Application of Granular Microbial Preparation and Silicon Dioxide Analcime for Bioremediation of Ecocide Areas
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characteristics of the Granular Microbial Preparation
2.2. The Characteristics of the Analcime Preparation
2.3. Model Soils
2.4. Model Solid Waste
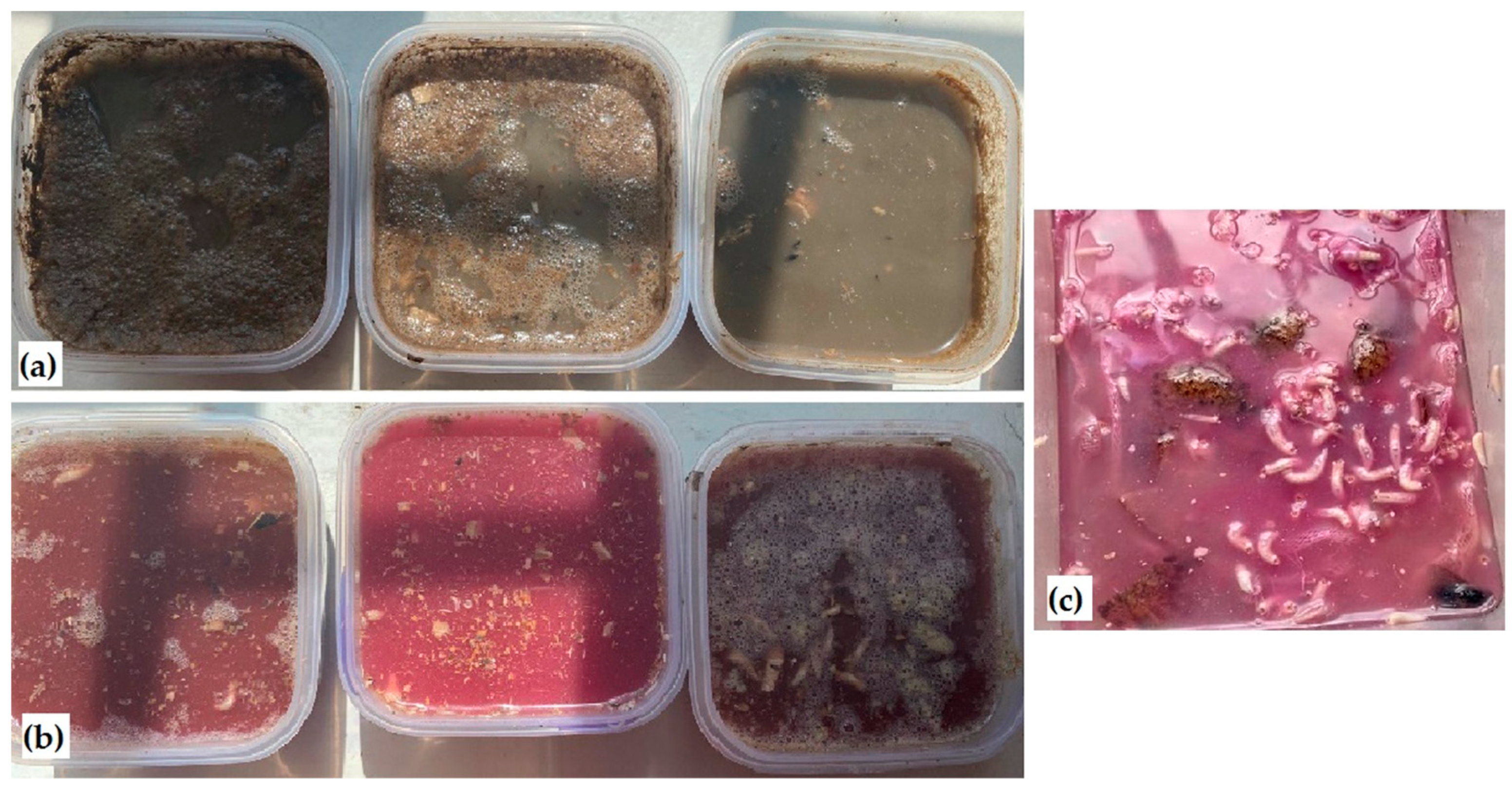
2.5. Creation of Model Ecosystems
2.6. Measurement of the Main Metabolic Parameters of Anaerobic Degradation of Organic Waste
2.7. Calculation of Fermentation Parameters to Evaluate the Efficiency of Decomposition of Model Organic Waste by GMP
2.8. Assessment of the Direct Toxicity of the Obtained Soil Mixtures Using Biological Tests with Plants of Cucumis sativus «Konkurent» and Amaranthus caudatus L.
