Modeling the Effects of Climate Change on the Current and Future Potential Distribution of Berberis vulgaris L. with Machine Learning
Abstract
1. Introduction
2. Materials and Methods
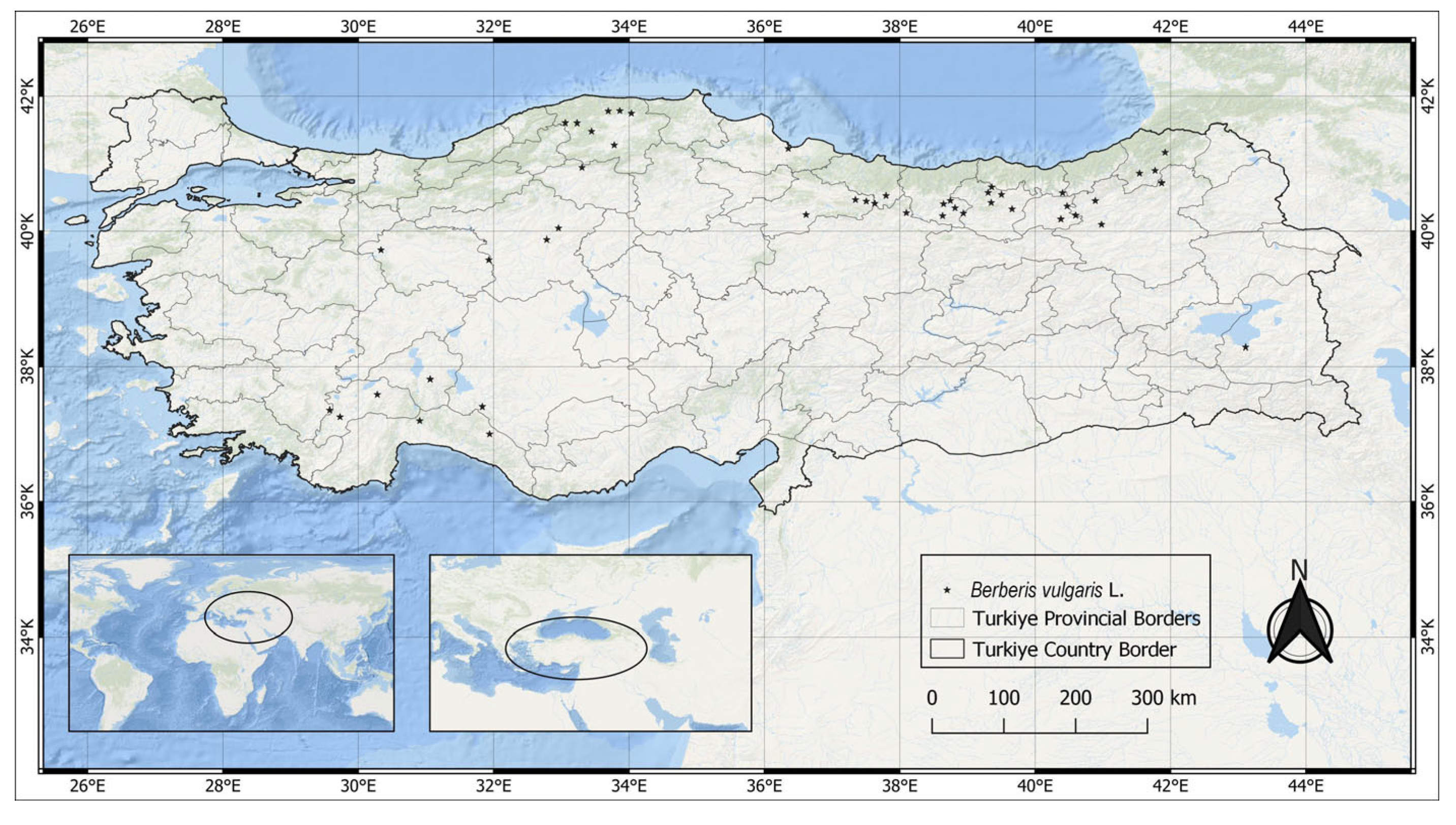
2.1. Study Region and Occurrence Data
2.2. Bioclimatic Variables and Modeling
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adhikari, D.; Barik, S.; Upadhaya, K. Habitat distribution modelling for reintroduction of Ilex khasiana Purk., a critically endangered tree species of northeastern India. Ecol. Eng. 2012, 40, 37–43. [Google Scholar] [CrossRef]
- Barnosky, A.D.; Matzke, N.; Tomiya, S.; Wogan, G.O.; Swartz, B.; Quental, T.B.; Marshall, C.; McGuire, J.L.; Lindsey, E.L.; Maguire, K.C. Has the Earth’s sixth mass extinction already arrived? Nature 2011, 471, 51–57. [Google Scholar] [CrossRef]
- Uzun, A.; Orucu, O.K. Prediction of present and future spread of Adenocarpus complicatus (L.) Gay species according to climate variables. Turk. J. For. 2020, 21, 498–508. [Google Scholar] [CrossRef]
- United Nations Convention to Combat Desertification (Secretariat). United Naions Convention to Combat Desertification in Those Countries Experiencing Serious Drought and/or Desertification, Particulary in Africa; United Nations: New York, NY, USA, 1999. [Google Scholar]
- Arslan, E.S.; Gülçin, D.; Sarikaya, A.G.; Olmez, Z.; Gulcu, S.; Sen, I.; Orucu, O.K. Modeling of the current and future potential distribution of Stinking juniper (Juniperus foetidissima Willd.) with machine learning techniques. Eur. J. Sci. Technol. 2021, 22, 1–12. [Google Scholar]
