Abstract
Medical devices are instrumental in servicing the healthcare sector and promoting well-being in modern societies. However, their production and use contribute significantly to greenhouse gas emissions, thus causing indirect harm to global health. With a share of approximately 4.4% of global emissions, the healthcare sector exhales CO2 throughout its value chain; sources of this range from direct electricity consumption and water heating in healthcare facilities to the supply chains delivering healthcare services and products. Within this context, the environmental impact of medical devices is present across their production, distribution, usage, and eventual disposal. Each step in the lifecycle of medical devices consumes energy and natural resources, and the end product, after its often single use, is discarded, generating plastic or electronic waste. This study aims to present the key findings from a scoping review of academic research on the topic, which focuses on reducing the environmental impact of medical devices and equipment. The review, conducted according to the PRISMA checklist for scoping reviews, examined 41 studies and categorised them based on the lifecycle stages of medical devices (design and development, manufacturing, usage, and end of life) and the sustainability aspects (economic, environmental, and social) discussed by the authors. The findings suggest that while efforts have been made to enhance economic and environmental sustainability throughout the design, development, and usage of medical devices, there is still room for improvement in mitigating their ecological impact at the end of their lifecycle and maximising their social impact by design.
1. Introduction
1.1. Global Emissions, Net Zero Policies, and the Healthcare Industry
In a landmark agreement signed in Paris in 2016, the world committed to curtail man-made climate change by limiting global temperature increases below 2 °C by the end of the century. To achieve this ambitious goal, Paris signatories set a voluntary emissions-based target mandating economies to reach Net Zero emissions by 2050 [1,2]. However, two factors affect these commitments: the breadth of complexity needed to decarbonise an economy and the limited timeframe. Having achieved exponential economic growth by burning hydrocarbons over the last two centuries, the sudden electrification of our economic systems is nearly impossible—despite its apparent necessity [3]. Finding a consensus in climate change mitigation, countries set on a Net Zero approach, consisting of decreasing emissions from obvious sources (e.g., power generation, agriculture, and real estate) while ‘offsetting’ emissions tied to processes requiring hydrocarbons (e.g., steel production, construction, and air travel). The intent is to create a positive balance of emissions while maintaining the same level of industrial output, effectively postponing the decarbonisation of some industries while technological advancements are being addressed [4,5].
Critics of the action taken thus far argue that it is ‘too little too late’, pointing to the business-as-usual attitude of industries promising Net Zero results without any incremental plan or relying too much on offsetting [6]. Our window of action is relatively short; within 25 years, economies must take extreme measures to reduce their emissions. Considering that the lifecycle of a coal power plant is 60 years, that of a car is 20, and that plastics may last hundreds of years (to name a few examples), it is reasonable to assume that we will fall short [7]. After all, 2023 marks the halfway point towards the first (2030) target of a 50% reduction in carbon dioxide equivalent (CO2e) emissions, a metric encompassing all hydrocarbons and fluoride emissions. The latest Intergovernmental Panel on Climate Change (IPCC) report claims that the current global efforts to meet this goal must be revised. Emissions are forecasted to increase by 10.6% by 2030 compared to 2010 [8]. Analyses projecting emissions after 2030 predict that CO2e outputs will plateau, yet this could lead to postponing the significant reductions necessary in this decade.
As of April 2023, global annual emissions have reached 37.12 billion tonnes (CO2e) with an atmospheric concentration of 418.27 ppm, a veritable tipping point [9,10,11]. Within this figure, the hospital and medical technology (MedTech) sector contributes to 4.4% of global emissions [12]. It is so that the different lifecycle stages of medical devices (MDs), i.e., the design, manufacturing, use, and end of life [13], produce greenhouse gas emissions and are linked to energy consumption, natural resource use, and waste production.
The healthcare sector alone of the Organization for Economic Cooperation and Development (OECD) countries produced 1.6 GtCO2e in 2019 [14]. In aggregate, more than a quarter of greenhouse gas emissions in the medical sector are imputable to the US, followed by China, Europe, Japan, and the Russian Federation [15]. Beyond these cumulative numbers, however, standard emission metrics are not immediately useful when contextualising the sector’s contribution to climate change. For instance, carbon intensity (i.e., a measure of CO2e emissions against economic output) would misleadingly place the healthcare industry as being very inefficient—especially in countries with socialised healthcare, which is as much an issue of accounting in the denominator as it is the carbon use of the industry [16].
