The Impact of Dissolved Organic Matter on Photodegradation Rates, Byproduct Formations, and Degradation Pathways for Two Neonicotinoid Insecticides in Simulated River Waters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Experiment Design
2.3. Instrumentation and Data Processes
2.4. Water Quality Sampling Analyses
2.5. Modeling Imidacloprid and Thiamethoxam Degradation Kinetics
2.6. Direct vs. Indirect Photolysis
2.7. Statistics
3. Results
3.1. Degradation Kinetics
3.1.1. Overview
3.1.2. MIS Treatments
3.1.3. SUW Treatments
3.1.4. NanopureTM Water Rates
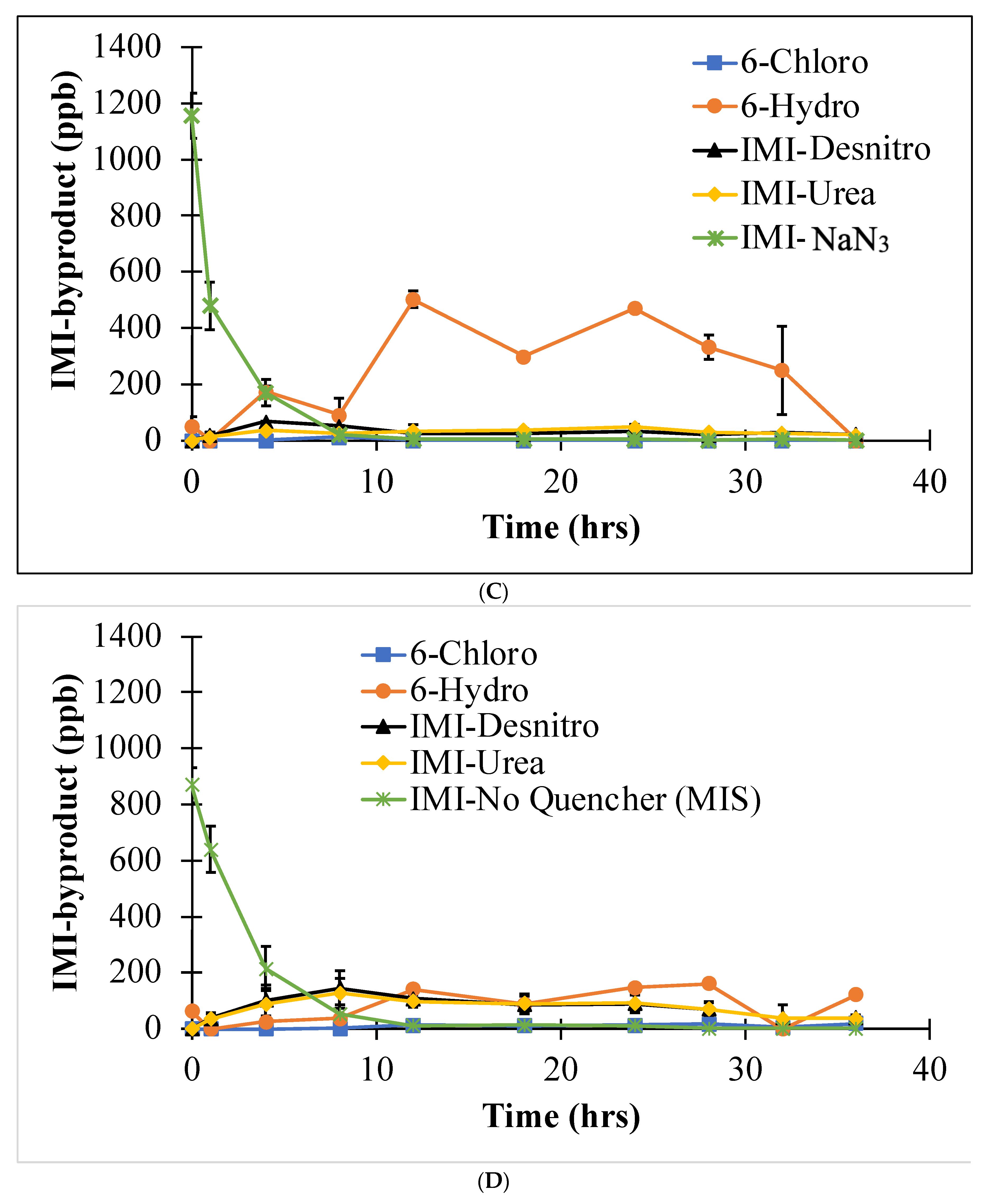
3.2. Byproduct Formation
3.2.1. Summary
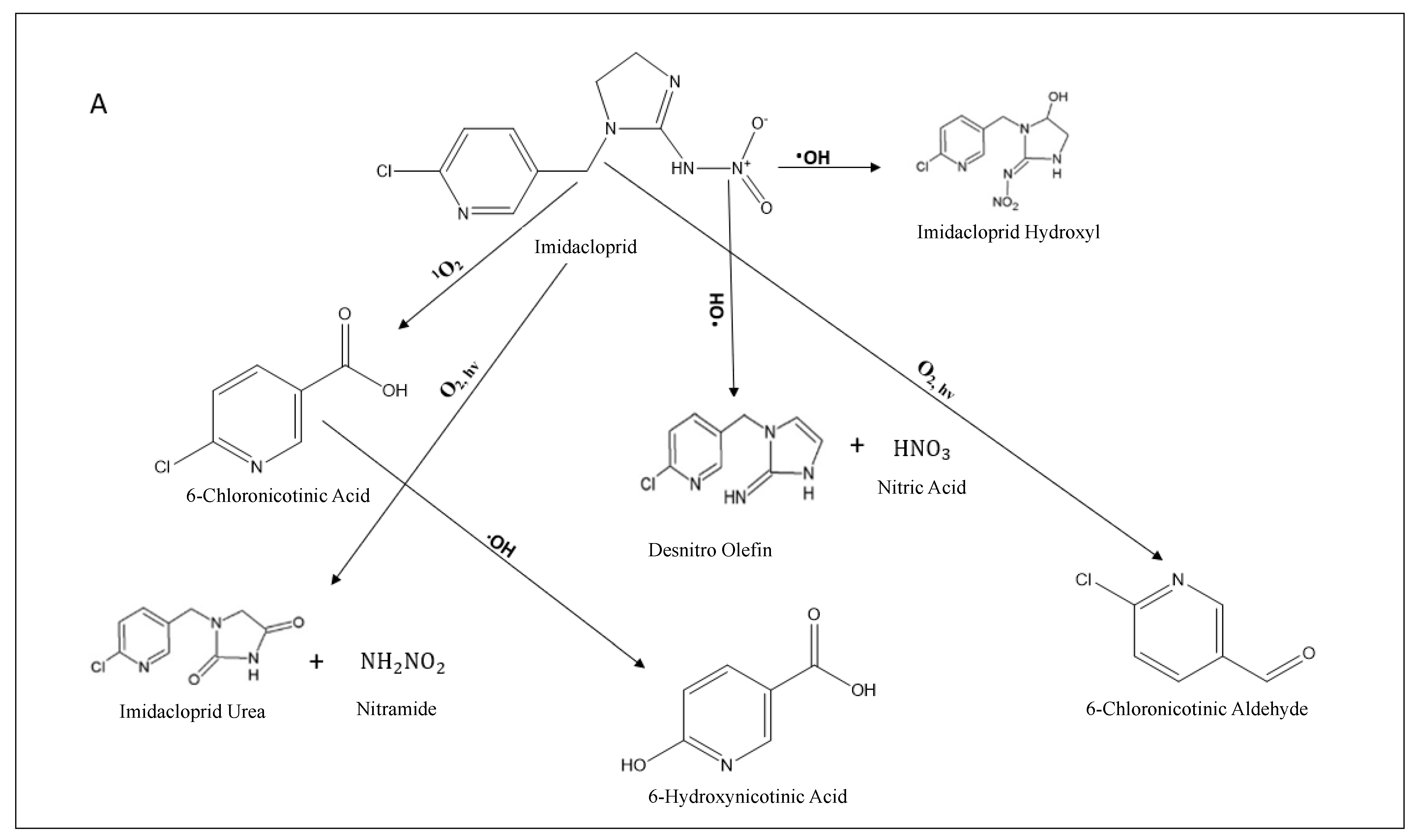
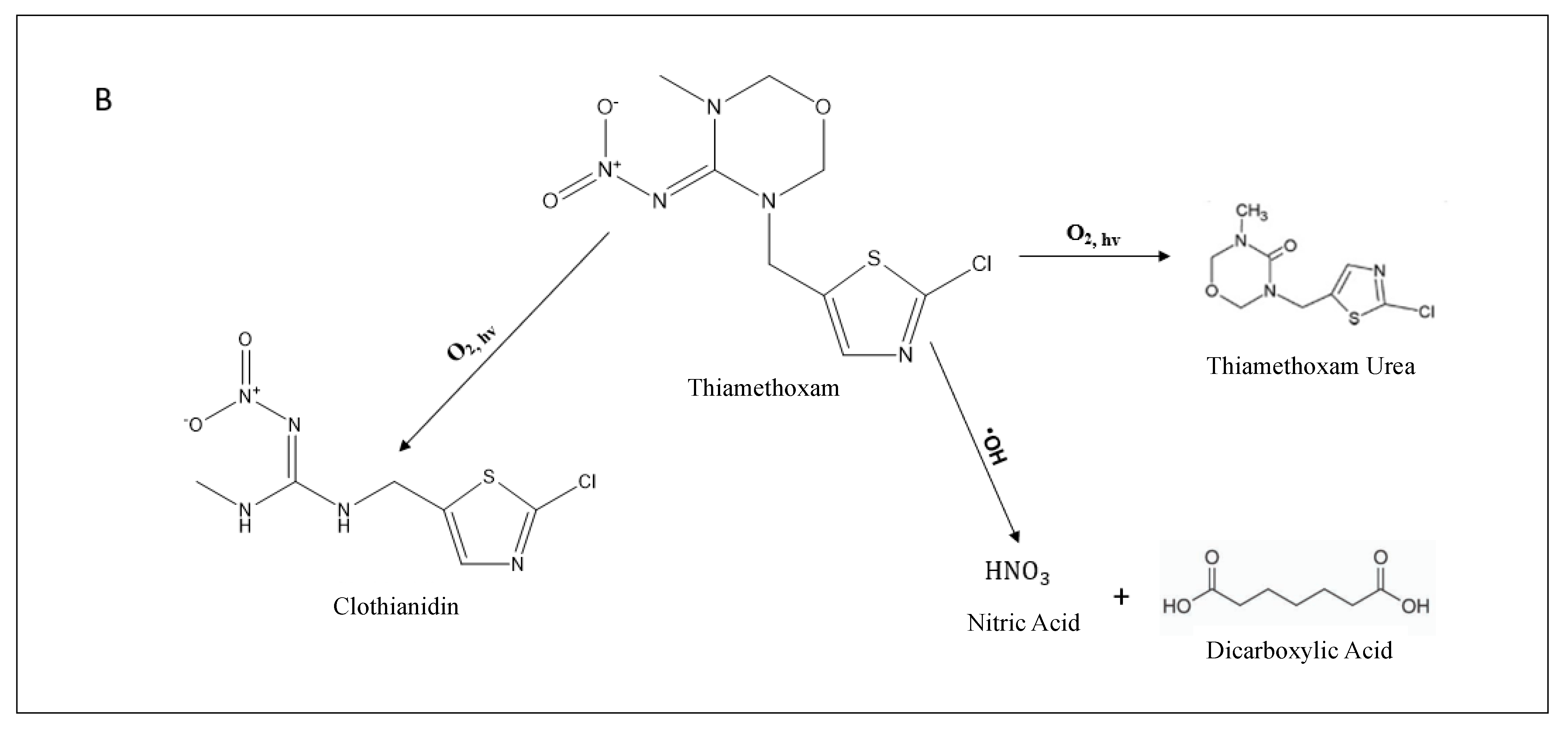
3.2.2. MIS DOM Degradation Pathways
3.2.3. SUW DOM Degradation Pathways
3.2.4. High-Purity NanopureTM Water Transformation Pathways
4. Discussion
4.1. Contributions of Direct and Indirect Photolysis
4.2. Effect of Dissolved Organic Matter on Photodegradation of Neonicotinoids
4.3. Reversion Processes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raschitor, A.; Romero, A.; Sanches, S.; Pereira, V.J.; Crespo, J.G.; Llanos, J. Degradation of neonicotinoids and caffeine from surface water by photolysis. Molecules 2021, 26, 7277. [Google Scholar] [CrossRef]
- Borsuah, J.F.; Messer, T.L.; Snow, D.D.; Comfort, S.D.; Mittelstet, A.R. Literature Review: Global Neonicotinoid Insecticide Occurrence in Aquatic Environments. Water 2020, 12, 3388. [Google Scholar] [CrossRef]
- Hladik, M.L.; Kolpin, D.W. First national-scale reconnaissance of neonicotinoid insecticides in streams across the USA. Environ. Chem. 2015, 13, 12. [Google Scholar] [CrossRef]
- De, A.; Bose, R.; Kumar, A.; Mozumdar, S. Targeted Delivery of Pesticides Using Biodegradable Polymeric Nanoparticles; Springer: New Delhi, India, 2014. [Google Scholar]
- Sharma, A.; Kumar, V.; Shahzad, B.; Tanveer, M.; Sidhu, G.P.S.; Handa, N.; Kohli, S.K.; Yadav, P.; Bali, A.S.; Parihar, R.D.; et al. Worldwide pesticide usage and its impacts on ecosystem. SN Appl. Sci. 2019, 1, 1446. [Google Scholar] [CrossRef]
- Loser, D.; Grillberger, K.; Hinojosa, M.G.; Blum, J.; Haufe, Y.; Danker, T.; Johansson, Y.; Möller, C.; Nicke, A.; Bennekou, S.H.; et al. Acute effects of the imidacloprid metabolite desnitro-imidacloprid on human nACh receptors relevant for neuronal signaling. Arch. Toxicol. 2021, 95, 3695–3716. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Han, Q.; Wang, Y.; He, Z. Five degradates of imidacloprid in source water, treated water, and tap water in Wuhan, central China. Sci. Total. Environ. 2020, 741, 140227. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency. Aquatic Life Benchmarks and Ecological Risk Assessments for Registered Pesticides; United States Environmental Protection Agency: Washington, DC, USA, 2016; p. 35.