- Chakraborty, A.; Joshi, P.; Sachdeva, K. Predicting distribution of major forest tree species to potential impacts of climate change in the central Himalayan region. Ecol. Eng. 2016, 97, 593–609. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, H.; Xu, C. Study on Taiwania cryptomerioides under climate change: MaxEnt modeling for predicting the potential geographical distribution. Glob. Ecol. Conserv. 2020, 24, e01313. [Google Scholar] [CrossRef]
- Atefi, M.; Ghavami, A.; Hadi, A.; Askari, G. The effect of barberry (Berberis vulgaris L.) supplementation on blood pressure: A systematic review and meta-analysis of the randomized controlled trials. Complement. Ther. Med. 2021, 56, 102608. [Google Scholar] [CrossRef] [PubMed]
- Maliwichi-Nyirenda, C.P.; Maliwichi, L.L.; Franco, M. Medicinal uses of Berberis holstii Engl. (Berberidaceae) in Malawi, the only African endemic barberry. J. Med. Plants Res. 2011, 5, 1367–1373. [Google Scholar]
- Ayna, O.F.; Arslanoglu, S.F. The Types of Berberis Growing in Anatolian Geography and Traditional Uses. Int. J. Life Sci. Biotechnol. 2019, 2, 36–42. [Google Scholar] [CrossRef]
- Mozaffarian, V. A Dictionary of Iranian Plant Names; Farhang Moaser: Tehran, Iran, 1996; pp. 396–398. [Google Scholar]
- Attuluri, M.L.V.S.; Darwin, R.C. Drug Bioavailability, Stability and Anticancer Effect of Berberine-loaded Magnetic Nanoparticles on MDA-MB-231 Cells in Breast Cancer. Indian J. Pharm. Educ. Res. 2022, 56, S444–S453. [Google Scholar] [CrossRef]
- Cansaran, A.; Kaya, O.F.; Yildirim, C. An Ethnobotanical Study (Amasya/Gümüşhacıköy) Between the Vicinity of Ovabaşı, Akpınar, Güllüce and Köseler Villages. Sci. Eng. J. Fırat. Univ. 2007, 19, 243–257. [Google Scholar]
- Kayacık, H. Special Systematics of Forest and Park Trees; Istanbul University Faculty of Forestry Publications: Istanbul, Turkey, 1968. [Google Scholar]
- Komarov, V.; Schischkin, B. Flora of the USSR Volume VII, Scientific Academy of the U.S.S.R. Leningrad: Moscow, Russia, 1937.