2.9. Data Analysis
3. Results
3.1. Dynamics of Metabolic Parameters during the Degradation of Solid Organic Waste by the GMP
3.2. Efficiency of Model Waste Degradation via the GMP
3.3. Evaluation of the Toxicity of Obtained Soil Mixtures Using Biological Tests with Plants
4. Discussion
- (1)
- Aerobic microorganisms consume organic compounds and create anaerobic (oxygen-free) conditions: CH3COOH + O2 → 2CO2 + H2O (Eh = −50…0 mV) [24];
- (2)
- Facultative anaerobic microorganisms under anaerobic conditions ferment organics and create low-potential (Eh = −300…−200 mV) obligate anaerobic conditions through the formation of reducing agents, for example, NH2-CH-SH2-COOH → H2 + NH3 + CO2 + H2O + S2− (Eh = −250 mV) [25];
- (3)
- Obligate anaerobic hydrolytic bacteria (Clostridium and others) perform the fermentation of animal and plant polymers with a significant reduction in their volume and weight [26]:
- Plant polymers: [C6H12O6]n → N·C6H12O6 + 2H2O → 2CH3COOH + 2CO2 + 4H2 (Eh = −280 mV);
- Animal polymers: [COOH-R-NH2]·n → N·COOH-R-NH2 → H2 + CO2 + NH4+ + NH3 (Eh = −250 mV);
- (4)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gross, M. Wars leave nature on the losing side. Feature 2023, 33, 879–881. [Google Scholar] [CrossRef]
- Dumont, A. A “Clear” War Crime against the Environment? The Destruction of the Nova Kakhovka Dam; Völkerrechtsblog: Berlin, Germany, 2023. [Google Scholar] [CrossRef]
- Gillett, M. The Kakhovka Dam and Ecocide: A Convergence of International Criminal Law, International Humanitarian Law, International Environmental Law, and International Human Rights Law? Verfassungsblog 2023. Available online: https://verfassungsblog.de/the-kakhovka-dam-and-ecocide/ (accessed on 13 October 2023).
- Nielsen, C.R. Ukraine, Ecocide, and Thinking about Environmental Justice in a Time of War. 2023. Available online: https://www.academia.edu/105081787/Ukraine_Ecocide_and_Thinking_About_Environmental_Justice_in_a_Time_of_War (accessed on 13 October 2023).
- Reflections on the Destruction of the Nova Kakhovka Dam from an International Law Perspective—Stavros Evdokimos Pantazopoulos. In Climate & Sustainability; Hellenic Foundation for European and Foreign Policy (ELIAMEP): Athens, Greece, 2023. Available online: https://www.eliamep.gr/wp-content/uploads/2023/06/Policy-paper-140-FINAL.pdf (accessed on 13 October 2023).
- Shumilova, O.; Tockner, K.; Sukhodolov, A. Impact of the Russia–Ukraine armed conflict on water resources and water infrastructure. Nat. Sustain. 2023, 6, 578–586. [Google Scholar] [CrossRef]
- Dupliak, V. Consequences of the Kakhovka Reservoir destruction for irrigation and water supply of the southern part of Ukraine. Probl. Water Supply Sewerage Hydraul. 2023, 44, 19–28. [Google Scholar] [CrossRef]
- Vyshnevskyi, V.; Shevchuk, S.; Komorin, V.; Oleynik, Y.; Gleick, P. The destruction of the Kakhovka dam and its consequences. Water Int. 2023, 48, 631–647. [Google Scholar] [CrossRef]
- Stokstad, E. After Ukrainian dam breach, war hampers study of ecological toll. Science 2023, 380, 1099. [Google Scholar] [CrossRef] [PubMed]
- The Extensive Destruction in Kherson Is One of the Worst Environmental Disasters in Recent Decades, Said the Ukrainian Prime Minister. Shocking Photos from the Evacuation Operation. 2023. Available online: https://www.news247.gr/kosmos/oukrania-i-xersona-vithistike-meta-tin-anatinaxi-tou-fragmatos-sigklonistika-kare-apo-tin-idatini-katastrofi/ (accessed on 13 October 2023).