- Wood, T.J.; Goulson, D. The environmental risks of neonicotinoid pesticides: A review of the evidence post 2013. Environ. Sci. Pollut. Res. 2017, 24, 17285–17325. [Google Scholar] [CrossRef] [PubMed]
- Bonmatin, J.-M.; Giorio, C.; Girolami, V.; Goulson, D.; Kreutzweiser, D.P.; Krupke, C.; Liess, M.; Long, E.; Marzaro, M.; Mitchell, E.A.D.; et al. Environmental fate and exposure; neonicotinoids and fipronil. Environ. Sci. Pollut. Res. 2015, 22, 35–67. [Google Scholar] [CrossRef]
- Mitchell, E.A.D.; Mulhauser, B.; Mulot, M.; Mutabazi, A.; Glauser, G.; Aebi, A. A worldwide survey of neonicotinoids in honey. Science 2017, 358, 109–111. [Google Scholar] [CrossRef]
- Yamamuro, M.; Komuro, T.; Kamiya, H.; Kato, T.; Hasegawa, H.; Kameda, Y. Neonicotinoids disrupt aquatic food webs and decrease fishery yields. Science 2019, 366, 620–623. [Google Scholar] [CrossRef]
- Wong, K.L.K.; Webb, D.T.; Nagorzanski, M.R.; Kolpin, D.W.; Hladik, M.L.; Cwiertny, D.M.; LeFevre, G.H. Chlorinated Byproducts of Neonicotinoids and Their Metabolites: An Unrecognized Human Exposure Potential? Environ. Sci. Technol. Lett. 2019, 6, 98–105. [Google Scholar] [CrossRef]
- Miles, J.C.; Hua, J.; Sepulveda, M.S.; Krupke, C.H.; Hoverman, J.T. Effects of clothianidin on aquatic communities: Evaluating the impacts of lethal and sublethal exposure to neonicotinoids. PLoS ONE 2017, 12, e0174171. [Google Scholar] [CrossRef]
- Anderson, J.; Dubetz, C.; Palace, V. Neonicotinoids in the Canadian aquatic environment: A literature review on current use products with a focus on fate, exposure, and biological effects. Sci. Total. Environ. 2015, 505, 409–422. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.C.; Carlson, J.C.; Low, J.E.; Challis, J.K.; Wong, C.S.; Knapp, C.W.; Hanson, M.L. Performance of a constructed wetland in Grand Marais, Manitoba, Canada: Removal of nutrients, pharmaceuticals, and antibiotic resistance genes from municipal wastewater. Chem. Central J. 2013, 7, 54. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, X.; Fan, L.; Su, L.; Zhao, Y. Photolysis mechanism of eleven insecticides under simulated sunlight irradiation: Kinetics, pathway and QSAR. Chemosphere 2023, 334, 138968. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Zhang, X.; Wang, J.; Wang, C.; Li, S.; Zhao, Y.H.; Martyniuk, C.J. Relationship between photolysis mechanism and photo-enhanced toxicity to Vibrio Fischeri for neonicotinoids with cyano-amidine and nitroguanidine structures. Aquat. Toxicol. 2023, 257, 106443. [Google Scholar] [CrossRef]