- Korkmaz, M.; Alpaslan, Z. Ethnobotanical properties of Ergan Mountain (Erzincan-Turkey). Bağbahçe Sci. J. 2014, 1, 1–31. [Google Scholar]
- Nandi, A.; Pal, A.K. Interpreting Machine Learning Models: Learn Model Interpretability and Explainability Methods; Apress: Berkeley, CA, USA, 2022. [Google Scholar]
- Jiang, H. Machine Learning Fundamentals: A Concise Introduction; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Eker, R.; Alkis, K.C.; Ucar, Z.; Aydın, A. Using Machine Learning in Forestry. Turk. J. For. 2023, 24, 150–177. [Google Scholar] [CrossRef]
- Bishop, C.M. Model-based machine learning. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2013, 371, 20120222. [Google Scholar] [CrossRef]
- Zhang, J.; Li, S. A Review of Machine Learning Based Species’ Distribution Modelling. In Proceedings of the 2017 International Conference on Industrial Informatics—Computing Technology, Intelligent Technology, Industrial Information Integration (ICIICII), Wuhan, China, 2–3 December 2017; p. 199. [Google Scholar] [CrossRef]
- Mitchell, T.M. Machine Learning; McGraw-Hill: New York, NY, USA, 1997. [Google Scholar]
- Scherrer, D.; Guisan, A. Ecological indicator values reveal missing predictors of species distributions. Sci. Rep. 2019, 9, 3061. [Google Scholar] [CrossRef]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudik, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Feng, X.; Park, D.S.; Walker, C.; Peterson, A.T.; Merow, C.; Papeş, M. A checklist for maximizing reproducibility of ecological niche models. Nat. Ecol. Evol. 2019, 3, 1382–1395. [Google Scholar] [CrossRef]
- Peterson, A.T.; Papes, M.; Eaton, M. Transferability and model evaluation in ecological niche modeling: A comparison of GARP and Maxent. Ecography 2007, 30, 550–560. [Google Scholar] [CrossRef]
- Arslan, E.S. Evaluation of urban road trees in terms of ecosystem services according to climate change scenarios and species distribution model: The case of Robinia pseudoacacia L. Turk. J. For. 2019, 20, 142–148. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Sarikaya, O.; Karaceylan, I.; Sen, I. Maximum entropy modeling (maxent) of current and future distributions of Ips mannsfeldi (Wachtl, 1879)(Curculionidae: Scolytinae) in Turkey. Appl. Ecol. Environ. Res. 2018, 16, 2527–2535. [Google Scholar] [CrossRef]
- Sérgio, C.; Figueira, R.; Draper, D.; Menezes, R.; Sousa, A.J. Modelling bryophyte distribution based on ecological information for extent of occurrence assessment. Biol. Conserv. 2007, 135, 341–351. [Google Scholar] [CrossRef]
- Tittensor, D.P.; Baco, A.R.; Brewin, P.E.; Clark, M.R.; Consalvey, M.; Hall-Spencer, J.; Rowden, A.A.; Schlacher, T.; Stocks, K.I.; Rogers, A.D. Predicting global habitat suitability for stony corals on seamounts. J. Biogeogr. 2009, 36, 1111–1128. [Google Scholar] [CrossRef]
