The Impact of Drying and Storage on the Characteristics of Two-Phase Olive Pomace
Abstract
1. Introduction
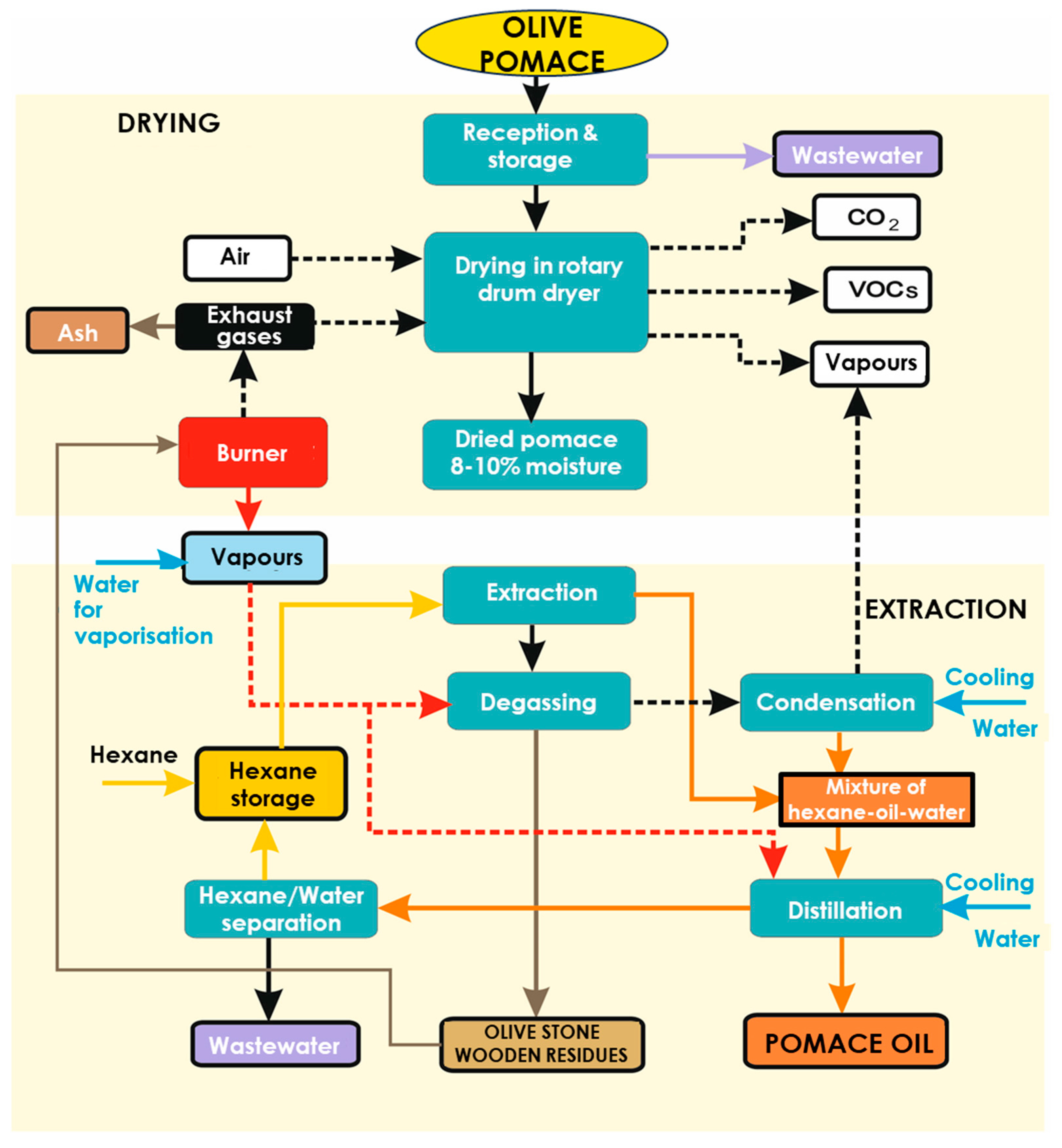
2. Materials and Methods—Methodological Approach
3. Results
3.1. Two-Phase Olive Pomace Composition
3.2. Effect of Storage and Drying on Two-Phase Olive Pomace Composition under Field Conditions
