF when compared to SmF, a greater production of biosurfactant by SSF is observed. However, disadvantages such as the complexity in establishing mathematical models, as well as the difficulties in purification and scaling up, show that there are still many advances to be achieved in this promising bioprocess for industrial application, considering sustainable concepts for a green economy.
Glycolipids are a type of biosurfactant with antifungal, larvicidal, and mosquitocidal properties, thus acting as agricultural defenders against pests and fungal infections [99,100]. Glycolipid-based biopesticides are applied to agricultural crops to control phytopathogenic bacteria, fungi, and pests [101].
The antibiotic properties of biosurfactants have been explored for some time and are well known. Antifungal properties stand out, since there is a more limited number of active ingredients for the treatment of these phytopathogens when compared to antibiotics. It is known that fungi such as mold/white rot (e.g., Sclerotinia sclerotiorum, Penicillium digitatum, Penicillium italicum) and black rot (e.g., Alternaria citri), among others, cause major losses for leguminous crops such as soybeans, beans, and citrus fruits. The routine use of a variety of synthetic fungicides, such as triazoles, triazolinthione, carbamates, dithiocarbamates, organochlorines, and organophosphates, has caused several environmental problems, such as the pollution of soil, groundwater, and water sources. Therefore, the adoption of biosurfactants with fungicidal effects proves to be a sustainable alternative for agricultural production.
Hultberg et al. [102] demonstrated the antifungal effect of biosurfactants produced by Pseudomonas fluorescerans against Pythium ultimum (causative agent of the damping off and root rot of plants), Fusarium oxysporum (causes wilting in crop plants), and Phytophthora cryptogea (causes rotting of fruits and flowers). Pseudomonas sp. have been reported as biocontrol agents against Verticillium microsclerotia, a causative agent of Verticillium wilt mainly in potatoes. The biosurfactant produced by this Pseudomonas sp. is considered to play a major role in the inhibition of the in vitro viability of Verticillium sp. [103].
Nalini and Parthasarathi [96] demonstrated the action of rhamnolipids produced by Serratia rubidaea SNAU02 as a biocontrol agent. In this study, the authors evaluated the use of mahua cake as a substrate for biosurfactant production via SSF. The rhamnolipid produced showed antifungal activity against Fusarium oxysporum and Colletotrichum gloeosporioides. The study suggested that this biosurfactant forms ion channels in the plasma membrane of the microorganism, creating pores in the membrane layer that affect the cell surface of plant pathogenic fungi.
The action of a rhamnolipid produced by Pseudomonas aeruginosa Tr20 in combination with a compatible strain of Trichoderma lixii TvR1 was also evaluated for the control of fusariosis and early blight in plants. Through these in vivo activities against the pathogens Fusarium oxysporum and Alternaria solani, a significant reduction in the incidence of fusariosis and early blight by 27.21% and 29.43%, respectively, was observed [104].
Lipopeptides are also important bacterial biosurfactants with agricultural applications. According to Hoff et al. [105], surfactin secreted by Bacillus velenzis in contact with plant roots acted as a bioactive secondary metabolite (BSM), acting as a signal and/or antimicrobial and being responsible for optimizing biofilm formation, motility, and early root colonization and increasing plant immunity.
With the COVID-19 pandemic, studies on the antiviral potential of biosurfactants increased [100,106,107,108,109]. In the agricultural sector, the antiviral effect of these molecules is of fundamental interest, since they can be used against phytopathogens such as Potyvirus (family Potyviridae), Tospovirus (family Bunyaviridae), Tobamovirus (family Virgaviridae), Closteovirus (family Closteoviridae), Begomovirus (Geminiviridae), and Polerovirus (family Luteoviridae), which affect crops of economic importance such as vegetables, soybeans, beans, sugar cane, potatoes, and citrus fruits [110,111,112,113,114,115]. In viruses, biosurfactants can act by disintegrating particles through interaction with lipid layers and denaturing proteins in structures such as the capsid and envelope [107].
In addition to their effects against pathogens, biosurfactants are also responsible for the disruption of the waxy cuticles of some insects [99]. The removal of these lipid structures can cause dehydration in the insect and also make it more susceptible to infection by biological control agents and the action of hydrolase enzymes, facilitating the death of the insect pest.
5. Phytohormones as Agricultural Bioinputs
5.1. Gibberellic Acids
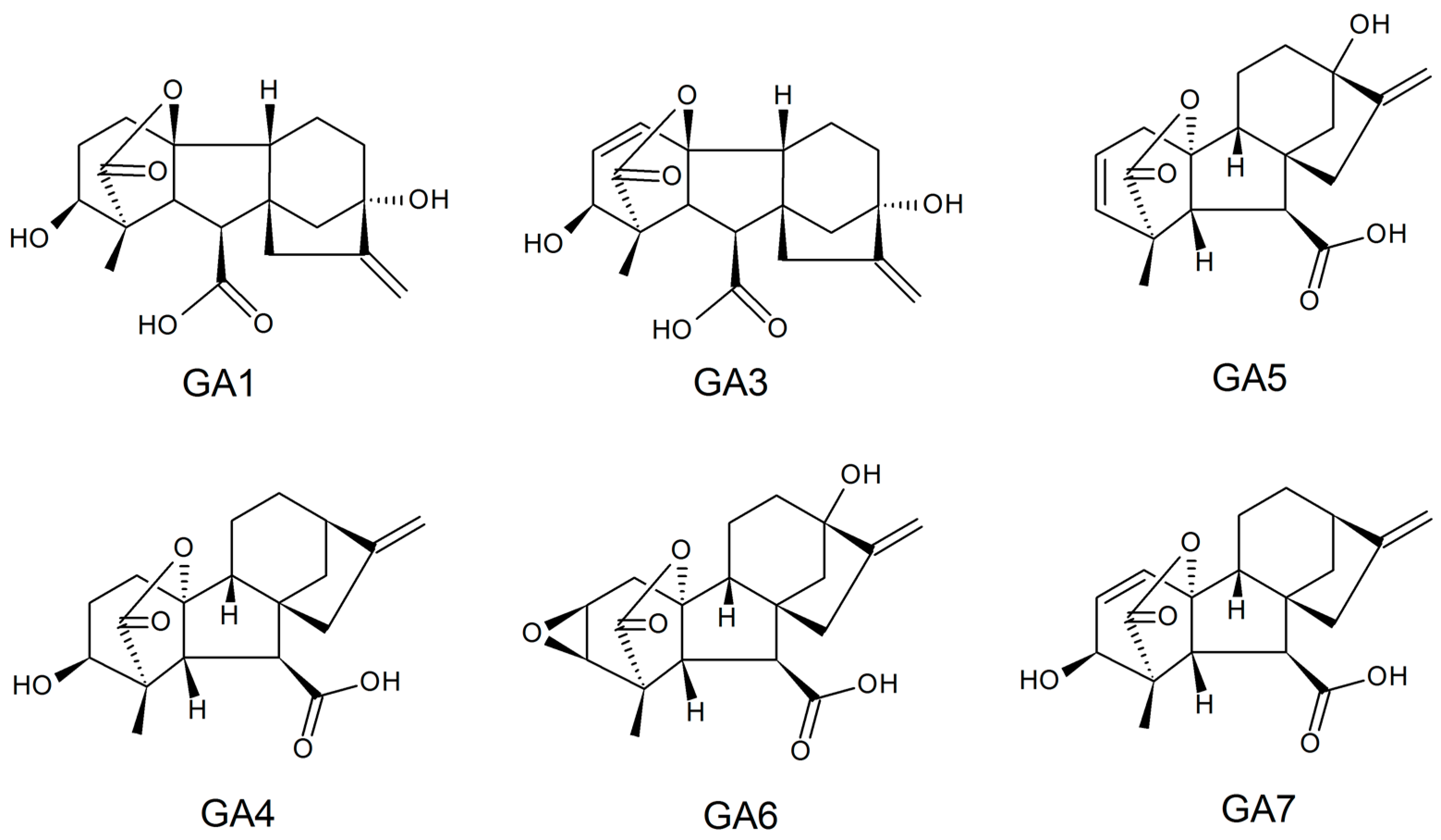
Gibberellins, or gibberellic acids (Gas), are a family of diterpenoid acid tetracyclics biosynthesized by plants, fungi, bacteria, and some algae genera that present hormonal effects in plant growth at low concentrations. These biomolecules exhibit structures with carboxylic acid, the presence or absence of unsaturated ring bonds, and different hydroxylation degrees; they have a molar mass of around 346.38 g/mol, are soluble in water (4.6 g/L in the room temperature), and have a melting point between 223 and 225 °C. Currently, 136 types of GA have been identified, and GA1, GA3, GA4, GA5, GA6, and GA7 are considered the most active. Their typical structures are represented in Figure 3 [116,117].
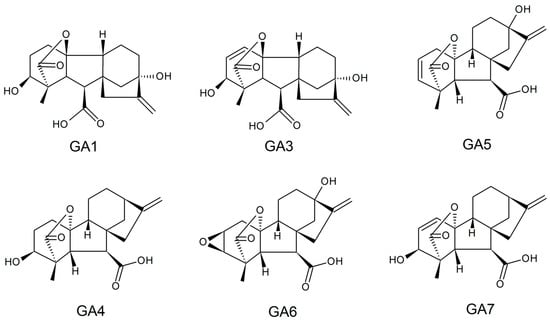
Figure 3.
Typical active structures reported for gibberellin.
GAs are important in plant physiology because they can promote stretching [118], germination and seed dormancy [119], flowering [120], gender development [121], leaf inhibition and senescence [122], and fruit aging [123]. Non-active forms of GA do not accumulate in plant tissues or cells but are rather transported in a root-to-shoot manner, or vice versa [124]. On the other hand, bioactive forms of GA such as GA4 accumulate in the sepals and petals during organ development [125]. Moreover, one study showed that GA levels are higher in the root elongation zone compared with the meristematic zone, pointing towards the hypothesis that GAs are synthesized locally or even transported from the surrounding tissues to compensate for hormone dilution during cellular growth [126,127].
Currently, GAs are bioinputs that have expanded the market in recent years, having been mainly used in horticulture [128,129,130,131]. Recent studies also show that gibberellin has potential applications as a biostimulant related to the main agricultural products of developing countries like corn [132,133], soybeans [134,135], sugarcane [136,137], and cotton [138]. Some studies have also shown that GA formulations (granular, pure, mixtures, and others) are used to protect seeds, flowers, and fruits against larvae and insects and that they act as herbicides [139,140].
According to the strategic importance of these bioinputs for achieving increasingly sustainable agriculture, the market trends show that production and profit are in ascendancy. The global market for GAs was valued at USD 500 million in 2017, and it is expected to reach revenues of 1167.8 million dollars in 2025, with an annual increase of 8.8% in CAGR [141]. The countries with the largest GA-producing companies are China, USA, and New Zealand.
Although the use of GAs is still restricted to small- and medium-sized plantations, the adoption of these bioinputs for farmers in developing countries, considered as “world granaries”, could further boost their use. Therefore, it is necessary to achieve high-yield production processes that use sustainable technologies and renewable raw materials such as lignocellulosic biomasses.
Currently, the industrial production of GAs has been predominantly carried out by SmF. However, diluting the sample in the fermentation broth requires a later concentration step, which makes the process more expensive [142]. It is also possible to produce GAs by chemical synthesis or by extraction from plants, but these methods are not economically viable. Thus, in recent years, the microbial production of GAs in SSF has presented itself as economically viable, whereby the main feedstock are agro-industrial byproducts [143]. In addition, in GA production via SSF, there is no concern with catabolic repression owing to the fact that carbon sources are present in solid state, majorly as carbohydrates; therefore, the microorganisms can possibly have better control over the available nutrients that can be absorbed by the excretion of cellulolytic enzymes.
GAs are produced via SSF generally using filamentous fungi as fermenting agents, the most common of which being Fusarium moniliforme and Gibberella fujikuroi [144]. In addition to filamentous fungi, bacteria can also produce GAs, especially those that promote plant growth and fulfill the role of nitrogen fixers, such as Azotobacter chroococcum, Bacillus licheniformis, P. fluorescens and Azospirillum lipoferum [144,145,146]. The use of bacteria that promote plant growth in the production of GAs comes with the advantage of obtaining a product for multifunctional agricultural application, acting as a biofertilizer and stimulant/regulator of plant growth [144].