- Wang, Y.S.; Xie, B.Y.; Wan, F.H.; Xiao, Q.M.; Dai, L.Y. The potential geographic distribution of Radopholus similis in China. Agric. Sci. China 2007, 6, 1444–1449. [Google Scholar] [CrossRef]
- Ward, D.F. Modelling the potential geographic distribution of invasive ant species in New Zealand. Biol. Invasions 2007, 9, 723–735. [Google Scholar] [CrossRef]
- Williams, J.N.; Seo, C.; Thorne, J.; Nelson, J.K.; Erwin, S.; O’Brien, J.M.; Schwartz, M.W. Using species distribution models to predict new occurrences for rare plants. Divers. Distrib. 2009, 15, 565–576. [Google Scholar] [CrossRef]
- Wollan, A.K.; Bakkestuen, V.; Kauserud, H.; Gulden, G.; Halvorsen, R. Modelling and predicting fungal distribution patterns using herbarium data. J. Biogeogr. 2008, 35, 2298–2310. [Google Scholar] [CrossRef]
- Yuan, H.S.; Wei, Y.L.; Wang, X.G. Maxent modeling for predicting the potential distribution of Sanghuang, an important group of medicinal fungi in China. Fungal Ecol. 2015, 17, 140–145. [Google Scholar] [CrossRef]
- Li, G.; Huang, J.; Guo, H.; Du, S. Projecting species loss and turnover under climate change for 111 Chinese tree species. For. Ecol. Manag. 2020, 477, 118488. [Google Scholar] [CrossRef]
- Pearson, R.G.; Raxworthy, C.J.; Nakamura, M.; Peterson, A.T. Predicting species distributions from small numbers of occurrence records: A test case using cryptic geckos in Madagascar. J. Biogeogr. 2007, 34, 102–117. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum Entropy Modeling of Species Geographic Distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Yi, Y.; Cheng, X.; Yang, Z.; Wieprecht, S.; Zhang, S.; Wu, Y. Evaluating the ecological infuence of hydraulic projects: A review of aquatic habitat suitability models. Renew. Sustain. Energy Rev. 2017, 68, 748–762. [Google Scholar] [CrossRef]
- Whittemore, A. Berberis; Flora of North America: St. Louis, MO, USA, 1997; Volume 3, pp. 276–286. [Google Scholar]
- Ministry of Agriculture and Forestry General Directorate of Forestry. BIYOD Biodiversity and Non-Wood Forest Products Database; Ministry of Agriculture and Forestry General Directorate of Forestry: Yenimahalle, Türkiye, 2020.
- Davis, P.H. Flora of Turkey the East Aegean Islands; Edinburgh University Press: Edinburgh, UK, 1965; Volume 1. [Google Scholar]
- GBIF. GBIF Occurrence Download. 2023. Available online: https://www.gbif.org/occurrence/download/0026674-231120084113126 (accessed on 9 November 2023).
- Akkemik, U. All Trees and Shrubs of Turkey; Türkiye İş Bank Publications: Istanbul, Türkiye, 2020. [Google Scholar]
- QGIS. QGIS 3.10.4 Coruna—A Free and Open GIS. Available online: https://www.qgis.org/en/site/forusers/visualchangelog322/index.html (accessed on 15 November 2023).
- WorldClim. WorldClim—Global Climate Data. 2019. Available online: www.worldclim.org (accessed on 7 November 2023).
