Tracing the Maternal Line in Glacial–Interglacial Migrations of Populus tremuloides: Finding Trees for Future Sustainable Forests by Searching in the Past
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection, DNA Extraction, and SSR Genotyping
2.2. Sequencing Plastid rpL16
2.3. Comparison of Datasets and Data Types Using Tree-Based Comparisons
2.4. Intronic Indel Comparisons
3. Results
3.1. Intraspecific Molecular Evolution of rpL16 in Populus tremuloides
3.2. Comparisons of SSR, SNV, and Indel Data
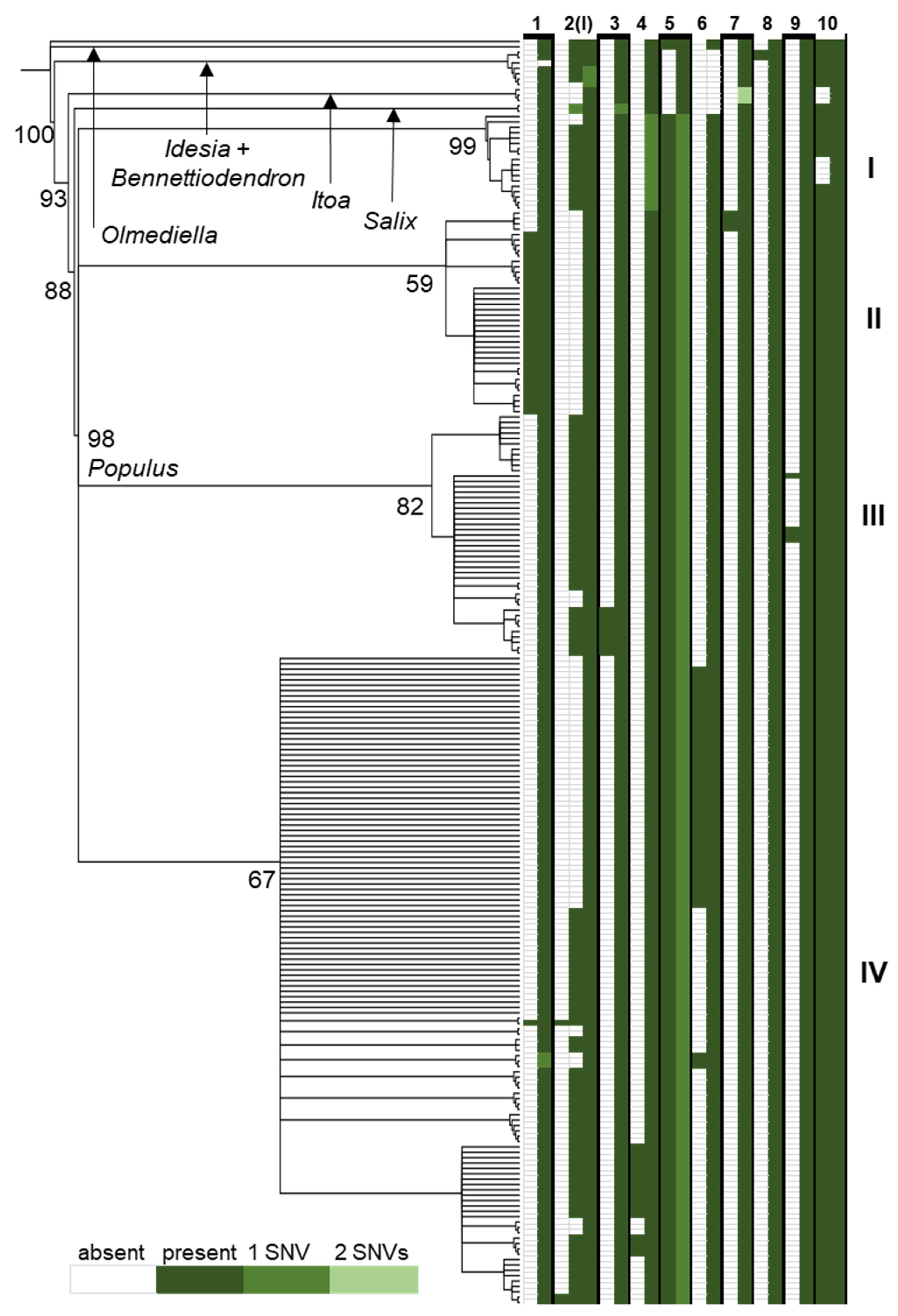
3.3. rpL16 Indel Sorting across Salicaceae
3.4. Intronic Indel Abundance in Salicaceae Plastomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Little, E.L., Jr. Atlas of United States Trees Volume 1: Conifers and Important Hardwoods; USDA Forest Service: Washington, DC, USA, 1971. [Google Scholar]
- Eckenwalder, J.E. Systematics and evolution of Populus. In Biology of Populus and Its Implications for Management and Conservation; Stettler, R.F., Bradshaw, H.D., Jr., Heilman, P.E., Hinckley, T.M., Eds.; NRC Research Press, Nation Research Council of Canada: Ottawa, ON, Canada, 1996; pp. 7–32. [Google Scholar]
- Mark, A.; Rumble, L.D.F.; Mills, T.R.; Dykstra, B.L. Do Pine Trees in Aspen Stands Increase Bird Diversity? USDA Forest Service Rocky Mountain Research Station: Fort Collins, CO, USA, 2001; pp. 185–192. [Google Scholar]
- Simonson, S.E.; Opler, P.A.; Stohlgren, T.J.; Chong, G.W. Rapid assessment of butterfly diversity in a montane landscape. Biodivers. Conserv. 2001, 10, 1369–1386. [Google Scholar] [CrossRef]
- Griffis-Kyle, K.L.; Beier, P. Small isolated aspen stands enrich bird communities in southwestern ponderosa pine forests. Biol. Conserv. 2003, 110, 375–385. [Google Scholar] [CrossRef]
- Cole, C.T.; Anderson, J.E.; Lindroth, R.L.; Waller, D.M. Rising concentrations of atmospheric CO2 have increased growth in natural stands of quaking aspen (Populus tremuloides). Glob. Chang. Biol. 2010, 16, 2186–2197. [Google Scholar] [CrossRef]
- Boča, A.; Jacobson, A.R.; Van Miegroet, H. Aspen soils retain more dissolved organic carbon than conifer soils in a sorption experiment. Front. For. Glob. Chang. 2020, 3, 594473. [Google Scholar] [CrossRef]
- DesRochers, A.; Driessche, R.V.D.; Thomas, B.R. Nitrogen fertilization of trembling aspen seedlings grown on soils of different pH. Can. J. For. Res. 2003, 33, 552–560. [Google Scholar] [CrossRef]
- LaMalfa, E.M.; Ryle, R. Differential snowpack accumulation and water dynamics in aspen and conifer communities: Implications for water yield and ecosystem function. Ecosystems 2008, 11, 569–581. [Google Scholar] [CrossRef]
- Fechner, G.H.; Barrows, J.S. Aspen Stands and Wildfire Fuel Breaks; U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station: Washington, DC, USA, 1976. [Google Scholar]
- DeByle, N.B.; Winokur, R.P. Aspen: Ecology and Management in the Western United States; USDA Forest Service General Technical Report RM-119; Rocky Mountain Forest and Range Experiment Station: Fort Collins, CO, USA, 1985; p. 283. [Google Scholar]