- Hashimoto, F.; Takanashi, H.; Nakajima, T.; Ueda, T.; Kadokawa, J.-I.; Ishikawa, H.; Miyamoto, N. Occurrence of imidacloprid and its transformation product (imidacloprid-nitroguanidine) in rivers during an irrigating and soil puddling duration. Microchem. J. 2020, 153, 104496. [Google Scholar] [CrossRef]
- Acero, J.L.; Real, F.J.; Benitez, F.J.; Matamoros, E. Degradation of neonicotinoids by UV irradiation: Kinetics and effect of real water constituents. Sep. Purif. Technol. 2019, 211, 218–226. [Google Scholar] [CrossRef]
- Malhotra, N.; Chen, K.H.-C.; Huang, J.-C.; Lai, H.-T.; Uapipatanakul, B.; Roldan, M.J.M.; Macabeo, A.P.G.; Ger, T.-R.; Hsiao, C.-D. Physiological Effects of Neonicotinoid Insecticides on Non-Target Aquatic Animals—An Updated Review. Int. J. Mol. Sci. 2021, 22, 9591. [Google Scholar] [CrossRef]
- Mourikes, V.E.; Márquez, R.S.; Deviney, A.; Neff, A.M.; Laws, M.J.; Flaws, J.A. Imidacloprid and Its Bioactive Metabolite, Desnitro-Imidacloprid, Differentially Affect Ovarian Antral Follicle Growth, Morphology, and Hormone Synthesis In Vitro. Toxics 2023, 11, 349. [Google Scholar] [CrossRef]
- Jacob, C.R.d.O.; Zanardi, O.Z.; Malaquias, J.B.; Silva, C.A.S.; Yamamoto, P.T. The impact of four widely used neonicotinoid insecticides on Tetragonisca angustula (Latreille) (Hymenoptera: Apidae). Chemosphere 2019, 224, 65–70. [Google Scholar] [CrossRef]
- DiBartolomeis, M.; Kegley, S.; Mineau, P.; Radford, R.; Klein, K. An assessment of acute insecticide toxicity loading (AITL) of chemical pesticides used on agricultural land in the United States. PLoS ONE 2019, 14, e0220029. [Google Scholar] [CrossRef]
- Finnegan, M.C.; Baxter, L.R.; Maul, J.D.; Hanson, M.L.; Hoekstra, P.F. Comprehensive characterization of the acute and chronic toxicity of the neonicotinoid insecticide thiamethoxam to a suite of aquatic primary producers, invertebrates, and fish. Environ. Toxicol. Chem. 2017, 36, 2838–2848. [Google Scholar] [CrossRef]
- Thompson, D.A.; Lehmler, H.-J.; Kolpin, D.W.; Hladik, M.L.; Vargo, J.D.; Schilling, K.E.; LeFevre, G.H.; Peeples, T.L.; Poch, M.C.; LaDuca, L.E.; et al. A critical review on the potential impacts of neonicotinoid insecticide use: Current knowledge of environmental fate, toxicity, and implications for human health. Environ. Sci. Process. Impacts 2020, 22, 1315–1346. [Google Scholar] [CrossRef]
