Reviewing Air Pollutants Generated during the Pyrolysis of Solid Waste for Biofuel and Biochar Production: Toward Cleaner Production Practices
Abstract
:1. Introduction
2. Factors Affecting the Types of Air Pollutants during Pyrolysis
2.1. Feedstock Composition
2.2. Pyrolysis Temperature
2.3. Residence Time
2.4. Heating Rate
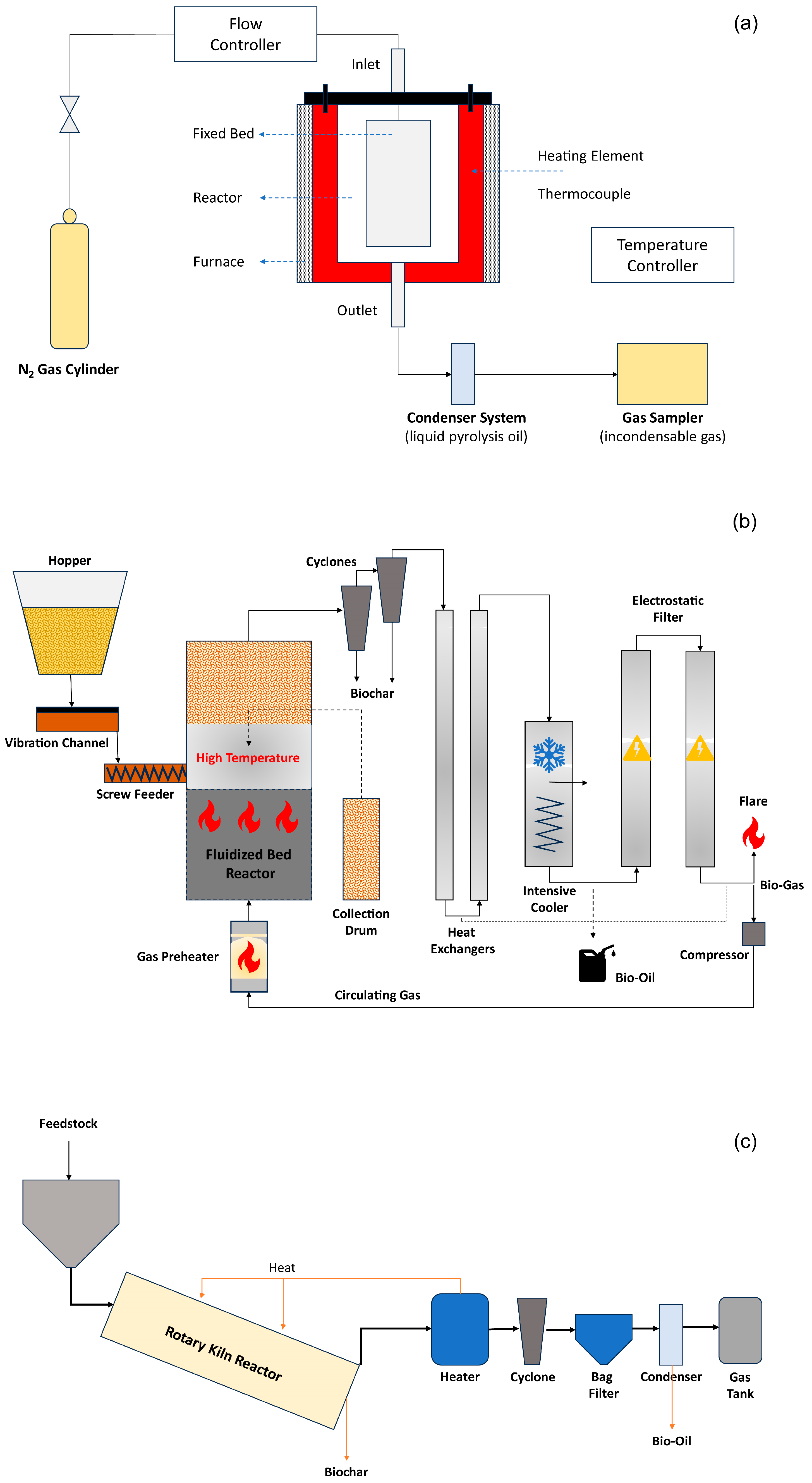
2.5. Reactor Design
2.6. Type of Pyrolysis
2.7. Gas Atmosphere
2.8. Catalysts
3. Environmental and Health Impacts of Air Pollutants
3.1. Particulate Matter (PM)
3.2. Volatile Organic Compounds (VOCs)
3.3. Polycyclic Aromatic Hydrocarbons (PAHs)
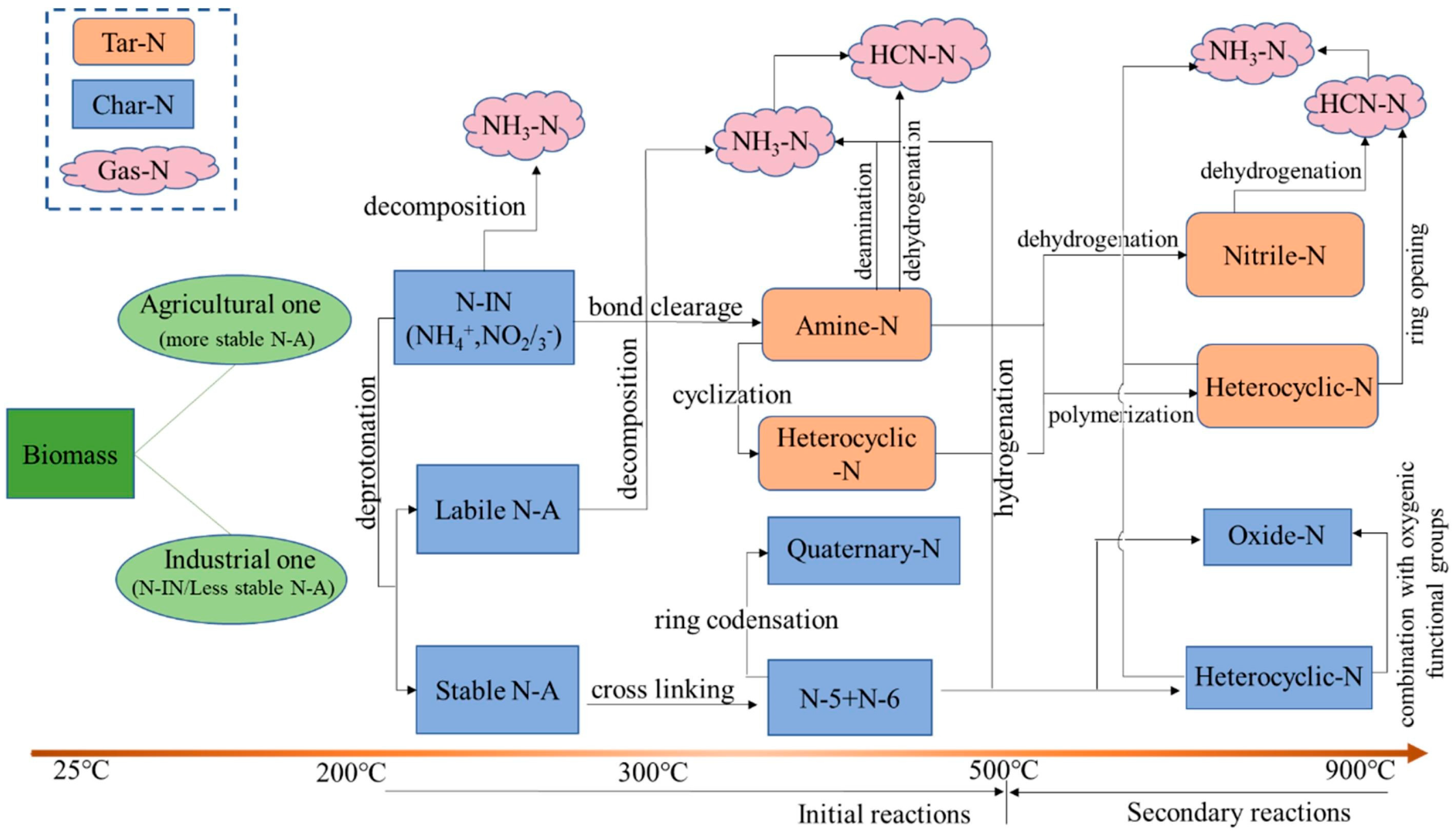
3.4. Nitrogen Oxides (NOx)
3.5. Carbon Monoxide (CO) and Carbon Dioxide (CO2)
3.6. Sulfur Compounds
4. Current State of Regulatory Expectations and Compliance in Various Regions
4.1. United States
4.2. European Union
4.3. Asia–Pacific Region
4.4. Global Trend
5. Strategies and Technologies Employed to Minimize Emissions during Pyrolysis
5.1. Improved Reactor Designs
5.2. Gas Cleaning and Filtration Systems
5.3. Catalytic Converters
5.4. Controlled Pyrolysis Conditions
5.5. Renewable Energy Integration
5.6. Realtime Monitoring and Control Systems
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oakleaf, J.R.; Kennedy, C.M.; Baruch-Mordo, S.; Gerber, J.S.; West, P.C.; Johnson, J.A.; Kiesecker, J. Mapping global development potential for renewable energy, fossil fuels, mining and agriculture sectors. Sci. Data 2019, 6, 101. [Google Scholar] [CrossRef] [PubMed]
- Vo, D.H. Sustainable agriculture & energy in the US: A link between ethanol production and the acreage for corn. Econ. Sociol. 2020, 13, 259–268. [Google Scholar]
- Kumar Sarangi, P.; Subudhi, S.; Bhatia, L.; Saha, K.; Mudgil, D.; Prasad Shadangi, K.; Srivastava, R.K.; Pattnaik, B.; Arya, R.K. Utilization of agricultural waste biomass and recycling toward circular bioeconomy. Environ. Sci. Pollut. Res. 2023, 30, 8526–8539. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Skelly, S. Physicochemical properties and applications of biochars derived from municipal solid waste: A review. Environ. Adv. 2023, 13, 100395. [Google Scholar] [CrossRef]
- Hoang, A.T.; Ong, H.C.; Fattah, I.R.; Chong, C.T.; Cheng, C.K.; Sakthivel, R.; Ok, Y.S. Progress on the lignocellulosic biomass pyrolysis for biofuel production toward environmental sustainability. Fuel Process. Technol. 2021, 223, 106997. [Google Scholar] [CrossRef]
- Escalante, J.; Chen, W.-H.; Tabatabaei, M.; Hoang, A.T.; Kwon, E.E.; Lin, K.-Y.A.; Saravanakumar, A. Pyrolysis of lignocellulosic, algal, plastic, and other biomass wastes for biofuel production and circular bioeconomy: A review of thermogravimetric analysis (TGA) approach. Renew. Sustain. Energy Rev. 2022, 169, 112914. [Google Scholar] [CrossRef]
- Li, S.; Chen, G. Thermogravimetric, thermochemical, and infrared spectral characterization of feedstocks and biochar derived at different pyrolysis temperatures. Waste Manag. 2018, 78, 198–207. [Google Scholar] [CrossRef]
- Li, S.; Galoustian, T.; Trejo, H. Biochar pyrolyzed with concentrated solar radiation for enhanced nitrate adsorption. J. Anal. Appl. Pyrolysis 2023, 174, 106131. [Google Scholar] [CrossRef]
- Djandja, O.S.; Wang, Z.-C.; Wang, F.; Xu, Y.-P.; Duan, P.-G. Pyrolysis of municipal sewage sludge for biofuel production: A review. Ind. Eng. Chem. Res. 2020, 59, 16939–16956. [Google Scholar] [CrossRef]
- Li, S.; Tasnady, D. Biochar for Soil Carbon Sequestration: Current Knowledge, Mechanisms, and Future Perspectives. C 2023, 9, 67. [Google Scholar] [CrossRef]
- Li, S.; Chan, C.Y. Will Biochar Suppress or Stimulate Greenhouse Gas Emissions in Agricultural Fields? Unveiling the Dice Game through Data Syntheses. Soil Syst. 2022, 6, 73. [Google Scholar] [CrossRef]
- Chen, X.; Che, Q.; Li, S.; Liu, Z.; Yang, H.; Chen, Y.; Wang, X.; Shao, J.; Chen, H. Recent developments in lignocellulosic biomass catalytic fast pyrolysis: Strategies for the optimization of bio-oil quality and yield. Fuel Process. Technol. 2019, 196, 106180. [Google Scholar] [CrossRef]
- Duanguppama, K.; Pannucharoenwong, N.; Echaroj, S.; Turakarn, C.; Chaiphet, K.; Rattanadecho, P. Processing of Leucaena Leucocepphala for renewable energy with catalytic fast pyrolysis. Energy Rep. 2022, 8, 466–479. [Google Scholar] [CrossRef]
- Park, Y.-K.; Ha, J.-M.; Oh, S.; Lee, J. Bio-oil upgrading through hydrogen transfer reactions in supercritical solvents. Chem. Eng. J. 2021, 404, 126527. [Google Scholar] [CrossRef]
- Li, S.; Chan, C.Y.; Sharbatmaleki, M.; Trejo, H.; Delagah, S. Engineered Biochar Production and Its Potential Benefits in a Closed-Loop Water-Reuse Agriculture System. Water 2020, 12, 2847. [Google Scholar] [CrossRef]
- Panahi, H.K.S.; Dehhaghi, M.; Ok, Y.S.; Nizami, A.-S.; Khoshnevisan, B.; Mussatto, S.I.; Aghbashlo, M.; Tabatabaei, M.; Lam, S.S. A comprehensive review of engineered biochar: Production, characteristics, and environmental applications. J. Clean. Prod. 2020, 270, 122462. [Google Scholar] [CrossRef]