To solve this, carbon emissions have been linked to a metric of longevity called Disability-Adjusted Life Years (DALYs)—a statistical measure representing the number of years lost due to morbidity and mortality [17]. Hence, instead of measuring climate change impacts in terms of gross domestic product (GDP) loss, an emitter is benchmarked against a loss of quality of life. The aggregate healthcare industry emissions may cause a loss of up to 3,060,000 DALYs (measured again in a loss in human health annually) [18]. To give a few examples, these can be due to climate-change-led undernutrition, malaria, respiratory diseases, and heat stress [18]. Converted into dollars using a global average GDP per capita, the loss in DALYs represents an economic cost between approximately 30 to 98 billion dollars [18].
1.2. Our Aim
A scoping review on sustainability across the MD lifecycle was performed with the following objectives in mind:
- To systematically identify research studies addressing sustainability in medical design, development, manufacturing, distribution, utilisation, management, maintenance, and decommissioning.
- To map the included studies to the different stages of the medical lifecycle, from design to decommissioning.
- To identify strengths and gaps in the current research on the topic and highlight areas that require further inquiry and possible solutions.
This review aligns with the objectives discussed at the United Nations Climate Change Conference 26, where 50 countries committed to low-carbon health services, and 14 of them further committed to Net-Zero-carbon health services by 2050 [18]. As the definition of sustainability is broad, this project was carried out by an interdisciplinary team of experts, including biomedical engineers, bioethicists, and sustainability experts. The article also aims to refer to the ethical facets of the issue of sustainability, in particular the MDs, which appeal not only to designers but also to governments in regulations aware of the values at stake.
1.3. Sustainability: A Catchall Term
Sustainability is a fuzzy concept that has evolved with time as its definition expanded and has different meanings based on context and uses [19]. It was first coined in the 17th century by Hans Carl Von Carlowitz and applied to forestry [20]. Later, one of the most recognised definitions is the one attributed to the 1987 Brundtland Commission (i.e., the United Nations Commission on Environment and Development) Report that defines sustainable development as a “development that meets the needs of the present without compromising the ability of future generations to meet their own needs” [21], which reminds individuals of Hans Jonas’ imperative of responsibility based on the precautionary principle [22]. Overall, sustainability is often seen as the intersection of three dimensions, namely environmental, social, and economic (hierarchically from the most to the least important), i.e., the so-called “triple bottom line” [23]. This paper has narrowed the concept of sustainability to include issues material to the lifecycle of medical devices (MDs) and the climate crisis, specifically decarbonisation, i.e., the ability to adapt to climate change impacts by reducing emissions and creating a Net Zero economy. Net Zero means balancing the amount of greenhouse gases emitted into the atmosphere and the amount removed (or offset for those inherently unsustainable manufacturing processes, e.g., steel and plastic production) to mitigate the impact of climate change [24].
2. Materials and Methods
2.1. Search Strategy and Selection Criteria
The scoping review, conducted following the PRISMA statement for scoping reviews, was relative to the following population (P), concept (C), and context (C) model [25,26]:
- P—MDs and equipment.
- C—sustainability (economic, social, and environmental) through the lifecycle of MDs.
- C—globally.
A search string was developed and iteratively refined by using keywords and logical operators. The final version of the search string was:
TITLE-ABS-KEY ((“medical device” OR “medical equipment”) AND (“sustainab*” OR “circular economy” OR “recycl*” OR “refurb*” OR “recondition*” OR (“reduce” AND “waste”) OR “ecologic*” OR “reuse” OR ”recover” OR “renewab*” OR “environment-friendly” OR “environmentally-safe” OR “environmentally sound” OR “ozone friendly” OR “environmental impact” OR “economically sound” OR “natural resourc*”) AND (lifecycle OR design OR development OR prototyping OR manufactur* OR use OR management OR maintenance OR decommission* OR obsolescence OR “end of life” OR “disposal” OR “packaging”).
This search string was run on two databases, namely Scopus and Web of Science Core Collection. The inclusion and exclusion criteria are presented in Table 1. Only Final or In Press articles published in the English language between 2013 and 2022 (included) were included. Other document types were excluded (e.g., Conference Paper, Review, Conference Review, Book Chapter, Book, Editorial, Letter, Note). Articles addressing medical technologies achieving their primary intended purposes through chemical or biological action, such as drugs and vaccines, were excluded too. The papers were screened by title, abstract, and full text by two authors. A third author reviewed the screening results and solved possible disagreements.