- Katagi, T. Direct photolysis mechanism of pesticides in water. J. Pestic. Sci. 2018, 43, 57–72. [Google Scholar] [CrossRef]
- Todey, S.A.; Fallon, A.M.; Arnold, W.A. Neonicotinoid insecticide hydrolysis and photolysis: Rates and residual toxicity. Environ. Toxicol. Chem. 2018, 37, 2797–2809. [Google Scholar] [CrossRef] [PubMed]
- Burrows, H.D.; Santaballa, J.A.; Steenken, S. Reaction pathways and mechanisms of photodegradation of pesticides. J. Photochem. Photobiol. B Biol. 2002, 67, 71–108. [Google Scholar] [CrossRef] [PubMed]
- Remucal, C.K. The role of indirect photochemical degradation in the environmental fate of pesticides: A review. Environ. Sci. Process. Impacts 2014, 16, 628–653. [Google Scholar] [CrossRef] [PubMed]
- Zeng, T.; Arnold, W.A. Pesticide Photolysis in Prairie Potholes: Probing Photosensitized Processes. Environ. Sci. Technol. 2013, 47, 6735–6745. [Google Scholar] [CrossRef]
- Aajoud, A.; Ravanel, P.; Tissut, M. Fipronil metabolism and dissipation in a simplified aquatic ecosystem. J. Agric. Food Chem. 2003, 51, 1347–1352. [Google Scholar] [CrossRef] [PubMed]
- de Urzedo, A.P.F.M.; Diniz, M.E.R.; Nascentes, C.C.; Catharino, R.R.; Eberlin, M.N.; Augusti, R. Photolytic degradation of the insecticide thiamethoxam in aqueous medium monitored by direct infusion electrospray ionization mass spectrometry. J. Mass Spectrom. 2007, 42, 1319–1325. [Google Scholar] [CrossRef]
- Wamhoff, H.; Schneider, V. Photodegradation of Imidacloprid. J. Agric. Food Chem. 1999, 47, 1730–1734. [Google Scholar] [CrossRef]
- Lin, A.Y.; Reinhard, M. Photodegradation of Common Environmental Pharmaceuticals and Estrogens in River Water. Environ. Toxicol. Chem. 2005, 24, 1303–1309. [Google Scholar] [CrossRef]
- Satiroff, J.A.; Messer, T.L.; Mittelstet, A.R.; Snow, D.D. Pesticide Occurrence and Persistence Entering Recreational Lakes in Watersheds of Varying Land Uses. Environ. Pollut. 2020, 273, 116399. [Google Scholar] [CrossRef] [PubMed]
- Snow, D.; Chakraborty, P.; Uralbekov, B.; Satybaldiev, B.; Sallach, J.; Hampton, L.T.; Jeffries, M.; Kolok, A.; Bartelt-Hunt, S. Legacy and current pesticide residues in Syr Darya, Kazakhstan: Contamination status, seasonal variation and preliminary ecological risk assessment. Water Res. 2020, 184, 116141. [Google Scholar] [CrossRef]
- SM 5310 D.; Total Organic Carbon (TOC) by Wet Oxidation Method. National Environmental Methods Index: Washington, DC, USA, 2000. Available online: https://www.nemi.gov/methods/method_summary/7614/ (accessed on 23 January 2024).