3.2.1. Evolution of Physicochemical Characteristics
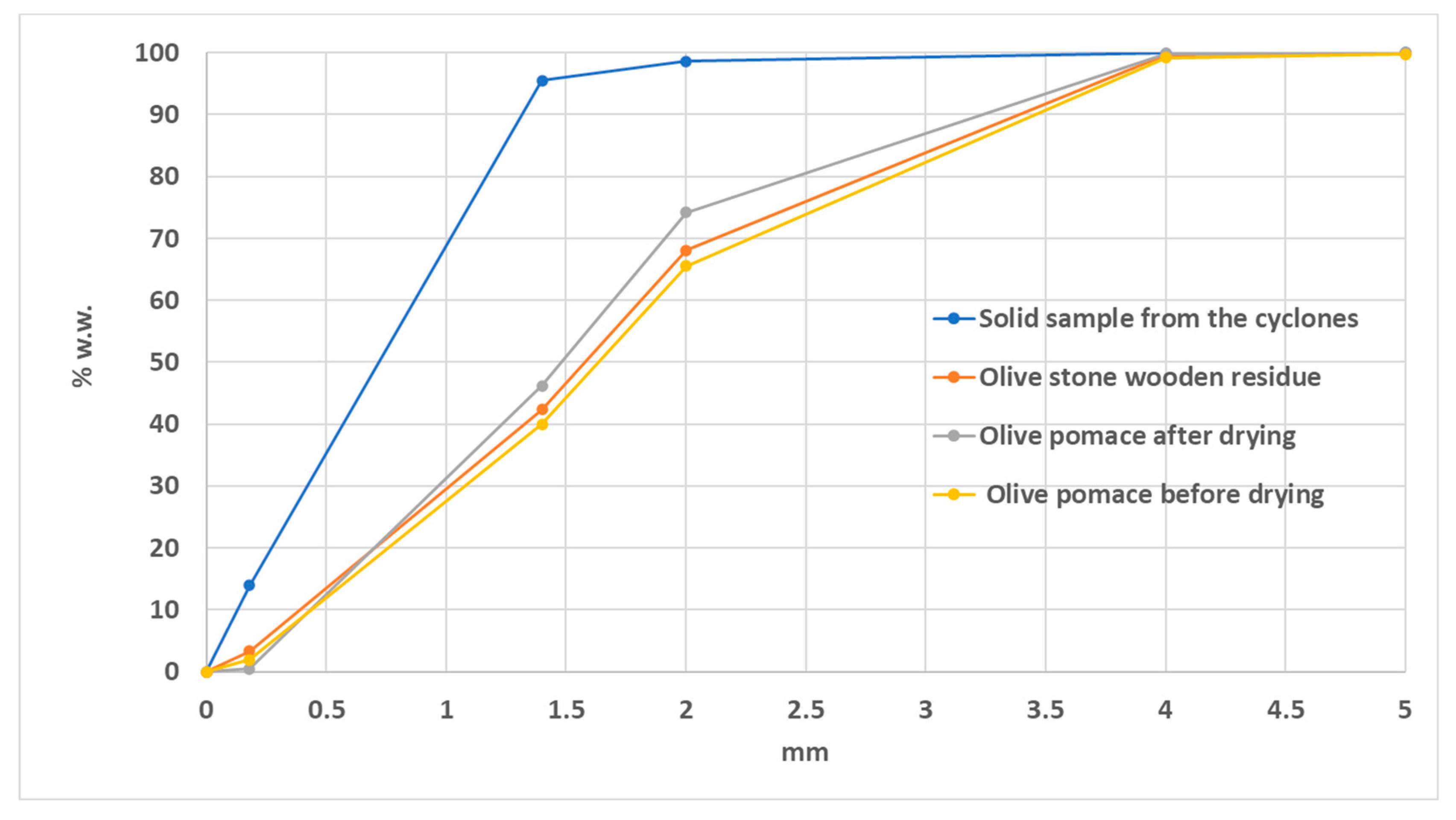
3.2.2. Particle Size Distribution
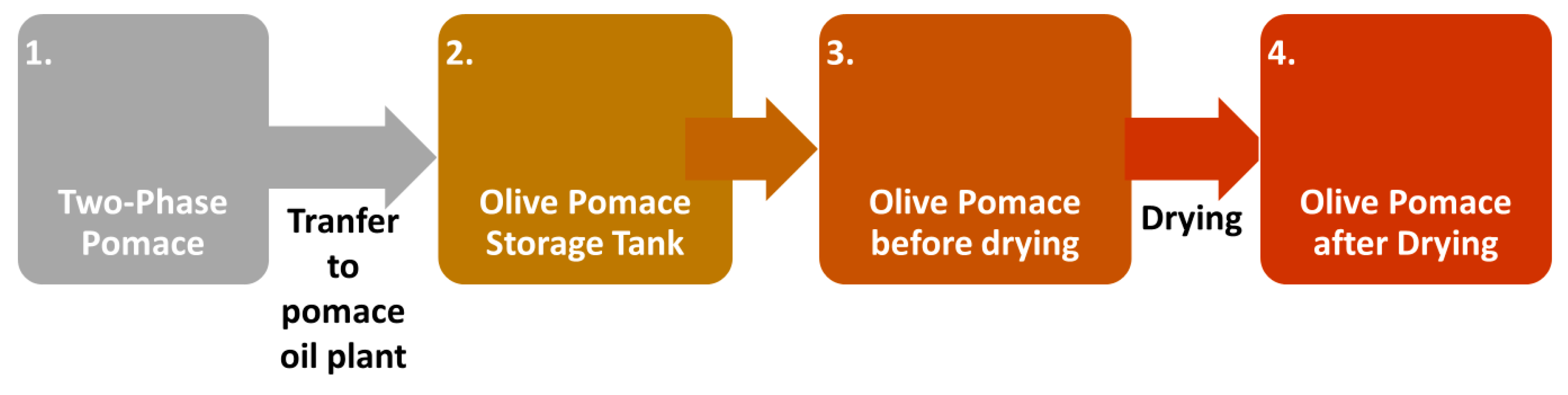
- Olive pomace before drying (Point 3).
- Olive pomace after drying (Point 4).
- Solid sample from the cyclones
- Olive stone wooden residue.