One important strategy for discovering new GA-producing microorganisms is the isolation of endophytes. These microorganisms symbiotically inhabit plant tissues, and their metabolites usually foster plant growth. Studies have pointed to Fusarium oxysporum strain and Porostereum spadiceum [134,147].
Regarding the selection of the feedstock, primarily, the water activity (aw) of the raw material must be considered for the survival of the selected microorganism. For instance, in Gibberella fujikuroi grown via wheat bran and soluble starch, it was observed that the minimum aw bore by the microorganism was 0.9 aw, whereas optimal growth and GA production was achieved between 0.985 aw and 0.995 aw [148]. Nonetheless, in the literature, there is still a lack of studies on GA production using yeast under SSF and controlling aw. In general, it is known that filamentous fungi thrive on substrates with a relative humidity between 50 and 70% (commonly used for SSF). Bacteria and yeasts require higher levels of moisture to grow. However, the elevated moisture content in the substrate hinders oxygen diffusion through the solid bed and facilitates contamination by other microorganisms, which, in turn, interferes with the fermentation performance. In this context, the use of filamentous fungi for the production of GAs via SSF is preferred.
Analogously, the nutritional elements and nature of the culture medium are also fundamentally important parameters for GA production. The main carbon sources used in GA production are known to be carbohydrates and polyols such as glucose, sucrose, galactose, mannitol, maltose, starch, and glycerol [149,150,151,152,153]. These substrates are found in large quantities in lignocellulosic and starchy-derived materials such as corn stalks, citric peel, wheat bran, sugarcane bagasse, citric pulp, soy bran, sugarcane bagasse, soy husk, cassava bagasse, and coffee husk [117].
Production of Gibberellic Acids via SSF
Table 6 shows some raw materials used for GA production via SSF.

Table 6.
Different residual substrates used in the production of gibberellins via SSF.
The use of agro-industry byproducts to obtain high-value-added products has been highlighted, and GAs could be alternatives to products in biological refineries.
N sources, mineral salts, and trace elements are also important in obtaining GAs. The use of agro-industrial byproducts with complex compositions dismisses supplementation with sources of N, mineral salts, vitamins, etc. If the addition of N and mineral salts is necessary, the use of liquid residual extracts such as corn steep liquor, plant meals, rice bran, or soy extract may be a viable and cheaper option than the addition of inorganic nitrogen salts solutions. It is interesting to use them as moisturizers, thus controlling the humidity of the process and supplementing it with nutrient sources. Another advantage of using organic N sources in fermentation is that during metabolism, the degradation of nitrogen compounds (hydrolysis reactions) will not drastically affect the pH. Due to these characteristics, complex organic nitrogen sources are favorable for the production of GAs.
However, even with the wide availability of N sources (e.g., organic and inorganic forms), it is not usual to vary nitrogen concentration while SSF occurs due to the difficulty of the operation, which makes it difficult to homogenize the system. Nonetheless, Panchal and Desai [154] evaluated the effect of supplementary N sources (e.g., NH4Cl, NH4NO3, (NH4)2SO4, and urea) in commercial wheat bran (CWB) fermentation for the production of GA3 under SSF with 70 mg of N (differing only in the nature) per 100 g CWB. The utilization of urea resulted in an enhancement in the productivity of the GA. Indeed, GA3 production was higher (234, 489, and 532 µg/g) compared to the assay without N supplementation (95, 125, and 163 µg/g) at 120, 144, and 168 h, respectively.
Parameters such as pH and temperature are also important factors in SSF for GA production. Controlling the pH in SSF for GA production is difficult, but this problem can be solved by the addition of buffers or solutions with pH between 3.5 and 5.8. Satpute et al. [143] tested SSF using pigeon pea at a pH range between 4.3 and 5.3, and it was shown that the optimal production of GA3 occurred at pH 4.76, probably because of the growth of the fungus. On the contrary, Rodrigues et al. [142] established an initial pH between 5.5 an 5.8, which corresponds to the natural pH of citric pulp used as a substrate/support for GA production. This strategy represented an interesting approach to lowering the cost of production of this bioinput.
On the other hand, Bai et al. [158] used enzymatic hydrolysate from steam-exploded corn stalks to produce GAs. The pH of the enzymatic hydrolysis employed in this study was 4.8; however, the pH of the biosynthesis process was 6. In this case, the temperature range used in SSF for GA production is between 25 and 34 °C, because the fungi used as fermenting agents are mesophiles. Satpute et al. [143] described the fermentation of pigeon pea pods at various temperatures (e.g., 20 °C, 29 °C, 37 °C, and 45 °C) and demonstrated that the best temperature for the production of gibberellin was 29 °C, which is very close to the ideal range of spore germination and growth and product formation in SSF using Fusarium proliferatum.
Aeration in SSF for GA production is only controlled via bioreactors. This parameter is important because GA biosynthesis is a process with several oxidative steps. Thus, the aeration control is critical for the optimization of the bioprocess. The effect of aeration was observed by Oliveira et al. [117], who compared the production of GAs in SSF using Erlenmeyer flasks and a column bioreactor with forced aeration (30 mL/min). In addition to having a higher productivity (in 120 h of fermentation), production with forced aeration led to a GA gain of 13.72% (w/w) in relation to the results obtained using the Erlenmeyer flasks. The granulometry of the substrates used in SSF can also contribute to aeration. It is known that substrates with a small particle size can cause the “packaging effect” in the bioreactor, making oxygenation difficult. However, the use of substrates with larger particle sizes does not favor the enzymatic process that occurs in SSF due to their large contact surfaces. Thus, a valid strategy is to mix substrates with different particle sizes; this strategy was used by Rodrigues et al. [142], who tested sizes between 0.8 and 2 mm in mixtures of different substrates (citric pulp, soy bran, sugarcane bagasse, soy husk, cassava bagasse, and coffee husk).
5.2. Auxins
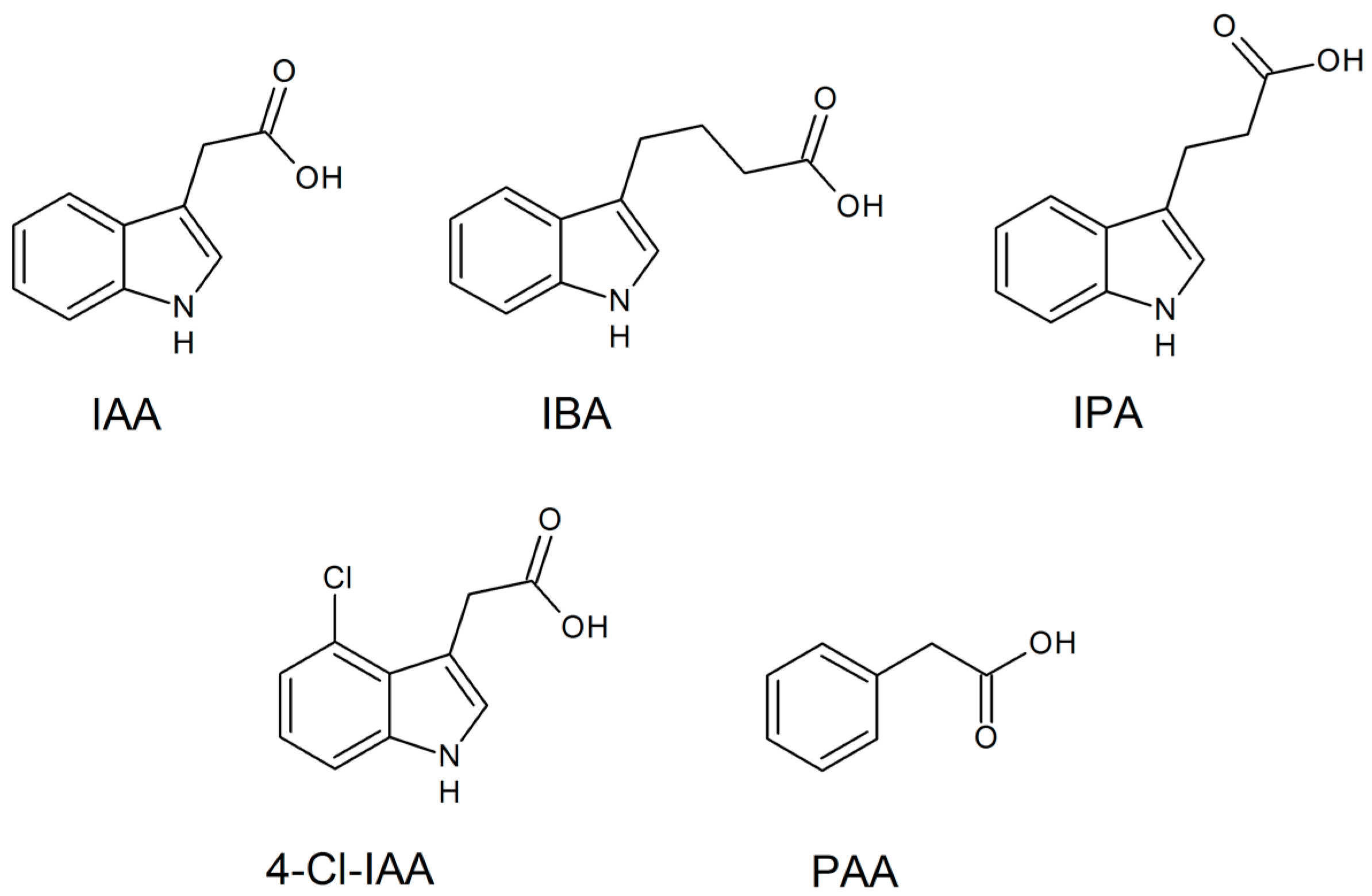
Auxins are compounds recognized as a family of aromatic molecules with a carboxylic acid group. They can be synthesized by plants and microorganisms and are responsible for controlling the plant growth response and development by means of cell differentiation, division, and expansion at low concentrations [160,161]. Commonly, these biomolecules are found in natural forms, as exemplified by indole-3-acetic acid (IAA), 4-Cl-IAA, phenylacetic acid (PAA), indole-3-butyric acid (IBA), and indole-3-propionic acid (IPA), and as exhibited in Figure 4 [161,162].
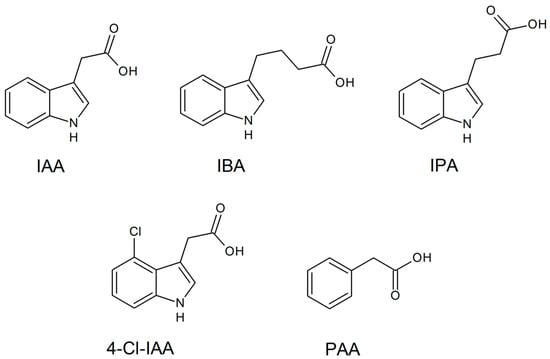
Figure 4.
Main active auxin forms found in plants.
There is also the occurrence of other types of auxins such as auxin a (auxenotriolic acid), auxin b (auxenolonic acid), benzofuran-3-acetic acid (BFA), and phenyl-butyric acid (PBA) in plants or other organisms, which is still being debated by experts or not fully understood. Also, synthetic auxins exist as active 2,4-dichlorophenoxyacetic acid (2,4-D) and naphthalene acetic acid (NAA), as well as the inactive precursor 2,4-dichlorophenoxybutyric acid (2,4-DB) [162]. In addition, auxins are hormones that partly regulate the processes of vascular tissue development, including tissue regeneration and vascular tissue connection, both of which are important for graft union [163].
Over the years, many studies have shown that auxin-regulated cell expansion plays an important role in plant development, specifically in primary root growth [164,165], lateral root development [166,167], organ development [168,169], tropism [170,171], apical dominance [172,173], etc. Because of their multifunctionality, auxins can be used in strategic plantations as plant growth promoters, and this approach has shown promising results in sugarcane [174], potato [175], and tomato plantations [176]. Auxins also help to obtain higher yields of good quality carrot seeds [177], improve the root morphology and growth of grafted cucumber seedlings [178], and can be used to alleviate abiotic stresses [179,180,181].
The most well-known compound used to treat weeds using auxin-based products in plantations is 2,4-dichlorophenoxyacetic acid (2,4-D), which was discovered in the 1940s and has been used extensively for more than 70 years worldwide. Arylex™ (developed mainly for cereal plantations) and Rinskor™ (used in rice fields) are new types of auxin herbicides produced on the market which have proven to be an attractive alternative to auxin application [182].