- Rogers, P.C.; Pinno, B.D.; Sebesta, J.; Albrectsen, B.R.; Li, G.Q.; Ivanova, N.; Kusbach, A.; Kuuluvainen, T.; Landhausser, S.M.; Liu, H.Y.; et al. A global view of aspen: Conservation science for widespread keystone systems. Glob. Ecol. Conserv. 2020, 21, e00828. [Google Scholar] [CrossRef]
- Gray, L.K.; Gylander, T.; Mbogga, M.S.; Chen, P.Y.; Hamann, A. Assisted migration to address climate change: Recommendations for aspen reforestation in western Canada. Ecol. Appl. 2011, 21, 1591–1603. [Google Scholar] [CrossRef]
- Callahan, C.M.; Rowe, C.A.; Ryel, R.J.; Shaw, J.D.; Madritch, M.D.; Mock, K.E. Continental-scale assessment of genetic diversity and population structure in quaking aspen (Populus tremuloides). J. Biogeog. 2013, 40, 1780–1791. [Google Scholar] [CrossRef]
- Latutrie, M.; Bergeron, Y.; Tremblay, F. Fine-scale assessment of genetic diversity of trembling aspen in northwestern North America. BMC Evol. Biol. 2016, 16, 231. [Google Scholar] [CrossRef]
- Schaal, B.A.; Olsen, K.M. Gene genealogies and population variation in plants. Proc. Natl. Acad. Sci. USA 2000, 97, 7024–7029. [Google Scholar] [CrossRef]
- Pluess, A.R. Pursuing glacier retreat: Genetic structure of a rapidly expanding Larix decidua population. Mol. Ecol. 2011, 20, 473–485. [Google Scholar] [CrossRef]
- Ding, C.; Schreiber, S.G.; Roberts, D.R.; Hamann, A.; Brouard, J.S. Post-glacial biogeography of trembling aspen inferred from habitat models and genetic variance in quantitative traits. Sci. Rep. 2017, 7, 4672. [Google Scholar] [CrossRef]
- Deacon, N.J.; Grossman, J.J.; Schweiger, A.K.; Armour, I.; Cavender-Bares, J. Genetic, morphological, and spectral characterization of relictual Niobrara River hybrid aspens (Populus × smithii). Am. J. Bot. 2017, 104, 1878–1890. [Google Scholar] [CrossRef]
- Tembrock, L.R.; Stevens, J.E.; Schuhmann, A.; Walton, J.A. Genetic Characterization and Comparison of Three Disjunct Populus Temuloides Michx (Salicaceae) Stands Across a Latitudinal Gradient; Natural Resource Report NPS/NRSS/IMD/NRR; National Park Service: Fort Collins, CO, USA, 2020; p. 74. [Google Scholar]
- Wang, D.; Wang, Z.; Kang, X.; Zhang, J. Genetic analysis of admixture and hybrid patterns of Populus hopeiensis and P. tomentosa. Sci. Rep. 2019, 9, 4821. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, L.; Zhang, Z.; Li, M.; Wang, D.; Zhang, X.; Xi, Z.; Keefover-Ring, K.; Smart, L.B.; DiFazio, S.P.; et al. Phylogenomics of the genus Populus reveals extensive interspecific gene flow and balancing selection. New Phytol. 2020, 225, 1370–1382. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, J.; Li, E.; Xu, S.; Zhan, Z.; Zhang, X.; Yang, Z.; Guo, F.; Liu, K.; Liu, D.; et al. Phylogenomics and biogeography of Populus based on comprehensive sampling reveal deep-level relationships and multiple intercontinental dispersals. Front. Plant Sci. 2022, 13, 813177. [Google Scholar] [CrossRef]
- Norell, M.A. Tree-based approaches to understanding history: Comments on ranks, rules, and the quality of the fossil record. Am. J. Sci. 1993, 293, 10. [Google Scholar] [CrossRef]