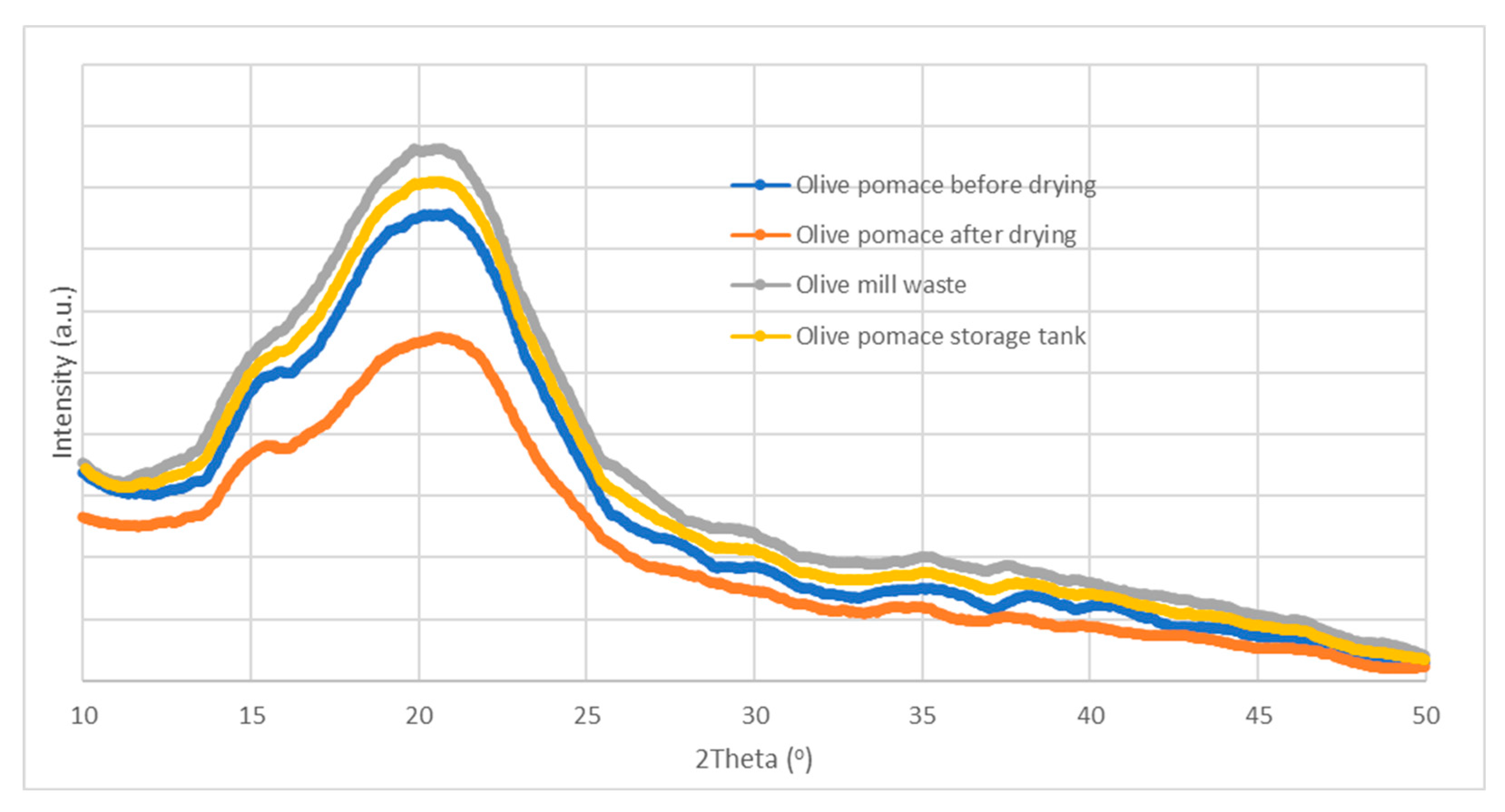
3.2.3. X-ray Diffraction Analysis
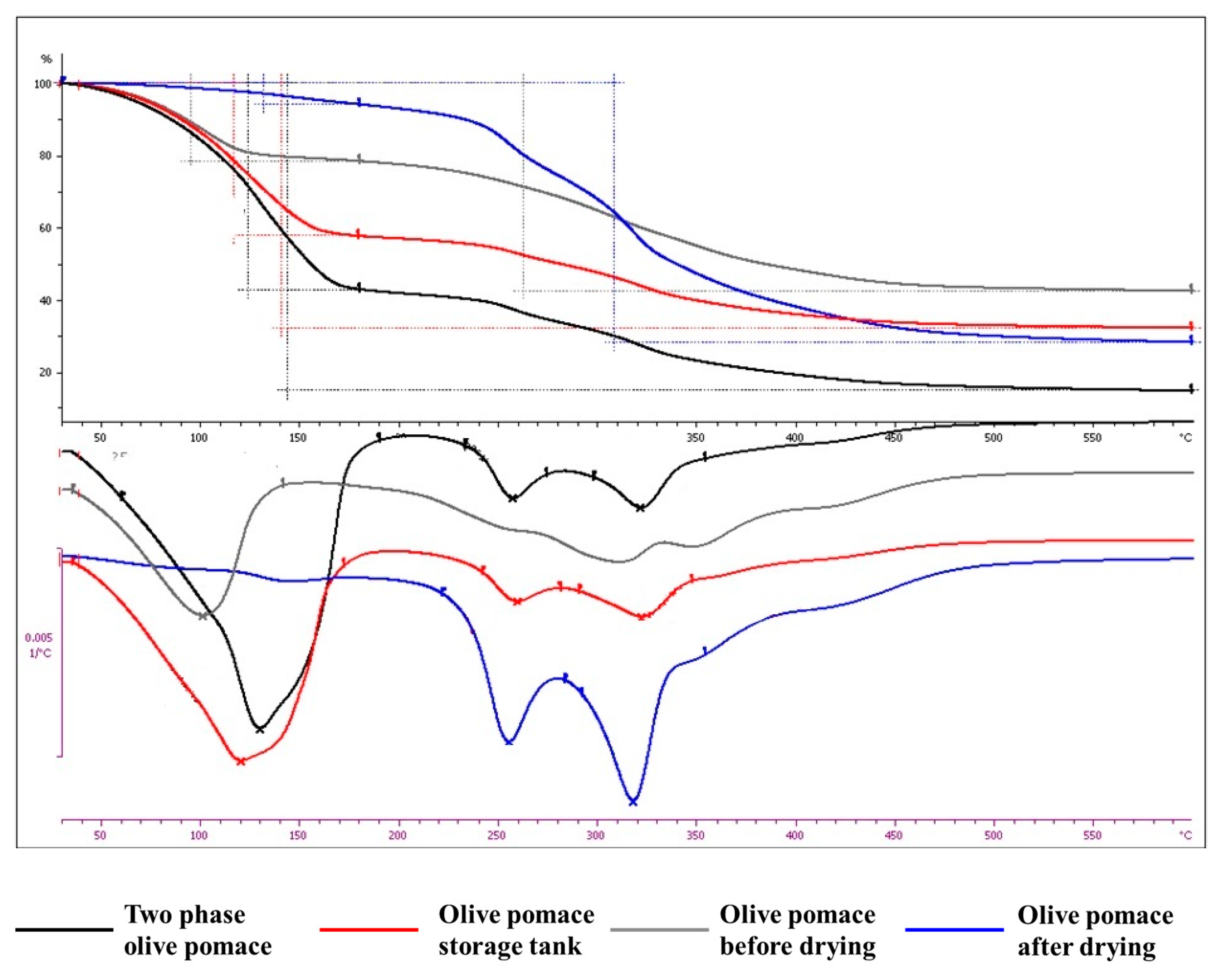
3.2.4. Thermogravimetric Analysis
- The thermal degradation in the operating temperature range of the dryer is very low (0.86–2.67%).
- The weight loss of fresh, ‘as received’ samples under air flow is proportional to the moisture content.