Due to studies carried out over the years showing the efficiency of using auxins in crops and improving their production, using this bioinput has been an important strategy for producers and their productions [177,178]. The production of auxins by chemical companies and laboratories started with the development and use of biostimulants with other phytohormones. Despite their great advantages, currently, synthetic auxins are largely employed rather than biosynthesized ones. Due to the environmental appeal and the commercial requirement for biodegradable products, auxin-producing microorganisms like fungi, bacteria, and algae are increasingly being researched and studied. The most well-known auxin of natural origin is indole-3-acetic acid (IAA), which has been widely studied in the literature. IAA is a molecule mainly produced by plants and microorganisms associated and not associated with plants. In the context of plant–microbe interactions, the presence of IAA plays a signaling role and helps to direct the growth and development of the plant [183].
In general, some Tryptophan (Trp)-independent routes have been described in the biosynthesis of IAA. For instance, a Trp-independent pathway that produced 90% of the IAA in the absence of tryptophan was observed by Prinsen et al. [184] in the nitrogen-fixing rhizobacteria Azospirillum brasilense Sp6 and its Tn5 mutant SpM7918. In addition, Li et al. [185] suggested the presence of an unknown tryptophan-independent pathway for IAA production by Arthrobacter pascens ZZ21 through endogenous precursors. Moreover, Roesch et al. [186] screened several microorganisms in order to discover promising Azospirillum spp. to promote plant growth through nitrogen fixation and auxin production in the southern fields of Brazil. Markedly, 30 isolates from rhizospheres, roots, and stems of maize were identified and evaluated as great candidates to synthesize auxin in the absence of tryptophan supplementation. However, nitrogen played a crucial role in regulating auxin pathways once the N-free media showed no trace of the product.
Suggestively, by comparing other studies regarding the isolation of potential auxin-producing bacteria such as Bacillus, Pseudomonas, Escherichia, Micrococcus, and Staphylococcus, it was presumed that bacteria that have strengthened N-fixing ability and are responsible for nitrogen cycling have more versatility upon auxin biosynthesis and may present more alternative routes to produce auxins [187]. Further investigations are necessary to elucidate the mechanism and cofactors required to obtain consistent and sufficient results.
On the contrary, Trp-dependent routes have been more frequently reported in the literature. For example, the indole-3-pyruvic acid (IPyA) pathways in some bacteria and fungi have been studied. This route involves the presence of decarboxylases to convert IPyA to indole-3-acetaldehyde (IAAld), which is oxidized to IAA by a dehydrogenase [188]. On the other hand, in the first step of the indole-3-acetamide (IAM) pathway, tryptophan is converted to IAM by tryptophan-2-monooxygenase. Subsequently, IAA is synthesized by IAM hydrolase from IAM. Interestingly, the genes found in plants share similarities with the enzymes that convert IAM to IAA in bacteria, which suggests the common evolution of this auxin biosynthesis pathway in bacteria and plants [189,190]. Moreover, the tryptophan side-chain oxidase (TSO) route indicates that the tryptophan is converted to IAAld, followed by the oxidation of IAAld to IAA. The decarboxylation of tryptophan to tryptamine (TAM) occurs in the TAM pathway. Then, an amine oxidase catalyzes the conversion of TAM to IAAld, which is oxidized to IAA by oxidation. In the case of the indole-3-acetonitrile (IAN) pathway, IAA can be obtained from the direct conversion of the intermediates by nitrilase or the conversion of IAM to IAA by nitrile hydratase [185,191]. For detailed information of the possible pathways currently available in the literature, we recommend Di et al.’s review [192].
Within this context, it is important to investigate distinct routes and means to synthesize IAA that function independently of tryptophan, broadening the spectrum of alternatives to afford higher productivities regarding IAA and making its industrial implementation economically viable. Regarding the already identified pathways of auxin-producing microorganisms, it is still necessary to conduct research aimed at unraveling the parameters and alternative routes for the synthesis of auxins as well as understanding the metabolism of auxin regulation, seeking high productivity, preferably through using byproducts from renewable sources as raw materials.
Production of Auxins in SSF
Despite its immense advantages in the use of SSF, the production of auxin as IAA using this fermentative method has been underexplored. However, Prado et al. [193] screened microorganism strains like Aspergillus spp., Bacillus spp., and Trichoderma spp. to investigate their capability to produce auxin (indole-3-acetic acid) and phytases through SSF in different substrates followed by the optimization of the process. The study revealed that all strains can produce auxin (comprising higher productivity with tryptophan supplementation). The highest indole derivative levels were observed in wheat bran as substrate by B. subtilis D strain (158 μg/mL), but the same strain did not produce auxins in cassava bagasse. These findings led the authors to investigate the physico-chemical characteristics of each substrate to set a reliable framework of the reasons for that response.
Presumably, the high starch and lower protein content could hinder auxin synthesis due to the N-limiting condition. Additionally, due to the fact that cassava bagasse was the substrate with the highest lignin composition and as indicated by the results, lignin has a strong negative correlation with the production of IAA, observed in the Bacillus and Trichoderma strains. Indeed, lignin stands as a physical barrier hampering the microbial and enzymatic access to the carbohydrate fraction of the biomass. In this regard, the selective degradation of lignin to carbon dioxide can be carried out using white rot fungi. For instance, Giri and Sharma [194] suggested pretreating wheat straw using Phanerochaete chrysosporium, known for its ability to degrade lignin, as an alternative to accessing cellulose and converting it into easily fermentable sugars. Nevertheless, during secondary fermentation, these precursors may contribute to the synthesis of IAA via this fungus, along with the supplementation of tryptophan.
Another study conducted by Prado et al. [195] evaluated the production of indole-3-acetic acid and its derivatives in 16 microbial strains. For the first time in the literature, this study reported high indole-3-acetic acid production using strain Aspergillus flavipes (ATCC® 16814™) through a tryptophan-dependent pathway in SSF, utilizing an optimized medium culture composed of soybean bran and tryptophan that achieved a yield of 71 ug mL−1. The highest indolic compound yield was 655 ug mL−1 using soybean bran as a substrate and the following conditions: particle size >1 mm and 15 mL of water supplemented with 1.5% of tryptophan. The fermentation broth was mixed in solid form (SF) and liquid form (LF) and used in the cultivation of hybrid Eucalyptus grandis and E. urophylla (clone IPB2). In response to the treatment, the plants showed an improvement in adventitious rooting rate, root length, both root fresh and dry mass, and root length and dry mass, respectively, 40 days after planting. Also, the plant biostimulant was confirmed to be non-toxic and eco-friendly by comparing the viability of (NIH/3t3) in the plants’ fibroblasts cultivated with indole-3-acetic and the control group (without exogenous phytohormone application). Another advantageous aspect of SSF is that the product can be applied as a compost directly in the soil, allowing it to be a promising technique for agriculture.
One of the major drawbacks of producing IAA in large quantities in fermentations is the dependence on supplementing the culture medium with tryptophan. The addition of tryptophan increased production by, on average, 10-fold in SSF involving tryptophan in almost all strains tested in one study carried out by Prado et al. [193]. Further, this trend was also observed in another study by Prado et al. [195] after optimizing the culture medium using A. flavipes as an IAA producer microorganism. The authors detected a 1716-fold increase in IAA production under SSF compared to the assays absent of tryptophan.
Although the product generated has huge potential for use in the formulation of bioinputs, the supplementation of the amino acid tryptophan to form IAA makes the production of this hormone more expensive, especially on an industrial scale. One of the possible solutions to this problem is the use of raw materials that contain tryptophan in a nutritional form, such as wheat or green pea, which contain 0.125 (g/g dry mass) and 0.180 (g/g dry mass), respectively [196].
Another alternative is the intensification of IAA synthesis in microorganisms through metabolic engineering. For instance, Romasi and Lee [197], cloned E. coli aminotransferase, indole-3-pyruvic acid decarboxylase, and indole-3-acetic acid dehydrogenase, which are three enzymes involved in the indole-3-pyruvic acid pathway. The results showed that the recombinant E. coli DH5α produced about 1.1 g/L of IAA after 48 h of cultivation in LB medium supplemented with 2 g/L of tryptophan. However, after the deletion of a gene that mediates indole formation from tryptophan, the yield of IAA obtained from the new strain E. coli IAA68 was 1.8 g/L of IAA, which corresponded to a 1.6-fold increase when compared to wild-type DH5α.
In contrast, Malhotra and Srivastava [198] expressed a heterologous indole acetamide IAM pathway consisting of the iaaM and iaaH genes in Azospirillum brasilense SM. Subsequently, the IAA levels increased by three-fold, and the sorghum roots as well as the dry weight of the plants improved when compared to the effect of the wild-type strain. Similarly, Kochar et al. [199] overexpressed the same pathway in Pseudomonas syringae subsp. Savastanoi, and they observed a mutant with a production 5-fold higher than the wild-type strain. However, the effect on the root growth and branching of sorghum was negative. The authors confirmed that there is a limit to the concentration of IAA that is beneficial for root growth, and extremely large amounts can be detrimental.
Similar to the addition of tryptophan as a precursor of IAA, it is important to consider other relevant factors and parameters in the process. Prado et al. [195] carried out a factorial design 23 in SSF, varying the percentage of tryptophan (0.5, 1.0, and 1.5) and particle size (in mm; 0.5, 1.0, and >1.0) and adding water (in mL; 5, 10, and 15). In this experiment, using the A. flavipes strain, it was observed that the amount of water and the use of tryptophan in the medium had a positive effect on the production of IAA in SSF. In addition, light and aeration influences have not been yet reported or described as parameters in the production of IAA in SSF.
6. Other Important Agricultural Bioinputs Obtained by SSF
In addition to the agricultural bioinputs obtained by SSF reported in this article, enzymes are also important products. Enzymes, along with inoculants and biological control agents, are commonly used in agriculture as a sustainable substitute for synthetic products. Agricultural enzymes help increase agricultural production, soil fertility, and food protection. They can also protect crops from various pests and diseases. The use of enzymes and inoculants increases the quality of commercial crops. Enzymes of agricultural importance, such as hydrolases, decompose plant residues and other organic matter that provide nutrients to plants and help in the initial stages of seed development, such as rooting and sprouting. Furthermore, the crop’s resistance to water stress and nutrient assimilation are improved. Thus, enzymes are essential for sustainable agricultural productivity and soil management. The most important enzymes used in plant growth and soil fertility include phosphatases, dehydrogenases, and ureases. Other enzymes used in agricultural applications include cellulases, proteases, phytases, sulfatases, and amylases [200,201,202].
7. Future Prospects
The aforementioned bioinputs are emerging microbial-based technologies that are expected to come to the fore in the coming decades. Several fermentation strategies have been developed, different bioproducts have been exploited, and, yet, productivity and competitiveness are still concerns in relation to synthetic agrochemicals. Nevertheless, the agricultural systems rely on basic monocultures, and approaches comprising agroecological systems may be one of the alternatives to validate the use of bioinputs in a sustainable manner. Furthermore, one of the main factors affecting the efficiency of bioinputs is their persistence in soil. In fact, upon application, inoculants and biological control agents may find harsh conditions to survive, facing competition and suppression by the adapted microbial populations [203]. As such, current studies are attempting to overcome these issues via the development of smart bioformulations. Bioformulations are highly complex materials consisting of the main bioinputs (e.g., enzymes, spores, viable cells, and metabolites) entrapped in an organic matrix, such as natural polymers (e.g., cellulose, lignin, starch, alginate, lectin, and others), which confers biodegradability and stabilization, and serves as a carrier/vehicle prolonging the shelf-life and persistence in soil under oscillatory environmental conditions. This will play a pivotal role in contributing to the commercialization of these bioinputs worldwide, increasing their affordability and broadening storage and application conditions.