- Haghighi Mood, S.; Pelaez-Samaniego, M.R.; Garcia-Perez, M. Perspectives of engineered biochar for environmental applications: A review. Energy Fuels 2022, 36, 7940–7986. [Google Scholar] [CrossRef]
- Yang, Q.; Zhou, H.; Bartocci, P.; Fantozzi, F.; Mašek, O.; Agblevor, F.A.; Wei, Z.; Yang, H.; Chen, H.; Lu, X. Prospective contributions of biomass pyrolysis to China’s 2050 carbon reduction and renewable energy goals. Nat. Commun. 2021, 12, 1698. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Tu, X.; Homm, G.; Weidenkaff, A. Plasma pyrolysis for a sustainable hydrogen economy. Nat. Rev. Mater. 2022, 7, 333–334. [Google Scholar] [CrossRef]
- Colpani, D.; Santos, V.O.; Araujo, R.O.; Lima, V.M.; Tenório, J.A.; Coleti, J.; Chaar, J.S.; de Souza, L.K. Bioenergy potential analysis of Brazil nut biomass residues through pyrolysis: Gas emission, kinetics, and thermodynamic parameters. Clean. Chem. Eng. 2022, 1, 100002. [Google Scholar] [CrossRef]
- Bulsink, P.; de Miguel Mercader, F.; Sandstrom, L.; Van De Beld, B.; Preto, F.; Zacher, A.; Oasmaa, A.; Dahmen, N.; Funke, A.; Bronson, B. Results of the International Energy Agency Bioenergy Round Robin on the Analysis of Heteroatoms in Biomass Liquefaction Oils. Energy Fuels 2020, 34, 11123–11133. [Google Scholar] [CrossRef]
- Hattori, T.; Nam, H.; Chapman, A. Multilateral energy technology cooperation: Improving collaboration effectiveness through evidence from International Energy Agency Technology Collaboration Programmes. Energy Strategy Rev. 2022, 43, 100920. [Google Scholar] [CrossRef]
- Sadiq, M.; Alshehhi, R.J.; Urs, R.R.; Mayyas, A.T. Techno-economic analysis of Green-H2@ Scale production. Renew. Energy 2023, 219, 119362. [Google Scholar] [CrossRef]
- Babu, S.; Singh, R.; Kumar, S.; Rathore, S.S.; Yadav, D.; Yadav, S.K.; Yadav, V.; Ansari, M.A.; Das, A.; Rajanna, G.A. Biochar implications in cleaner agricultural production and environmental sustainability. Environ. Sci. Adv. 2023, 2, 1042–1059. [Google Scholar] [CrossRef]
- Ayaz, M.; Feizienė, D.; Tilvikienė, V.; Akhtar, K.; Stulpinaitė, U.; Iqbal, R. Biochar role in the sustainability of agriculture and environment. Sustainability 2021, 13, 1330. [Google Scholar] [CrossRef]
- Elias, D.M.O.; Ooi, G.T.; Ahmad Razi, M.F.; Robinson, S.; Whitaker, J.; McNamara, N.P. Effects of Leucaena biochar addition on crop productivity in degraded tropical soils. Biomass Bioenergy 2020, 142, 105710. [Google Scholar] [CrossRef]
- Li, S.; Harris, S.; Anandhi, A.; Chen, G. Predicting biochar properties and functions based on feedstock and pyrolysis temperature: A review and data syntheses. J. Clean. Prod. 2019, 215, 890–902. [Google Scholar] [CrossRef]
- Li, S.; Chen, G. Contemporary strategies for enhancing nitrogen retention and mitigating nitrous oxide emission in agricultural soils: Present and future. Environ. Dev. Sustain. 2020, 22, 2703–2741. [Google Scholar] [CrossRef]
- Hasnain, M.; Munir, N.; Abideen, Z.; Zulfiqar, F.; Koyro, H.W.; El-Naggar, A.; Caçador, I.; Duarte, B.; Rinklebe, J.; Yong, J.W.H. Biochar-plant interaction and detoxification strategies under abiotic stresses for achieving agricultural resilience: A critical review. Ecotoxicol. Environ. Saf. 2023, 249, 114408. [Google Scholar] [CrossRef]
- Lyu, H.; Tang, J.; Cui, M.; Gao, B.; Shen, B. Biochar/iron (BC/Fe) composites for soil and groundwater remediation: Synthesis, applications, and mechanisms. Chemosphere 2020, 246, 125609. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, S.; Ju, M.; Liu, L. Preparation and modification of biochar materials and their application in soil remediation. Appl. Sci. 2019, 9, 1365. [Google Scholar] [CrossRef]
- Chiang, H.-L.; Lo, C.-C.; Ma, S.-Y. Characteristics of exhaust gas, liquid products, and residues of printed circuit boards using the pyrolysis process. Environ. Sci. Pollut. Res. 2010, 17, 624–633. [Google Scholar] [CrossRef]
- Chen, H.; Shan, R.; Zhao, F.; Gu, J.; Zhang, Y.; Yuan, H.; Chen, Y. A review on the NOx precursors release during biomass pyrolysis. Chem. Eng. J. 2023, 451, 138979. [Google Scholar] [CrossRef]
- Chew, K.W.; Chia, S.R.; Chia, W.Y.; Cheah, W.Y.; Munawaroh, H.S.H.; Ong, W.-J. Abatement of hazardous materials and biomass waste via pyrolysis and co-pyrolysis for environmental sustainability and circular economy. Environ. Pollut. 2021, 278, 116836. [Google Scholar] [CrossRef]
- Tao, W.; Yang, X.; Li, Y.; Zhu, R.; Si, X.; Pan, B.; Xing, B. Components and persistent free radicals in the volatiles during pyrolysis of lignocellulose biomass. Environ. Sci. Technol. 2020, 54, 13274–13281. [Google Scholar] [CrossRef]
- USEPA Advance Notice of Proposed Rulemaking on Pyrolysis and Gasification Units. Available online: https://www.epa.gov/stationary-sources-air-pollution/advance-notice-proposed-rulemaking-pyrolysis-and-gasification (accessed on 23 October 2023).