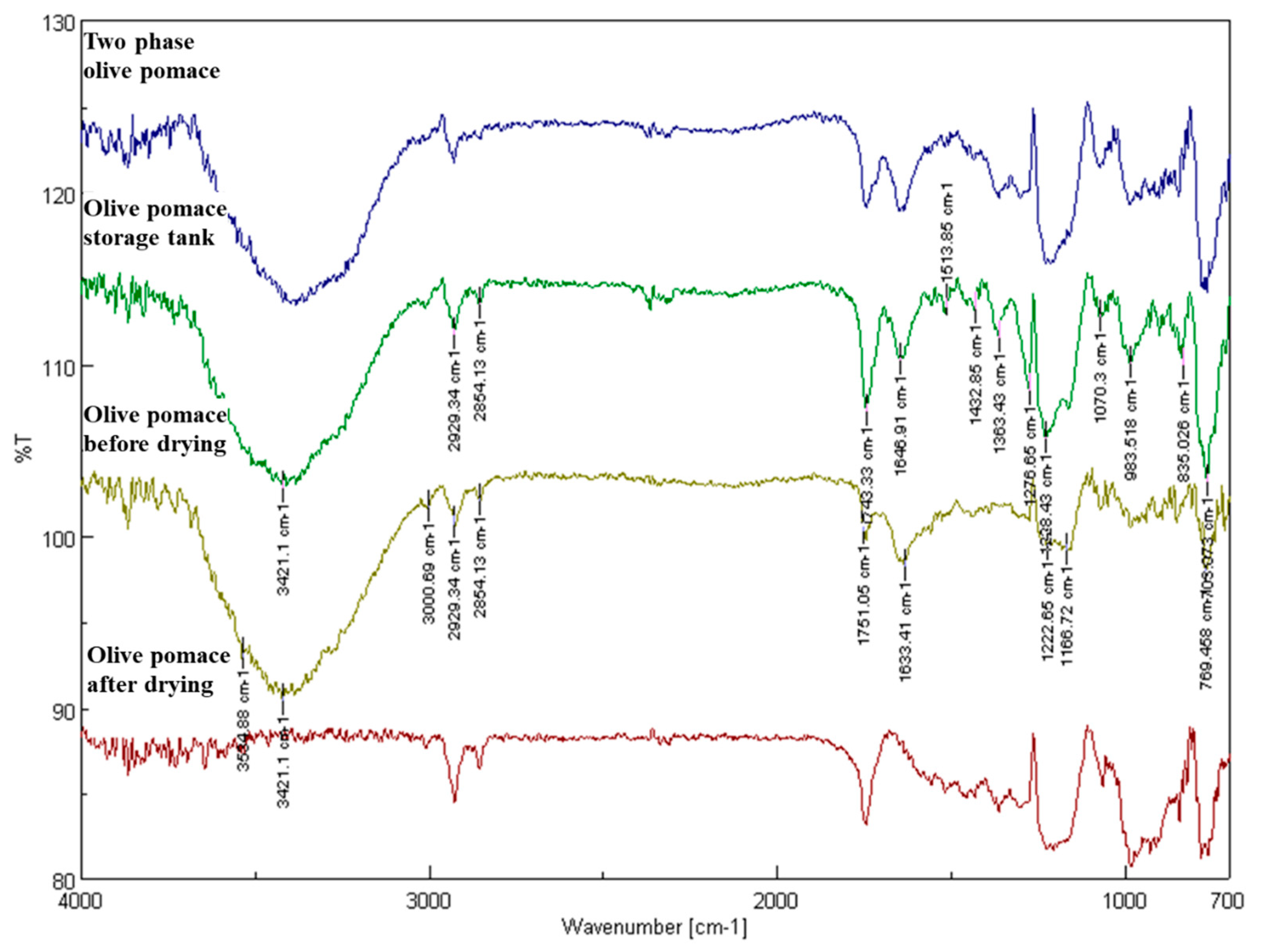
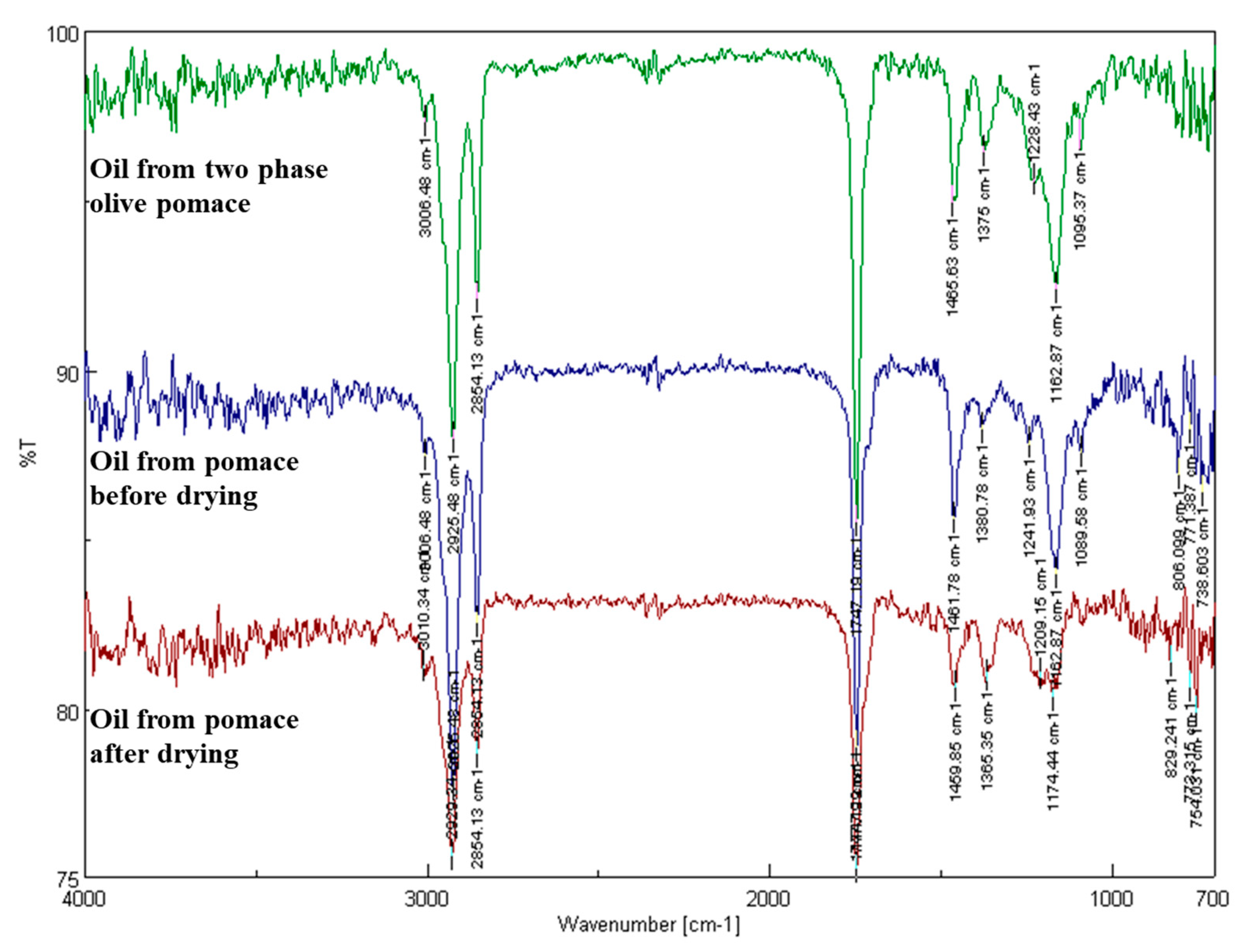
3.2.5. Analysis of Fresh, ‘As Received’ Olive Pomace Samples by Fourier Transform Infrared Spectroscopy
- at 1365.35 cm−1, corresponding to the CH2-CH2 groups and
- at 1174.44 cm−1, corresponding to the C-O bond of the ester groups,
- as well as at 1459.85 cm−1, corresponding to the group CH2-CH3.