8. Conclusions
As can be seen, in recent decades agricultural bioinputs have emerged as fundamental pieces in the search for more sustainable and efficient agricultural practices. The importance of these bioproducts spans economic, environmental, and geopolitical dimensions, reflecting a broad approach to addressing global food production challenges. These bioproducts are of fundamental importance for the development of the bioeconomy and circular economy, promoting the sustainable development of modern civilizations. As reported, inoculants, biological control agents, biosurfactants, and phytohormones play a crucial role in maximizing agricultural productivity by contributing to food security and increasing profitability for farmers. The use of agricultural bioinputs also reduces dependence on synthetic agrochemicals, minimizing adverse impacts on the biosphere and having a lower impact on biogeochemical cycles, contributing to more sustainable agricultural practices and mitigating climate change. Geopolitically, bioinputs are also important, as they give countries strategic advantages, reducing dependence on imports of chemical products and associated technologies. This strengthens the resilience of national agricultural systems, protecting countries from fluctuations in commodity prices and ensuring food sovereignty.
It should also be highlighted that SSF, as a production process for agricultural bioinputs, represents an innovative and effective approach in the production of agricultural bioinputs mainly associated with the context of biorefineries. By taking advantage of organic waste to promote the growth of beneficial microorganisms and the production of substances that influence plant growth, this process contributes to promoting soil health, increasing agricultural productivity, and reducing the environmental impact associated with conventional practices. FES stands out as a valuable component in the search for more sustainable and resilient agricultural systems.
Agricultural bioinputs are indispensable biotechnological products for a sustainable and less unequal future in food production, guaranteeing food security for different global civilizations.
Author Contributions
Writing—original draft, conceptualization, methodology, and writing—review and editing: T.M.R., P.R.F.M. and R.A.M.D.C. Validation, formal analysis, and data curation: F.G.B. Writing—original draft preparation: D.R.-R. Resources and supervision: S.S.d.S. All authors have read and agreed to the published version of the manuscript.
Funding
The authors gratefully acknowledge Coordenação de Aperfeiçoamento de Pessoal de Nível Superior–Brasil (CAPES) Finance Code 001; Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Process number #16/10636-8 and #16/14852-7; and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) with process number 141639/2023-7 for their financial support.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The information contained in this review was surveyed from Google Scholar, Web of Science and Scopus database platforms.
Acknowledgments
The authors acknowledge the Laboratory of Bioprocesses and Sustainable Products.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zvelebil, M.; Pluciennik, M. Historical origins of agriculture. In Role Food, Agriculture, Forestry and Fisheries in Human Nutrition, 1st ed.; Squires, V.R., Ed.; EOLSS Publications: Oxford, UK, 2011; Volume 3, pp. 41–78. [Google Scholar]
- Our World in Data. Available online: https://ourworldindata.org/population-growth (accessed on 18 December 2023).
- Zimdahl, R.L. Agriculture: A Brief History. In Agricultural Ethics—An Invitation, 1st ed.; Springer: Cham, Switzerland, 2020; pp. 23–50. [Google Scholar]
- Meena, R.S.; Kumar, S.; Datta, R.; Lal, R.; Vijayakumar, V.; Brtnicky, M.; Sharma, M.P.; Yadav, G.S.; Jhariya, M.K.; Jangir, C.K.; et al. Impact of Agrochemicals on Soil Microbiota and Management: A Review. Land 2020, 9, 34. [Google Scholar] [CrossRef]
- Srivastava, R.K. Influence of Sustainable Agricultural Practices on Healthy Food Cultivation. In Environmental Biotechnology; Gothandam, K., Ranjan, S., Dasgupta, N., Lichtfouse, E., Eds.; Springer: Cham, Switzerland, 2020; Volume 2, pp. 95–124. [Google Scholar]
- Rengalakshmi, R.; Manjula, M.; Prabavathy, V.R.; Jegan, S.; Selvamukilan, B. Building bioeconomy in agriculture: Harnessing soil microbes for sustaining ecosystem services. In Towards a Sustainable Bioeconomy: Principles, Challenges and Perspectives; Leal Filho, W., Pociovălișteanu, D., Borges de Brito, P., Borges de Lima, I., Eds.; Springer: Cham, Switzerland, 2018; pp. 261–277. [Google Scholar]
- Markets and Markets. Available online: https://www.marketsandmarkets.com/Market-Reports/agricultural-biological-market-100393324.html (accessed on 18 December 2023).
- Cerda, A.; Mejias, L.; Rodríguez, P.; Rodríguez, A.; Artola, A.; Font, X.; Gea, T.; Sánchez, A. Valorisation of digestate from biowaste through solid-state fermentation to obtain value added bioproducts: A first approach. Bioresour. Technol. 2019, 271, 409–416. [Google Scholar] [CrossRef]
- Chundakkadu, K. Solid-State Fermentation Systems—An Overview. Crit. Rev. Biotechnol. 2005, 25, 1–30. [Google Scholar] [CrossRef]
- Abu Yazid, N.; Barrena, R.; Komilis, D.; Sánchez, A. Solid-State Fermentation as a Novel Paradigm for Organic Waste Valorization: A Review. Sustainability 2017, 9, 224. [Google Scholar] [CrossRef]
- Pathak, D.V.; Kumar, M. Microbial inoculants as biofertilizers and biopesticides. In Microbial Inoculants in Sustainable Agricultural Productivity; Singh, D., Singh, H., Prabha, R., Eds.; Springer: New Delhi, India, 2016; Volume 1, pp. 197–209. [Google Scholar]
- Palmieri, D.; Ianiri, G.; Del Grosso, C.; Barone, G.; De Curtis, F.; Castoria, R.; Lima, G. Advances and Perspectives in the Use of Biocontrol Agents against Fungal Plant Diseases. Horticulturae 2022, 8, 577. [Google Scholar] [CrossRef]
- Mazoyon, C.; Firmin, S.; Bensaddek, L.; Pecourt, A.; Chabot, A.; Faucon, M.-P.; Sarazin, V.; Dubois, F.; Duclercq, J. Optimizing Crop Production with Bacterial Inputs: Insights into Chemical Dialogue between Sphingomonas sediminicola and Pisum sativum. Microorganisms 2023, 11, 1847. [Google Scholar] [CrossRef]
- Sudheer, S.; Johny, L.; Srivastava, S.; Adholeya, A. The trade-in-trade: Multifunctionalities, current market and challenges for arbuscular mycorrhizal fungal inoculants. Symbiosis 2023, 89, 259–272. [Google Scholar] [CrossRef]
- Nimsi, K.A.; Manjusha, K.; Kathiresan, K.; Arya, H. Plant growth-promoting yeasts (PGPY), the latest entrant for use in sustainable agriculture: A review. J. Appl. Microbiol. 2023, 134, lxac088. [Google Scholar] [CrossRef]
- Mattedi, A.; Sabbi, E.; Farda, B.; Djebaili, R.; Mitra, D.; Ercole, C.; Cacchio, P.; Del Gallo, M.; Pellegrini, M. Solid-State Fermentation: Applications and Future Perspectives for Biostimulant and Biopesticides Production. Microorganisms 2023, 11, 1408. [Google Scholar] [CrossRef]
- Pandey, A.K.; Edgard, G.; Negi, S. Optimization of concomitant production of cellulase and xylanase from Rhizopus oryzae SN5 through EVOP-factorial design technique and application in Sorghum Stover based bioethanol production. Renew. Energy 2016, 98, 51–56. [Google Scholar] [CrossRef]
- Ali, S.R.; Anwar, Z.; Irshad, M.; Mukhtar, S.; Warraich, N.T. Bio-synthesis of citric acid from single and co-culture-based fermentation technology using agro-wastes. J. Radiat. Res. Appl. Sci. 2016, 9, 57–62. [Google Scholar] [CrossRef]
- Costa, J.A.V.; Treichel, H.; Kumar, V.; Pandey, A. Advances in Solid-State Fermentation. In Current Developments in Biotechnology and Bioengineering, 1st ed.; Pandey, A., Larroche, C., Carlos Soccol, C.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–17. [Google Scholar] [CrossRef]
- Sadh, P.K.; Duhan, S.; Duhan, J.S. Agro-industrial wastes and their utilization using solid state fermentation: A review. Bioresour. Bioprocess. 2018, 5, 1. [Google Scholar] [CrossRef]
- Vassilev, N.; de Oliveira Mendes, G. Solid-state fermentation and plant-beneficial microorganisms. In Current Developments in Biotechnology and Bioengineering, 1st ed.; Pandey, A., Larroche, C., Carlos Soccol, C.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 435–450. [Google Scholar] [CrossRef]
- Manan, M.A.; Webb, C. Design aspects of solid state fermentation as applied to microbial bioprocessing. J. Appl. Biotechnol. Bioeng. 2017, 4, 91. [Google Scholar] [CrossRef]
- Itoh, Y.; Tada, K.; Kanno, T.; Horiuchi, J.I. Selective production of lactic acid in continuous anaerobic acidogenesis by extremely low pH operation. J. Biosci. Bioeng. 2012, 114, 537–539. [Google Scholar] [CrossRef]
- Sakthiselvan, P.; Meenambiga, S.S.; Madhumathi, R. Kinetic Studies on Cell Growth. In Cell Growth, 1st ed.; Vikas, B., Fasullo, M., Eds.; IntechOpen: London, UK, 2020; Volume 1, p. 13. [Google Scholar]
- Chen, Y.; Lin, Y.; Tian, X.; Li, Q.; Chu, J. Real-time dynamic analysis with low-field nuclear magnetic resonance of residual oil and sophorolipids concentrations in the fermentation process of Starmerella bombicola. J. Microbiol. Methods 2019, 157, 9–15. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, R.; An, Z.; Chen, J.; Liu, X. Optimization of temperature shift-based fermentation process for sophorolipids production using response surface methodology. In AIP Conference Proceedings; AIP Publishing: Melville, NY, USA, 2019; Volume 2110. [Google Scholar] [CrossRef]
- Gueri, M.V.D.; Schirmer, W.N.; Torres, L.M.G.; Furtado, A.C. Pré-tratamentos de resíduos lignocelulósicos visando ao aumento da geração de metano nos processos de digestão anaeróbia: Uma revisão. Rev. Geama 2021, 7, 13–27. [Google Scholar]
- Kumar, R.; Kim, T.H.; Basak, B.; Patil, S.M.; Kim, H.H.; Ahn, Y.; Yadav, K.K.; Cabral-Pinto, M.S.M.; Jeon, B.H. Emerging approaches in lignocellulosic biomass pretreatment and anaerobic bioprocesses for sustainable biofuels production. J. Clean. Prod. 2022, 333, 130180. [Google Scholar] [CrossRef]
- Shakouri, B.; Babaeipour, V.; Mashreghi, M. Increasing Protein Content of Tomato Pomace using Solid-State Fermentation with Industrial Bakery Yeasts. Appl. Food Biotechnol. 2023, 10, 47–59. [Google Scholar] [CrossRef]
- Laditi, M.A.; Nwoke, O.C.; Jemo, M.; Abaidoo, R.C.; Ogunjobi, A.A. Evaluation of microbial inoculants as biofertilizers for the improvement of growth and yield of soybean and maize crops in savanna soils. Afr. J. Agric. Res. 2012, 7, 405–413. [Google Scholar] [CrossRef]
- Tsiafouli, M.A.; Thébault, E.; Sgardelis, S.P.; de Ruiter, P.C.; van der Putten, W.H.; Birkhofer, K.; Hemerik, L.; de Vries, F.T.; Bardgett, R.D.; Brady, M.V.; et al. Intensive Agriculture Reduces Soil Biodiversity across Europe. Glob. Chang. Biol. 2015, 21, 973–985. [Google Scholar] [CrossRef]
- Hungria, M.; Campo, R.J.; Souza, E.M.; Pedrosa, F.O. Inoculation with selected strains of Azospirillum brasilense and A. lipoferum improves yields of maize and wheat in Brazil. Plant Soil 2010, 331, 413–425. [Google Scholar] [CrossRef]
- Abdullahi, I.N.; Chuwang, P.Z.; Isah, A.D. Effect of biofertilizer application on growth of Vitellaria paradoxa seedlings. J. Res. Environ. Sci. Toxicol. 2012, 1, 294–297. [Google Scholar]
- Malusá, E.; Vassilev, N. A contribution to set a legal framework for biofertilizers. Appl. Microbiol. Biotechnol. 2014, 98, 6599–6607. [Google Scholar] [CrossRef]
- Johnston-Monje, D.; Lundberg, D.S.; Lazarovits, G.; Reis, V.M.; Raizada, M.N. Bacterial populations in juvenile maize rhizospheres originate from both seed and soil. Plant Soil 2016, 405, 337–355. [Google Scholar] [CrossRef]
- Bizos, G.; Papatheodorou, E.M.; Chatzistathis, T.; Ntalli, N.; Aschonitis, V.G.; Monokrousos, N. The Role of Microbial Inoculants on Plant Protection, Growth Stimulation, and Crop Productivity of the Olive Tree (Olea europea L.). Plants 2020, 9, 743. [Google Scholar] [CrossRef]
- Kowalska, J.; Krzymińska, J.; Tyburski, J. Yeasts as a Potential Biological Agent in Plant Disease Protection and Yield Improvement—A Short Review. Agriculture 2022, 12, 1404. [Google Scholar] [CrossRef]
- Savita Sharma, A. Fungi as Biological Control Agents. In Biofertilizers for Sustainable Agriculture and Environment; Giri, B., Prasad, R., Wu, Q.S., Varma, A., Eds.; Springer: Cham, Switzerland, 2019; Volume 55, pp. 395–411. [Google Scholar] [CrossRef]
- Koul, B.; Chopra, M.; Lamba, S. Microorganisms as biocontrol agents for sustainable agriculture. In Relationship Between Microbes and the Environment for Sustainable Ecosystem Services; Samuel, J., Kumar, A., Singh, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; Volume 1, pp. 45–68. [Google Scholar] [CrossRef]
- Mordor Intelligence. Available online: https://www.mordorintelligence.com/industry-reports/agricultural-inoculants-market (accessed on 17 December 2023).