- US EPA. EPA 300 Determination of Inorganic Anions by Ion Chromatography; US EPA: Washington, DC, USA, 1993.
- Kurwadkar, S.; Evans, A.; DeWinne, D.; White, P.; Mitchell, F. Modeling photodegradation kinetics of three systemic neonicotinoids—Dinotefuran, imidacloprid, and thiamethoxam—In aqueous and soil environment. Environ. Toxicol. Chem. 2016, 35, 1718–1726. [Google Scholar] [CrossRef] [PubMed]
- Redlich, D.; Shahin, N.; Ekici, P.; Friess, A.; Parlar, H. Kinetical study of the photoinduced degradation of imidacloprid in aquatic media. Clean 2007, 35, 452–458. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, W.; Wang, F.; Su, Y.; Feng, Y.; Chen, P.; Ma, J.; Su, H.; Yao, K.; Liu, Y.; et al. Study of the simulated sunlight photolysis mechanism of ketoprofen: The role of superoxide anion radicals, transformation byproducts, and ecotoxicity assessment. Environ. Sci. Process. Impacts 2017, 19, 1176–1184. [Google Scholar] [CrossRef] [PubMed]
- Kobkeatthawin, T.; Trakulmututa, J.; Amornsakchai, T.; Kajitvichyanukul, P.; Smith, S.M. Identification of Active Species in Photodegradation of Aqueous Imidacloprid over g-C3N4/TiO2 Nanocomposites. Catalysts 2022, 12, 120. [Google Scholar] [CrossRef]
- Zhang, P.; Shao, Y.; Xu, X.; Huang, P.; Sun, H. Phototransformation of biochar-derived dissolved organic matter and the effects on photodegradation of imidacloprid in aqueous solution under ultraviolet light. Sci. Total. Environ. 2020, 724, 137913. [Google Scholar] [CrossRef] [PubMed]