- Huang, D.I.; Hefer, C.A.; Kolosova, N.; Douglas, C.J.; Cronk, Q.C.B. Whole plastome sequencing reveals deep plastid divergence and cytonuclear discordance between closely related balsam poplars, Populus balsamifera and P. trichocarpa (Salicaceae). New Phytol. 2014, 204, 693–703. [Google Scholar] [CrossRef]
- Barnes, B.V. Phenotypic variation of trembling aspen in western North America. For. Sci. 1975, 21, 319–328. [Google Scholar]
- Elena, S.F.; Wilke, C.O.; Ofria, C.; Lenski, R.E. Effects of population size and mutation rate on the evolution of mutational robustness. Evolution 2007, 61, 666–674. [Google Scholar] [CrossRef]
- Thompson, S.L.; Lamothe, M.; Meirmans, P.G.; Perinet, P.; Isabel, N. Repeated unidirectional introgression towards Populus balsamifera in contact zones of exotic and native poplars. Mol. Ecol. 2010, 19, 132–145. [Google Scholar] [CrossRef]
- Macaya-Sanz, D.; Suter, L.; Joseph, J.; Barbara, T.; Alba, N.; Gonzalez-Martinez, S.C.; Widmer, A.; Lexer, C. Genetic analysis of post-mating reproductive barriers in hybridizing European Populus species. Heredity 2011, 107, 478–486. [Google Scholar] [CrossRef]
- Chhatre, V.E.; Evans, L.M.; DiFazio, S.P.; Keller, S.R. Adaptive introgression and maintenance of a trispecies hybrid complex in range-edge populations of Populus. Mol. Ecol. 2018, 27, 4820–4838. [Google Scholar] [CrossRef]
- Shaw, J.; Lickey, E.B.; Beck, J.T.; Farmer, S.B.; Liu, W.; Miller, J.; Siripun, K.C.; Winder, C.T.; Schilling, E.E.; Small, R.L. The tortoise and the hare II: Relative utility of 21 noncoding chloroplast DNA sequences for phylogenetic analysis. Am. J. Bot. 2005, 92, 142–166. [Google Scholar] [CrossRef]
- Katoh, K.; Misawa, K.; Kuma, K.; Miyata, T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Perrier, X.; Flori, A.; Bonnot, F. Data analysis methods. In Genetic Diversity of Cultivated Topical Plants; Hamon, P., Sguin, M., Perrier, X., Glaszmann, J.C., Eds.; Enfield, Science Publishers: Montpellier, France, 2003; pp. 43–76. [Google Scholar]
- Perrier, X.; Jacquemoud-Collet, J.P. DARwin Software. Available online: https://darwin.cirad.fr/ (accessed on 21 December 2023).
- Dice, L.R. Measures of the amount of ecologic association between species. Ecology 1945, 26, 297–302. [Google Scholar] [CrossRef]
- Tamura, K.; Nei, M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 1993, 10, 512–526. [Google Scholar] [CrossRef]
- Robbins, E.H.J.; Kelly, S. The evolutionary constraints on angiosperm chloroplast adaptation. Genome Biol. Evol. 2023, 15, evad101. [Google Scholar] [CrossRef]
- Forsythe, E.S.; Grover, C.E.; Miller, E.R.; Conover, J.L.; Arick, M.A., II; Chavarro, M.C.F.; Leal-Bertioli, S.C.M.; Peterson, D.G.; Sharbrough, J.; Wendel, J.F.; et al. Organellar transcripts dominate the cellular mRNA pool across plants of varying ploidy levels. Proc. Natl. Acad. Sci. USA 2022, 119, e2204187119. [Google Scholar] [CrossRef]