- Markets and Markets. Agricultural Inoculants Market. Available online: https://www.marketsandmarkets.com/Market-Reports/agricultural-inoculants-market-152735696.html (accessed on 17 December 2023).
- Marcelino, P.R.F.; Milani, K.M.L.; Mali, S.; dos Santos, O.J.A.P.; de Oliveira, A.L.M. Formulations of polymeric biodegradable low-cost foam by melt extrusion to deliver plant growth-promoting bacteria in agricultural systems. Appl. Microbiol. Biotechnol. 2016, 100, 7323–7338. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, X.; Li, Z. Growth of Rhizobium leguminosarum in a periodic pressure oscillating, solid-state fermentation of wheat straw. Biotechnol. Lett. 2001, 23, 827–829. [Google Scholar] [CrossRef]
- Molina-Peñate, E.; del Carmen Vargas-García, M.; Artola, A.; Sánchez, A. Filling in the gaps in biowaste biorefineries: The use of the solid residue after enzymatic hydrolysis for the production of biopesticides through solid-state fermentation. Waste Manag. 2023, 161, 92–103. [Google Scholar] [CrossRef]
- Alias, C.; Bulgari, D.; Gobbi, E. It Works! Organic-Waste-Assisted Trichoderma spp. Solid-State Fermentation on Agricultural Digestate. Microorganisms 2022, 10, 164. [Google Scholar] [CrossRef]
- Liu, Q.; Meng, X.; Li, T.; Raza, W.; Liu, D.; Shen, Q. The Growth Promotion of Peppers (Capsicum annuum L.) by Trichoderma guizhouense NJAU4742-Based Biological Organic Fertilizer: Possible Role of Increasing Nutrient Availabilities. Microorganisms 2020, 8, 1296. [Google Scholar] [CrossRef] [PubMed]
- Shengping, X.; Liantian, M.; Yujie, M.; Yan, D.; Hongbo, Y. Optimizing Bacillus circulans Xue-113168 for Biofertilizer Production and Its Effects on Crops. Afr. J. Biotechnol. 2016, 15, 2795–2803. [Google Scholar] [CrossRef]
- Berikashvili, V.; Sokhadze, K.; Kachlishvili, E.; Elisashvili, V.; Chikindas, M.L. Bacillus amyloliquefaciens Spore Production Under Solid-State Fermentation of Lignocellulosic Residues. Probiotics Antimicrob. Proteins 2018, 10, 755–761. [Google Scholar] [CrossRef] [PubMed]
- El-Bendary, M.A. Production of Mosquitocidal bacillus sphaericus by Solid State Fermentation Using Agricultural Wastes. World J. Microbiol. Biotechnol. 2010, 26, 153–159. [Google Scholar] [CrossRef]
- de Sá Santos, P.; Abati, K.; Mendoza, N.V.R.; Mascarin, G.M.; Júnior, I.D. Nutritional impact of low-cost substrates on biphasic fermentation for conidia production of the fungal biopesticide Metarhizium anisopliae. Bioresour. Technol. Rep. 2021, 13, 100619. [Google Scholar] [CrossRef]
- Sala, A.; Vittone, S.; Barrena, R.; Sanchez, A.; Artola, A. Scanning agro-industrial wastes as substrates for fungal biopesticide production: Use of Beauveria bassiana and Trichoderma harzianum in solid-state fermentation. J. Environ. Manag. 2021, 295, 113113. [Google Scholar] [CrossRef] [PubMed]
- Bulgari, D.; Alias, C.; Peron, G.; Ribaudo, G.; Gianoncelli, A.; Savino, S.; Boureghda, H.; Bouznad, Z.; Monti, E.; Gobbi, E. Solid-State Fermentation of Trichoderma spp.: A New Way to Valorize the Agricultural Digestate and Produce Value-Added Bioproducts. J. Agric. Food Chem. 2023, 71, 3994–4004. [Google Scholar] [CrossRef]
- Shinde, R.; Shahi, D.K.; Mahapatra, P.; Naik, S.K.; Thombare, N.; Singh, A.K. Potential of lignocellulose degrading microorganisms for agricultural residue decomposition in soil: A review. J. Environ. Manag. 2022, 320, 115843. [Google Scholar] [CrossRef]
- Vyas, P.; Gulati, A. Organic acid production in vitro and plant growth promotion in maize under controlled environment by phosphate-solubilizing fluorescent Pseudomonas. BMC Microbiol. 2009, 9, 174. [Google Scholar] [CrossRef]
- Orozco-Mosqueda, M.d.C.; Santoyo, G.; Glick, B.R. Recent Advances in the Bacterial Phytohormone Modulation of Plant Growth. Plants 2023, 12, 606. [Google Scholar] [CrossRef]
- Guo, N.; Zhang, S.; Gu, M.; Xu, G. Function, transport, and regulation of amino acids: What is missing in rice? Crop J. 2021, 9, 530–542. [Google Scholar] [CrossRef]
- Veliz, E.A.; Martínez-Hidalgo, P.; Hirsch, A.M. Chitinase-producing bacteria and their role in biocontrol. AIMS Microbiol. 2017, 3, 689. [Google Scholar] [CrossRef]
- Amparo Asensi-Fabado, M.; Munne-Bosch, S. Vitamins in plants: Occurrence, biosynthesis and antioxidant function. Trends Plant Sci. 2010, 15, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Römheld, V.; Marschner, H. Function of micronutrients in plants. Micronutr. Agric. 1991, 4, 297–328. [Google Scholar] [CrossRef]
- Singh, R.; Hans, M.; Kumar, S.; Yadav, Y.K. Thermophilic Anaerobic Digestion: An Advancement towards Enhanced Biogas Production from Lignocellulosic Biomass. Sustainability 2023, 15, 1859. [Google Scholar] [CrossRef]
- Raposo, F.; De la Rubia, M.A.; Fernández-Cegrí, V.; Borja, R. Anaerobic digestion of solid organic substrates in batch mode: An overview relating to methane yields and experimental procedures. Renew. Sustain. Energy Rev. 2012, 16, 861–877. [Google Scholar] [CrossRef]
- Sukphun, P.; Sittijunda, S.; Reungsang, A. Volatile Fatty Acid Production from Organic Waste with the Emphasis on Membrane-Based Recovery. Fermentation 2021, 7, 159. [Google Scholar] [CrossRef]
- Monlau, F.; Sambusiti, C.; Ficara, E.; Aboulkas, A.; Barakat, A.; Carrère, H. New opportunities for agricultural digestate valorization: Current situation and perspectives. Energy Environ. Sci. 2015, 8, 2600–2621. [Google Scholar] [CrossRef]
- Vassilev, N.; Baca, M.T.; Vassileva, M.; Franco, I.; Azcon, R. Rock phosphate solubilization by Aspergillus niger grown on sugar-beet waste medium. Appl. Microbiol. Biotechnol. 1995, 44, 546–549. [Google Scholar] [CrossRef]
- Ajala, A.S.; Adeoye, A.O.; Olaniyan, S.A.; Fasonyin, O.T. A Study on Effect of Fermentation Conditions on Citric Acid Production from Cassava Peels. Sci. Afr. 2020, 8, e00396. [Google Scholar] [CrossRef]
- Devi, V.; Sumathy, V.J.H. Production of biofertilizer from fruit waste. Eur. J. Pharm. Med. Res. 2017, 4, 436–443. [Google Scholar]
- Mikkelsen, R.L. Humic materials for agriculture. Better Crops 2005, 89, 6–10. [Google Scholar]
- Burlakovs, J.; Kļaviņš, M.; Osinska, L.; Purmalis, O. The impact of humic substances as remediation agents to the speciation forms of metals in soil. APCBEE Procedia 2013, 5, 192–196. [Google Scholar] [CrossRef]
- Huelva López, R.; Martínez Balmori, D.; Calderín García, A.; Hernández González, O.L.; Guridi Izquierdo, F. Propiedades químicas y química-físicas de derivados estructurales de ácidos húmicos obtenidos de vermicompost. Actividad biológica. Rev. Cienc. Téc. Agropecu. 2013, 22, 56–60. [Google Scholar]
- Yel, E.; Ahmetli, G. Environmental dilemma of humic substances: Being adsorbents and being carcinogens. Int. J. Environ. Sci. Dev. 2015, 6, 73. [Google Scholar] [CrossRef]
- de Melo, B.A.G.; Motta, F.L.; Santana, M.H.A. Humic acids: Structural properties and multiple functionalities for novel technological developments. Mater. Sci. Eng. C 2016, 62, 967–974. [Google Scholar] [CrossRef]
- Volpi, M.P.C.; Corzo, I.J.M.; Bastos, R.G.; Santana, M.H.A. Production of humic acids by solid-state fermentation of Trichoderma reesei in raw oil palm empty fruit bunch fibers. 3 Biotech 2019, 9, 393. [Google Scholar] [CrossRef]
- Ji, J.L.; Chen, F.; Liu, S.; Yang, Y.; Hou, C.; Wang, Y.Z. Co-production of biogas and humic acid using rice straw and pig manure as substrates through solid-state anaerobic fermentation and subsequent aerobic composting. J. Environ. Manag. 2022, 320, 115860. [Google Scholar] [CrossRef] [PubMed]
- Mendes, G.O.; Dias, C.S.; Silva, I.R.; Júnior, J.I.R.; Pereira, O.L.; Costa, M.D. Fungal rock phosphate solubilization using sugarcane bagasse. World J. Microbiol. Biotechnol. 2013, 29, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Vassilev, N.; Vassileva, M.; Lopez, A.; Martos, V.; Reyes, A.; Maksimovic, I.; Eichler-Lobermann, B.; Malusa, E. Unexploited potential of some biotechnological techniques for biofertilizer production and formulation. Appl. Microbiol. Biotechnol. 2015, 99, 4983–4996. [Google Scholar] [CrossRef] [PubMed]
- Cameotra, S.S.; Makkar, R.S. Biosurfactant-enhanced bioremediation of hydrophobic pollutants. Pure Appl. Chem. 2010, 82, 97–116. [Google Scholar] [CrossRef]
- Marcelino, P.R.F.; Gonçalves, F.; Jimenez, I.M.; Carneiro, B.C.; Santos, B.B.; da Silva, S.S. Sustainable production of biosurfactants and their applications. In Lignocellulosic Biorefining Technologies, 1st ed.; Ingle, A.P., Chandel, A.K., Silva, S.S., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2020; Volume 1, pp. 159–183. [Google Scholar] [CrossRef]
- Dierickx, S.; Castelein, M.; Remmery, J.; De Clercq, V.; Lodens, S.; Baccile, N.; De Maeseneire, S.L.; Roelants, S.L.K.W.; Soetaert, W.K. From bumblebee to bioeconomy: Recent developments and perspectives for Sophorolipid biosynthesis. Biotechnol. Adv. 2022, 54, 107788. [Google Scholar] [CrossRef]
- Zeng, Z.; Liu, Y.; Zhong, H.; Xiao, R.; Zeng, G.; Liu, Z.; Cheng, M.; Lai, C.; Zhang, C.; Liu, G.; et al. Mechanisms for rhamnolipids-mediated biodegradation of hydrophobic organic compounds. Sci. Total Environ. 2018, 634, 1–11. [Google Scholar] [CrossRef]
- Kallimanis, A.; Frillingos, S.; Drainas, C.; Koukkou, A.I. Taxonomic identification, phenanthrene uptake activity, and membrane lipid alterations of the PAH degrading Arthrobacter sp. strain Sphe3. Appl. Microbiol. Biotechnol. 2007, 76, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Murínová, S.; Dercová, K. Response mechanisms of bacterial degraders to environmental contaminants on the level of cell walls and cytoplasmic membrane. Int. J. Microbiol. 2014, 2014, 873081. [Google Scholar] [CrossRef]