- Schwartz, N.R.; Paulsen, A.D.; Blaise, M.J.; Wagner, A.L.; Yelvington, P.E. Analysis of emissions from combusting pyrolysis products. Fuel 2020, 274, 117863. [Google Scholar] [CrossRef]
- Winchell, L.J.; Ross, J.J.; Brose, D.A.; Pluth, T.B.; Fonoll, X.; Norton Jr, J.W.; Bell, K.Y. Pyrolysis and gasification at water resource recovery facilities: Status of the industry. Water Environ. Res. 2022, 94, e10701. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, M.; Hayafune, Y.; Sugamiya, R.; Nakagawa, Y.; Makishima, K. Pyrolysis of municipal solid waste in Japan. J. Energy Resour. Technol. 1984, 106, 377–382. [Google Scholar] [CrossRef]
- Liu, W.-J.; Li, W.-W.; Jiang, H.; Yu, H.-Q. Fates of chemical elements in biomass during its pyrolysis. Chem. Rev. 2017, 117, 6367–6398. [Google Scholar] [CrossRef]
- Ortuño, N.; Conesa, J.A.; Moltó, J.; Font, R. Pollutant emissions during pyrolysis and combustion of waste printed circuit boards, before and after metal removal. Sci. Total Environ. 2014, 499, 27–35. [Google Scholar] [CrossRef]
- Itoh, T.; Fujiwara, N.; Iwabuchi, K.; Narita, T.; Mendbayar, D.; Kamide, M.; Niwa, S.; Matsumi, Y. Effects of pyrolysis temperature and feedstock type on particulate matter emission characteristics during biochar combustion. Fuel Process. Technol. 2020, 204, 106408. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Su, L.; Li, X.; Duan, L.; Wang, C.; Huang, T. Hazardous Air Pollutant Formation from Pyrolysis of Typical Chinese Casting Materials. Environ. Sci. Technol. 2011, 45, 6539–6544. [Google Scholar] [CrossRef]
- Acharya, S.; Vadher, J.; Kanjariya, P. Identification and quantification of gases releasing from furan no bake binder. Arch. Foundry Eng. 2016, 16, 5–10. [Google Scholar] [CrossRef]
- Kwon, E.; Castaldi, M.J. Fundamental Understanding of the Thermal Degradation Mechanisms of Waste Tires and Their Air Pollutant Generation in a N2 Atmosphere. Environ. Sci. Technol. 2009, 43, 5996–6002. [Google Scholar] [CrossRef] [PubMed]
- Settle, A.E.; Berstis, L.; Rorrer, N.A.; Roman-Leshkóv, Y.; Beckham, G.T.; Richards, R.M.; Vardon, D.R. Heterogeneous Diels–Alder catalysis for biomass-derived aromatic compounds. Green Chem. 2017, 19, 3468–3492. [Google Scholar] [CrossRef]
- Lee, B.-K. Sources, distribution and toxicity of polyaromatic hydrocarbons (PAHs) in particulate matter. In Air Pollution; IntechOpen: London, UK, 2010. [Google Scholar]
- Butler, T.J.; Vermeylen, F.M.; Rury, M.; Likens, G.E.; Lee, B.; Bowker, G.E.; McCluney, L. Response of ozone and nitrate to stationary source NOx emission reductions in the eastern USA. Atmos. Environ. 2011, 45, 1084–1094. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, S.; Zhang, Q.; Zhao, Y.; Liu, M.; Zhang, D.; Cai, X.; Wang, N.; Wang, W. Structural characterization and transformation of nitrogen compounds in waste tire pyrolysis oils. J. Chromatogr. A 2023, 1702, 464093. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, J.; Chen, J. Behavior of sulfur during pyrolysis of waste tires: A critical review. J. Energy Inst. 2022, 102, 302–314. [Google Scholar] [CrossRef]
- Joseph, A.M.; George, B.; Madhusoodanan, K.; Alex, R. Current status of sulphur vulcanization and devulcanization chemistry: Process of vulcanization. Rubber Sci. 2015, 28, 82–121. [Google Scholar]
- Dunnigan, L.; Morton, B.J.; Hall, P.A.; Kwong, C.W. Production of biochar and bioenergy from rice husk: Influence of feedstock drying on particulate matter and the associated polycyclic aromatic hydrocarbon emissions. Atmos. Environ. 2018, 190, 218–225. [Google Scholar] [CrossRef]
- Zhao, B.; O’Connor, D.; Zhang, J.; Peng, T.; Shen, Z.; Tsang, D.C.; Hou, D. Effect of pyrolysis temperature, heating rate, and residence time on rapeseed stem derived biochar. J. Clean. Prod. 2018, 174, 977–987. [Google Scholar] [CrossRef]
- Kim, S.-S.; Ly, H.V.; Choi, G.-H.; Kim, J.; Woo, H.C. Pyrolysis characteristics and kinetics of the alga Saccharina japonica. Bioresour. Technol. 2012, 123, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, K.; Xie, L.; Zhu, H.; Ji, S.; Shu, X. Effects of residence time on characteristics of biochars prepared via co-pyrolysis of sewage sludge and cotton stalks. J. Anal. Appl. Pyrolysis 2019, 142, 104659. [Google Scholar] [CrossRef]
- Li, S.; Barreto, V.; Li, R.; Chen, G.; Hsieh, Y.P. Nitrogen retention of biochar derived from different feedstocks at variable pyrolysis temperatures. J. Anal. Appl. Pyrolysis 2018, 133, 136–146. [Google Scholar] [CrossRef]
- Mohanty, P.; Nanda, S.; Pant, K.K.; Naik, S.; Kozinski, J.A.; Dalai, A.K. Evaluation of the physiochemical development of biochars obtained from pyrolysis of wheat straw, timothy grass and pinewood: Effects of heating rate. J. Anal. Appl. Pyrolysis 2013, 104, 485–493. [Google Scholar] [CrossRef]
- Dudek, M.; Świechowski, K.; Manczarski, P.; Koziel, J.A.; Białowiec, A. The effect of biochar addition on the biogas production kinetics from the anaerobic digestion of brewers’ spent grain. Energies 2019, 12, 1518. [Google Scholar] [CrossRef]
- Demirbas, A. Effects of temperature and particle size on bio-char yield from pyrolysis of agricultural residues. J. Anal. Appl. Pyrolysis 2004, 72, 243–248. [Google Scholar] [CrossRef]
- Karaosmanoǧlu, F.; Işıḡıgür-Ergüdenler, A.; Sever, A. Biochar from the straw-stalk of rapeseed plant. Energy Fuels 2000, 14, 336–339. [Google Scholar] [CrossRef]
- Garcia-Nunez, J.A.; Pelaez-Samaniego, M.R.; Garcia-Perez, M.E.; Fonts, I.; Abrego, J.; Westerhof, R.J.M.; Garcia-Perez, M. Historical Developments of Pyrolysis Reactors: A Review. Energy Fuels 2017, 31, 5751–5775. [Google Scholar] [CrossRef]
- Lewandowski, W.M.; Januszewicz, K.; Kosakowski, W. Efficiency and proportions of waste tyre pyrolysis products depending on the reactor type—A review. J. Anal. Appl. Pyrolysis 2019, 140, 25–53. [Google Scholar] [CrossRef]
- Sekar, M.; Ponnusamy, V.K.; Pugazhendhi, A.; Nižetić, S.; Praveenkumar, T. Production and utilization of pyrolysis oil from solidplastic wastes: A review on pyrolysis process and influence of reactors design. J. Environ. Manag. 2022, 302, 114046. [Google Scholar] [CrossRef]
- Aysu, T.; Küçük, M.M. Biomass pyrolysis in a fixed-bed reactor: Effects of pyrolysis parameters on product yields and characterization of products. Energy 2014, 64, 1002–1025. [Google Scholar] [CrossRef]
- Liu, S.; Chu, L.; Chen, M.; Zhang, W.; Xin, W. Modeling and analysis of the pyrolysis of bio-oil aqueous fraction in a fixed-bed reactor. Fuel 2014, 133, 1–6. [Google Scholar] [CrossRef]
- Khan, S.R.; Zeeshan, M.; Khokhar, M.F.; Zeshan; Ahmad, I. A comprehensive study on upgradation of pyrolysis products through co-feeding of waste tire into rice straw under broad range of co-feed ratios in a bench-scale fixed bed reactor. Biomass Convers. Biorefin. 2021, 13, 4751–4765. [Google Scholar] [CrossRef]
- Kersten, S.R.; Wang, X.; Prins, W.; van Swaaij, W.P. Biomass pyrolysis in a fluidized bed reactor. Part 1: Literature review and model simulations. Ind. Eng. Chem. Res. 2005, 44, 8773–8785. [Google Scholar] [CrossRef]
- Afacan, O.; Gogebakan, Y.; Selçuk, N. Modeling of NOx emissions from fluidized bed combustion of high volatile lignites. Combust. Sci. Technol. 2007, 179, 227–247. [Google Scholar] [CrossRef]
- Li, S.-Q.; Yao, Q.; Chi, Y.; Yan, J.-H.; Cen, K.-F. Pilot-scale pyrolysis of scrap tires in a continuous rotary kiln reactor. Ind. Eng. Chem. Res. 2004, 43, 5133–5145. [Google Scholar] [CrossRef]
- De Conto, D.; Silvestre, W.; Baldasso, C.; Godinho, M. Performance of rotary kiln reactor for the elephant grass pyrolysis. Bioresour. Technol. 2016, 218, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yu, J.; He, W.; Huang, J.; Xu, J.; Li, G. Recovery of carbon black from waste tire in continuous commercial rotary kiln pyrolysis reactor. Sci. Total Environ. 2021, 772, 145507. [Google Scholar] [CrossRef]