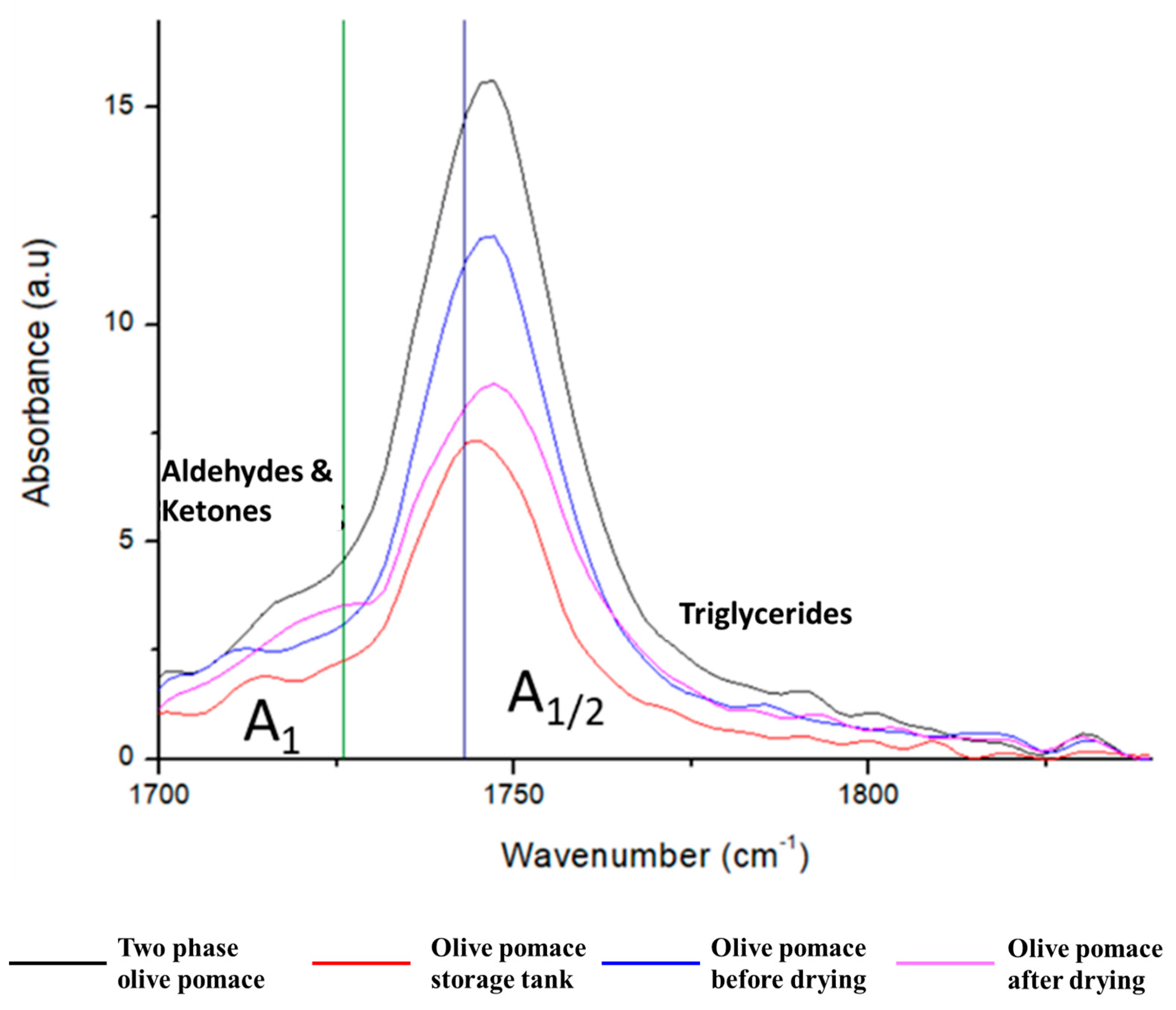
3.2.6. Pomace Oil Acidity
3.2.7. Analysis of Odours by TD-GC-MS
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Statista. Production Volume of Olive Oil Worldwide from 2012/13 to 2022/23. Available online: https://www.statista.com/statistics/613466/olive-oil-production-volume-worldwide/ (accessed on 5 August 2023).
- Olive oil times. Europe Confirms Steep Decline in Olive Oil Production. Available online: https://www.oliveoiltimes.com/production/europe-confirms-steep-decline-in-olive-oil-production/122348 (accessed on 4 July 2023).
- International Olive Council. The World of Olive Oil. Available online: https://www.internationaloliveoil.org/the-world-of-olive-oil/ (accessed on 11 September 2023).