- Sana, S.; Datta, S.; Biswas, D.; Sengupta, D. Assessment of synergistic antibacterial activity of combined biosurfactants revealed by bacterial cell envelop damage. Biochim. Biophys. Acta Biomembr. 2018, 1860, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Kaczorek, E.; Pacholak, A.; Zdarta, A.; Smułek, W. The Impact of Biosurfactants on Microbial Cell Properties Leading to Hydrocarbon Bioavailability Increase. Colloids Interfaces 2018, 2, 35. [Google Scholar] [CrossRef]
- Boller, T.; Felix, G. A renaissance of elicitors: Perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu. Rev. Plant Biol. 2009, 60, 379–406. [Google Scholar] [CrossRef] [PubMed]
- Nair, D.N.; Padmavathy, S. Impact of endophytic microorganisms on plants, environment and humans. Sci. World J. 2014, 2014, 250693. [Google Scholar] [CrossRef]
- Singh, P.; Patil, Y.; Rale, V. Biosurfactant production: Emerging trends and promising strategies. J. Appl. Microbiol. 2019, 126, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Castiglioni, G.L.; Bertolin, T.E.; Costa, J.A. V Solid-state biosurfactant production by Aspergillus fumigatus using agricultural residues as substrate. Quím. Nova 2009, 32, 292–295. [Google Scholar] [CrossRef]
- Mnif, I.; Besbes, S.; Ellouze-Ghorbel, R.; Ellouze-Chaabouni, S.; Ghribi, D. Improvement of bread dough quality by Bacillus subtilis SPB1 biosurfactant addition: Optimized extraction using response surface methodology. J. Sci. Food Agric. 2013, 93, 3055–3064. [Google Scholar] [CrossRef]
- Velioglu, Z.; Urek, R.O. Optimization of cultural conditions for biosurfactant production by Pleurotus djamor in solid state fermentation. J. Biosci. Bioeng. 2015, 120, 526–531. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, F.; Wei, Z.; Ran, W.; Shen, Q. The usage of rice straw as a major substrate for the production of surfactin by Bacillus amyloliquefaciens XZ-173 in solid-state fermentation. J. Environ. Manag. 2013, 127, 96–102. [Google Scholar] [CrossRef]
- Ghribi, D.; Abdelkefi-Mesrati, L.; Mnif, I.; Kammoun, R.; Ayadi, I.; Saadaoui, I.; Maktouf, S.; Chaabouni-Ellouze, S. Investigation of antimicrobial activity and statistical optimization of Bacillus subtilis SPB1 biosurfactant production in solid-state fermentation. BioMed Res. Int. 2012, 2012, 373682. [Google Scholar] [CrossRef]
- Jiménez-Peñalver, P.; Castillejos, M.; Koh, A.; Gross, R.; Sánchez, A.; Font, X.; Gea, T. Production and characterization of sophorolipids from stearic acid by solid-state fermentation, a cleaner alternative to chemical surfactants. J. Clean. Prod. 2018, 172, 2735–2747. [Google Scholar] [CrossRef]
- Nalini, S.; Parthasarathi, R.; Prabudoss, V. Production and characterization of lipopeptide from Bacillus cereus SNAU01 under solid state fermentation and its potential application as anti-biofilm agent. Biocatal. Agric. Biotechnol. 2016, 5, 123–132. [Google Scholar] [CrossRef]
- Kiran, G.S.; Thomas, T.A.; Selvin, J.; Sabarathnam, B.; Lipton, A.P. Optimization and characterization of a new lipopeptide biosurfactant produced by marine Brevibacterium aureum MSA13 in solid state culture. Bioresour. Technol. 2010, 101, 2389–2396. [Google Scholar] [CrossRef]
- Zouari, R.; Ellouze-Chaabouni, S.; Ghribi-Aydi, D. Optimization of Bacillus subtilis SPB1 biosurfactant production under solid-state fermentation using by-products of a traditional olive mill factory. Achiev. Life Sci. 2014, 8, 162–169. [Google Scholar] [CrossRef]
- Nalini, S.; Parthasarathi, R. Optimization of rhamnolipid biosurfactant production from Serratia rubidaea SNAU02 under solid-state fermentation and its biocontrol efficacy against Fusarium wilt of eggplant. Ann. Agrar. Sci. 2018, 16, 108–115. [Google Scholar] [CrossRef]
- Slivinski, C.T.; Mallmann, E.; de Araújo, J.M.; Mitchell, D.A.; Krieger, N. Production of surfactin by Bacillus pumilus UFPEDA 448 in solid-state fermentation using a medium based on okara with sugarcane bagasse as a bulking agent. Process Biochem. 2012, 47, 1848–1855. [Google Scholar] [CrossRef]
- Banat, I.M.; Carboue, Q.; Saucedo-Castaneda, G.; de Jesus Cazares-Marinero, J. Biosurfactants: The green generation of speciality chemicals and potential production using Solid-State fermentation (SSF) technology. Bioresour. Technol. 2021, 320, 124222. [Google Scholar] [CrossRef]
- Franco Marcelino, P.R.; da Silva, V.L.; Rodrigues Philippini, R.; Von Zuben, C.J.; Contiero, J.; Dos Santos, J.C.; da Silva, S.S. Biosurfactants produced by Scheffersomyces stipitis cultured in sugarcane bagasse hydrolysate as new green larvicides for the control of Aedes aegypti, a vector of neglected tropical diseases. PLoS ONE 2017, 12, e0187125. [Google Scholar] [CrossRef]
- Barbosa, F.G.; Ribeaux, D.R.; Rocha, T.; Costa, R.A.; Guzmán, R.R.; Marcelino, P.R.; Lacerda, T.M.; Silva, S.S.D. Biosurfactants: Sustainable and versatile molecules. J. Braz. Chem. Soc. 2022, 33, 870–893. [Google Scholar] [CrossRef]
- Mnif, I.; Ghribi, D. Glycolipid biosurfactants: Main properties and potential applications in agriculture and food industry. J. Sci. Food Agric. 2016, 96, 4310–4320. [Google Scholar] [CrossRef]
- Hultberg, M.; Bergstrand, K.J.; Khalil, S.; Alsanius, B. Characterization of biosurfactant-producing strains of fluorescent pseudomonads in a soilless cultivation system. Antonie van Leeuwenhoek 2008, 94, 329–334. [Google Scholar] [CrossRef]
- Debode, J.; Maeyer, K.D.; Perneel, M.; Pannecoucque, J.; Backer, G.D.; Höfte, M. Biosurfactants are involved in the biological control of Verticillium microsclerotia by Pseudomonas spp. J. Appl. Microbiol. 2007, 103, 1184–1196. [Google Scholar] [CrossRef]
- Sachdev, S.; Bauddh, K.; Singh, R.P. Prospective of biosurfactant in management of fusarium wilt and early blight of Lycopersicon esculentum. Plant Stress 2023, 7, 100126. [Google Scholar] [CrossRef]
- Hoff, G.; Arguelles Arias, A.; Boubsi, F.; Pršić, J.; Meyer, T.; Ibrahim, H.M.; Steels, S.; Luzuriaga, P.; Legras, A.; Franzil, L.; et al. Surfactin stimulated by pectin molecular patterns and root exudates acts as a key driver of the Bacillus-plant mutualistic interaction. mBio 2021, 12, e01774-21. [Google Scholar] [CrossRef]
- Çelik, P.A.; Manga, E.B.; Çabuk, A.; Banat, I.M. Biosurfactants’ Potential Role in Combating COVID-19 and Similar Future Microbial Threats. Appl. Sci. 2021, 11, 334. [Google Scholar] [CrossRef]
- Smith, M.L.; Gandolfi, S.; Coshall, P.M.; Rahman, P.K. Biosurfactants: A Covid-19 perspective. Front. Microbiol. 2020, 11, 1341. [Google Scholar] [CrossRef]
- Antony, S.; Sukumaran, T.U.; Rathinam, P.; Reshmy, R.; Binod, P.; Pandey, A.; Sindhu, R. Biosurfactants in respiratory viruses and the Coronavirus disease 2019 pandemic. In Green Sustainable Process for Chemical and Environmental Engineering and Science; Inamuddin Adetunji, C.O., Ahamed, M.I., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 439–450. [Google Scholar] [CrossRef]
- Raza, Z.A.; Shahzad, Q.; Rehman, A.; Taqi, M.; Ayub, A. Biosurfactants in the sustainable eradication of SARS COV-2 from the environmental surfaces. 3 Biotech 2022, 12, 273. [Google Scholar] [CrossRef]
- Rybicki, E.P. A Top Ten list for economically important plant viruses. Arch. Virol. 2015, 160, 17–20. [Google Scholar] [CrossRef]
- Islam, W.; Adnan, M.; Tayyab, M.; Hussain, M.; Islam, S.U. Phyto-metabolites; an impregnable shield against plant viruses. Nat. Prod. Commun. 2018, 13, 1934578X1801300131. [Google Scholar] [CrossRef]
- Crouzet, J.; Arguelles-Arias, A.; Dhondt-Cordelier, S.; Cordelier, S.; Pršić, J.; Hoff, G.; Mazeyrat-Gourbeyre, F.; Baillieul, F.; Clément, C.; Ongena, M.; et al. Biosurfactants in plant protection against diseases: Rhamnolipids and lipopeptides case study. Front. Bioeng. Biotechnol. 2020, 8, 1014. [Google Scholar] [CrossRef]
- Manjunatha, L.; Rajashekara, H.; Uppala, L.S.; Ambika, D.S.; Patil, B.; Shankarappa, K.S.; Nath, V.S.; Kavitha, T.R.; Mishra, A.K. Mechanisms of Microbial Plant Protection and Control of Plant Viruses. Plants 2022, 11, 3449. [Google Scholar] [CrossRef]
- Raheel, M.; Aatif, H.M.; Ali, S.; Shakeel, Q.; Ahmad, A.; Shaheen, M.R. Biological control activity of biosurfactant against plant pathogens. In Applications of Biosurfactant in Agriculture; Inamuddin Adetunji, C.O., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 17–28. [Google Scholar] [CrossRef]
- Tatineni, S.; Hein, G.L. Plant viruses of agricultural importance: Current and future perspectives of virus disease management strategies. Phytopathology® 2023, 113, 117–141. [Google Scholar] [CrossRef]
- Csukasi, F.; Merchante, C.; Valpuesta, V. Modification of plant hormone levels and signaling as a tool in plant biotechnology. Biotechnol. J. 2009, 4, 1293–1304. [Google Scholar] [CrossRef]
- De Oliveira, J.; Rodrigues, C.; Vandenberghe, L.P.S.; Câmara, M.C.; Libardi, N.; Soccol, C.R. Gibberellic Acid Production by Different Fermentation Systems Using Citric Pulp as Substrate/Support. BioMed Res. Int. 2017, 2017, 5191046. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, J.; Lu, W.; Deng, D. Gibberellin in plant height control: Old player, new story. Plant Cell Rep. 2017, 36, 391–398. [Google Scholar] [CrossRef]
- Urbanova, T.; Leubner-Metzger, G. Gibberellins and seed germination. In Annual Plant Reviews; Hedden, P., Thomas, S.G., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2016; Volume 49, pp. 253–284. [Google Scholar] [CrossRef]
- Bao, S.; Hua, C.; Shen, L.H.Y. New insights into gibberellin signaling in regulating flowering in Arabidopsis. J. Integr. Plant Biol. 2020, 62, 118–131. [Google Scholar] [CrossRef]