- Avise, J.C.; Arnold, J.; Ball, R.M.; Bermingham, E.; Lamb, T.; Neigel, J.E.; Reeb, C.A.; Saunders, N.C. Intraspecific phylogeography—The mitochondrial-DNA bridge between population-genetics and systematics. An. Rev. Ecol. Systemat. 1987, 18, 489–522. [Google Scholar] [CrossRef]
- Cuenca, A.; Escalante, A.E.; Pinero, D. Long-distance colonization, isolation by distance, and historical demography in a relictual Mexican pinyon pine (Pinus nelsonii Shaw) as revealed by paternally inherited genetic markers (cpSSRs). Mol. Ecol. 2003, 12, 2087–2097. [Google Scholar] [CrossRef]
- He, W.; Chen, C.; Xiang, K.; Wang, J.; Zheng, P.; Tembrock, L.R.; Jin, D.; Wu, Z. The history and diversity of rice domestication as resolved from 1464 complete plastid genomes. Front. Plant Sci. 2021, 12, 781793. [Google Scholar] [CrossRef]
- Wang, Z.S.; Du, S.H.; Dayanandan, S.; Wang, D.S.; Zeng, Y.F.; Zhang, J.G. Phylogeny reconstruction and hybrid analysis of Populus (Salicaceae) based on nucleotide sequences of multiple single-copy nuclear genes and plastid fragments. PLoS ONE 2014, 9, e103645. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, S.; Wang, J.; Shen, H.; Ai, B.; Gao, W.; Zhang, C.; Fei, Q.; Yuan, D.; Wu, Z.; et al. Chloroplast genomes in Populus (Salicaceae): Comparisons from an intensively sampled genus reveal dynamic patterns of evolution. Sci. Rep. 2021, 11, 9471. [Google Scholar] [CrossRef]
- Cartwright, R.A. Problems and solutions for estimating indel rates and length distributions. Mol. Biol. Evol. 2009, 26, 473–480. [Google Scholar] [CrossRef]
- Loewenthal, G.; Rapoport, D.; Avram, O.; Moshe, A.; Wygoda, E.; Itzkovitch, A.; Israeli, O.; Azouri, D.; Cartwright, R.A.; Mayrose, I.; et al. A probabilistic model for indel evolution: Differentiating insertions from deletions. Mol. Biol. Evol. 2021, 38, 5769–5781. [Google Scholar] [CrossRef]
- Golenberg, E.M.; Clegg, M.T.; Durbin, M.L.; Doebley, J.; Ma, D.P. Evolution of a noncoding region of the chloroplast genome. Mol. Phylogenet. Evol. 1993, 2, 52–64. [Google Scholar] [CrossRef]
- Tian, D.; Wang, Q.; Zhang, P.; Araki, H.; Yang, S.; Kreitman, M.; Nagylaki, T.; Hudson, R.; Bergelson, J.; Chen, J.Q. Single-nucleotide mutation rate increases close to insertions/deletions in eukaryotes. Nature 2008, 455, 105–108. [Google Scholar] [CrossRef]
- Abdullah; Mehmood, F.; Shahzadi, I.; Ali, Z.; Islam, M.; Naeem, M.; Mirza, B.; Lockhart, P.J.; Ahmed, I.; Waheed, M.T. Correlations among oligonucleotide repeats, nucleotide substitutions, and insertion–deletion mutations in chloroplast genomes of plant family Malvaceae. J. Systemat. Evol. 2021, 59, 388–402. [Google Scholar] [CrossRef]
- Pan, Z.L.; Li, Z.T.; Zhang, J.P.; Bai, S.J.; Zhao, W.; Tong, C.F. Investigation of genome-wide InDel distribution and segregation in Populus with restriction-site associated DNA sequencing data. Trop. Plant Biol. 2022, 15, 171–180. [Google Scholar] [CrossRef]
- Messer, P.W.; Arndt, P.F. The majority of recent short DNA insertions in the human genome are tandem duplications. Mol. Biol. Evol. 2007, 24, 1190–1197. [Google Scholar] [CrossRef]