- Romero-Gámez, M.; Castro-Rodríguez, J.; Suárez-Rey, E.M. Optimization of olive growing practices in Spain from a life cycle assessment perspective. J. Clean. Prod. 2017, 149, 25–37. [Google Scholar] [CrossRef]
- Palazzo, A.L.; Aristone, O. Peri-urban matters. changing olive growing patterns in central Italy. Sustainability 2017, 9, 638. [Google Scholar] [CrossRef]
- Alburquerque, J.A.; Gonzalvez, J.; Garcia, D.; Cegarra, J. Agrochemical characterisation of alperujo, a solid by-product of the two-phase centrifugation method for olive oil extraction. Bioresour. Technol. 2004, 91, 195–200. [Google Scholar] [CrossRef]
- Christoforou, E.; Fokaides, P. A review of olive mill solid wastes to energy utilization techniques. Waste Manag. 2016, 49, 346–363. [Google Scholar] [CrossRef]
- Sierra, J.; Martí, E.; Garau, M.A.; Cruañas, R. Effects of the agronomic use of olive oil mill wastewater: Field experiment. Sci. Total Environ. 2007, 378, 90–94. [Google Scholar] [CrossRef]
- Ribeiro, T.B.; Oliveira, A.L.; Costa, C.; Nunes, J.; Vicente, A.A.; Pintado, M. Total and sustainable valorisation of olive pomace using a fractionation approach. Appl. Sci. 2020, 10, 6785. [Google Scholar] [CrossRef]
- Sánchez-Sánchez, C.; González-González, A.; Cuadros-Salcedo, F.; Cuadros-Blázquez, F. Two-phase Olive mill waste: A circular economy solution to an imminent problem in Southern Europe. J. Clean. Prod. 2020, 274, 122789. [Google Scholar] [CrossRef]
- Moral, P.S.; Méndez, M.V.R. Production of pomace olive oil. Grasas y Aceites 2006, 57, 47–55. [Google Scholar]
- Deutsche Gesellschaft für Internationale Zusammenarbeit GmbH. Guidance to Develop By-Products and End-of-Waste Criteria and Proposals for Draft Legislation in Greece. 2021. Available online: https://environment.ec.europa.eu/topics/waste-and-recycling/waste-framework-directive_en#end-of-waste-criteria (accessed on 8 November 2023).
- De Marco, I.; Riemma, S.; Iannone, R. Global Warming Potential analysis of olive pomace processing. Chem. Eng. Trans. 2017, 57, 601–606. [Google Scholar] [CrossRef]
- Doymaz, I.; Gorel, O.; Akgun, N.A. Drying characteristics of the solid by-product of olive oil extraction. Biosyst. Eng. 2004, 88, 213–219. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Ash in Biomass. Natl. Renew. Energy Lab. (NREL) 2005, 8. Available online: https://www.nrel.gov/docs/gen/fy08/42622.pdf (accessed on 10 January 2022).
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D.L.A.P. Determination of structural carbohydrates and lignin in biomass: Laboratory Analytical Procedure (LAP) (NREL/TP-510-42618). Natl. Renew. Energy Lab. (NREL) 2012, 1617, 17. [Google Scholar]
- Sluiter, A.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Extractives in Biomass. Natl. Renew. Energy Lab. (NREL) 2005, 1617, 12. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Hyman, D.; Payne, D.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, J.; Wolfe, J. Determination of total solids in biomass and total dissolved solids in liquid process samples. Natl. Renew. Energy Lab. (NREL) 2008, 3–5. Available online: https://www.nrel.gov/docs/gen/fy08/42621.pdf (accessed on 10 January 2022).
- Sluiter, J.; Sluiter, A. Summative Mass Closure: Laboratory Analytical Procedure (LAP) Review and Integration: Feedstocks; Issue Date: April 2010; Revision Date: July 2011 (Version 07-08-2011). 2010. Available online: https://www.nrel.gov/docs/gen/fy11/48087.pdf (accessed on 10 January 2022).
- OIL AND GREASE. Standard Methods For the Examination of Water and Wastewater, 23rd. Available online: https://www.standardmethods.org/ (accessed on 10 January 2022).
- Nielsen, S.S. Food Analysis Laboratory Manual; Springer: West Lafayette, IN, USA, 2017. [Google Scholar] [CrossRef]
- Hewavitharana, G.G.; Perera, D.N.; Navaratne, S.B.; Wickramasinghe, I. Extraction methods of fat from food samples and preparation of fatty acid methyl esters for gas chromatography: A review. Arab. J. Chem. 2020, 13, 6865–6875. [Google Scholar] [CrossRef]
- Moreno, M.C.M.M.; Olivares, D.M.; Lopez, F.J.A.; Martinez, V.P.; Reig, F.B. Study of the formation of carbonyl compounds in edible oils and fats by 1H-NMR and FTIR. J. Mol. Struct. 1999, 482–483, 557–561. [Google Scholar] [CrossRef]
- Frangipane, M.T.; Costantini, L.; Merendino, N.; Massantini, R. Antioxidant Profile and Sensory Analysis in Olive Oils of Different Quality Grades. Agriculture 2023, 13, 993–1006. [Google Scholar] [CrossRef]
- Fella, P.; Stylianou, M.; Agapiou, A. A green sorptive extraction method (HiSorb-TD-GC-MS) for determining the extra virgin olive oil (EVOO) aroma profile. Pure Appl. Chem. 2023, 595–610. [Google Scholar] [CrossRef]
- Cecchi, L.; Migliorini, M.; Giambanelli, E.; Cane, A.; Mulinacci, N.; Zanoni, B. Volatile Profile of Two-Phase Olive Pomace (Alperujo) by HS-SPME-GC-MS as a Key to Defining Volatile Markers of Sensory Defects Caused by Biological Phenomena in Virgin Olive Oil. J. Agric. Food Chem. 2021, 69, 5155–5166. [Google Scholar] [CrossRef]