- Pawełkowicz, M.E.; Skarzyńska, A.; Pląder, W.; Przybecki, Z. Genetic and molecular bases of cucumber (Cucumis sativus L.) sex determination. Mol. Breed. 2019, 39, 50. [Google Scholar] [CrossRef]
- Jan, S.; Abbas, N.; Ashraf, M.; Ahmad, P. Roles of potential plant hormones and transcription factors in controlling leaf senescence and drought tolerance. Protoplasma 2019, 256, 313–329. [Google Scholar] [CrossRef]
- Ning, H.T.; Subroto, G. Effect of Hormone Concentration and frequency of administration of Gibberellins on Growth and Yield of Tomato Fruit. Agric. Sci. 2018, 1, 104–115. [Google Scholar]
- Regnault, T.; Davière, J.M.; Wild, M.; Sakvarelidze-Achard, L.; Heintz, D.; Carrera Bergua, E.; Diaz, I.L.; Gong, F.; Hedden, P.; Achard, P. The gibberellin precursor GA12 acts as a long-distance growth signal in Arabidopsis. Nat. Plants 2015, 1, 15073. [Google Scholar] [CrossRef]
- Pimenta Lange, M.J.; Lange, T. Ovary-derived precursor gibberellin A9 is essential for female flower development in cucumber. Development 2016, 143, 4425–4429. [Google Scholar] [CrossRef]
- Band, L.R.; Úbeda-Tomás, S.; Dyson, R.J.; Middleton, A.M.; Hodgman, T.C.; Owen, M.R.; Jensen, O.E.; Bennett, M.J.; King, J.R. Growth-induced hormone dilution can explain the dynamics of plant root cell elongation. Proc. Natl. Acad. Sci. USA 2012, 109, 7577–7582. [Google Scholar] [CrossRef]
- Binenbaum, J.; Weinstain, R.; Shani, E. Gibberellin localization and transport in plants. Trends Plant Sci. 2018, 23, 410–421. [Google Scholar] [CrossRef]
- Li, H.; Wu, H.; Qi, Q.; Li, H.; Li, Z.; Chen, S.; Ding, Q.; Wang, Q.; Yan, Z.; Gai, Y.; et al. Gibberellins Play a Role in Regulating Tomato Fruit Ripening. Plant Cell Physiol. 2019, 60, 1619–1629. [Google Scholar] [CrossRef]
- Mushtaq, S.; Amjad, M.; Ziaf, K.; Afzal, I. Gibberellins application timing modulates growth, physiology, and quality characteristics of two onion (Allium cepa L.) cultivars. Environ. Sci. Pollut. Res. 2018, 25, 25155–25161. [Google Scholar] [CrossRef]
- Chen, S.; Wang, X.J.; Tan, G.F.; Zhou, W.Q.; Wang, G.L. Gibberellin and the plant growth retardant Paclobutrazol altered fruit shape and ripening in tomato. Protoplasma 2020, 257, 853–861. [Google Scholar] [CrossRef]
- Kang, S.M.; Radhakrishnan, R.; You, Y.H.; Khan, A.L.; Park, J.M.; Lee, S.M.; Lee, I.J. Cucumber performance is improved by inoculation with plant growth-promoting microorganisms. Acta Agric. Scand. Sect. B Soil Plant Sci. 2015, 65, 36–44. [Google Scholar] [CrossRef]
- Lima, S.F.; Jesus, A.A.; Vendruscolo, E.P.; Oliveira, T.R.; Maria Andrade, G.O.; Simon, C.A. Development and production of sweet corn applied with biostimulant as seed treatment. Hortic. Bras. 2019, 6, 94–100. [Google Scholar] [CrossRef]
- Oliveira, F.d.A.; de Medeiros, J.F.; de Cunha, R.C.; da Souza, M.W.d.L.; Lima, L.A. Use of biostimulants in relieving salt stress in popcorn. Rev. Ciênc. Agron. 2016, 47, 307–315. [Google Scholar] [CrossRef][Green Version]
- Hamayun, M.; Hussain, A.; Khan, S.A.; Kim, H.Y.; Khan, A.L.; Waqas, M.; Irshad, M.; Iqbal, A.; Rehman, G.; Jan, S.; et al. Gibberellins producing endophytic fungus Porostereum spadiceum AGH786 rescues growth of salt affected soybean. Front. Microbiol. 2017, 8, 686. [Google Scholar] [CrossRef]
- Peres, A.R.; Kappes, C.; Portugal, J.R.; de Sá, M.E.; Meirelles, F.C. Physiological quality of soybean seeds with application of biostimulant. Aust. J. Crop Sci. 2017, 11, 162–168. [Google Scholar] [CrossRef]
- de Moraes, E.R.; Mageste, J.G.; Lana, R.M.Q.; da Silva, R.V.; de Camargo, R. Sugarcane: Organo-Mineral Fertilizers and Biostimulants. In Sugarcane—Technology and Research; de Oliveira, A.B., Ed.; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Nguyen, C.T.; Dang, L.H.; Nguyen, D.T.; Tran, K.P.; Giang, B.L.; Tran, N.Q. Effect of GA3 and Gly plant growth regulators on productivity and sugar content of sugarcane. Agriculture 2019, 9, 136. [Google Scholar] [CrossRef]
- Hussain, N.; Anwar, A.; Yasmeen, A.; Arif, M.; Naz, S.; Bibi, M.; Iqbal, J.; Qadir, I.; Salim, M.N.; Latif, S. Resource use efficiency of cotton in improved vs conventional planting geometry with exogenous application of bio-stimulant and synthetic growth retardant. Braz. J. Biol. 2020, 6984, 18–26. [Google Scholar] [CrossRef]
- Alvarenga, R.; Moraes, J.C.; Auad, A.M.; Coelho, M.; Nascimento, A.M. Induction of resistance of corn plants to Spodoptera frugiperda (J. E. Smith, 1797) (Lepidoptera: Noctuidae) by application of silicon and gibberellic acid. Bull. Entomol. Res. 2017, 107, 527–533. [Google Scholar] [CrossRef]
- Berli, F.J.; Pharis, R.P.; Bottini, A.R.; Alonso, R.E. Use of Gibberellin A5 to Increase the Yield and Quality of Wine Grapes. U.S. Patent Application No. 16/138,335, 24 January 2019. [Google Scholar]
- Grand View Research. Available online: https://www.grandviewresearch.com/press-release/global-gibberellins-market (accessed on 18 December 2023).
- Rodrigues, C.; Vandenberghe, L.P.D.S.; Teodoro, J.; Oss, J.F.; Pandey, A.; Soccol, C.R. A new alternative to produce gibberellic acid by solid state fermentation. Braz. Arch. Biol. Technol. 2009, 52, 181–188. [Google Scholar] [CrossRef]
- Satpute, D.; Sharma, V.; Murarkar, K.; Bhotmange, M.; Dharmadhikari, D. Solid-state fermentation for production of gibberellic acid using agricultural residues. Int. J. Environ. Pollut. 2010, 43, 201–213. [Google Scholar] [CrossRef]
- Kumar, P.K.R.; Lonsane, B.K. Solid state fermentation: Physical and nutritional factors influencing gibberellic acid production. Appl. Microbiol. Biotechnol. 1990, 34, 145–148. [Google Scholar] [CrossRef]
- Piccoli, P.; Lucangeli, C.D.; Bottini, R.; Schneider, G. Hydrolysis of [17, 17–2H2] gibberellin A 20-glucoside and [17, 17–2H2] gibberellin A 20-glucosyl ester by Azospirillum lipoferum cultured in a nitrogen-free biotin-based chemically-defined medium. Plant Growth Regul. 1997, 23, 179–182. [Google Scholar] [CrossRef]
- Dobert, R.C.; Rood, S.B.; Blevins, D.G. Gibberellins and the legume-rhizobium symbiosis: I. Endogenous gibberellins of lima bean (Phaseolus lunatus L.) stems and nodules. Plant Physiol. 1992, 98, 221–224. [Google Scholar] [CrossRef]
- Rhouma, M.; Ben Kriaa, M.; Nasr, Y.; Ben Mellouli, L.; Kammoun, R. A New Endophytic Fusarium Oxysporum Gibberellic Acid: Optimization of Production Using Combined Strategies of Experimental Designs and Potency on Tomato Growth under Stress Condition. BioMed Res. Int. 2020, 2020, 4587148. [Google Scholar] [CrossRef]
- Corona, A.; Sáez, D.; Agosin, E. Effect of water activity on gibberellic acid production by Gibberella fujikuroi under solid-state fermentation conditions. Process Biochem. 2005, 40, 2655–2658. [Google Scholar] [CrossRef]
- Kumar, P.K.R.; Lonsane, B.K. Immobilized growing cells of Gibberella fujikuroi P-3 for production of gibberellic acid and pigment in batch and semi-continuous cultures. Appl. Microbiol. Biotechnol. 1988, 28, 537–542. [Google Scholar] [CrossRef]
- Pastrana, L.M.; Gonzalez, M.; Pintado, J.; Murado, M.A. Interactions affecting gibberellic acid production in solid-state culture: A factorial study. Enzym. Microb. Technol. 1995, 17, 784–790. [Google Scholar] [CrossRef]
- Tomasini, A.; Fajardo, C.; Barrios-González, J. Gibberellic acid production using different solid-state fermentation systems. World J. Microbiol. Biotechnol. 1997, 13, 203–206. [Google Scholar] [CrossRef]
- Machado, C.M.; Soccol, C.R.; de Oliveira, B.H.; Pandey, A. Gibberellic acid production by solid-state fermentation in coffee husk. Appl. Biochem. Biotechnol. 2002, 102, 179–191. [Google Scholar] [CrossRef]
- Camara, M.C.; Vandenberghe, L.P.; Rodrigues, C.; de Oliveira, J.; Faulds, C.; Bertrand, E.; Soccol, C.R. Current advances in gibberellic acid (GA 3) production, patented technologies and potential applications. Planta 2018, 248, 1049–1062. [Google Scholar] [CrossRef] [PubMed]
- Panchal, R.R.; Desai, P.V. Study of Gibberellic Acid Production by Solid State Fermentation Using Fusarium Moniliforme Sheldon. Int. J. Appl. Sci. Biotechnol. 2016, 4, 402–407. [Google Scholar] [CrossRef]
- Rodrigues, C.; Vandenberghe, L.P.; Goyzueta, L.D.; Soccol, C.R. Production, extraction and purification of gibberellic acid by solid state fermentation using citric pulp and soy husk. BAOJ Chem. 2016, 2, 14. [Google Scholar]
- Pinheiro, U.V.; Wancura, J.H.; Brondani, M.; da Silva, C.M.; Mainardi, M.A.; Gai, R.M.; Jahn, S.L. Production of Gibberellic Acid by Solid-State Fermentation Using Wastes from Rice Processing and Brewing Industry. Appl. Biochem. Biotechnol. 2023, 1–16. [Google Scholar] [CrossRef] [PubMed]
- El-Sheikh, M.A.; Rajaselvam, J.; Abdel-Salam, E.M.; Vijayaraghavan, P.; Alatar, A.A.; Biji, G.D. Paecilomyces sp. ZB is a cell factory for the production of gibberellic acid using a cheap substrate in solid state fermentation. Saudi J. Biol. Sci. 2020, 27, 2431–2438. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Liu, F.; Li, S.; Li, P.; Chang, C.; Fang, S. Solid-state Fermentation Process for Gibberellin Production Using Enzymatic Hydrolysate Corn Stalks. BioResources 2020, 15, 429–443. [Google Scholar] [CrossRef]
- Werle, L.B.; Abaide, E.R.; Felin, T.H.; Kuhn, K.R.; Tres, M.V.; Zabot, G.L.; Kuhl, R.C.; Jahn, S.L.; Mazutti, M.A. Gibberellic acid production from Gibberella fujikuroi using agro-industrial residues. Biocatal. Agric. Biotechnol. 2020, 25, 101608. [Google Scholar] [CrossRef]
- Azcón, R.; Barea, J.M. Synthesis of auxins, gibberellins and cytokinins by Azotobacter vinelandii and Azotobacter beijerinckii related to effects produced on tomato plants. Plant Soil 1975, 43, 609–619. [Google Scholar] [CrossRef]
- Kasahara, H. Current aspects of auxin biosynthesis in plants. Biosci. Biotechnol. Biochem. 2016, 80, 34–42. [Google Scholar] [CrossRef]