- Schiml, S.; Fauser, F.; Puchta, H. Repair of adjacent single-strand breaks is often accompanied by the formation of tandem sequence duplications in plant genomes. Proc. Natl. Acad. Sci. USA 2016, 113, 7266–7271. [Google Scholar] [CrossRef]
- Wolter, F.; Schindele, P.; Beying, N.; Scheben, A.; Puchta, H. Different DNA repair pathways are involved in single-strand break-induced genomic changes in plants. Plant Cell 2021, 33, 3454–3469. [Google Scholar] [CrossRef]
- Houde, P.; Braun, E.L.; Narula, N.; Minjares, U.; Mirarab, S. Phylogenetic signal of indels and the neoavian radiation. Diversity 2019, 11, 108. [Google Scholar] [CrossRef]
- Morley, S.A.; Ahmad, N.; Nielsen, B.L. Plant organelle genome replication. Plants 2019, 8, 358. [Google Scholar] [CrossRef]
- Reddy, A.S.; Rogers, M.F.; Richardson, D.N.; Hamilton, M.; Ben-Hur, A. Deciphering the plant splicing code: Experimental and computational approaches for predicting alternative splicing and splicing regulatory elements. Front. Plant Sci. 2012, 3, 18. [Google Scholar] [CrossRef]
- Abramowitz, A.; Gos, M. Splicing mutations in human genetic disorders: Examples, detection, and confirmation. J. App. Genet. 2018, 59, 253–268. [Google Scholar] [CrossRef]
- Hampe, A.; Jump, A.S. Climate relicts: Past, present, future. Annu. Rev. Ecol. Evol. Syst. 2011, 42, 313–333. [Google Scholar] [CrossRef]
- Cobo-Simon, I.; Mendez-Cea, B.; Jump, A.S.; Seco, J.; Gallego, F.J.; Linares, J.C. Understanding genetic diversity of relict forests. Linking long-term isolation legacies and current habitat fragmentation in Abies pinsapo Boiss. For. Ecol. Manag. 2020, 461, 117947. [Google Scholar] [CrossRef]
- Steffen, W.; Richardson, K.; Rockstrom, J.; Cornell, S.E.; Fetzer, I.; Bennett, E.M.; Biggs, R.; Carpenter, S.R.; de Vries, W.; de Wit, C.A.; et al. Sustainability. Planetary boundaries: Guiding human development on a changing planet. Science 2015, 347, 1259855. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Tanaka, R. Chlorophyll metabolism. Curr. Opin. Plant Biol. 2006, 9, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Blonder, B.; Graae, B.J.; Greer, B.; Haagsma, M.; Helsen, K.; Kapás, R.E.; Pai, H.; Rieksta, J.; Sapena, D.; Still, C.J.; et al. Remote sensing of ploidy level in quaking aspen (Populus tremuloides Michx.). J. Ecol. 2020, 108, 175–188. [Google Scholar] [CrossRef]
- Bogdanova, V.S. Genetic and molecular genetic basis of nuclear-plastid incompatibilities. Plants 2019, 9, 23. [Google Scholar] [CrossRef]
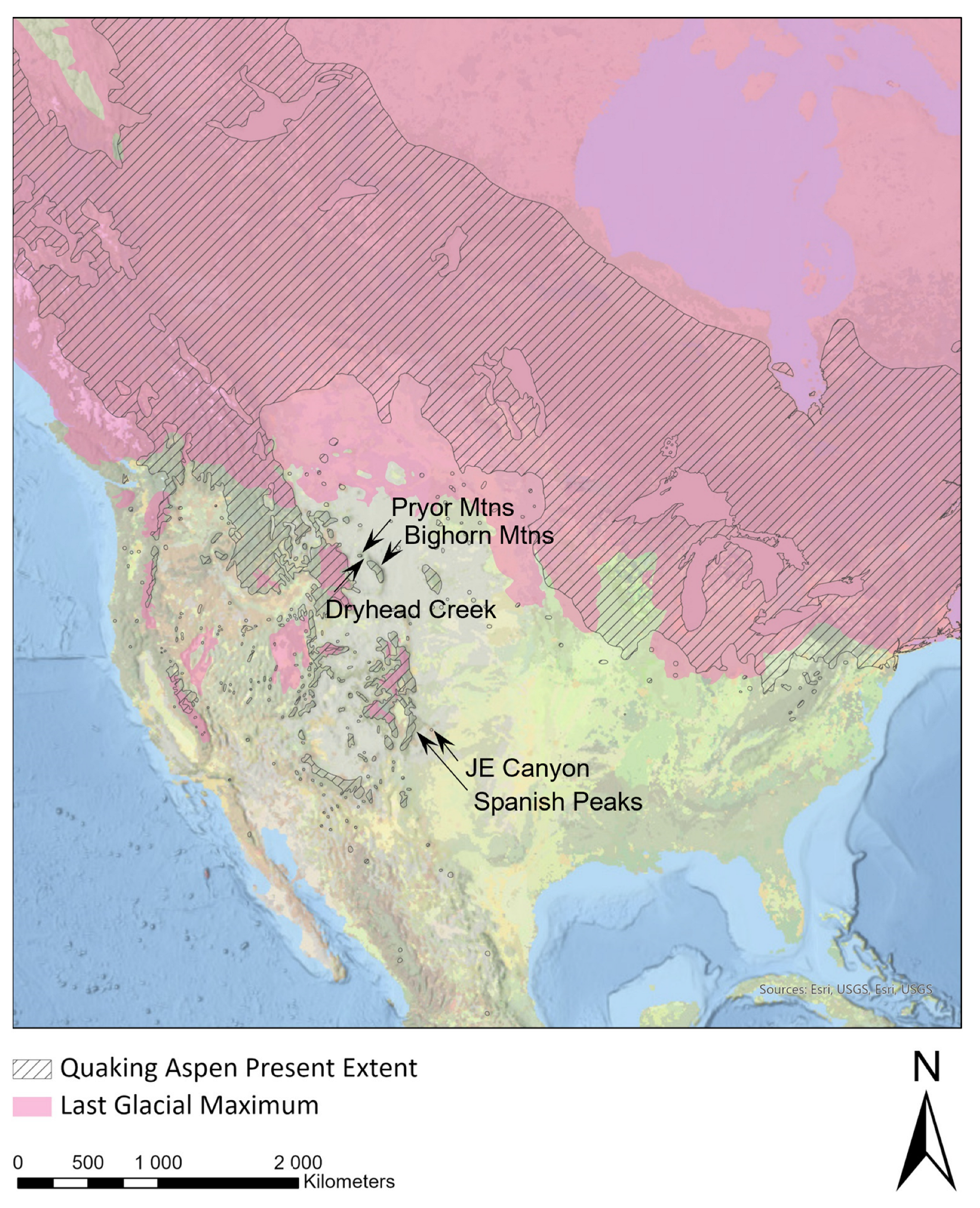
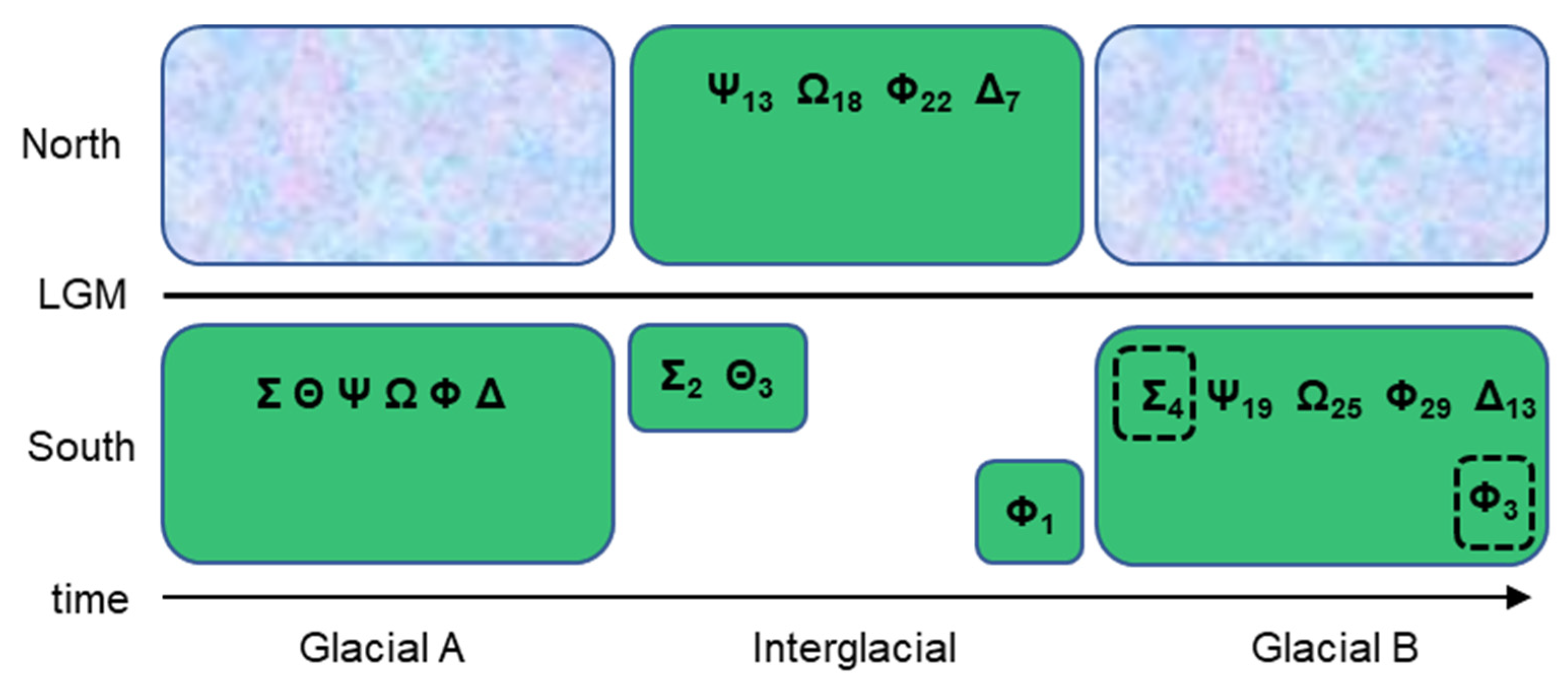
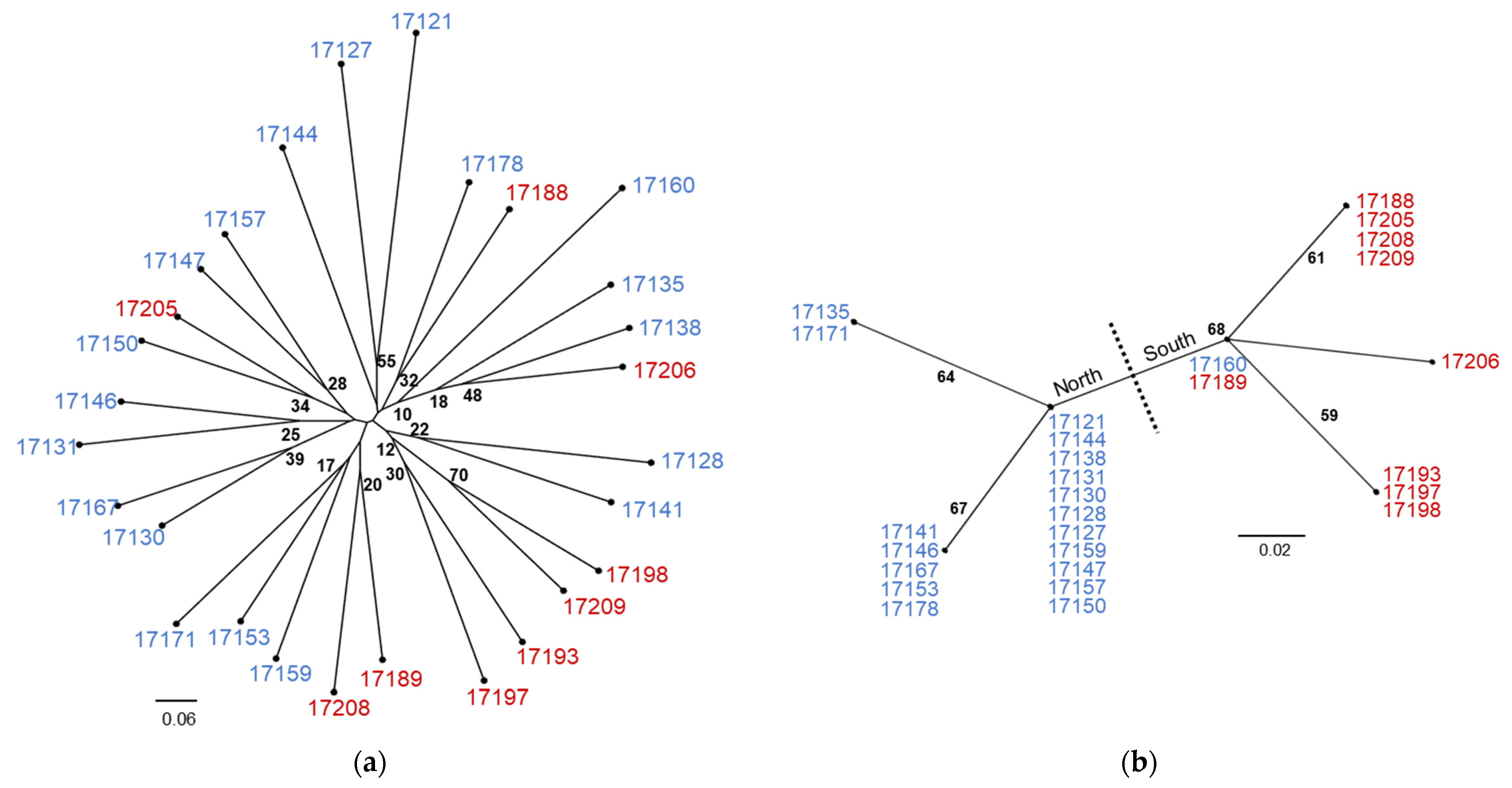
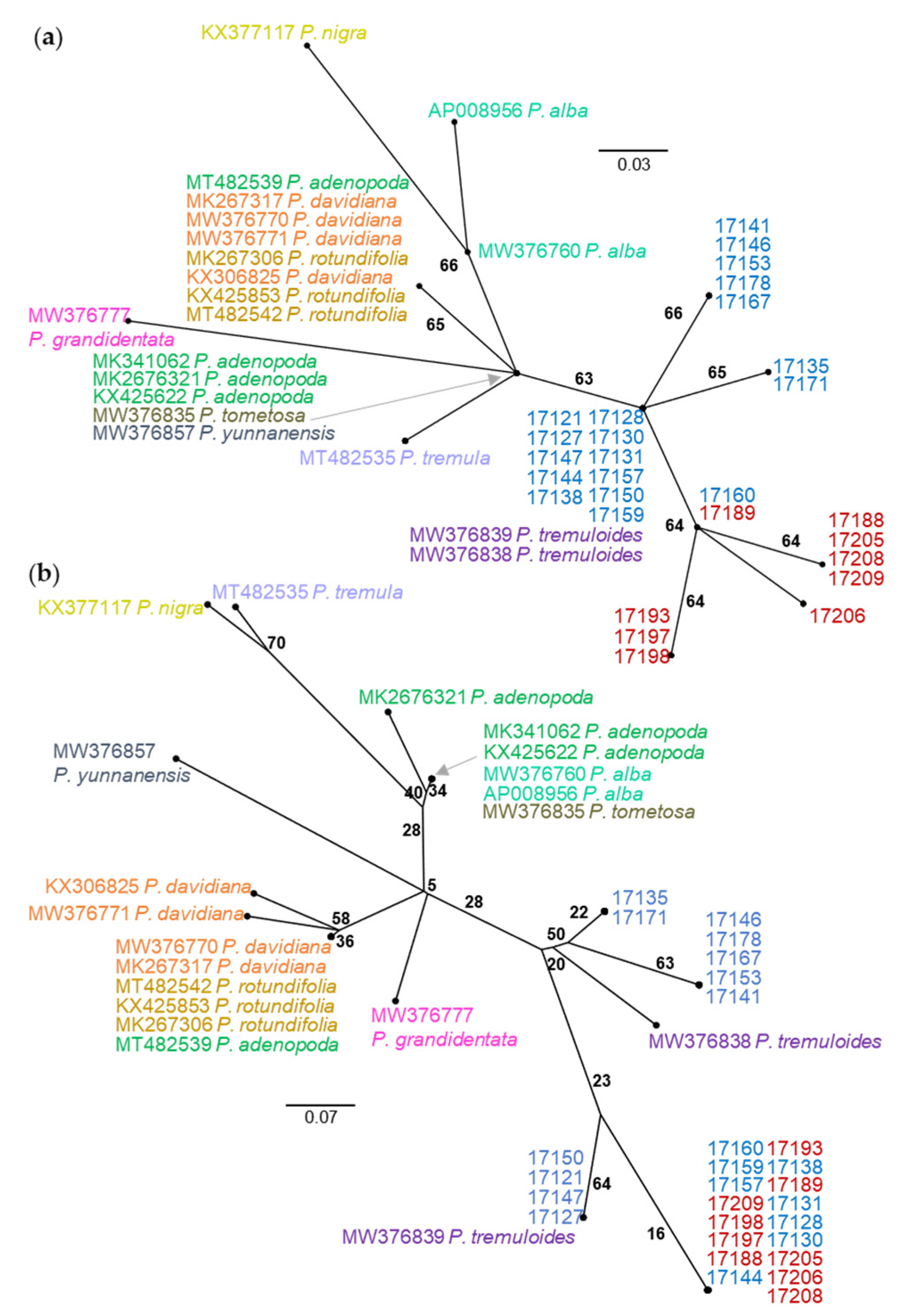

| Species | Accession | Site | A | B | C | D | E | F | G | H | I | J | K | L | M | 386 | 393 | 402 | 450 | 473 | 567 | 638 | 702 | 706 | 753 | 808 | 837 | 846 | 871 | 995 | 1022 | Type |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P. tremuloides | 17121 | N, P | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | C | C | A | C | T | G | C | C | T | G | G | A | C | T | C | A | 1 |