- Tanoh, T.S.; Ait Oumeziane, A.; Lemonon, J.; Escudero Sanz, F.J.; Salvador, S. Green waste/wood pellet pyrolysis in a pilot-scale rotary kiln: Effect of temperature on product distribution and characteristics. Energy Fuels 2020, 34, 3336–3345. [Google Scholar] [CrossRef]
- Krishnamoorthy, V.; Yeboah, Y.D.; Pisupati, S.V. Influence of pyrolysis gas on volatile yield and CO2 reaction kinetics of the char samples generated in a high-pressure, high-temperature flow reactor. Energies 2018, 12, 107. [Google Scholar] [CrossRef]
- Pienihäkkinen, E.; Lindfors, C.; Ohra-aho, T.; Lehtonen, J.; Granström, T.; Yamamoto, M.; Oasmaa, A. Fast Pyrolysis of Hydrolysis Lignin in Fluidized Bed Reactors. Energy Fuels 2021, 35, 14758–14769. [Google Scholar] [CrossRef]
- Xue, Y.; Zhou, S.; Brown, R.C.; Kelkar, A.; Bai, X. Fast pyrolysis of biomass and waste plastic in a fluidized bed reactor. Fuel 2015, 156, 40–46. [Google Scholar] [CrossRef]
- Zhou, H.; Wu, C.; Meng, A.; Zhang, Y.; Williams, P.T. Effect of interactions of biomass constituents on polycyclic aromatic hydrocarbons (PAH) formation during fast pyrolysis. J. Anal. Appl. Pyrolysis 2014, 110, 264–269. [Google Scholar] [CrossRef]
- Safdari, M.-S.; Rahmati, M.; Amini, E.; Howarth, J.E.; Berryhill, J.P.; Dietenberger, M.; Weise, D.R.; Fletcher, T.H. Characterization of pyrolysis products from fast pyrolysis of live and dead vegetation native to the Southern United States. Fuel 2018, 229, 151–166. [Google Scholar] [CrossRef]
- Wang, W.; Wen, C.; Li, C.; Wang, M.; Li, X.; Zhou, Y.; Gong, X. Emission reduction of particulate matter from the combustion of biochar via thermal pre-treatment of torrefaction, slow pyrolysis or hydrothermal carbonisation and its co-combustion with pulverized coal. Fuel 2019, 240, 278–288. [Google Scholar] [CrossRef]
- Liu, J.; Hou, Q.; Ju, M.; Ji, P.; Sun, Q.; Li, W. Biomass pyrolysis technology by catalytic fast pyrolysis, catalytic co-pyrolysis and microwave-assisted pyrolysis: A review. Catalysts 2020, 10, 742. [Google Scholar] [CrossRef]
- Canel, M.; Mısırlıoğlu, Z.; Canel, E.; Bozkurt, P.A. Distribution and comparing of volatile products during slow pyrolysis and hydropyrolysis of Turkish lignites. Fuel 2016, 186, 504–517. [Google Scholar] [CrossRef]
- Enam, R.N.; Tahir, M.; Hasan Rizvi, H.; Rafique, A.; Mustafa, S.M.N. A sustainable way to generate energy through biomass flash pyrolysis in south asia: A green energy technology. Int. J. Energy Econ. Policy 2022, 12, 274–279. [Google Scholar] [CrossRef]
- Ruiz, M.; Martin, E.; Blin, J.; Van de Steene, L.; Broust, F. Understanding the Secondary Reactions of Flash Pyrolysis Vapors inside a Hot Gas Filtration Unit. Energy Fuels 2017, 31, 13785–13795. [Google Scholar] [CrossRef]
- Yan, M.; Zhang, S.; Wibowo, H.; Grisdanurak, N.; Cai, Y.; Zhou, X.; Kanchanatip, E.; Antoni. Biochar and pyrolytic gas properties from pyrolysis of simulated municipal solid waste (SMSW) under pyrolytic gas atmosphere. Waste Dispos. Sustain. Energy 2020, 2, 37–46. [Google Scholar] [CrossRef]
- Xue, Y.; Johnston, P.; Bai, X. Effect of catalyst contact mode and gas atmosphere during catalytic pyrolysis of waste plastics. Energy Convers. Manag. 2017, 142, 441–451. [Google Scholar] [CrossRef]
- Barbooti, M.M.; Mohamed, T.J.; Hussain, A.A.; Abas, F.O. Optimization of pyrolysis conditions of scrap tires under inert gas atmosphere. J. Anal. Appl. Pyrolysis 2004, 72, 165–170. [Google Scholar] [CrossRef]
- Baker, R.R. A review of pyrolysis studies to unravel reaction steps in burning tobacco. J. Anal. Appl. Pyrolysis 1987, 11, 555–573. [Google Scholar] [CrossRef]
- Li, J.; Duan, H.; Yu, K.; Liu, L.; Wang, S. Characteristic of low-temperature pyrolysis of printed circuit boards subjected to various atmosphere. Resour. Conserv. Recycl. 2010, 54, 810–815. [Google Scholar] [CrossRef]
- Chang, L.; Xie, Z.; Xie, K.-C.; Pratt, K.C.; Hayashi, J.-i.; Chiba, T.; Li, C.-Z. Formation of NOx precursors during the pyrolysis of coal and biomass. Part VI. Effects of gas atmosphere on the formation of NH3 and HCN. Fuel 2003, 82, 1159–1166. [Google Scholar] [CrossRef]
- Pröll, T.; Al Afif, R.; Schaffer, S.; Pfeifer, C. Reduced local emissions and long-term carbon storage through pyrolysis of agricultural waste and application of pyrolysis char for soil improvement. Energy Procedia 2017, 114, 6057–6066. [Google Scholar] [CrossRef]
- Guoxin, H.; Hao, H.; Yanhong, L. Hydrogen-rich gas production from pyrolysis of biomass in an autogenerated steam atmosphere. Energy Fuels 2009, 23, 1748–1753. [Google Scholar] [CrossRef]
- Demirbas, A. Hydrogen-rich gases from biomass via pyrolysis and air-steam gasification. Energy Sources Part A 2009, 31, 1728–1736. [Google Scholar] [CrossRef]
- Alkhatib, M.A.F.; Muyibi, S.A.; Amode, J.O. Optimization of activated carbon production from empty fruit bunch fibers in one-step steam pyrolysis for cadmium removal from aqueous solution. Environmentalist 2011, 31, 349–357. [Google Scholar] [CrossRef]
- Zhang, G.; Zhu, C.; Ge, Y.; Liu, X.; Xu, G. Fluidized bed combustion in steam-rich atmospheres for high-nitrogen fuel: Nitrogen distribution in char and volatile and their contributions to NOx. Fuel 2016, 186, 204–214. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, S.; Liu, H.; Yang, J.; Liu, X.; Xu, G. NOx emission characteristics of fluidized bed combustion in atmospheres rich in oxygen and water vapor for high-nitrogen fuel. Fuel 2015, 139, 346–355. [Google Scholar] [CrossRef]
- Sørmo, E.; Krahn, K.M.; Flatabø, G.Ø.; Hartnik, T.; Arp, H.P.H.; Cornelissen, G. Distribution of PAHs, PCBs, and PCDD/Fs in products from full-scale relevant pyrolysis of diverse contaminated organic waste. J. Hazard. Mater. 2024, 461, 132546. [Google Scholar] [CrossRef]
- Soler, A.; Conesa, J.A.; Iñiguez, M.E.; Ortuño, N. Pollutant formation in the pyrolysis and combustion of materials combining biomass and e-waste. Sci. Total Environ. 2018, 622–623, 1258–1264. [Google Scholar] [CrossRef] [PubMed]
- Florentino-Madiedo, L.; Díaz-Faes, E.; García, R.; Barriocanal, C. Influence of binder type on greenhouse gases and PAHs from the pyrolysis of biomass briquettes. Fuel Process. Technol. 2018, 171, 330–338. [Google Scholar] [CrossRef]
- Sahoo, K.; Kumar, A.; Chakraborty, J.P. A comparative study on valuable products: Bio-oil, biochar, non-condensable gases from pyrolysis of agricultural residues. J. Mater. Cycles Waste Manag. 2021, 23, 186–204. [Google Scholar] [CrossRef]
- Guo, S.; Xiong, X.; Che, D.; Liu, H.; Sun, B. Effects of sludge pyrolysis temperature and atmosphere on characteristics of biochar and gaseous products. Korean J. Chem. Eng. 2021, 38, 55–63. [Google Scholar] [CrossRef]
- Yin, Y.; Gao, Y.; Li, A. Self-activation of biochar from furfural residues by recycled pyrolysis gas. Waste Manag. 2018, 77, 312–321. [Google Scholar] [CrossRef]
- Severy, M.A.; Carter, D.J.; Palmer, K.D.; Eggink, A.J.; Chamberlin, C.E.; Jacobson, A.E. Performance and emissions control of commercial-scale biochar production unit. Appl. Eng. Agric. 2018, 34, 73–84. [Google Scholar] [CrossRef]
- Imran, A.; Bramer, E.A.; Seshan, K.; Brem, G. An overview of catalysts in biomass pyrolysis for production of biofuels. Biofuel Res. J. 2018, 5, 872–885. [Google Scholar] [CrossRef]
- Samolada, M.; Papafotica, A.; Vasalos, I. Catalyst evaluation for catalytic biomass pyrolysis. Energy Fuels 2000, 14, 1161–1167. [Google Scholar] [CrossRef]
- Liang, J.; Shan, G.; Sun, Y. Catalytic fast pyrolysis of lignocellulosic biomass: Critical role of zeolite catalysts. Renew. Sustain. Energy Rev. 2021, 139, 110707. [Google Scholar] [CrossRef]
- Yao, D.; Yang, H.; Chen, H.; Williams, P.T. Co-precipitation, impregnation and so-gel preparation of Ni catalysts for pyrolysis-catalytic steam reforming of waste plastics. Appl. Catal. B Environ. 2018, 239, 565–577. [Google Scholar] [CrossRef]