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km Spatial Resolution Climate Surfaces for Global Land Areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. A J. R. Meteorol. Soc. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Hausfather, Z.; Drake, H.F.; Abbott, T.; Schmidt, G.A. Evaluating the performance of past climate model projections. Geophys. Res. Lett. 2020, 47, e2019GL085378. [Google Scholar] [CrossRef]
- Husband, B.C. Review of Quantitative Methods for Conservation Biology. In The Quarterly Review of Biology; Ferson, S., Burgman, M., Eds.; Springer: New York, NY, USA, 2002; Volume 77, pp. 94–165. [Google Scholar]
- Fielding, A.H.; Bell, J.F. A review of methods for assessment of prediction errors in conservation presence/absence models. Environ. Conserv. 1997, 24, 38–49. [Google Scholar] [CrossRef]
- Hundessa, S.; Li, S.; Liu, D.L.; Guo, J.; Guo, Y.; Zhang, W.; Williams, G. Projecting environmental suitable areas for malaria transmission in China under climate change scenarios. Environ. Res. 2018, 162, 203–210. [Google Scholar] [CrossRef]
- Khanum, R.; Mumtaz, A.S.; Kumar, S. Predicting impacts of climate change on medicinal asclepiads of Pakistan using Maxent modeling. Acta Oecologica 2013, 49, 23–31. [Google Scholar] [CrossRef]
- Shcheglovitova, M.; Anderson, R.P. Estimating optimal complexity for ecological niche models: A jack knife approach for species with small sample sizes. Eco Log. Model. 2013, 269, 9–17. [Google Scholar] [CrossRef]
- Ma, J.; Lei, D.; Ren, Z.; Tan, C.; Xia, D.; Guo, H. Automated Machine Learning-Based Landslide Susceptibility Mapping for the Three Gorges Reservoir Area, China. Math. Geosci. 2023. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, J.; Xia, D.; Jiang, S.; Ren, Z.; Tan, C.; Lei, D.; Guo, H. Toward the reliable prediction of reservoir landslide displacement using earthworm optimization algorithm-optimized support vector regression (EOA-SVR). Nat. Hazards 2023. [Google Scholar] [CrossRef]
- Gao, J.; Heng, F.; Yuan, Y.; Liu, Y. A novel machine learning method for multiaxial fatigue life prediction: Improved adaptive neuro-fuzzy inference system. Int. J. Fatigue 2024, 178, 108007. [Google Scholar] [CrossRef]
- Tuttu, G.; Aytas, I.; Bulut, S. Estimation of the distribution areas of Crataegus× bornmuelleri Zabel ex KI Chr. & Ziel. depending on climate change. In Proceedings of the International Eurasia Climate Change Congress, Van, Türkiye, 29 September–1 October 2022; Van Yuzuncu Yil University Press Publication: Van, Türkiye, 2022; p. 53. [Google Scholar]
- Ngarega, B.K.; Masocha, V.F.; Schneider, H. Forecasting the effects of bioclimatic characteristics and climate change on the potential distribution of Colophospermum mopane in southern Africa using Maximum Entropy (Maxent). Ecol. Inform. 2021, 65, 101419. [Google Scholar] [CrossRef]
- Martin, G.; Magengelele, N.L.; Paterson, I.D.; Sutton, G.F. Climate modelling suggests a review of the legal status of Brazilian pepper Schinus terebinthifolia in South Africa is required. South Afr. J. Bot. 2020, 132, 95–102. [Google Scholar] [CrossRef]