| P. tremuloides | 17127 | N, P | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | C | C | A | C | T | G | C | C | T | G | G | A | C | T | C | A | 1 |
| P. tremuloides | 17128 | N, P | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | C | C | A | C | T | G | C | C | T | G | G | A | C | T | C | A | 2 |
| P. tremuloides | 17130 | N, P | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | C | C | A | C | T | G | C | C | T | G | G | A | C | T | C | A | 2 |
| P. tremuloides | 17131 | N, P | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | C | C | A | C | T | G | C | C | T | G | G | A | C | T | C | A | 2 |
| P. tremuloides | 17135 | N, P | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | C | C | A | C | T | G | C | C | T | G | G | G | C | T | C | A | 3 |
| P. tremuloides | 17138 | N, P | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | C | C | A | C | T | G | C | C | T | G | G | A | C | T | C | A | 2 |
| P. tremuloides | 17141 | N, P | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | C | C | A | C | T | G | C | C | T | G | G | A | C | T | A | A | 4 |
| P. tremuloides | 17144 | N, P | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | C | C | A | C | T | G | C | C | T | G | G | A | C | T | C | A | 2 |
| P. tremuloides | 17146 | N, P | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | C | C | A | C | T | G | C | C | T | G | G | A | C | T | A | A | 4 |
| P. tremuloides | 17147 | N, P | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | C | C | A | C | T | G | C | C | T | G | G | A | C | T | C | A | 1 |
| P. tremuloides | 17150 | N, P | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | C | C | A | C | T | G | C | C | T | G | G | A | C | T | C | A | 1 |
| P. tremuloides | 17153 | N, P | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | C | C | A | C | T | G | C | C | T | G | G | A | C | T | A | A | 4 |
| P. tremuloides | 17157 | N, P | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | C | C | A | C | T | G | C | C | T | G | G | A | C | T | C | A | 2 |
| P. tremuloides | 17159 | N, P | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | C | C | A | C | T | G | C | C | T | G | G | A | C | T | C | A | 2 |
| P. tremuloides | 17160 | N, B | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | C | C | A | C | T | G | C | C | T | G | G | A | C | T | C | G | 5 |
| P. tremuloides | 17163 * | N, B | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | C | C | A | C | T | G | C | C | T | G | G | A | C | T | C | G | 5 |
| P. tremuloides | 17167 | N, B | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | C | C | A | C | T | G | C | C | T | G | G | A | C | T | A | A | 4 |
| P. tremuloides | 17171 | N, B | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | C | C | A | C | T | G | C | C | T | G | G | G | C | T | C | A | 3 |
| P. tremuloides | 17174 * | N, B | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | C | C | A | C | T | G | C | C | T | G | G | A | C | T | A | A | 4 |
| P. tremuloides | 17178 | N, B | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | C | C | A | C | T | G | C | C | T | G | G | A | C | T | A | A | 4 |