- Kowalska, S. Ekobójstwo—Wielowymiarowe zagrożenie dla środowiska. Stud. Prawnoustr. 2023, 61, 145–160. [Google Scholar] [CrossRef]
- Lin, F.; Li, X.; Jia, N.; Feng, F.; Huang, H.; Huang, J.; Fan, S.; Ciais, P.; Song, X.-P. The impact of Russia-Ukraine conflict on global food security. Glob. Food Secur. 2023, 36, 100661. [Google Scholar] [CrossRef]
- Kiosya, Y. Ukrainian Zoos Amidst the War—The Threats and Challenges of Running a Zoo during the Military Conflict. J. Appl. Anim. Ethics Res. 2023, 5, 9–26. [Google Scholar] [CrossRef]
- Sanina, I.V.; Lyuta, N.G. Environmental consequences of the Kakhovka hydroelectric power plant dam explosion and ways to improve water supply to the population. Miner. Resour. Ukr. 2023, 2, 50–55. [Google Scholar]
- Xenarios, S. Water at time of war. Nat. Sustain. 2023, 6, 485–486. [Google Scholar] [CrossRef]
- Holt, E. Thousands at risk after Ukrainian dam destruction. World Rep. 2023, 401, 2028. [Google Scholar] [CrossRef]
- Korzhov, Y.; Honcharova, O. Key factors of the expected deterioration of the ecological condition of the lower Dnieper in the modern period due to the technogenic violation of the regulated river waters flow regime. Sworld-Us Conf. Proc. 2023, 1, 44–47. [Google Scholar]
- Dodds, K.; Taylor, Z.; Akbari, A.; Broto, V.C.; Detterbeck, K.; Inverardi-Ferri, C. The Russian invasion of Ukraine: Implications for politics, territory and governance. Territ. Politics Gov. 2023, 11, 1519–1536. [Google Scholar] [CrossRef]
- Chang, H.; Pallathadka, A.; Sauer, J.; Grimm, N.B.; Zimmerman, R.; Cheng, C.; Herreros-Cantis, P. Assessment of urban flood vulnerability using the social-ecological-technological systems framework in six US cities. Sustain. Cities Soc. 2021, 68, 102786. [Google Scholar] [CrossRef]
- Financial Management of Flood Risk. 2016. Available online: https://www.oecd.org/daf/fin/insurance/Financial-Management-of-Flood-Risk.pdf (accessed on 13 October 2023).
- Tashyrev, O.; Prekrasna, I. Express Method for Redox Potential and Ph Measuring in Microbial Cultures. Int. J. Bioautom. 2014, 18, 217–230. Available online: http://www.biomed.bas.bg/bioautomation/2014/vol_18.3/files/18.3_05.pdf (accessed on 13 October 2023).
- Acree, W.E. Basic Gas Chromatography (McNair, Harold M.; Miller, James M.). J. Chem. Educ. 1998, 75, 1094. [Google Scholar] [CrossRef]
- Agarwal, A.; D’Souza, P.; Johnson, T.S.; Dethe, S.M.; Chandrasekaran, C. Use of in vitro bioassays for assessing botanicals. Curr. Opin. Biotechnol. 2014, 25, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Folayan, A.; Dosunmu, A.; Oriji, A. Aerobic and anaerobic biodegradation of synthetic drilling fluids in marine deep-water offshore environments: Process variables and empirical investigations. Energy Rep. 2023, 9, 2153–2168. [Google Scholar] [CrossRef]
- Deng, L.; Liu, Y.; Wang, W. Anaerobic Digestion Microorganisms. In Biogas Technology; Springer: Singapore, 2020. [Google Scholar]
- Pereira, M.A. Anaerobic Biodegradation of Long Chain Fatty Acids Biomethanisation of Biomass-Associated LCFA as a Challenge for the Anaerobic Treatment of Effluents with High Lipid/LCFA Content. 2003. Available online: https://hdl.handle.net/1822/4650 (accessed on 13 October 2023).
- Thauer, R.K. Biochemistry of methanogenesis: A tribute to Marjory Stephenson: 1998 Marjory Stephenson prize lecture. Microbiology 1998, 144, 2377–2406. [Google Scholar] [CrossRef]
- Christy, M.; Gopinath, L.R.; Indran, D. A review on anaerobic decomposition and enhancement of biogas production through enzymes and microorganisms. Renew. Sustain. Energy Rev. 2014, 34, 167–173. [Google Scholar] [CrossRef]
- Ekta, P.; Nainesh, R.; Modi, N.; Nainesh, D.; Modi, R. A review of phytoremediation. J. Pharmacogn. Phytochem. 2018, 7, 1485–1489. Available online: https://www.phytojournal.com/archives/2018/vol7issue4/PartY/7-3-558-344.pdf (accessed on 13 October 2023).