- Li, Y.; Shao, W.; Jiang, J. Predicting the potential global distribution of Sapindus mukorossi under climate change based on MaxEnt modelling. Environ. Sci. Pollut. Res. 2022, 29, 21751–21768. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Xu, G.; Guo, K.; Du, S. Mapping the Global Potential Geographical Distribution of Black Locust (Robinia pseudoacacia L.) Using Herbarium Data and a Maximum Entropy Model. Forests 2014, 5, 2773. [Google Scholar] [CrossRef]
- Barve, N.; Barve, V.; Jiménez-Valverde, A.; Lira-Noriega, A.; Maher, S.P.; Peterson, A.T.; Soberón, J.; Villalobos, F. The crucial role of the accessible area in ecological niche modeling and species distribution modeling. Ecol. Model. 2011, 222, 1810–1819. [Google Scholar] [CrossRef]
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudík, M.; Ferrier, S. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
- Kariyawasam, C.S.; Kumar, L.; Ratnayake, S.S. Invasive Plants Distribution Modeling: A Tool for Tropical Biodiversity Conservation With Special Reference to Sri Lanka. Trop. Conserv. Sci. 2019, 12, 1940082919864269. [Google Scholar] [CrossRef]
- Miller, J. Species Distribution Modeling. Geogr. Compass 2010, 4, 490–509. [Google Scholar] [CrossRef]
- Muller, J.J.; Nagel, L.M.; Palik, B.J. Forest adaptation strategies aimed at climate change: Assessing the performance of future climate-adapted tree species in a northern Minnesota pine ecosystem. For. Ecol. Manag. 2019, 451, 117539. [Google Scholar] [CrossRef]
- Moukrim, S.; Lahssini, S.; Rhazi, M.; Alaoui, H.M.; Benabou, A.; Wahby, I.; Rhazi, L. Climate change impacts on potential distribution of multipurpose agroforestry species: Argania spinosa (L.) Skeels as case study. Agrofor. Syst. 2019, 93, 1209–1219. [Google Scholar] [CrossRef]
- Wiens, J.J. Climate-Related Local Extinctions Are Already Widespread among Plant and Animal Species. PLoS Biol. 2016, 14, e2001104. [Google Scholar] [CrossRef] [PubMed]









| Berberis vulgaris | x | y | Province-District | Precipitation (mm) | Temperature (°C) | Altitude (m) |
|---|---|---|---|---|---|---|
| 1 | 31.92793 | 39.57613 | Eskişehir-Sivrihisar | 32.08333 | 12.15833 | 745 |
| 2 | 41.8686 | 40.71457 | Erzurum-Oltu | 41.08333 | 7.85 | 1315 |
| 3 | 41.77139 | 40.89483 | Artvin-Yusufeli | 51.58333 | 7.55 | 1539 |
| 4 | 41.54225 | 40.85444 | Artvin-Yusufeli | 64.58334 | 11.83333 | 730 |
| 5 | 37.79682 | 40.52826 | Ordu-Mesudiye | 52.16667 | 7.83333 | 1329 |
| 6 | 33.44638 | 41.47991 | Kastamonu-Daday | 50.58333 | 8.85 | 922 |
| 7 | 32.95726 | 40.04383 | Ankara-Altındağ | 33.58333 | 11.18333 | 991 |
| 8 | 40.88837 | 40.45238 | Erzurum-İspir | 41.66667 | 8.86667 | 1358 |
| 9 | 33.69235 | 41.7753 | Kastamonu-Küre | 57.41667 | 6.08333 | 1428 |
| 10 | 37.50486 | 40.44489 | Tokat-Reşadiye | 49.25 | 6.8 | 1503 |
| 11 | 32.78359 | 39.87803 | Ankara-Çankaya | 31.66667 | 10.89167 | 980 |