| P. tremuloides | 17185 * | N, B | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | C | T | A | C | T | G | C | C | T | G | G | A | C | T | C | G | 6 |
| P. tremuloides | 17188 | S, SP | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | C | T | A | C | T | G | C | C | T | G | G | A | C | T | C | G | 6 |
| P. tremuloides | 17189 | S, SP | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | C | C | A | C | T | G | C | C | T | G | G | A | C | T | C | G | 5 |
| P. tremuloides | 17193 | S, SP | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | C | C | A | C | T | G | C | C | T | G | G | A | C | C | C | G | 7 |
| P. tremuloides | 17196 * | S, SP | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | C | T | A | C | T | G | C | C | T | G | G | A | C | T | C | G | 6 |
| P. tremuloides | 17197 | S, SP | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | C | C | A | C | T | G | C | C | T | G | G | A | C | C | C | G | 7 |
| P. tremuloides | 17198 | S, JE | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | C | C | A | C | T | G | C | C | T | G | G | A | C | C | C | G | 7 |
| P. tremuloides | 17205 | S, SP | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | C | T | A | C | T | G | C | C | T | G | G | A | C | T | C | G | 6 |
| P. tremuloides | 17206 | S, SP | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | C | C | A | C | T | G | C | C | T | G | G | A | T | T | C | G | 8 |
| P. tremuloides | 17208 | S, SP | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | C | T | A | C | T | G | C | C | T | G | G | A | C | T | C | G | 6 |
| P. tremuloides | 17209 | S, SP | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | C | T | A | C | T | G | C | C | T | G | G | A | C | T | C | G | 6 |
| P. tremuloides | MW376839 | IL | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | C | C | A | C | T | G | C | C | T | G | G | A | C | T | C | G | 9 |
| P. tremuloides | MW376838 | NY | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | C | C | A | C | T | G | C | C | T | G | G | A | C | T | C | G | 10 |
| P. grandidentata | MW376777 | N/A | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | C | C | A | T | T | A | C | C | C | A | G | A | C | T | C | G | 11 |
| P. adenopoda | KX425622 | N/A | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | C | C | A | C | T | G | C | C | C | G | G | A | C | T | C | G | 12 |
| P. adenopoda | MK341062 | N/A | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | C | C | A | C | T | G | C | C | C | G | G | A | C | T | C | G | 12 |
| P. adenopoda | MK267321 | N/A | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | C | C | A | C | T | G | C | C | C | G | G | A | C | T | C | G | 13 |
| P. adenopoda | MK482539 | N/A | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | C | C | A | C | A | G | C | C | C | G | G | A | C | T | C | G | 14 |
| P. tometosa | MW376835 | N/A | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | C | C | A | C | T | G | C | C | C | G | G | A | C | T | C | G | 13 |
| P. alba | MW376760 | N/A | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | C | C | G | C | T | G | C | C | C | G | A | A | C | T | C | G | 15 |