- Tian, X.; Wang, Y.; Zeng, Z.; Dai, L.; Xu, J.; Cobb, K.; Ke, L.; Zou, R.; Liu, Y.; Ruan, R. Research progress on the role of common metal catalysts in biomass pyrolysis: A state-of-the-art review. Green Chem. 2022, 24, 3922–3942. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, W.; Qiu, J.; Li, B. Effect of Na, Ca and Fe on the evolution of nitrogen species during pyrolysis and combustion of model chars. Fuel 2003, 82, 1839–1844. [Google Scholar] [CrossRef]
- Li, S.; Wang, C.; Luo, Z.; Zhu, X. Investigation on the catalytic behavior of alkali metals and alkaline earth metals on the biomass pyrolysis assisted with real-time monitoring. Energy Fuels 2020, 34, 12654–12664. [Google Scholar] [CrossRef]
- Banks, S.W.; Nowakowski, D.J.; Bridgwater, A.V. Impact of potassium and phosphorus in biomass on the properties of fast pyrolysis bio-oil. Energy Fuels 2016, 30, 8009–8018. [Google Scholar] [CrossRef]
- Case, P.A.; Wheeler, M.C.; DeSisto, W.J. Formate assisted pyrolysis of pine sawdust for in-situ oxygen removal and stabilization of bio-oil. Bioresour. Technol. 2014, 173, 177–184. [Google Scholar] [CrossRef]
- Mukherjee, A.; Agrawal, M. World air particulate matter: Sources, distribution and health effects. Environ. Chem. Lett. 2017, 15, 283–309. [Google Scholar] [CrossRef]
- Pothirat, C.; Chaiwong, W.; Liwsrisakun, C.; Bumroongkit, C.; Deesomchok, A.; Theerakittikul, T.; Limsukon, A.; Tajaroenmuang, P.; Phetsuk, N. Influence of particulate matter during seasonal smog on quality of life and lung function in patients with chronic obstructive pulmonary disease. Int. J. Environ. Res. Public Health 2019, 16, 106. [Google Scholar] [CrossRef]
- Petzold, A.; Weingartner, E.; Hasselbach, J.; Lauer, P.; Kurok, C.; Fleischer, F. Physical properties, chemical composition, and cloud forming potential of particulate emissions from a marine diesel engine at various load conditions. Environ. Sci. Technol. 2010, 44, 3800–3805. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.H.; Wang, J.; Xu, Q. Characterization of particulate matter formed during sewage sludge pyrolysis. Fuel 2018, 224, 210–218. [Google Scholar]
- Rai, P.K. Multifaceted health impacts of particulate matter (PM) and its management: An overview. Environ. Skept. Crit. 2015, 4, 1. [Google Scholar]
- Wang, X.; Zhang, J.; Bai, S.; Zhang, L.; Li, Y.; Mikulčić, H.; Chen, J.; Wang, L.; Tan, H. Effect of pyrolysis upgrading temperature on particulate matter emissions from lignite semi-char combustion. Energy Convers. Manag. 2019, 195, 384–391. [Google Scholar] [CrossRef]
- Hester, R.; Harrison, R.; Derwent, R.G. Sources, Distributions, and Fates of VOCs in the Atmosphere; Royal Society of Chemistry: London, UK, 1995; p. 387. [Google Scholar]
- Soni, V.; Singh, P.; Shree, V.; Goel, V. Effects of VOCs on human health. In Air Pollution and Control; Springer: Berlin/Heidelberg, Germany, 2018; pp. 119–142. [Google Scholar]
- Wu, W.; Zhao, B.; Wang, S.; Hao, J. Ozone and secondary organic aerosol formation potential from anthropogenic volatile organic compounds emissions in China. J. Environ. Sci. 2017, 53, 224–237. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Kong, S.; Chen, N.; Niu, Z.; Zhang, Y.; Jiang, S.; Yan, Y.; Qi, S. Source apportionment of volatile organic compounds: Implications to reactivity, ozone formation, and secondary organic aerosol potential. Atmos. Res. 2021, 249, 105344. [Google Scholar] [CrossRef]
- Li, J.; Deng, S.; Tohti, A.; Li, G.; Yi, X.; Lu, Z.; Liu, J.; Zhang, S. Spatial characteristics of VOCs and their ozone and secondary organic aerosol formation potentials in autumn and winter in the Guanzhong Plain, China. Environ. Res. 2022, 211, 113036. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, Z.; Wang, G.; Wang, Z.; Li, M.; Liang, W.; Gao, J.; Wang, W.; Chen, D.; Feng, Y. Machine learning and theoretical analysis release the non-linear relationship among ozone, secondary organic aerosol and volatile organic compounds. J. Environ. Sci. 2022, 114, 75–84. [Google Scholar] [CrossRef]
- Gilman, J.; Lerner, B.; Kuster, W.; Goldan, P.; Warneke, C.; Veres, P.; Roberts, J.; De Gouw, J.; Burling, I.; Yokelson, R. Biomass burning emissions and potential air quality impacts of volatile organic compounds and other trace gases from fuels common in the US. Atmos. Chem. Phys. 2015, 15, 13915–13938. [Google Scholar] [CrossRef]
- Li, L.; Chen, Y.; Zeng, L.; Shao, M.; Xie, S.; Chen, W.; Lu, S.; Wu, Y.; Cao, W. Biomass burning contribution to ambient volatile organic compounds (VOCs) in the Chengdu–Chongqing Region (CCR), China. Atmos. Environ. 2014, 99, 403–410. [Google Scholar] [CrossRef]
- Bălă, G.-P.; Râjnoveanu, R.-M.; Tudorache, E.; Motișan, R.; Oancea, C. Air pollution exposure—The (in) visible risk factor for respiratory diseases. Environ. Sci. Pollut. Res. 2021, 28, 19615–19628. [Google Scholar] [CrossRef] [PubMed]
- Sahle-Demessie, E.; Mezgebe, B.; Dietrich, J.; Shan, Y.; Harmon, S.; Lee, C.C. Material recovery from electronic waste using pyrolysis: Emissions measurements and risk assessment. J. Environ. Chem. Eng. 2021, 9, 104943. [Google Scholar] [CrossRef] [PubMed]
- Spinelle, L.; Gerboles, M.; Kok, G.; Persijn, S.; Sauerwald, T. Review of portable and low-cost sensors for the ambient air monitoring of benzene and other volatile organic compounds. Sensors 2017, 17, 1520. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.; Campbell, C.; Naidenko, O.V. Cumulative risk analysis of carcinogenic contaminants in United States drinking water. Heliyon 2019, 5, e02314. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, N.; Cuadras, A.; Rovira, E.; Borrull, F.; Marcé, R.M. Chronic risk assessment of exposure to volatile organic compounds in the atmosphere near the largest Mediterranean industrial site. Environ. Int. 2012, 39, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Hakim, M.; Broza, Y.Y.; Barash, O.; Peled, N.; Phillips, M.; Amann, A.; Haick, H. Volatile organic compounds of lung cancer and possible biochemical pathways. Chem. Rev. 2012, 112, 5949–5966. [Google Scholar] [CrossRef] [PubMed]
- Lundstedt, S.; White, P.A.; Lemieux, C.L.; Lynes, K.D.; Lambert, I.B.; Öberg, L.; Haglund, P.; Tysklind, M. Sources, fate, and toxic hazards of oxygenated polycyclic aromatic hydrocarbons (PAHs) at PAH-contaminated sites. AMBIO J. Hum. Environ. 2007, 36, 475–485. [Google Scholar] [CrossRef]
- McGrath, T.; Sharma, R.; Hajaligol, M. An experimental investigation into the formation of polycyclic-aromatic hydrocarbons (PAH) from pyrolysis of biomass materials. Fuel 2001, 80, 1787–1797. [Google Scholar] [CrossRef]
- Zhu, L.; Chen, Y.; Zhou, R. Distribution of polycyclic aromatic hydrocarbons in water, sediment and soil in drinking water resource of Zhejiang Province, China. J. Hazard. Mater. 2008, 150, 308–316. [Google Scholar] [CrossRef]
- Lindgren, J.F.; Hassellöv, I.-M.; Dahllöf, I. PAH effects on meio-and microbial benthic communities strongly depend on bioavailability. Aquat. Toxicol. 2014, 146, 230–238. [Google Scholar] [CrossRef]
- Odinga, E.S.; Gudda, F.O.; Waigi, M.G.; Wang, J.; Gao, Y. Occurrence, formation and environmental fate of polycyclic aromatic hydrocarbons in biochars. Fundam. Res. 2021, 1, 296–305. [Google Scholar] [CrossRef]
- Girardin, V.; Grung, M.; Meland, S. Polycyclic aromatic hydrocarbons: Bioaccumulation in dragonfly nymphs (Anisoptera), and determination of alkylated forms in sediment for an improved environmental assessment. Sci. Rep. 2020, 10, 10958. [Google Scholar] [CrossRef]