| 12 | 39.65868 | 40.32847 | Gümüşhane-Centre | 44.58333 | 5.20833 | 1819 |
| 13 | 39.30508 | 40.57435 | Gümüşhane-Torul | 47.58333 | 10.725 | 943 |
| 14 | 37.34488 | 40.46817 | Tokat-Reşadiye | 48.75 | 7.70833 | 1543 |
| 15 | 39.35059 | 40.4239 | Gümüşhane-Centre | 41 | 9.05833 | 1305 |
| 16 | 33.86361 | 41.77972 | Kastamonu-Devrekani | 56.58333 | 6.875 | 1290 |
| 17 | 33.78121 | 41.27519 | Kastamonu-Centre | 44.16667 | 8.3 | 1107 |
| 18 | 33.3042 | 40.94056 | Çankırı-Kurşunlu | 48.83333 | 6.53333 | 1285 |
| 19 | 30.90709 | 37.19898 | Antalya-Serik | 59.58333 | 16.98333 | 258 |
| 20 | 29.57781 | 37.35591 | Denizli-Acıpayam | 46 | 11.69167 | 1054 |
| 21 | 40.98007 | 40.09898 | Erzurum-Aziziye | 43.16667 | 4.16667 | 1946 |
| 22 | 30.33495 | 39.72538 | Eskişehir-Tepebaşı | 38.66667 | 10.86667 | 905 |
| 23 | 38.097 | 40.27174 | Sivas-Suşehri | 49.41667 | 10.13333 | 1204 |
| 24 | 37.62837 | 40.41298 | Tokat-Reşadiye | 49 | 5.94167 | 1602 |
| 25 | 38.6325 | 40.22757 | Giresun-Alucra | 48.75 | 4.51667 | 1970 |
| 26 | 38.64615 | 40.4052 | Giresun-Şebinkarahisar | 48.25 | 8.35 | 1414 |
| 27 | 38.81601 | 40.34509 | Giresun-Alucra | 47.66667 | 6.21667 | 1753 |
| 28 | 38.93972 | 40.26588 | Giresun-Alucra | 46.33333 | 6.34167 | 1684 |
| 29 | 30.28079 | 37.58223 | Burdur-Centre | 44.08333 | 10.70833 | 1271 |
| 30 | 31.06106 | 37.81084 | Isparta-Aksu | 50.33333 | 10.23333 | 1247 |
| 31 | 31.06174 | 37.81049 | Isparta-Aksu | 50.33333 | 10.23333 | 1247 |
| 32 | 29.72987 | 37.25625 | Burdur-Tefenni | 45.33333 | 10.925 | 1295 |
| 33 | 34.03415 | 41.74219 | Kastamonu-Devrekani | 55.83333 | 6.425 | 1360 |
| 34 | 33.23236 | 41.59983 | Kastamonu-Azdavay | 56 | 7.7 | 993 |
| 35 | 40.37922 | 40.17859 | Bayburt-Centre | 41.25 | 5.86667 | 1711 |
| 36 | 40.5992 | 40.23474 | Bayburt-Centre | 50.75 | 3.075 | 2145 |
| 37 | 40.37926 | 40.17865 | Bayburt-Centre | 41.25 | 5.86667 | 1711 |
| 38 | 40.5992 | 40.23474 | Bayburt-Centre | 50.75 | 3.075 | 2145 |
| 39 | 40.40522 | 40.57008 | Trabzon-Çaykara | 49.91667 | 4.58333 | 1900 |
| 40 | 39.50235 | 40.54477 | Gümüşhane-Torul | 44.75 | 6.65833 | 1552 |
| 41 | 39.35762 | 40.65752 | Gümüşhane-Torul | 48.75 | 6.45 | 1763 |
| 42 | 31.83252 | 37.40472 | Konya-Seydişehir | 54.83333 | 11.14167 | 1127 |
| 43 | 33.0583 | 41.60131 | Kastamonu-Pınarbaşı | 57.41667 | 9.33333 | 770 |
| 44 | 36.35301 | 41.22309 | Samsun-Canik | 53.41667 | 11.76667 | 583 |
| 45 | 36.61503 | 40.24437 | Tokat-Centre | 38.66667 | 9.46667 | 1048 |
| 46 | 40.47138 | 40.37269 | Bayburt-Centre | 39.5 | 7.15833 | 1632 |
| 47 | 41.91951 | 41.16631 | Artvin-Ardanuç | 69.33334 | 9.74167 | 1414 |
| 48 | 43.10971 | 38.28423 | Van-Gevaş | 45.75 | 7.75 | 1951 |
| 49 | 31.94009 | 37.00053 | Antalya-Akseki | 58.75 | 8.51667 | 1960 |
| 50 | 38.74682 | 40.45486 | Giresun-Alucra | 51.25 | 4.36667 | 1937 |
| Bio 1 | Annual Mean Temperature |
| Bio 2 | Mean Diurnal Range (Mean of Monthly (Max Temp.–Min Temp.) |
| Bio 3 | Isothermality (WC2/WC7) (×100) |
| Bio 4 | Temperature Seasonality (Standard Deviation × 100) |