- Morillo, J.A.; Antizar-Ladislao, B.; Monteoliva-Sánchez, M.; Ramos-Cormenzana, A.; Russell, N.J. Bioremediation and biovalorisation of olive-mill wastes. Appl. Microbiol. Biotechnol. 2009, 82, 25–39. [Google Scholar] [CrossRef]
- Doula, M.K.; Moreno-Ortego, J.L.; Tinivella, F.; Inglezakis, V.J.; Sarris, A.; Komnitsas, K. Olive mill waste: Recent advances for the sustainable development of olive oil industry. Olive Mill Waste Recent Adv. Sustain. Manag. 2017, 29–56. [Google Scholar] [CrossRef]
- González-Martínez, L.; Hernández, D.; Astudillo, C.A.; Silva, A.F.; Gabriel, D. Data extracted from olive oil mill waste exposed to ambient conditions. Data Brief 2019, 26, 104555. [Google Scholar] [CrossRef]
- Maniscalco, M.P.; Volpe, M.; Volpe, R.; Messineo, A. Promoting energy recovery from recalcitrant agro-industrial wastes through anaerobic digestion: A review on olive mill residues. AIP Conf. Proc. 2018, 2040, 140012. [Google Scholar] [CrossRef]
- Serrano, A.; Fermoso, F.G.; Rodríguez-Gutierrez, G.; Fernandez-Bolaños, J.; Borja, R. Biomethanization of olive mill solid waste after phenols recovery through low-temperature thermal pre-treatment. Waste Manag. 2017, 61, 229–235. [Google Scholar] [CrossRef]
- Hernández, D.; Astudillo, C.A.; Fernández-Palacios, E.; Cataldo, F.; Tenreiro, C. Evolution of physical-chemical parameters, D.G., microbial diversity and VOC emissions of olive oil mill waste exposed to ambient conditions in open reservoirs. Waste Manag. 2018, 79, 501–509. [Google Scholar] [CrossRef]
- Fernández-Rodríguez, M.J.; de la Lama-Calvente, D.; Jiménez-Rodríguez, A.; Borja, R.; Rincón, B. Evolution of control parameters in biochemical methane potential tests of olive mill solid waste (OMSW), thermal pre-treated OMSW, and its co-digestion with Dunaliella salina. J. Appl. Phycol. 2021, 33, 419–429. [Google Scholar] [CrossRef]
- Mahasneh, Z.M.; Abdelnour, S.; Ebrahim, A.; Almasodi, A.G.; Moustafa, M.; Alshaharni, M.O.; Algopish, U.; Tellez-Isaias, G.; El-Hack, M.E.A. Olive oil and its derivatives for promoting performance, health, and struggling thermal stress effects on broilers. Poult. Sci. 2024, 103, 103348. [Google Scholar] [CrossRef]
- Jamrah, A.; Al-Zghoul, T.M.; Darwish, M.M. A comprehensive review of combined processes for olive mill wastewater treatments. Case Stud. Chem. Environ. Eng. 2023, 8, 100493. [Google Scholar] [CrossRef]
- Manthos, G.; Zagklis, D.; Papavasileiou, V.; Gkountou, N.A.; Saita, Z.; Zafiri, C.; Kornaros, M. High-rate upflow anaerobic sludge blanket bioreactor for the treatment of olive mill effluents: Laboratory and pilot scale systems investigation. Renew. Energy 2023, 217, 119215. [Google Scholar] [CrossRef]
- Hernández, D.; Quinteros-Lama, H.; Tenreiro, C.; Gabriel, D. Assessing concentration changes of odorant compounds in the thermal-mechanical drying phase of sediment-likewastes from olive oil extraction. Appl. Sci. 2019, 9, 519. [Google Scholar] [CrossRef]
- De Castro, A.; Asencio, E.; Ruiz-Méndez, V.; Romero, C.; Brenes, M. Production of 4-ethylphenol in alperujo by Lactobacillus pentosus. J. Sci. Food Agric. 2014, 95, 2222–2227. [Google Scholar] [CrossRef]
- Nagata, Y. Measurement of Odor Threshold by Triangle Odor Bag Method. Environ. Sci. 2003, 118, 118–127. [Google Scholar]


| Parameter | Sample 1 | Sample 2 | Sample 3 | Sample 4 | Sample 5 | Mean Values | Literature [26,27,28,29,30,31,32,33,34,35,36] |
|---|---|---|---|---|---|---|---|
| pH | 4.71 ± 0.02 | 4.77 ± 0.06 | 4.55 ± 0.05 | 4.67 ± 0.05 | 4.81 ± 0.06 | 4.70 ± 0.05 | 5.78 ± 1.05 |
| Conductivity (μS/cm) | 1425 ± 5 | 2417 ± 9 | 3251 ± 75 | 13680 ± 2020 | 2686 ± 101 | 4692 ± 441 | 3220 ± 2020 |
| Moisture (% w.b. *) | 63.68 ± 1.52 | 62.36 ± 2.32 | 71.92 ± 0.59 | 60.36 ± 0.10 | 68.34 ± 0.11 | 65.33 ± 0.93 | na * |
| Total solids, TS (% w.b. *) | 36.33 ± 1.52 | 37.64 ± 2.32 | 28.08 ± 0.59 | 39.64 ± 0.10 | 31.66 ± 0.11 | 34.67 ± 0.93 | 36.75 ± 13.65 |
| Volatile solids, vs. (% TS) | 99.02 ± 1.26 | 99.32 ± 0.13 | 98.66 ± 0.01 | 97.25 ± 1.15 | 99.25 ± 1.01 | 99.00 ± 0.07 | 83.25 ± 4.15 |
| Composition of the solid phase (% d.b. *) | |||||||
| Water soluble solids | 20.69 ± 0.16 | 24.18 ± 1.58 | 20.47 ± 3.28 | 15.89 ± 1.18 | 14.66 ± 1.79 | 19.18 ± 1.6 | na * |
| Cellulose | 5.46 ± 1.18 | 6.01 ± 0.83 | 14.06 ± 0.68 | 8.93 ± 0.58 | 8.96 ± 0.23 | 8.68 ± 0.7 | 24.65 ± 12.55 |
| Hemicellulose | 27.33 ± 0.29 | 26.96 ± 0.29 | 17.96 ± 0.29 | 27.50 ± 2.13 | 29.75 ± 1.13 | 26.43 ± 0.96 | 27.40 ± 15.20 |
| Acid insoluble residue | 43.07 ± 2.92 | 44.6 ± 1.15 | 38.88 ± 1.74 | 42.42 ± 2.19 | 47.64 ± 1.6 | 43.32 ± 2.11 | 36.10 ± 16.30 |
| Soluble lignin | 1.33 ± 0.17 | 0.94 ± 0.06 | 1.63 ± 0.12 | 0.94 ± 0.08 | 0.9 ± 0.05 | 1.15 ± 0.11 | na * |