- Giokas, D.L.; Vlessidis, A.G. Application of a novel chemometric approach to the determination of aqueous photolysis rates of organic compounds in natural waters. Talanta 2007, 71, 288–295. [Google Scholar] [CrossRef]
- Scholes, R.C.; Prasse, C.; Sedlak, D.L. The Role of Reactive Nitrogen Species in Sensitized Photolysis of Wastewater-Derived Trace Organic Contaminants. Environ. Sci. Technol. 2019, 53, 6483–6491. [Google Scholar] [CrossRef]
- Thuyet, D.Q.; Watanabe, H.; Yamazaki, K.; Takagi, K. Photodegradation of imidacloprid and fipronil in rice–Paddy water. Bull. Environ. Contam. Toxicol. 2011, 86, 548–553. [Google Scholar] [CrossRef]
- Žabar, R.; Dolenc, D.; Jerman, T.; Franko, M.; Trebše, P. Photolytic and photocatalytic degradation of 6-chloronicotinic acid. Chemosphere 2011, 85, 861–868. [Google Scholar] [CrossRef]
- Julian, J.P.; Doyle, M.W.; Powers, S.M.; Stanley, E.H.; Riggsbee, J.A. Optical water quality in rivers. Water Resour. Res. 2008, 44, 1–19. [Google Scholar] [CrossRef]
- Latch, D.E.; McNeill, K. Microheterogeneity of Singlet Oxygen Distributions in Irradiated Humic Acid Solutions. Science 2006, 311, 1743–1747. [Google Scholar] [CrossRef]
- Averett, R.C.; Leenheer, J.A.; McKnight, D.M.; Thorn, K.A. Humic Substances in the Suwannee River, Georgia: Interactions, Properties, and Proposed Structures; US Government Printing Office: Washington, DC, USA, 1994. [CrossRef]
- IHSS. International Humic Substances Society. 2022. Available online: https://humic-substances.org/source-materials-for-ihss-samples/ (accessed on 23 January 2024).