- Recabarren-Villalón, T.; Ronda, A.C.; Oliva, A.L.; Cazorla, A.L.; Marcovecchio, J.E.; Arias, A.H. Seasonal distribution pattern and bioaccumulation of Polycyclic aromatic hydrocarbons (PAHs) in four bioindicator coastal fishes of Argentina. Environ. Pollut. 2021, 291, 118125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, Y.; Wang, C.; Feng, Z.; Hao, Z.; Yu, W.; Wang, T.; Zou, X. Polycyclic aromatic hydrocarbons (PAHs) in marine organisms from two fishing grounds, South Yellow Sea, China: Bioaccumulation and human health risk assessment. Mar. Pollut. Bull. 2020, 153, 110995. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, H.; Zhang, X.; Xing, W.; Wang, Y.; Bai, P.; Zhang, L.; Hayakawa, K.; Toriba, A.; Tang, N. Exposure to atmospheric particulate matter-bound polycyclic aromatic hydrocarbons and their health effects: A review. Int. J. Environ. Res. Public Health 2021, 18, 2177. [Google Scholar] [CrossRef] [PubMed]
- Låg, M.; Øvrevik, J.; Refsnes, M.; Holme, J.A. Potential role of polycyclic aromatic hydrocarbons in air pollution-induced non-malignant respiratory diseases. Respir. Res. 2020, 21, 299. [Google Scholar] [CrossRef] [PubMed]
- Rota, M.; Bosetti, C.; Boccia, S.; Boffetta, P.; La Vecchia, C. Occupational exposures to polycyclic aromatic hydrocarbons and respiratory and urinary tract cancers: An updated systematic review and a meta-analysis to 2014. Arch. Toxicol. 2014, 88, 1479–1490. [Google Scholar] [CrossRef]
- Kameda, Y.; Shirai, J.; Komai, T.; Nakanishi, J.; Masunaga, S. Atmospheric polycyclic aromatic hydrocarbons: Size distribution, estimation of their risk and their depositions to the human respiratory tract. Sci. Total Environ. 2005, 340, 71–80. [Google Scholar] [CrossRef]
- Mallah, M.A.; Mallah, M.A.; Liu, Y.; Xi, H.; Wang, W.; Feng, F.; Zhang, Q. Relationship between polycyclic aromatic hydrocarbons and cardiovascular diseases: A systematic review. Front. Public Health 2021, 9, 763706. [Google Scholar] [CrossRef]
- Collins, J.; Brown, J.; Dawson, S.; Marty, M. Risk assessment for benzo [a] pyrene. Regul. Toxicol. Pharmacol. 1991, 13, 170–184. [Google Scholar] [CrossRef]
- Boström, C.-E.; Gerde, P.; Hanberg, A.; Jernström, B.; Johansson, C.; Kyrklund, T.; Rannug, A.; Törnqvist, M.; Victorin, K.; Westerholm, R. Cancer risk assessment, indicators, and guidelines for polycyclic aromatic hydrocarbons in the ambient air. Environ. Health Perspect. 2002, 110 (Suppl. S3), 451–488. [Google Scholar]
- Shen, H.; Tao, S.; Liu, J.; Huang, Y.; Chen, H.; Li, W.; Zhang, Y.; Chen, Y.; Su, S.; Lin, N.; et al. Global lung cancer risk from PAH exposure highly depends on emission sources and individual susceptibility. Sci. Rep. 2014, 4, 6561. [Google Scholar] [CrossRef]
- Zhan, H.; Yin, X.; Huang, Y.; Yuan, H.; Wu, C. NOx precursors evolving during rapid pyrolysis of lignocellulosic industrial biomass wastes. Fuel 2017, 207, 438–448. [Google Scholar] [CrossRef]
- Zhuang, X.; Song, Y.; Wang, X.; Zhan, H.; Yin, X.; Wu, C.; Wang, P. Pyrolysis of hydrothermally pretreated biowastes: The controllability on the formation of NOx precursors. Chem. Eng. J. 2020, 393, 124727. [Google Scholar] [CrossRef]
- Li, C.-Z.; Tan, L.L. Formation of NOx and SOx precursors during the pyrolysis of coal and biomass. Part III. Further discussion on the formation of HCN and NH3 during pyrolysis. Fuel 2000, 79, 1899–1906. [Google Scholar] [CrossRef]
- Boningari, T.; Smirniotis, P.G. Impact of nitrogen oxides on the environment and human health: Mn-based materials for the NOx abatement. Curr. Opin. Chem. Eng. 2016, 13, 133–141. [Google Scholar] [CrossRef]
- Pitari, G.; Iachetti, D.; Di Genova, G.; De Luca, N.; Søvde, O.A.; Hodnebrog, Ø.; Lee, D.S.; Lim, L.L. Impact of Coupled NOx/Aerosol Aircraft Emissions on Ozone Photochemistry and Radiative Forcing. Atmosphere 2015, 6, 751–782. [Google Scholar] [CrossRef]
- Asghar, U.; Rafiq, S.; Anwar, A.; Iqbal, T.; Ahmed, A.; Jamil, F.; Khurram, M.S.; Akbar, M.M.; Farooq, A.; Shah, N.S.; et al. Review on the progress in emission control technologies for the abatement of CO2, SOx and NOx from fuel combustion. J. Environ. Chem. Eng. 2021, 9, 106064. [Google Scholar] [CrossRef]
- Zhan, H.; Yin, X.-L.; Huang, Y.-Q.; Zhang, X.-H.; Yuan, H.-Y.; Xie, J.-J.; Wu, C.-Z. Characteristics of NOx precursors and their formation mechanism during pyrolysis of herb residues. J. Fuel Chem. Technol. 2017, 45, 279–288. [Google Scholar] [CrossRef]
- Xu, L.; Tsona, N.T.; You, B.; Zhang, Y.; Wang, S.; Yang, Z.; Xue, L.; Du, L. NOx enhances secondary organic aerosol formation from nighttime γ-terpinene ozonolysis. Atmos. Environ. 2020, 225, 117375. [Google Scholar] [CrossRef]
- Liu, C.; Shi, K. A review on methodology in O3-NOx-VOC sensitivity study. Environ. Pollut. 2021, 291, 118249. [Google Scholar] [CrossRef]
- Kowalska, M.; Skrzypek, M.; Kowalski, M.; Cyrys, J. Effect of NOx and NO2 Concentration Increase in Ambient Air to Daily Bronchitis and Asthma Exacerbation, Silesian Voivodeship in Poland. Int. J. Environ. Res. Public Health 2020, 17, 754. [Google Scholar] [CrossRef]
- Li, A.; Zhou, Q.; Xu, Q. Prospects for ozone pollution control in China: An epidemiological perspective. Environ. Pollut. 2021, 285, 117670. [Google Scholar] [CrossRef]
- Wu, I.P.; Liao, S.-L.; Lai, S.-H.; Wong, K.-S. The respiratory impacts of air pollution in children: Global and domestic (Taiwan) situation. Biomed. J. 2022, 45, 88–94. [Google Scholar] [CrossRef]
- Kinoshita, H.; Türkan, H.; Vucinic, S.; Naqvi, S.; Bedair, R.; Rezaee, R.; Tsatsakis, A. Carbon monoxide poisoning. Toxicol. Rep. 2020, 7, 169–173. [Google Scholar] [CrossRef]
- Rehman, A.; Ma, H.; Ahmad, M.; Irfan, M.; Traore, O.; Chandio, A.A. Towards environmental Sustainability: Devolving the influence of carbon dioxide emission to population growth, climate change, Forestry, livestock and crops production in Pakistan. Ecol. Indic. 2021, 125, 107460. [Google Scholar] [CrossRef]
- Wang, Q.; Hua, Z.; Guan, J. Structure of Wangqing oil shale and mechanism of carbon monoxide release during its pyrolysis. Energy Sci. Eng. 2019, 7, 2398–2409. [Google Scholar] [CrossRef]
- Yaqoob, H.; Teoh, Y.H.; Jamil, M.A.; Gulzar, M. Potential of tire pyrolysis oil as an alternate fuel for diesel engines: A review. J. Energy Inst. 2021, 96, 205–221. [Google Scholar] [CrossRef]
- Dey, S.; Dhal, G.C.; Mohan, D.; Prasad, R. Application of hopcalite catalyst for controlling carbon monoxide emission at cold-start emission conditions. J. Traffic Transp. Eng. 2019, 6, 419–440. [Google Scholar] [CrossRef]
- Al-Wabel, M.I.; Ahmad, M.; Usman, A.R.A.; Akanji, M.; Rafique, M.I. Advances in Pyrolytic Technologies with Improved Carbon Capture and Storage to Combat Climate Change. In Environment, Climate, Plant and Vegetation Growth; Fahad, S., Hasanuzzaman, M., Alam, M., Ullah, H., Saeed, M., Ali Khan, I., Adnan, M., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 535–575. [Google Scholar]
- Ma, C.; Zhao, Y.; Chen, H.; Liu, Y.; Huang, R.; Pan, J. Biochars derived from by-products of microalgae pyrolysis for sorption of gaseous H2S. J. Environ. Chem. Eng. 2022, 10, 107370. [Google Scholar] [CrossRef]
- Czajczyńska, D.; Krzyżyńska, R.; Jouhara, H. Hydrogen sulfide removal from waste tyre pyrolysis gas by inorganics. Int. J. Hydrogen Energy 2024, 52, 785–799. [Google Scholar] [CrossRef]
- Batterman, S.; Grant-Alfieri, A.; Seo, S.-H. Low level exposure to hydrogen sulfide: A review of emissions, community exposure, health effects, and exposure guidelines. Crit. Rev. Toxicol. 2023, 53, 244–295. [Google Scholar] [CrossRef]
- Idris, R.; Chong, W.W.F.; Ali, A.; Idris, S.; Tan, W.H.; Md Salim, R.; Mong, G.R.; Chong, C.T. Pyrolytic oil with aromatic-rich hydrocarbons via microwave-induced in-situ catalytic co-pyrolysis of empty fruit bunches with a waste truck tire. Energy Convers. Manag. 2021, 244, 114502. [Google Scholar] [CrossRef]
- Esworthy, R.; McCarthy, J.E. The National Ambient Air Quality Standards (NAAQS) for Particulate Matter (PM): EPA’s 2006 Revisions and Associated Issues, 2013; Library of Congress, Congressional Research Service: Washington, DC, USA, 2013.