| Bio 5 | Max Temperature of Warmest Month |
| Bio 6 | Min Temperature of Coldest Month |
| Bio 7 | Temperature Annual Range (WC5–WC6) |
| Bio 8 | Mean Temperature of Wettest Quarter |
| Bio 9 | Mean Temperature of Driest Quarter |
| Bio 10 | Mean Temperature of Warmest Quarter |
| Bio 11 | Mean Temperature of Coldest Quarter |
| Bio 12 | Annual Precipitation |
| Bio 13 | Precipitation of Wettest Month |
| Bio 14 | Precipitation of Driest Month |
| Bio 15 | Precipitation Seasonality (Coefficient of Variation) |
| Bio 16 | Precipitation of Wettest Quarter |
| Bio 17 | Precipitation of Driest Quarter |
| Bio 18 | Precipitation of Warmest Quarter |
| Bio 19 | Precipitation of Coldest Quarter |
| SSP2 4.5 | SSP5 8.5 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Current | % | 2041–2060 | % | 2081–2100 | % | 2041–2060 | % | 2081–2100 | % | |
| Not suitable | 566,141.9 | 72.65 | 654,925 | 84.04 | 659,050 | 84.57 | 637,449.1 | 81.80 | 725,712.1 | 93.13 |
| Less suitable | 121,749.9 | 15.62 | 39,944.76 | 5.13 | 40,174.39 | 5.16 | 48,162.23 | 6.18 | 20,318 | 2.61 |
| Suitable | 45,963.45 | 5.90 | 25,279.3 | 3.24 | 23,314.31 | 2.99 | 26,987.42 | 3.46 | 13,130.71 | 1.68 |
| Very suitable | 45,413.82 | 5.83 | 59,120.05 | 7.59 | 56,730.46 | 7.28 | 66,670.39 | 8.56 | 20,108.29 | 2.58 |
| Total | 779,269.1 | 100 | 779,269.1 | 100 | 779,269.1 | 100 | 779,269.1 | 100 | 779,269.1 | 100 |
| SSP2 4.5 | SSP5 8.5 | |||||||
|---|---|---|---|---|---|---|---|---|
| Change | 2041–2060 | % | 2081–2100 | % | 2041–2060 | % | 2081–2100 | % |
| Gain | 81,463.731 | 10.45 | 80,263.758 | 10.30 | 84,300.667 | 10.82 | 37,092.284 | 4.76 |
| Loss | 168,278.165 | 21.59 | 169,765.384 | 21.79 | 153,488.538 | 19.70 | 196,525.05 | 25.22 |
| Stable | 18,751.607 | 2.41 | 17,535.724 | 2.25 | 30,563.106 | 3.92 | 7300.523 | 0.94 |
| Unsuitable | 510,775.642 | 65.55 | 511,704.259 | 65.66 | 510,916.836 | 65.56 | 53,8351.23 | 69.08 |
| Total | 779,269.145 | 100 | 779,269.145 | 100 | 779,269.145 | 100 | 779,269.145 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarikaya, A.G.; Uzun, A. Modeling the Effects of Climate Change on the Current and Future Potential Distribution of Berberis vulgaris L. with Machine Learning. Sustainability 2024, 16, 1230. https://doi.org/10.3390/su16031230
Sarikaya AG, Uzun A. Modeling the Effects of Climate Change on the Current and Future Potential Distribution of Berberis vulgaris L. with Machine Learning. Sustainability. 2024; 16(3):1230. https://doi.org/10.3390/su16031230
Chicago/Turabian StyleSarikaya, Ayse Gul, and Almira Uzun. 2024. "Modeling the Effects of Climate Change on the Current and Future Potential Distribution of Berberis vulgaris L. with Machine Learning" Sustainability 16, no. 3: 1230. https://doi.org/10.3390/su16031230
APA StyleSarikaya, A. G., & Uzun, A. (2024). Modeling the Effects of Climate Change on the Current and Future Potential Distribution of Berberis vulgaris L. with Machine Learning. Sustainability, 16(3), 1230. https://doi.org/10.3390/su16031230