- Xiang, L.; Harindintwali, J.D.; Wang, F.; Redmile-Gordon, M.; Chang, S.X.; Fu, Y.; He, C.; Muhoza, B.; Brahushi, F.; Bolan, N.; et al. Integrating Biochar, Bacteria, and Plants for Sustainable Remediation of Soils Contaminated with Organic Pollutants. Environ. Sci. Technol. 2022, 56, 16546–16566. [Google Scholar] [CrossRef] [PubMed]
- Kafle, A.; Timilsina, A.; Gautam, A.; Adhikari, K.; Bhattarai, A.; Aryal, N. Phytoremediation: Mechanisms, plant selection and enhancement by natural and synthetic agents. Environ. Adv. 2022, 8, 100203. [Google Scholar] [CrossRef]
- Prabakaran, K.; Li, J.; Kumar, A.; Leng, Z.; Zou, C.; Du, D. Managing environmental contamination through phytoremediation by invasive plants: A review. Ecol. Eng. 2019, 138, 28–37. [Google Scholar] [CrossRef]
- Rostami, S.; Azhdarpoor, A. The application of plant growth regulators to improve phytoremediation of contaminated soils: A review. Chemosphere 2019, 220, 818–827. [Google Scholar] [CrossRef]
- Havryliuk, O.; Hovorukha, V.; Savitsky, O.; Trilis, V.; Kalinichenko, A.; Dołhańczuk-Śródka, A.; Janecki, D.; Tashyrev, O. Anaerobic Degradation of Environmentally Hazardous Aquatic Plant Pistia stratiotes and Soluble Cu(II) Detoxification by Methanogenic Granular Microbial Preparation. Energies 2021, 14, 3849. [Google Scholar] [CrossRef]
- Havryliuk, O.; Hovorukha, V.; Gladka, G.; Tymoshenko, A.; Kyrylov, S.; Shabliy, O.; Bida, I.; Mariychuk, R.; Tashyrev, O. A Noxious Weed Ambrosia artemisiifolia L. (Ragweed) as Sustainable Feedstock for Methane Production and Metals Immobilization. Sustainability 2023, 15, 6696. [Google Scholar] [CrossRef]
- Havryliuk, O.; Hovorukha, V.; Bida, I.; Gladka, G.; Tymoshenko, A.; Kyrylov, S.; Mariychuk, R.; Tashyrev, O. Anaerobic Degradation of the Invasive Weed Solidago canadensis L. (goldenrod) and Copper Immobilization by a Community of Sulfate-Reducing and Methane-Producing Bacteria. Plants 2023, 12, 198. [Google Scholar] [CrossRef]
- Hovorukha, V.; Havryliuk, O.; Gladka, G.; Tashyrev, O.; Kalinichenko, A.; Sporek, M.; Dołhańczuk-Śródka, A. Hydrogen Dark Fermentation for Degradation of Solid and Liquid Food Waste. Energies 2021, 14, 1831. [Google Scholar] [CrossRef]
- Hovorukha, V.; Havryliuk, O.; Gladka, G.; Bida, I.; Danko, Y.; Shabliy, O.; Tashyrev, O. Gaseous fuel obtaining via fermentation of organic landfill waste. Ecol. Eng. Environ. Prot. 2021, 1, 36–48. [Google Scholar] [CrossRef]
- Zaimenko, N.; Pavliuchenko, N.; Didyk, N.; Ellanska, N.; Yunosheva, O. Application of Siliceous Mineral Analcite for Optimizing the Physiological, Biochemical, Allelopathic, and Microbiological Properties of Plant-Soil System. Sci. Innov. 2022, 18, 44–55. [Google Scholar] [CrossRef]
- Silambarasan, S.; Logeswari, P.; Valentine, A. Pseudomonas citronellolis strain SLP6 enhances the phytoremediation efficiency of Helianthus annuus in copper contaminated soils under salinity stress. Plant Soil 2020, 457, 241–253. [Google Scholar] [CrossRef]
- Zhang, X.; Su, C.; Liu, X.; Liu, Z.; Liang, X.; Zhang, Y.; Feng, Y. Effect of plant-growth-promoting rhizobacteria on phytoremediation efficiency of Scirpus triqueter in pyrene-Ni co-contaminated soils. Chemosphere 2019, 241, 125027. [Google Scholar] [CrossRef] [PubMed]
- Meifang, J.; Qiqi, L.; Meixia, Y.; Aiping, L.; Maozi, L. The physiological mechanism of the tolerance of Eichhornia crassipes (Mart.) Solms to cadmium. Int. J. Phytoremediat. 2021, 23, 1077–1084. [Google Scholar]