Table 1.
Inclusion and exclusion criteria.
2.2. Data Extraction
Relevant data were extracted by five authors and collected in an ad hoc Excel sheet (see the Supplementary Materials). The extracted variables of interest included the related medical technology (if any), the lifecycle stage, the proposed innovation, the impact metrics (if any), the impact type, and the associated SDGs. In particular, the lifecycle stages were divided as presented by Sousa et al. [13]:
- Design and development. This stage includes the product design and development methodologies (e.g., ecodesign), material/product design and development, feasibility assessment, and prototyping.
- Manufacture. This stage includes sourcing raw materials, supplier selection, production, and distribution.
- Use. This stage includes operation, reuse, and reprocessing.
- End of life. This stage includes decommissioning, disposal, and recycling.
2.3. Data Synthesis
The narrative synthesis method was applied to synthesise the extracted data [27,28]. This allowed us to organise the results following the four stages mentioned above. Each subsection analyses and compares similarities and contrasts found in our literature review. This unlocks further considerations presented and contextualised in the broader literature in the discussions section.
3. Results
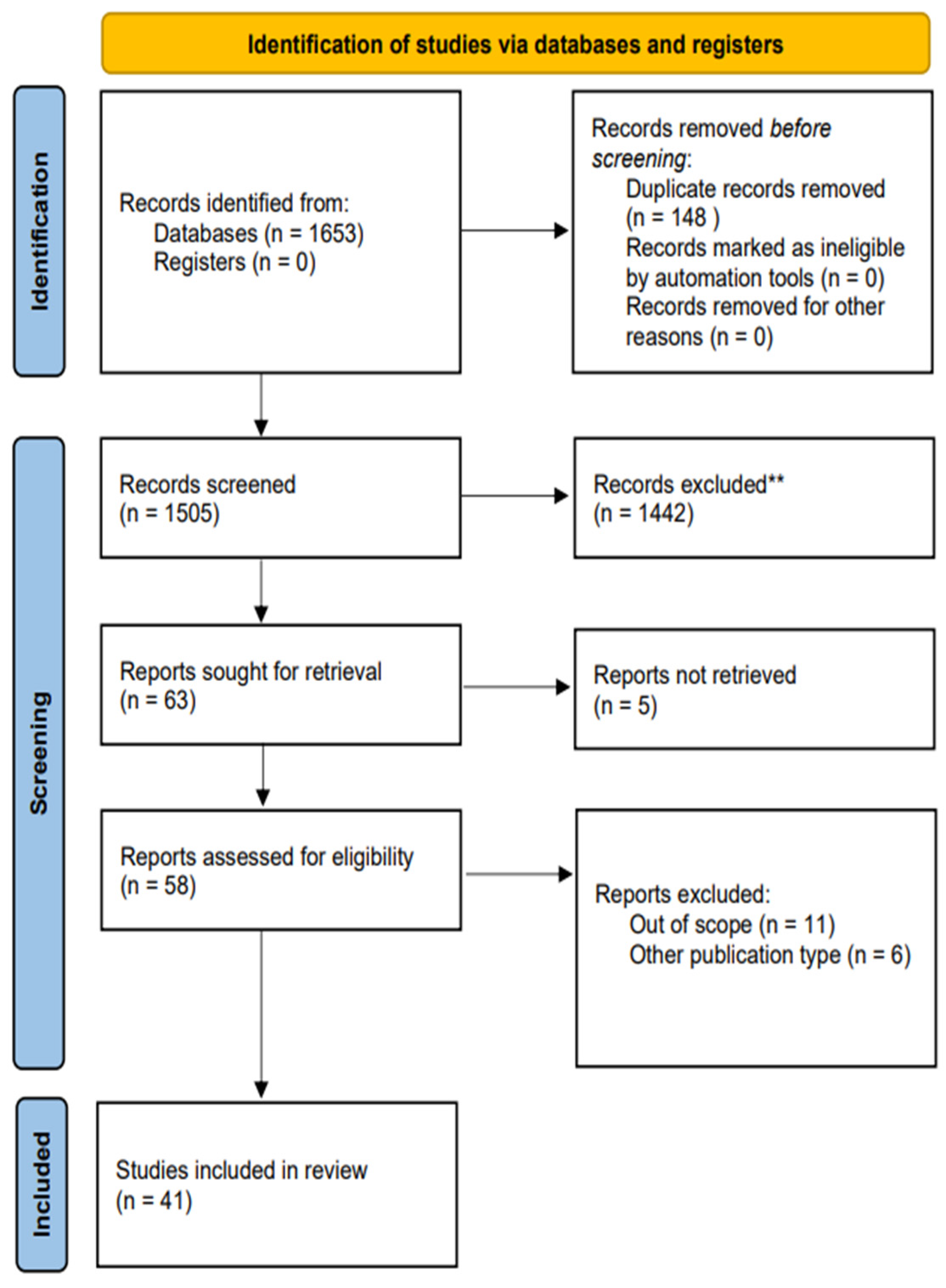
The initial search conducted yielded 1653 potential sources. After excluding duplicates and screening, 63 records met the criteria regarding the title and abstract, proceeding to a full-text examination. Five articles could not be