- Zhao, C.; Pelaez, M.; Duan, X.; Deng, H.; O’Shea, K.; Fatta-Kassinos, D.; Dionysiou, D.D. Role of pH on photolytic and photocatalytic degradation of antibiotic oxytetracycline in aqueous solution under visible/solar light: Kinetics and mechanism studies. Appl. Catal. B Environ. 2013, 134–135, 83–92. [Google Scholar] [CrossRef]
- Sharpless, C.M.; Blough, N.V. The importance of charge-transfer interactions in determining chromophoric dissolved organic matter (CDOM) optical and photochemical properties. Environ. Sci. Process. Impacts 2014, 16, 654–671. [Google Scholar] [CrossRef] [PubMed]
- Webb, D.T.; Nagorzanski, M.R.; Powers, M.M.; Cwiertny, D.M.; Hladik, M.L.; LeFevre, G.H. Differences in Neonicotinoid and Metabolite Sorption to Activated Carbon Are Driven by Alterations to the Insecticidal Pharmacophore. Environ. Sci. Technol. 2020, 54, 14694–14705. [Google Scholar] [CrossRef] [PubMed]
- Qu, S.; Kolodziej, E.P.; Long, S.A.; Gloer, J.B.; Patterson, E.V.; Baltrusaitis, J.; Jones, J.D.; Benchetler, P.V.; Cole, E.A.; Kimbrough, K.C.; et al. Trenbolone: Unrecognized Risks. Science 2013, 342, 347–352. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4096139/ (accessed on 23 January 2024). [CrossRef] [PubMed]
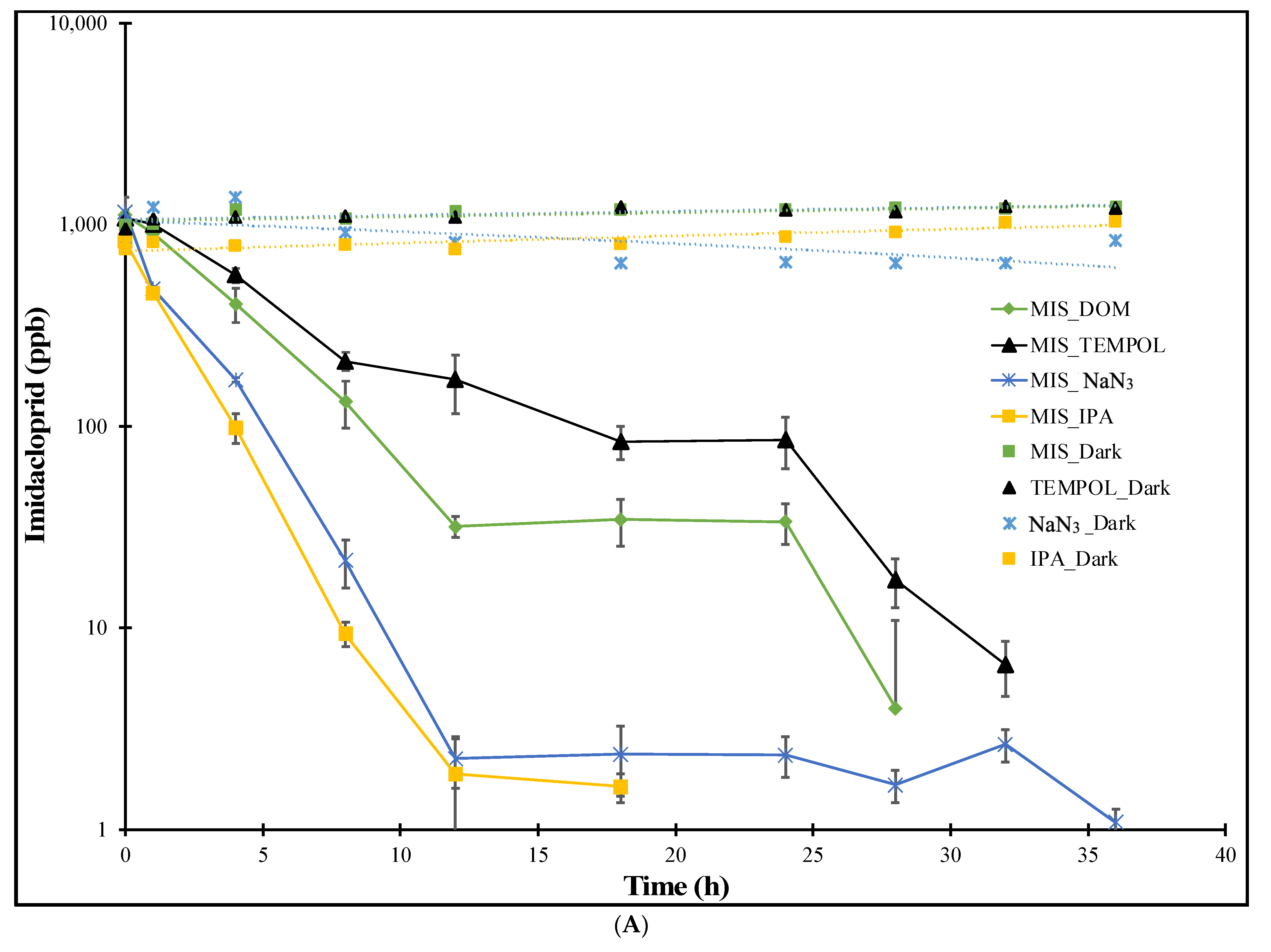
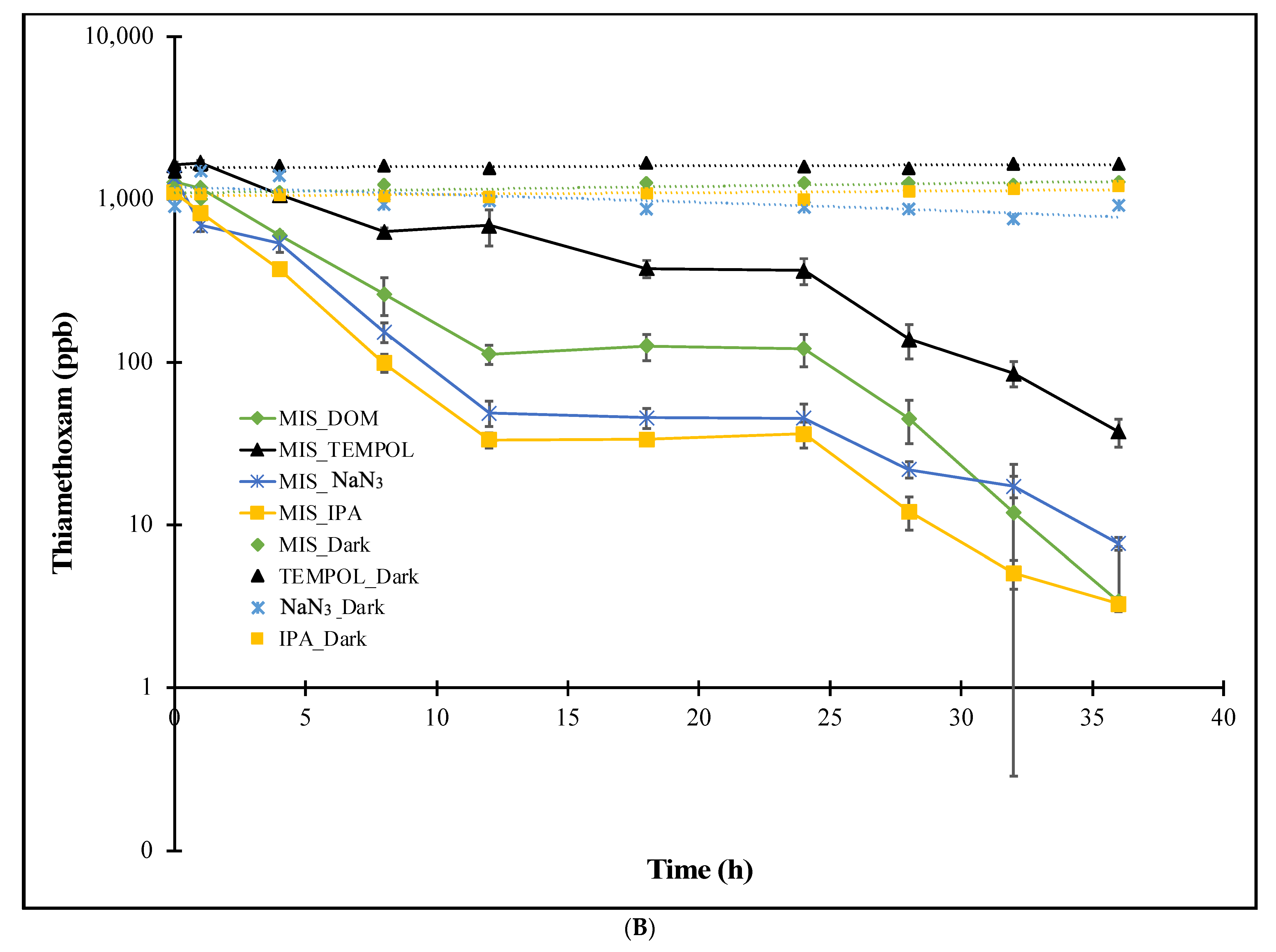

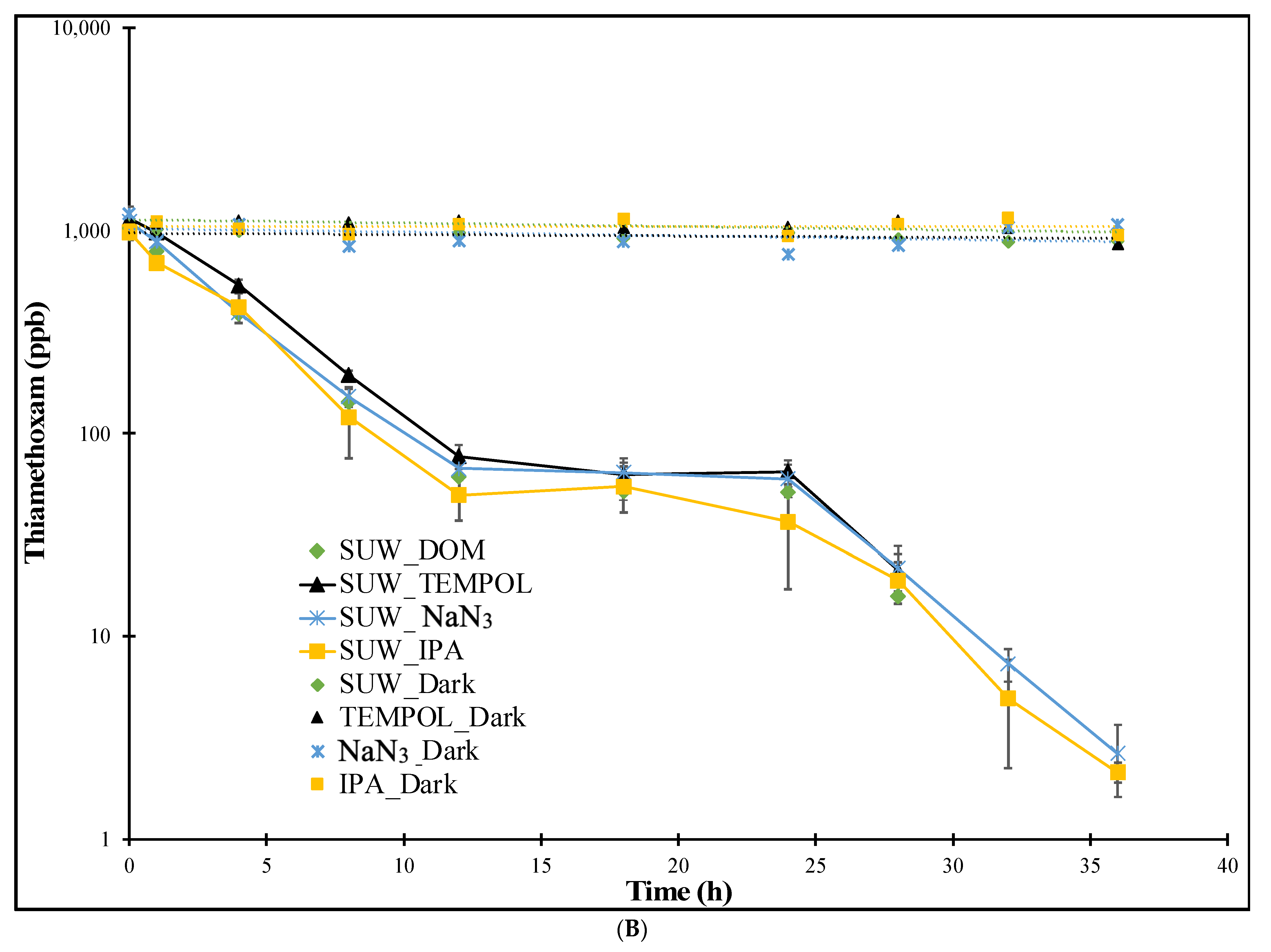
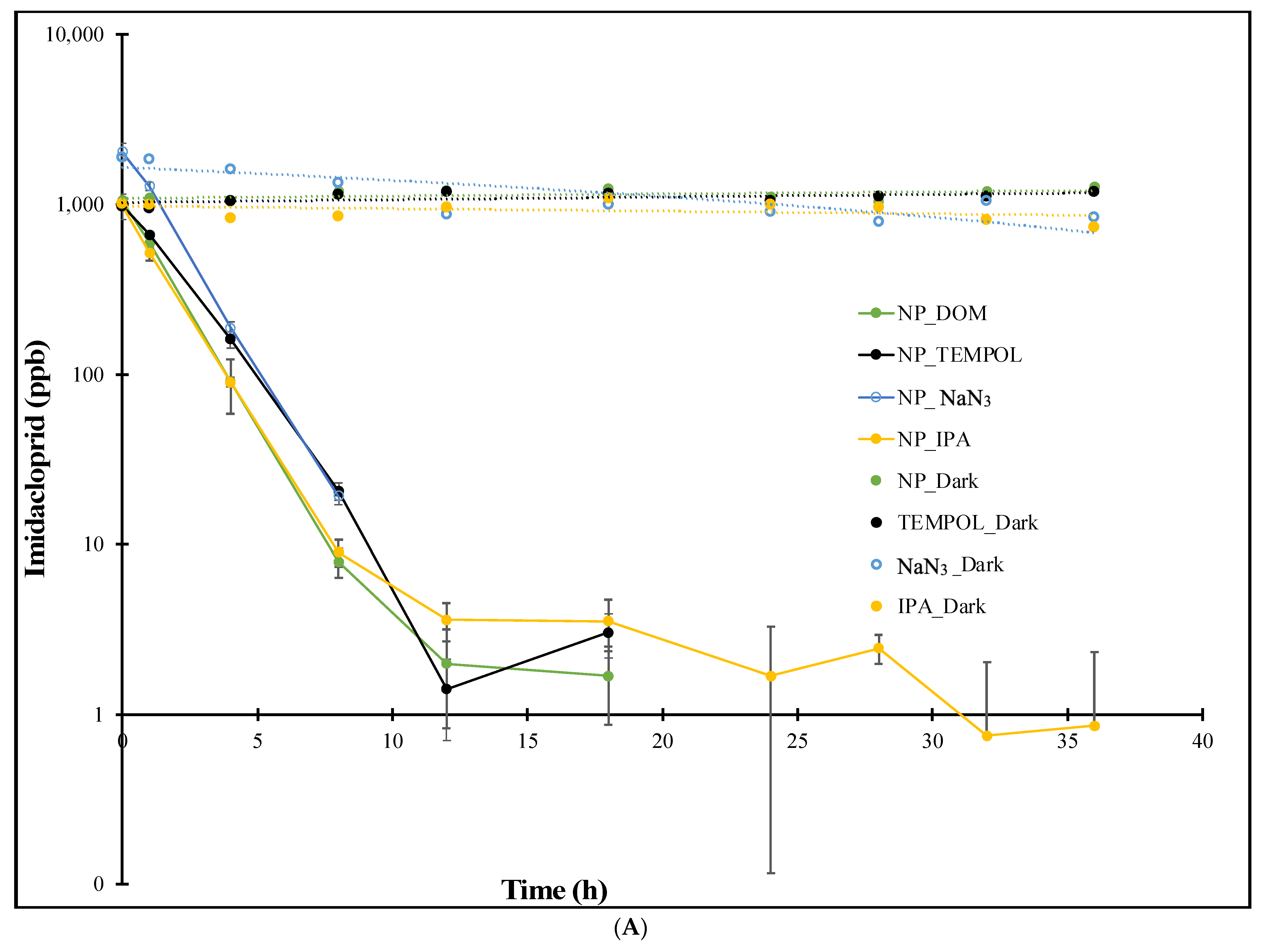






| Experiment | Imidacloprid k (h−1) | Thiamethoxam k (h−1) |
|---|---|---|
| NP | 0.531 ± 0.043 | 0.340 ± 0.033 |
| NP + NaN3 | 0.587 ± 0.023 | 0.312 ± 0.026 |