| P. alba | AP008956 | N/A | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | C | C | A | C | T | G | C | C | C | G | A | A | C | T | C | G | 16 |
| P. nigra | KX377117 | N/A | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | T | C | A | C | T | G | T | C | C | G | A | A | C | T | C | G | 17 |
| P. tremula | MT482535 | N/A | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | C | C | A | C | T | G | C | T | C | G | G | A | C | T | C | G | 18 |
| P. davidiana | MK267317 | N/A | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | C | C | A | C | T | G | C | C | C | G | G | A | C | T | C | G | 19 |
| P. davidiana | MW376770 | N/A | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | C | C | A | C | T | G | C | C | C | G | G | A | C | T | C | G | 19 |
| P. davidiana | KX306825 | N/A | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | C | C | A | C | T | G | C | C | C | G | G | A | C | T | C | G | 20 |
| P. davidiana | MW376771 | N/A | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | C | C | A | C | T | G | C | C | C | G | G | A | C | T | C | G | 21 |
| P. rotundifolia | MK267306 | N/A | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | C | C | A | C | A | G | C | C | C | G | G | A | C | T | C | G | 22 |
| P. rotundifolia | MT482542 | N/A | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | C | C | A | C | A | G | C | C | C | G | G | A | C | T | C | G | 22 |
| P. rotundifolia | KX425853 | N/A | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | C | C | A | C | A | G | C | C | C | G | G | A | C | T | C | G | 22 |
| P. yunnanensis | MW376857 | N/A | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | C | C | A | C | A | G | C | C | C | G | G | A | C | T | C | G | 23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tembrock, L.R.; Zink, F.A.; Zhang, G.; Schuhmann, A.; Gu, C.; Wu, Z. Tracing the Maternal Line in Glacial–Interglacial Migrations of Populus tremuloides: Finding Trees for Future Sustainable Forests by Searching in the Past. Sustainability 2024, 16, 949. https://doi.org/10.3390/su16030949
Tembrock LR, Zink FA, Zhang G, Schuhmann A, Gu C, Wu Z. Tracing the Maternal Line in Glacial–Interglacial Migrations of Populus tremuloides: Finding Trees for Future Sustainable Forests by Searching in the Past. Sustainability. 2024; 16(3):949. https://doi.org/10.3390/su16030949
Chicago/Turabian StyleTembrock, Luke R., Frida A. Zink, Guozhe Zhang, Andrea Schuhmann, Cuihua Gu, and Zhiqiang Wu. 2024. "Tracing the Maternal Line in Glacial–Interglacial Migrations of Populus tremuloides: Finding Trees for Future Sustainable Forests by Searching in the Past" Sustainability 16, no. 3: 949. https://doi.org/10.3390/su16030949
APA StyleTembrock, L. R., Zink, F. A., Zhang, G., Schuhmann, A., Gu, C., & Wu, Z. (2024). Tracing the Maternal Line in Glacial–Interglacial Migrations of Populus tremuloides: Finding Trees for Future Sustainable Forests by Searching in the Past. Sustainability, 16(3), 949. https://doi.org/10.3390/su16030949