retrieved and, consequently, were not selected for further review. Once full-text revision was finalised, 41 articles satisfied the requirements imposed and comprised the analysis. A graphical representation of the selection process is illustrated in Figure 1.
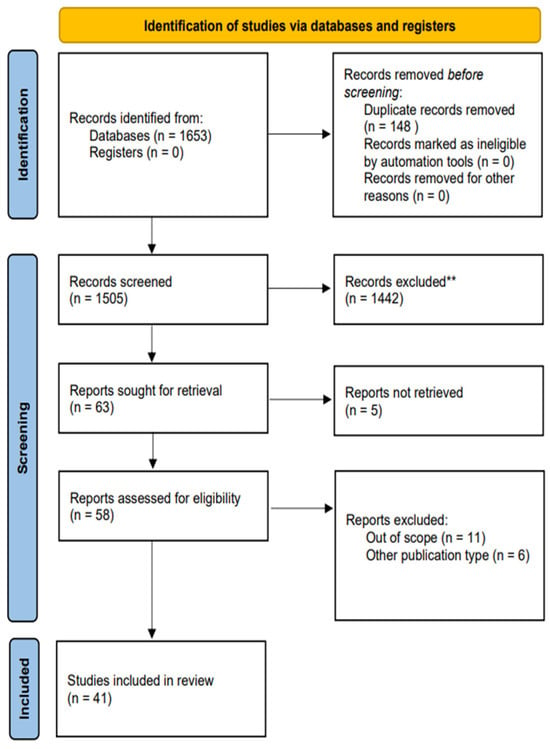
Figure 1.
PRISMA flow diagram. Study selection process used divided into 3 phases: identification, screening, and inclusion. ** based on our inclusion and exclusion criteria.
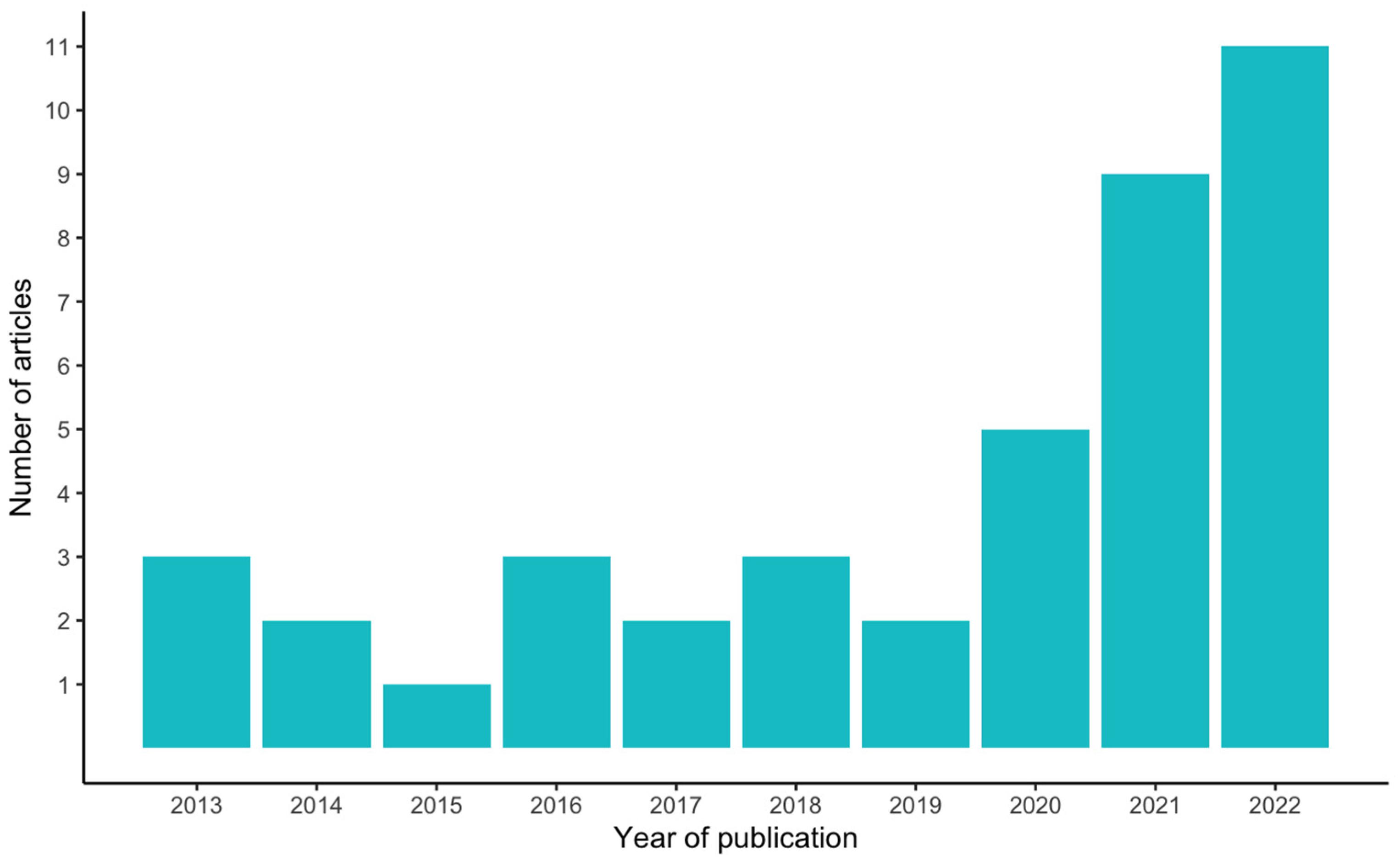
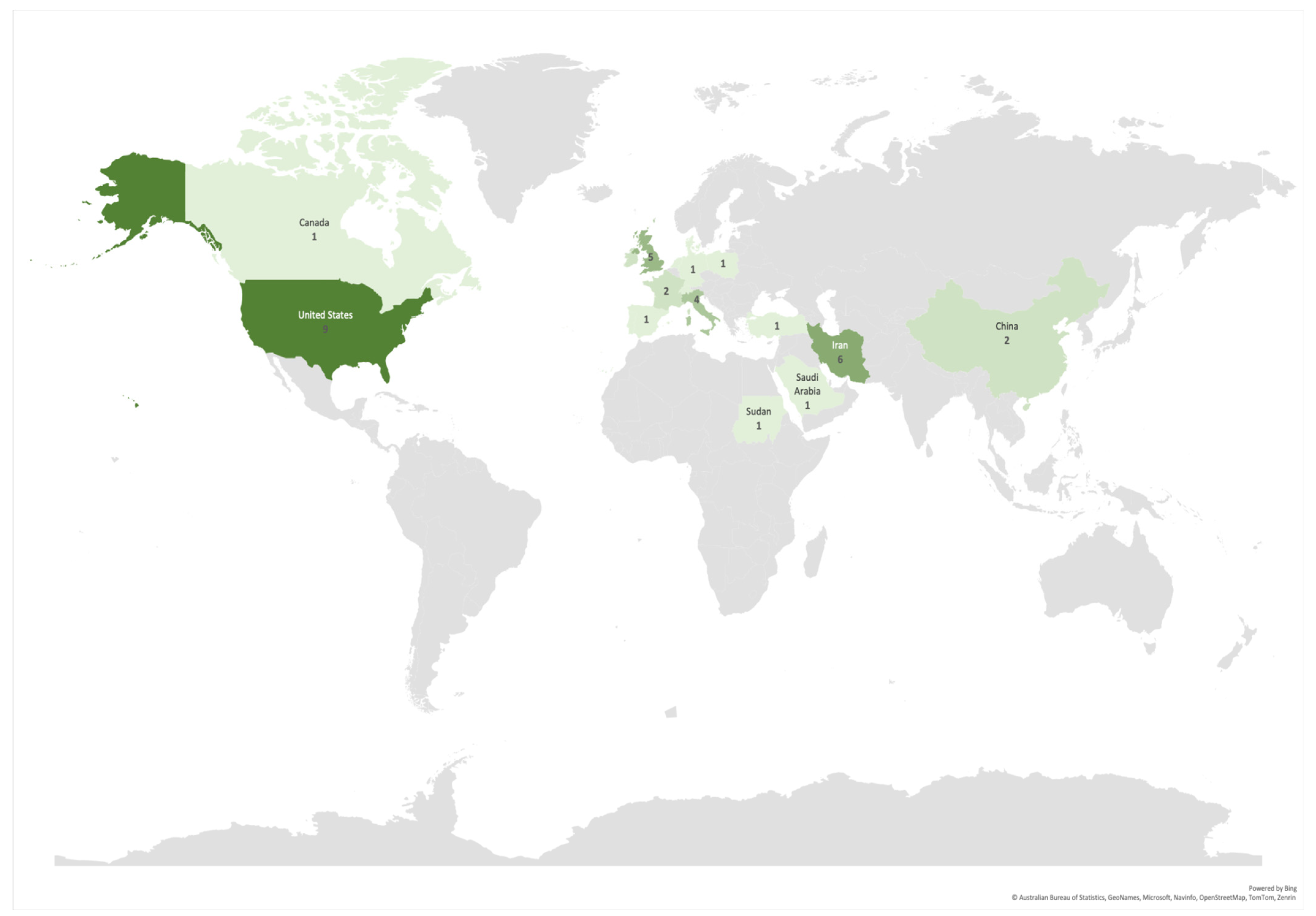
More than 60% of the articles (25) included in this study were published in the last three years (2020–2022), an acceleration in these studies compared to the seven years prior (2013–2019), during which less than 40% of the articles (16) were