| Total Nitrogen | 0.94 ± 0.07 | 0.84 ± 0.16 | 1.1 ± 0.21 | 0.89 ± 0. 06 | 0.89 ± 0.34 | 0.96 ± 0.15 | 1.56 ± 0.49 |
| Oil | 9.77 ± 0.54 | 15.05 ± 0.62 | 15.6 ± 0.75 | 1 3.71 ± 0.45 | 14.49 ± 0.61 | 13.72 ± 0.64 | 11.95 ± 7.85 |
| Parameter | Two Phase Olive Pomace (Point 1) | Olive Pomace Storage Tank (Point 2) | Olive Pomace before Drying (Point 3) | Olive Pomace after Drying (Point 4) |
|---|---|---|---|---|
| pH | 4.67 ± 0.05 | 4.81 ± 0.06 | 4.71 ± 0.03 | 4.87 ± 0.06 |
| Conductivity (μS/cm) | 13680 ± 2020 | 2686 ± 101 | 2425 ± 51 | 2477 ± 91 |
| Moisture (% w.b. *) | 60.36 ± 0.10 | 68.34 ± 0.11 | 45.07 ± 0.37 | 5.24 ± 0.12 |
| Total solids, TS (% w.b.) | 39.64 ± 0.10 | 31.66 ± 0.11 | 54.93 ± 0.37 | 94.76 ± 0.12 |
| Volatile solids, VS (% TS) | 97.25 ± 1.15 | 99.25 ± 1.01 | 99.43 ± 0.17 | 99.47 ± 0.02 |
| Composition of the solid phase (% d.b. *) | ||||
| Water soluble solids | 15.89 ± 1.18 | 14.66 ± 1.79 | 12.61 ± 1.47 | 20.23 ± 0.20 |
| Cellulose | 8.93 ± 0.58 | 8.96 ± 0.23 | 9.14 ± 0.23 | 6.67 ± 0.02 |
| Hemicellulose | 27.50 ± 2.13 | 29.75 ± 1.13 | 27.09 ± 3.63 | 20.78 ± 1.03 |
| Acid insoluble residue | 42.42 ± 2.19 | 47.64 ± 1.6 | 47.15 ± 1.56 | 46.73 ± 5.95 |
| Soluble lignin | 0.94 ± 0.08 | 0.9 ± 0.05 | 0.99 ± 0.18 | 1.56 ± 0.09 |
| Total Nitrogen | 0.89 ± 0.06 | 0.89 ± 0.34 | 0.91 ± 0.14 | 1.05 ± 0.16 |
| Oil | 13.71 ± 0.45 | 14.49 ± 0.61 | 12.17 ± 0.65 | 14.00 ± 0.52 |
| Weight Loss (%) | |||||||
|---|---|---|---|---|---|---|---|
| Dried under Nitrogen Flow | ‘As Received’ under Air Flow | ||||||
| Moisture (%) | 30–150 (°C) | 150–600 (°C) | 30–600 (°C) | 30–180 (°C) | 180–600 (°C) | 30–600 (°C) | |
| Two phase olive pomace | 59.38 | 2.67 | 72.24 | 74.91 | 57.33 | 28.14 | 85.47 |
| Olive pomace storage tank | 65.05 | 0.86 | 73.07 | 73.93 | 42.51 | 24.47 | 67.98 |
| Olive pomace before drying | 39.97 | 0.98 | 71.43 | 72.41 | 21.72 | 36.01 | 57.73 |
| Olive pomace after drying | 6.36 | 2.72 | 68.06 | 70.78 | 5.99 | 66 | 71.99 |
| Two Phase Olive Pomace | Olive Pomace Storage Tank | Olive Pomace before Drying | Olive Pomace after Drying | |
|---|---|---|---|---|
| A1/A1/2 | 0.12 | 0.16 | 0.13 | 0.23 |
| Free Acidity | K232 | K270 | ||||
|---|---|---|---|---|---|---|
| (%) | (a.u. *) | (a.u.) | ||||
| Two-phase olive pomace | 3.69 ± 0.21 | 0.835 | 1.275 | |||
| Olive pomace storage tank | 7.60 ± 0.10 | 1.126 | 1.591 | |||
| Olive pomace before drying | 7.96 ± 0.14 | 1.093 | 1.644 | |||
| Olive pomace after drying | 8.88 ± 0.12 | 1.103 | 1.538 | |||
| (%) | Limit | (a.u.) | Limit | (a.u.) | Limit | |
| Virgin | 1.41 | <2 | 0.343 | <2.5 | 0.510 | <0.25 |
| Extra Virgin | 0.42 | <0.8 | 0.352 | <2.6 | 0.447 | <0.22 |
| Ret. Time (min) | Ingredient | Odour | Odour Threshold (ppb) | Two-Phase Olive Pomace (ppb) | Olive Pomace Storage Tank (ppb) | Olive Pomace before Drying (ppb) | Olive Pomace after Drying (ppb) |
|---|---|---|---|---|---|---|---|
| 14.69 | Ethyl Acetate | 870 | 261 | 1084 | 811 | 12 | |
| 17.892 | Acetic acid | 60 | 330 | 1173 | 492 | 478 | |
| 20.22 | Propanoic acid, ethyl ester | Fruity | 7 | 4 | 399 | 334 | 9 |
| 20.943 | Butanoic acid, methyl ester | 2 | 461 | 377 | 3 | ||
| 25.32 | Butanoic acid, ethyl ester | Fruity | 0.04 | 14 | 1759 | 1558 | 7 |
| 27.898 | Butanoic acid | 0.19 | 4 | 633 | 558 | 265 | |
| 35.663 | Hexanoic acid, ethyl ester | Apple, fruity, sweetish, aniseed | 57 | 485 | 253 | 0 | |
| 37.053 | D-Limonene | 38 | 0 | 0 | 464 | 386 | |
| 48.428 | Phenol, 2-ethyl | 0 | 103 | 319 | 187 | ||
| 42.79 | Cyclohexanecarboxylic acid, ethyl ester | 0 | 430 | 286 | 20 | ||
| 53.707 | Phenol, 4-ethyl-2-methoxy | Smoky, gammon-like | 0 | 181 | 208 | 60 | |
| 47.969 | 2-Methoxy-5-methylphenol | 0 | 150 | 206 | 57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christofi, A.; Fella, P.; Agapiou, A.; Barampouti, E.M.; Mai, S.; Moustakas, K.; Loizidou, M. The Impact of Drying and Storage on the Characteristics of Two-Phase Olive Pomace. Sustainability 2024, 16, 1116. https://doi.org/10.3390/su16031116
Christofi A, Fella P, Agapiou A, Barampouti EM, Mai S, Moustakas K, Loizidou M. The Impact of Drying and Storage on the Characteristics of Two-Phase Olive Pomace. Sustainability. 2024; 16(3):1116. https://doi.org/10.3390/su16031116
Chicago/Turabian StyleChristofi, Andreas, Panagiota Fella, Agapios Agapiou, Elli Maria Barampouti, Sofia Mai, Konstantinos Moustakas, and Maria Loizidou. 2024. "The Impact of Drying and Storage on the Characteristics of Two-Phase Olive Pomace" Sustainability 16, no. 3: 1116. https://doi.org/10.3390/su16031116
APA StyleChristofi, A., Fella, P., Agapiou, A., Barampouti, E. M., Mai, S., Moustakas, K., & Loizidou, M. (2024). The Impact of Drying and Storage on the Characteristics of Two-Phase Olive Pomace. Sustainability, 16(3), 1116. https://doi.org/10.3390/su16031116