- Kumar, R.; Gupta, P. Air pollution control policies and regulations. In Plant Responses to Air Pollution; Springer: Berlin/Heidelberg, Germany, 2016; pp. 133–149. [Google Scholar]
- Hester, R.E.; Harrison, R.M.; Lloyd, A.C. California’s approach to air quality management. In Air Quality Management; Harrison, R.M., Hester, R.E., Eds.; The Royal Society of Chemistry: London, UK, 1997; Volume 8. [Google Scholar]
- Balmes, J.R. California’s Integrated Approach to Air Quality and Climate Change. In Climate Change and Global Public Health; Pinkerton, K.E., Rom, W.N., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 541–548. [Google Scholar]
- Thoma, E.D.; Wright, R.S.; George, I.; Krause, M.; Presezzi, D.; Villa, V.; Preston, W.; Deshmukh, P.; Kauppi, P.; Zemek, P.G. Pyrolysis processing of PFAS-impacted biosolids, a pilot study. J. Air Waste Manag. Assoc. 2022, 72, 309–318. [Google Scholar] [CrossRef]
- Sari, R.M.; Gea, S.; Wirjosentono, B.; Hendrana, S.; Hutapea, Y.A. Improving quality and yield production of coconut shell charcoal through a modified pyrolysis reactor with tar scrubber to reduce smoke pollution. Pol. J. Environ. Stud. 2020, 29, 1815–1824. [Google Scholar] [CrossRef]
- Sabegh, M.Y.; Norouzi, O.; Jafarian, S.; Tavasoli, A. Pyrolysis of marine biomass to produce bio-oil and its upgrading using a novel multi-metal catalyst prepared from the spent car catalytic converter. Bioresour. Technol. 2018, 249, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhao, T.; Liu, K.; Sun, B.; Bai, C. Optimization of structure parameters in a coal pyrolysis filtration system based on CFD and quadratic regression orthogonal combination and a genetic algorithm. Eng. Appl. Comput. Fluid Mech. 2021, 15, 815–829. [Google Scholar] [CrossRef]
- Kaivosoja, T.; Virén, A.; Tissari, J.; Ruuskanen, J.; Tarhanen, J.; Sippula, O.; Jokiniemi, J. Effects of a catalytic converter on PCDD/F, chlorophenol and PAH emissions in residential wood combustion. Chemosphere 2012, 88, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Mikulski, M.; Hunicz, J.; Duda, K.; Kazimierski, P.; Suchocki, T.; Rybak, A. Tyre pyrolytic oil fuel blends in a modern compression ignition engine: A comprehensive combustion and emissions analysis. Fuel 2022, 320, 123869. [Google Scholar] [CrossRef]
- Buss, W.; Hilber, I.; Graham, M.C.; Mašek, O. Composition of PAHs in biochar and implications for biochar production. ACS Sustain. Chem. Eng. 2022, 10, 6755–6765. [Google Scholar] [CrossRef]
- Han, J.; Kim, H. The reduction and control technology of tar during biomass gasification/pyrolysis: An overview. Renew. Sustain. Energy Rev. 2008, 12, 397–416. [Google Scholar] [CrossRef]
- Raza, M.; Inayat, A.; Ahmed, A.; Jamil, F.; Ghenai, C.; Naqvi, S.R.; Shanableh, A.; Ayoub, M.; Waris, A.; Park, Y.-K. Progress of the pyrolyzer reactors and advanced technologies for biomass pyrolysis processing. Sustainability 2021, 13, 11061. [Google Scholar] [CrossRef]
- Atsonios, K.; Kougioumtzis, M.A.; Grammelis, P.; Kakaras, E. Process integration of a polygeneration plant with biomass/coal co-pyrolysis. Energy Fuels 2017, 31, 14408–14422. [Google Scholar] [CrossRef]
- Guerreiro, C.B.; Foltescu, V.; De Leeuw, F. Air quality status and trends in Europe. Atmos. Environ. 2014, 98, 376–384. [Google Scholar] [CrossRef]
- Calvo, V.L.V.; Giner-Santonja, G.; Alonso-Fariñas, B.; Aguado, J.M. The effect of the European Industrial Emissions Directive on the air emission limit values set by competent authorities in the permitting procedure: The case of the Spanish cement industry. Sci. Total Environ. 2021, 773, 145491. [Google Scholar] [CrossRef]
- Van Caneghem, J.; Van Acker, K.; De Greef, J.; Wauters, G.; Vandecasteele, C. Waste-to-energy is compatible and complementary with recycling in the circular economy. Clean Technol. Environ. Policy 2019, 21, 925–939. [Google Scholar] [CrossRef]
- Aouadj, S.; Zebirate, S.; Smail, R.; Saidi, F. Optimization of the technical and environmental performance of the renewable energies. Case of the hybrid powerplant “SPPI” of HassiR’mel in the central highlands of Algeria. Environ. Eng. Res. 2021, 26, 200056. [Google Scholar] [CrossRef]
- Coelho, S.T.; Diaz-Chavez, R. Best Available Technologies (BAT) for WtE in developing countries. In Municipal Solid Waste Energy Conversion in Developing Countries; Elsevier: Amsterdam, The Netherlands, 2020; pp. 63–105. [Google Scholar]
- Conti, M.E.; Ciasullo, R.; Tudino, M.B.; Matta, E.J. The industrial emissions trend and the problem of the implementation of the Industrial Emissions Directive (IED). Air Qual. Atmos. Health 2015, 8, 151–161. [Google Scholar] [CrossRef]
- O’Malley, V. The Integrated Pollution Prevention and Control (IPPC) Directive and its implications for the environment and industrial activities in Europe. Sens. Actuators B Chem. 1999, 59, 78–82. [Google Scholar] [CrossRef]
- Barthe, P.; Chaugny, M.; Roudier, S.; Delgado Sancho, L. Best Available Techniques (BAT) Reference Document for the Refining of Mineral Oil and Gas; European Commission: Brussels, Belgium, 2015; Volume 754. [Google Scholar]
- Cai, S.; Wang, Y.; Zhao, B.; Wang, S.; Chang, X.; Hao, J. The impact of the “air pollution prevention and control action plan” on PM2.5 concentrations in Jing-Jin-Ji region during 2012–2020. Sci. Total Environ. 2017, 580, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Zhang, M.; Wang, Q.; Wang, Y.; Zuo, J. Evolution analysis of environmental standards: Effectiveness on air pollutant emissions reduction. J. Clean. Prod. 2017, 149, 511–520. [Google Scholar] [CrossRef]
- Feng, Y.; Ning, M.; Lei, Y.; Sun, Y.; Liu, W.; Wang, J. Defending blue sky in China: Effectiveness of the “Air Pollution Prevention and Control Action Plan” on air quality improvements from 2013 to 2017. J. Environ. Manag. 2019, 252, 109603. [Google Scholar] [CrossRef]
- Ganguly, T.; Selvaraj, K.L.; Guttikunda, S.K. National Clean Air Programme (NCAP) for Indian cities: Review and outlook of clean air action plans. Atmos. Environ. X 2020, 8, 100096. [Google Scholar] [CrossRef]
- Purohit, P.; Amann, M.; Kiesewetter, G.; Rafaj, P.; Chaturvedi, V.; Dholakia, H.H.; Koti, P.N.; Klimont, Z.; Borken-Kleefeld, J.; Gomez-Sanabria, A.; et al. Mitigation pathways towards national ambient air quality standards in India. Environ. Int. 2019, 133, 105147. [Google Scholar] [CrossRef]
- Lee, S.-I.; Park, H.-J.; Jeong, Y.-J.; Seo, B.-S.; Kwak, J.-H.; Yang, H.I.; Xu, X.; Tang, S.; Cheng, W.; Lim, S.-S. Biochar-induced reduction of N2O emission from East Asian soils under aerobic conditions: Review and data analysis. Environ. Pollut. 2021, 291, 118154. [Google Scholar] [CrossRef]
- Li, L.; Fu, Z.; He, K.; Tan, Y.; Feng, Q.; Wu, J. Comparative study on ambient air quality standards of countries along the belt and road. Strateg. Study Chin. Acad. Eng. 2019, 21, 82–91. [Google Scholar] [CrossRef]
- Brickler, C.A.; Wu, Y.; Li, S.; Anandhi, A.; Chen, G. Comparing Physicochemical Properties and Sorption Behaviors of Pyrolysis-Derived and Microwave-Mediated Biochar. Sustainability 2021, 13, 2359. [Google Scholar] [CrossRef]
- Márquez, A.; Ortiz, I.; Sánchez-Hervás, J.M.; Monte, M.C.; Negro, C.; Blanco, Á. Global trends of pyrolysis research: A bibliometric analysis. Environ. Sci. Pollut. Res. 2023, 31, 931–947. [Google Scholar] [CrossRef] [PubMed]
- Al-Salem, S.M. Thermal pyrolysis of high density polyethylene (HDPE) in a novel fixed bed reactor system for the production of high value gasoline range hydrocarbons (HC). Process Saf. Environ. Prot. 2019, 127, 171–179. [Google Scholar] [CrossRef]
- Hu, E.; Tian, Y.; Yang, Y.; Dai, C.; Li, M.; Li, C.; Shao, S. Pyrolysis behaviors of corn stover in new two-stage rotary kiln with baffle. J. Anal. Appl. Pyrolysis 2022, 161, 105398. [Google Scholar] [CrossRef]