- Lu, H.; Xia, C.; Chinnathambi, A.; Nasif, O.; Narayanan, M.; Shanmugam, S.; Chi, N.; Pugazhendhi, A.; On-Uma, R.; Jutamas, K.; et al. Optimistic influence of multi-metal tolerant Bacillus species on phytoremediation potential of Chrysopogon zizanioides on metal contaminated soil. Chemosphere 2023, 311, 136889. [Google Scholar] [CrossRef]
- Zhou, H.; Wei, L.; Wang, D.; Zhang, W. Environmental impacts and optimizing strategies of municipal sludge treatment and disposal routes in China based on life cycle analysis. Environ. Int. 2022, 166, 107378. [Google Scholar] [CrossRef]
- Kelessidis, A.; Stasinakis, A.S. Comparative study of the methods used for treatment and final disposal of sewage sludge in European countries. Waste Manag. 2012, 32, 1186–1195. [Google Scholar] [CrossRef] [PubMed]
- Antonkiewicz, J.; Popławska, A.; Kołodziej, B.; Ciarkowska, K.; Gambuś, F.; Bryk, M.; Babula, J. Application of ash and municipal sewage sludge as macronutrient sources in sustainable plant biomass production. J. Environ. Manag. 2020, 264, 110450. [Google Scholar] [CrossRef]
- Risto, P.; Watkins, G.; Dahl, O. Characterisation of municipal sewage sludge as a soil improver and a fertilizer product. Ecol. Chem. Eng. S 2019, 26, 547–557. [Google Scholar] [CrossRef]
- Seiple, T.E.; Coleman, A.M.; Skaggs, R.L. Municipal wastewater sludge as a sustainable bioresource in the United States. J. Environ. Manag. 2017, 197, 673–680. [Google Scholar] [CrossRef]
- Świerczek, L.; Cieślik, B.M.; Konieczka, P. Challenges and opportunities related to the use of sewage sludge ash in cement-based building materials—A review. J. Clean. Prod. 2021, 287, 125054. [Google Scholar] [CrossRef]
- Li, X.; He, C.; Lv, Y.; Jian, S.; Liu, G.; Jiang, W.; Jiang, D. Utilization of municipal sewage sludge and waste glass powder in production of lightweight aggregates. Constr. Build. Mater. 2020, 256, 119413. [Google Scholar] [CrossRef]





| № | Factors | ||||
|---|---|---|---|---|---|
| Chernozem | Sand | Analcime | Fish and Potatoes | GMP | |
| 1 | + 1 | − 2 | + | + | + |
| 2 | − | + | + | + | + |
| 3 | + | + | + | + | + |
| 4 | + | − | − | + | − |
| 5 | − | + | − | + | − |
| 6 | + | + | − | + | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Havryliuk, O.; Bida, I.; Hovorukha, V.; Bielaieva, Y.; Liubinska, A.; Gladka, G.; Kalinichenko, A.; Zaimenko, N.; Tashyrev, O.; Dziuba, O. Application of Granular Microbial Preparation and Silicon Dioxide Analcime for Bioremediation of Ecocide Areas. Sustainability 2024, 16, 1097. https://doi.org/10.3390/su16031097
Havryliuk O, Bida I, Hovorukha V, Bielaieva Y, Liubinska A, Gladka G, Kalinichenko A, Zaimenko N, Tashyrev O, Dziuba O. Application of Granular Microbial Preparation and Silicon Dioxide Analcime for Bioremediation of Ecocide Areas. Sustainability. 2024; 16(3):1097. https://doi.org/10.3390/su16031097
Chicago/Turabian StyleHavryliuk, Olesia, Iryna Bida, Vira Hovorukha, Yana Bielaieva, Alla Liubinska, Galyna Gladka, Antonina Kalinichenko, Nataliia Zaimenko, Oleksandr Tashyrev, and Oksana Dziuba. 2024. "Application of Granular Microbial Preparation and Silicon Dioxide Analcime for Bioremediation of Ecocide Areas" Sustainability 16, no. 3: 1097. https://doi.org/10.3390/su16031097
APA StyleHavryliuk, O., Bida, I., Hovorukha, V., Bielaieva, Y., Liubinska, A., Gladka, G., Kalinichenko, A., Zaimenko, N., Tashyrev, O., & Dziuba, O. (2024). Application of Granular Microbial Preparation and Silicon Dioxide Analcime for Bioremediation of Ecocide Areas. Sustainability, 16(3), 1097. https://doi.org/10.3390/su16031097