| NP + IPA | 0.623 ± 0.021 | 0.379 ± 0.016 |
| NP + TEMPOL | 0.543 ± 0.068 | 0.274 ± 0.004 |
| MIS DOM | 0.296 ± 0.005 | 0.203 ± 0.006 |
| MIS DOM + NaN3 | 0.485 ± 0.038 | 0.251 ± 0.061 |
| MIS DOM + IPA | 0.515 ± 0.050 | 0.291 ± 0.006 |
| MIS DOM + TEMPOL | 0.156 ± 0.032 | 0.027 ± 0.017 |
| SUW DOM | 0.372 ± 0.008 | 0.230 ± 0.003 |
| SUW DOM + NaN3 | 0.437 ± 0.011 | 0.218 ± 0.005 |
| SUW DOM + IPA | 0.465 ± 0.021 | 0.250 ± 0.019 |
| SUW DOM + TEMPOL | 0.380 ± 0.023 | 0.225 ± 0.010 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borsuah, J.F.; Messer, T.L.; Snow, D.D.; Comfort, S.D.; Bartelt-Hunt, S. The Impact of Dissolved Organic Matter on Photodegradation Rates, Byproduct Formations, and Degradation Pathways for Two Neonicotinoid Insecticides in Simulated River Waters. Sustainability 2024, 16, 1181. https://doi.org/10.3390/su16031181
Borsuah JF, Messer TL, Snow DD, Comfort SD, Bartelt-Hunt S. The Impact of Dissolved Organic Matter on Photodegradation Rates, Byproduct Formations, and Degradation Pathways for Two Neonicotinoid Insecticides in Simulated River Waters. Sustainability. 2024; 16(3):1181. https://doi.org/10.3390/su16031181
Chicago/Turabian StyleBorsuah, Josephus F., Tiffany L. Messer, Daniel D. Snow, Steven D. Comfort, and Shannon Bartelt-Hunt. 2024. "The Impact of Dissolved Organic Matter on Photodegradation Rates, Byproduct Formations, and Degradation Pathways for Two Neonicotinoid Insecticides in Simulated River Waters" Sustainability 16, no. 3: 1181. https://doi.org/10.3390/su16031181