- Enders, T.A.; Strader, L.C. Auxin activity: Past, present, and future. Am. J. Bot. 2015, 102, 180–196. [Google Scholar] [CrossRef]
- Nanda, A.K.; Melnyk, C.W. The role of plant hormones during grafting. J. Plant Res. 2018, 131, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Bruno, L.; Pacenza, M.; Forgione, I.; Lamerton, L.R.; Greco, M.; Chiappetta, A.; Bitonti, M.B. In Arabidopsis thaliana cadmium impact on the growth of primary root by altering SCR expression and auxin-cytokinin cross-talk. Front. Plant Sci. 2017, 8, 1323. [Google Scholar] [CrossRef] [PubMed]
- Fukaki, H.; Okushima, Y.; Tasaka, M. Auxin-mediated lateral root formation in higher plants. Int. Rev. Cytol. 2007, 256, 111–137. [Google Scholar] [CrossRef] [PubMed]
- Fukaki, H.; Tasaka, M. Hormone interactions during lateral root formation. Plant Mol. Biol. 2009, 69, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Jing, H.; Strader, L.C. Interplay Auxin and Cytokinin in Lateral Root Development. Int. J. Mol. Sci. 2019, 20, 486. [Google Scholar] [CrossRef] [PubMed]
- Cucinotta, M.; Manrique, S.; Guazzotti, A.; Quadrelli, N.E.; Mendes, M.A.; Benkova, E.; Colombo, L. Cytokinin response factors integrate auxin and cytokinin pathways for female reproductive organ development. Development 2016, 143, 4419–4424. [Google Scholar] [CrossRef]
- Heisler, M.G.; Byrne, M.E. Progress in understanding the role of auxin in lateral organ development in plants. Curr. Opin. Plant Biol. 2020, 53, 73–79. [Google Scholar] [CrossRef]
- Harmer, S.L.; Brooks, C.J. Growth-mediated plant movements: Hidden in plain sight. Curr. Opin. Plant Biol. 2018, 41, 89–94. [Google Scholar] [CrossRef]
- Nakajima, Y.; Nara, Y.; Kobayashi, A.; Sugita, T.; Miyazawa, Y.; Fujii, N.; Takahashi, H. Auxin transport and response requirements for root hydrotropism differ between plant species. J. Exp. Bot. 2017, 68, 3441–3456. [Google Scholar] [CrossRef] [PubMed]
- Barbier, F.F.; Dun, E.A.; Beveridge, C.A. Apical dominance. Curr. Biol. 2017, 27, R864–R865. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Takei, K.; Kojima, M.; Sakakibara, H.; Mori, H. Auxin controls local cytokinin biosynthesis in the nodal stem in apical dominance. Plant J. 2006, 45, 1028–1036. [Google Scholar] [CrossRef]
- Solangi, S.K.; Qureshi, S.T.; Khan, I.A.; Raza, S. Establishment of in vitro callus in sugarcane (Saccharum officinarum L.) varieties influenced by different auxins. Afr. J. Biotechnol. 2016, 15, 1541–1550. [Google Scholar] [CrossRef]
- Ahmed, A.A.A.; Alkharpotly, A.A.; Gabal, A.A.A.; Abido, A.I.A. Potato growth and yield as affected by foliar application with NAA auxin and 6-BA cytokinin. J. Plant Prod. 2021, 12, 591–596. [Google Scholar] [CrossRef]
- Pramanik, K.; Mohapatra, P.P. Role of auxin on growth, yield and quality of tomato-A review. Int. J. Curr. Microbiol. Appl. Sci 2017, 6, 1624–1636. [Google Scholar] [CrossRef]
- Noor, A.; Ziaf, K.; Naveed, M.; Khan, K.S.; Ghani, M.A.; Ahmad, I.; Anwar, R.; Siddiqui, M.H.; Shakeel, A.; Khan, A.I. L-Tryptophan-Dependent Auxin-Producing Plant-Growth-Promoting Bacteria Improve Seed Yield and Quality of Carrot by Altering the Umbel Order. Horticulturae 2023, 9, 954. [Google Scholar] [CrossRef]
- Balliu, A.B.; Sallaku, G. Exogenous auxin improves root morphology and restores growth of grafted cucumber seedlings. Hortic. Sci. 2017, 44, 82–90. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Petropoulos, S.A.; Sun, W. Survey of the Influences of Microbial Biostimulants on Horticultural Crops: Case Studies and Successful Paradigms. Horticulturae 2023, 9, 193. [Google Scholar] [CrossRef]
- Koza, N.A.; Adedayo, A.A.; Babalola, O.O.; Kappo, A.P. Microorganisms in Plant Growth and Development: Roles in Abiotic Stress Tolerance and Secondary Metabolites Secretion. Microorganisms 2022, 10, 1528. [Google Scholar] [CrossRef] [PubMed]
- Jankovska-Bortkevič, E.; Katerova, Z.; Todorova, D.; Jankauskienė, J.; Mockevičiūtė, R.; Sergiev, I.; Jurkonienė, S. Effects of Auxin-Type Plant Growth Regulators and Cold Stress on the Endogenous Polyamines in Pea Plants. Horticulturae 2023, 9, 244. [Google Scholar] [CrossRef]
- Epp, J.B.; Alexander, A.L.; Balko, T.W.; Buysse, A.M.; Brewster, W.K.; Bryan, K.; Daeuble, J.F.; Fields, S.C.; Gast, R.E.; Green, R.A.; et al. The discovery of Arylex™ active and Rinskor™ active: Two novel auxin herbicides. Bioorganic Med. Chem. 2016, 24, 362–371. [Google Scholar] [CrossRef]
- Matsuda, R.; Handayani, M.L.; Sasaki, H.; Takechi, K.; Takano, H.; Takio, S. Production of indoleacetic acid by strains of the epiphytic bacteria Neptunomonas spp. isolated from the red alga Pyropia yezoensis and the seagrass Zostera marina. Arch. Microbiol. 2018, 200, 255–265. [Google Scholar] [CrossRef]
- Prinsen, E.; Costacurta, A.; Michiels, K.; Vanderleyden, J.; Van Onckelen, H. Azospirillum brasilense indole-3-acetic acid biosynthesis: Evidence for a non-tryptophan dependent pathway. Mol. Plant Microbe Interact. 1993, 6, 609. [Google Scholar] [CrossRef]
- Li, M.; Guo, R.; Yu, F.; Chen, X.; Zhao, H.; Li, H.; Wu, J. Indole-3-Acetic Acid Biosynthesis Pathways in the Plant-Beneficial Bacterium Arthrobacter pascens ZZ21. Int. J. Mol. Sci. 2018, 19, 443. [Google Scholar] [CrossRef]
- Roesch, L.F.W.; de Quadros, P.D.; Camargo, F.A.; Triplett, E.W. Screening of diazotrophic bacteria Azopirillum spp. for nitrogen fixation and auxin production in multiple field sites in southern Brazil. World J. Microbiol. Biotechnol. 2007, 23, 1377–1383. [Google Scholar] [CrossRef]
- Ali, B.; Sabri, A.N.; Ljung, K.; Hasnain, S. Auxin production by plant associated bacteria: Impact on endogenous IAA content and growth of Triticum aestivum L. Lett. Appl. Microbiol. 2009, 48, 542–547. [Google Scholar] [CrossRef]
- Sardar, P.; Kempken, F. Characterization of indole-3-pyruvic acid pathway-mediated biosynthesis of auxin in Neurospora crassa. PLoS ONE 2018, 13, e0192293. [Google Scholar] [CrossRef]
- Khasin, M.; Cahoon, R.R.; Nickerson, K.W.; Riekhof, W.R. Molecular machinery of auxin synthesis, secretion, and perception in the unicellular chlorophyte alga Chlorella sorokiniana UTEX 1230. PLoS ONE 2018, 13, e0205227. [Google Scholar] [CrossRef] [PubMed]
- Keswani, C.; Singh, S.P.; Cueto, L.; García-Estrada, C.; Mezaache-Aichour, S.; Glare, T.R.; Borriss, R.; Pratap, S.; Blázquez, M.A.; Sansinenea, E. Auxins of microbial origin and their use in agriculture. Appl. Microbiol. Biotechnol. 2020, 104, 8549–8565. [Google Scholar] [CrossRef] [PubMed]
- Duca, D.; Lorv, J.; Patten, C.L.; Rose, D.; Glick, B.R. Indole-3-acetic acid in plant–microbe interactions. Antonie van Leeuwenhoek 2014, 106, 85–125. [Google Scholar] [CrossRef] [PubMed]
- Di, D.W.; Zhang, C.; Luo, P.; An, C.W.; Guo, G.Q. The biosynthesis of auxin: How many paths truly lead to IAA? Plant Growth Regul. 2016, 78, 275–285. [Google Scholar] [CrossRef]
- do Prado, D.Z.; Okino-Delgado, C.H.; Zanutto-Elgui, M.R.; da Silva, R.B.G.; Pereira, M.S.; Jahn, L.; Ludwig-Muller, J.; da Silva, M.R.; Velini, E.D.; Fleuri, L.F. Screening of Aspergillus, Bacillus and Trichoderma strains and influence of substrates on auxin and phytases production through solid-state fermentation. Biocatal. Agric. Biotechnol. 2019, 19, 101165. [Google Scholar] [CrossRef]
- Giri, R.; Meena, V.; Sharma, R.K. Production of indole acetic acid by a wood degrading fungus Phanerochaete chrysosporium. J. Food Chem. Nanotechnol. 2020, 6, 97–101. [Google Scholar] [CrossRef]
- do Prado, D.Z.; Oliveira, S.L.; Okino-Delgado, C.H.; Auer, S.; Ludwig-Müller, J.; da Silva, M.R.; Fernandes, C.J.C.; Carbonari, C.A.; Zambuzzi, W.F.; Fleuri, L.F. Aspergillus flavipes as a novel biostimulant for rooting-enhancement of Eucalyptus. J. Clean. Prod. 2019, 234, 681–689. [Google Scholar] [CrossRef]
- Elek, Á.; Borbely, M.; Győri, Z. Tryptophan content of some food raw materials. Cereal Res. Commun. 2007, 35, 373–376. [Google Scholar] [CrossRef]
- Romasi, E.F.; Lee, J. Development of indole-3-acetic acid-producing Escherichia coli by functional expression of IpdC, AspC, and Iad1. J. Microbiol. Biotechnol. 2013, 23, 1726–1736. [Google Scholar] [CrossRef]
- Malhotra, M.; Srivastava, S. An ipdC gene knock-out of Azospirillum brasilense strain SM and its implications on indole-3-acetic acid biosynthesis and plant growth promotion. Antonie van Leeuwenhoek 2008, 93, 425–433. [Google Scholar] [CrossRef]
- Kochar, M.; Upadhyay, A.; Srivastava, S. Indole-3-acetic acid biosynthesis in the biocontrol strain Pseudomonas fluorescens Psd and plant growth regulation by hormone overexpression. Res. Microbiol. 2011, 162, 426–435. [Google Scholar] [CrossRef]
- Piotrowska-Długosz, A. Significance of enzymes and their application in agriculture. In Biocatalysis: Enzymatic Basics and Applications; Husain, Q., Ullah, M., Eds.; Springer: Cham, Switzerland, 2019; pp. 277–308. [Google Scholar] [CrossRef]
- Sobucki, L.; Ramos, R.F.; Meireles, L.A.; Antoniolli, Z.I.; Jacques, R.J.S. Contribution of enzymes to soil quality and the evolution of research in Brazil. Rev. Bras. Ciênc. Solo 2021, 45, e0210109. [Google Scholar] [CrossRef]
- Arya, P.S.; Yagnik, S.M.; Raval, V.H. Role of microbial enzymes in agricultural industry. In Biotechnology of Microbial Enzymes, Brahmachari; Academic Press: Cambridge, MA, USA, 2023; pp. 525–550. [Google Scholar] [CrossRef]
- Nagpal, S.; Sharma, P.; Kumawat, K.C. Microbial bioformulations: Revisiting role in sustainable agriculture. In Biofertilizers; Rakshit, A., Meena, V.S., Parihar, M., Singh, H.B., Singh, A.K., Eds.; Woodhead Publishing: Sawston, UK, 2021; pp. 329–346. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).