- Ou, W.; Liu, T.; Wang, C.; Xiao, R.; Zeng, D. DEM simulation of biomass pyrolysis in a novel interconnected screw reactor. Int. J. Chem. React. Eng. 2023, 21, 937–949. [Google Scholar] [CrossRef]
- Yang, S.; Dong, R.; Du, Y.; Wang, S.; Wang, H. Numerical study of the biomass pyrolysis process in a spouted bed reactor through computational fluid dynamics. Energy 2021, 214, 118839. [Google Scholar] [CrossRef]
- Sia, S.Q.; Wang, W.-C. Numerical simulations of fluidized bed fast pyrolysis of biomass through computational fluid dynamics. Renew. Energy 2020, 155, 248–256. [Google Scholar] [CrossRef]
- Zheng, C.; Liu, X.; Xu, X.; Yan, P.; Chang, Q.; Wang, Y.; Gao, X. Experimental study on electrostatic removal of high-carbon particle in high temperature coal pyrolysis gas. Proc. Combust. Inst. 2019, 37, 2959–2965. [Google Scholar] [CrossRef]
- Chen, Q.; Fang, M.; Cen, J.; Zhao, Y.; Wang, Q.; Wang, Y. Electrostatic precipitation under coal pyrolysis gas at high temperatures. Powder Technol. 2020, 362, 1–10. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, D.; Lv, P.; Liu, Z.; Cheng, T.; Wang, B. Fine particles removal of pyrolysis gasification flue gas from rural domestic waste: Laboratory research, molecular dynamics simulation, and applications. Environ. Res. 2023, 236, 116732. [Google Scholar] [CrossRef] [PubMed]
- Albert, C.; Liu, L.; Haug, J.; Wu, H.; He, R.; Hong, J. Real-time Multiple-particle Tracking in Ultrasonic Spray Pyrolysis. Manuf. Lett. 2022, 33, 9–16. [Google Scholar] [CrossRef]
- Duan, J.; Gao, S.; Lu, Y.; Wang, W.; Zhang, P.; Li, C. Study and optimization of flow field in a novel cyclone separator with inner cylinder. Adv. Powder Technol. 2020, 31, 4166–4179. [Google Scholar] [CrossRef]
- Lima, W.F.; Huebner, R. Optimization of Air Distribution in a Baghouse Filter Using Computational Fluid Dynamics. Eng. Technol. Appl. Sci. Res. 2019, 9, 4452–4456. [Google Scholar] [CrossRef]
- Liu, X.; Shen, H.; Nie, X. Study on the filtration performance of the baghouse filters for ultra-low emission as a function of filter pore size and fiber diameter. Int. J. Environ. Res. Public Health 2019, 16, 247. [Google Scholar] [CrossRef]
- Jacob, S.; Hemanath, D. Analysis of nano material based Zeolite catalytic converter and urea injection in single cylinder engine fuelled with diesel and plastic pyrolysis oil. Mater. Today Proc. 2022, 69, 888–895. [Google Scholar] [CrossRef]
- Sharma, G.S.; Sugavaneswaran, M.; Prakash, R. NOx reduction in IC engines through after treatment catalytic converter. In NOx Emission Control Technologies in Stationary and Automotive Internal Combustion Engines; Elsevier: Amsterdam, The Netherlands, 2022; pp. 223–253. [Google Scholar]
- Harrison, R.M.; Allan, J.; Carruthers, D.; Heal, M.R.; Lewis, A.C.; Marner, B.; Murrells, T.; Williams, A. Non-exhaust vehicle emissions of particulate matter and VOC from road traffic: A review. Atmos. Environ. 2021, 262, 118592. [Google Scholar] [CrossRef]
- Abumounshar, N.; Raj, A.; Ibrahim, S. Novel processes for lean acid gas utilization for sulfur production with high efficiency. Chem. Eng. Sci. 2022, 248, 117194. [Google Scholar] [CrossRef]
- Mong, G.R.; Chong, C.T.; Chong, W.W.F.; Ng, J.-H.; Ong, H.C.; Ashokkumar, V.; Tran, M.-V.; Karmakar, S.; Goh, B.H.H.; Yasin, M.F.M. Progress and challenges in sustainable pyrolysis technology: Reactors, feedstocks and products. Fuel 2022, 324, 124777. [Google Scholar] [CrossRef]
- He, M.; Xu, Z.; Sun, Y.; Chan, P.; Lui, I.; Tsang, D.C. Critical impacts of pyrolysis conditions and activation methods on application-oriented production of wood waste-derived biochar. Bioresour. Technol. 2021, 341, 125811. [Google Scholar] [CrossRef]
- Han, H.; Buss, W.; Zheng, Y.; Song, P.; Khalid Rafiq, M.; Liu, P.; Mašek, O.; Li, X. Contaminants in biochar and suggested mitigation measures—A review. Chem. Eng. J. 2022, 429, 132287. [Google Scholar] [CrossRef]
- Bargmann, I.; Rillig, M.C.; Buss, W.; Kruse, A.; Kuecke, M. Hydrochar and Biochar Effects on Germination of Spring Barley. J. Agron. Crop Sci. 2013, 199, 360–373. [Google Scholar] [CrossRef]
- Buss, W.; Mašek, O. High-VOC biochar—Effectiveness of post-treatment measures and potential health risks related to handling and storage. Environ. Sci. Pollut. Res. 2016, 23, 19580–19589. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Deng, Y.; Dai, M.; Jiang, X.; Li, S.; Fu, H.; Peng, C. Migration and transformation of heavy metals in Chinese medicine residues during the process of traditional pyrolysis and solar pyrolysis. Chemosphere 2022, 293, 133658. [Google Scholar] [CrossRef]
- Li, L.; Yao, Z.; You, S.; Wang, C.-H.; Chong, C.; Wang, X. Optimal design of negative emission hybrid renewable energy systems with biochar production. Appl. Energy 2019, 243, 233–249. [Google Scholar] [CrossRef]
- Zhang, H.; Ju, S.; Jin, X.; Yuan, Y.; Wu, Y.; Nadda, A.K.; Pugazhendhi, A.; Cai, L.; Xia, C. A review of sensor applications towards precise control of pyrolysis of solid waste and biomasses. Renew. Sustain. Energy Rev. 2022, 169, 112915. [Google Scholar] [CrossRef]
- Rebholz, J.; Grossmann, K.; Pham, D.; Pokhrel, S.; Mädler, L.; Weimar, U.; Barsan, N. Selectivity Enhancement by Using Double-Layer MOX-Based Gas Sensors Prepared by Flame Spray Pyrolysis (FSP). Sensors 2016, 16, 1437. [Google Scholar] [CrossRef] [PubMed]
- Aboughaly, M.; Gabbar, H.A.; Damideh, V.; Hassen, I. RF-ICP Thermal Plasma for Thermoplastic Waste Pyrolysis Process with High Conversion Yield and Tar Elimination. Processes 2020, 8, 281. [Google Scholar] [CrossRef]

| Feedstock | Pyro. Temp. (°C) | Residence Time (min) | Heating Rate (°C/min) | Atm. Gas | Reactor Type | Gaseous Contaminant | Ref. |
|---|---|---|---|---|---|---|---|
| Sewage sludge | 500–800 | 20 | - | - | Rotary kiln | PAHs 0.22–421 µg/m3 | [95] |
| Xylan, lignin, cellulose | 800 | 2.6 s | - | N2 100 mL/min | Fixed bed | PAHs 11.9–48.8 µg/g | [76] |
| Wood pellets, e-waste | 850 | 6–15 | 12–25 | N2 500 mL/min | Fixed bed | CO 227.6 mg/g CO2 107.7 mg/g VOCs 91.9 mg/g | [96] |
| Coal, pine sawdust | 300–1000 | 60 | 3 | N2 100 mL/min | Fixed bed | CO 1.9–7.2% CO2 2.3–21.1% CH4 3.4–19.2% | [97] |
| Municipal solid waste | 600–800 | 6 | - | N2 200 mL/min | Fixed bed | CO 5.8 mol/kg CH4 3.2 mol/kg | [83] |
| Wheat straw | 350–650 | - | 20 | N2 100 mL/min | Fixed bed | CO 19–39% CO2 15–64% | [98] |
| Municipal sludge | 300–700 | 60 | 10 | N2 1 L/min | Fixed bed | H2S ~900 mg/m3 SO2 ~220 mg/m3 NO ~140 mg/m3 CO ~26,000 mg/m3 | [99] |
| Municipal sludge | 300–700 | 60 | 10 | CO2 1 L/min | Fixed bed | H2S ~620 mg/m3 SO2 ~280 mg/m3 NO ~120 mg/m3 CO ~24,000 mg/m3 | [99] |
| Furfural residues | 500 | 60 | 10 | N2 60 mL/min | Fixed bed | CO 34.66–62.29% CO2 12.17–48.26% | [100] |
| Rice husk | 400–800 | - | - | N2 250 mL/min | Screw reactor | CO 20–25% CO2 8–27% CH4 2–6% PM 21.5% | [52] |
| Dairy manure | 200–500 | 60 | - | - | Fixed bed | PM 12.5 ± 2.7 mg/g | [42] |
| Conifer chip | 300–500 | 120 | - | - | Fixed bed | CO 28,000 ppm VOCs 634 ppm | [101] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S. Reviewing Air Pollutants Generated during the Pyrolysis of Solid Waste for Biofuel and Biochar Production: Toward Cleaner Production Practices. Sustainability 2024, 16, 1169. https://doi.org/10.3390/su16031169
Li S. Reviewing Air Pollutants Generated during the Pyrolysis of Solid Waste for Biofuel and Biochar Production: Toward Cleaner Production Practices. Sustainability. 2024; 16(3):1169. https://doi.org/10.3390/su16031169
Chicago/Turabian StyleLi, Simeng. 2024. "Reviewing Air Pollutants Generated during the Pyrolysis of Solid Waste for Biofuel and Biochar Production: Toward Cleaner Production Practices" Sustainability 16, no. 3: 1169. https://doi.org/10.3390/su16031169