published (Figure 2). The authors of the selected articles were based in 18 different countries, yet almost 50% of the articles (20) were written by authors hailing from just three countries, namely the USA, Iran, and the UK (nine, six, and five articles, respectively) (Figure 3).
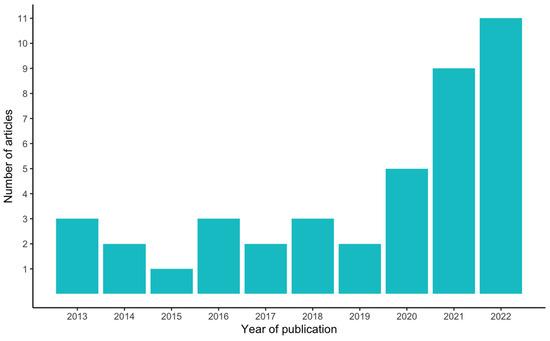
Figure 2.
Number of articles per year of publication.
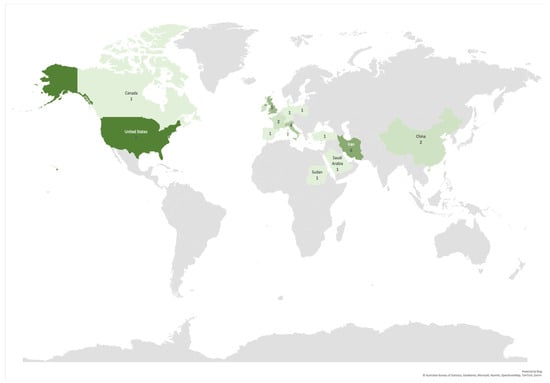
Figure 3.
Number of articles per corresponding author’s countries. Darkest color denotes a greater number of contributions. Countries with included articles in descending order were the USA (9); Iran (6); UK (5); Italy (4); China, France, and Ireland (2 each); and Canada, Denmark, Germany, Netherlands, Poland, Portugal, Saudi Arabia, Spain, Sudan, Switzerland, and Turkey (1 each).
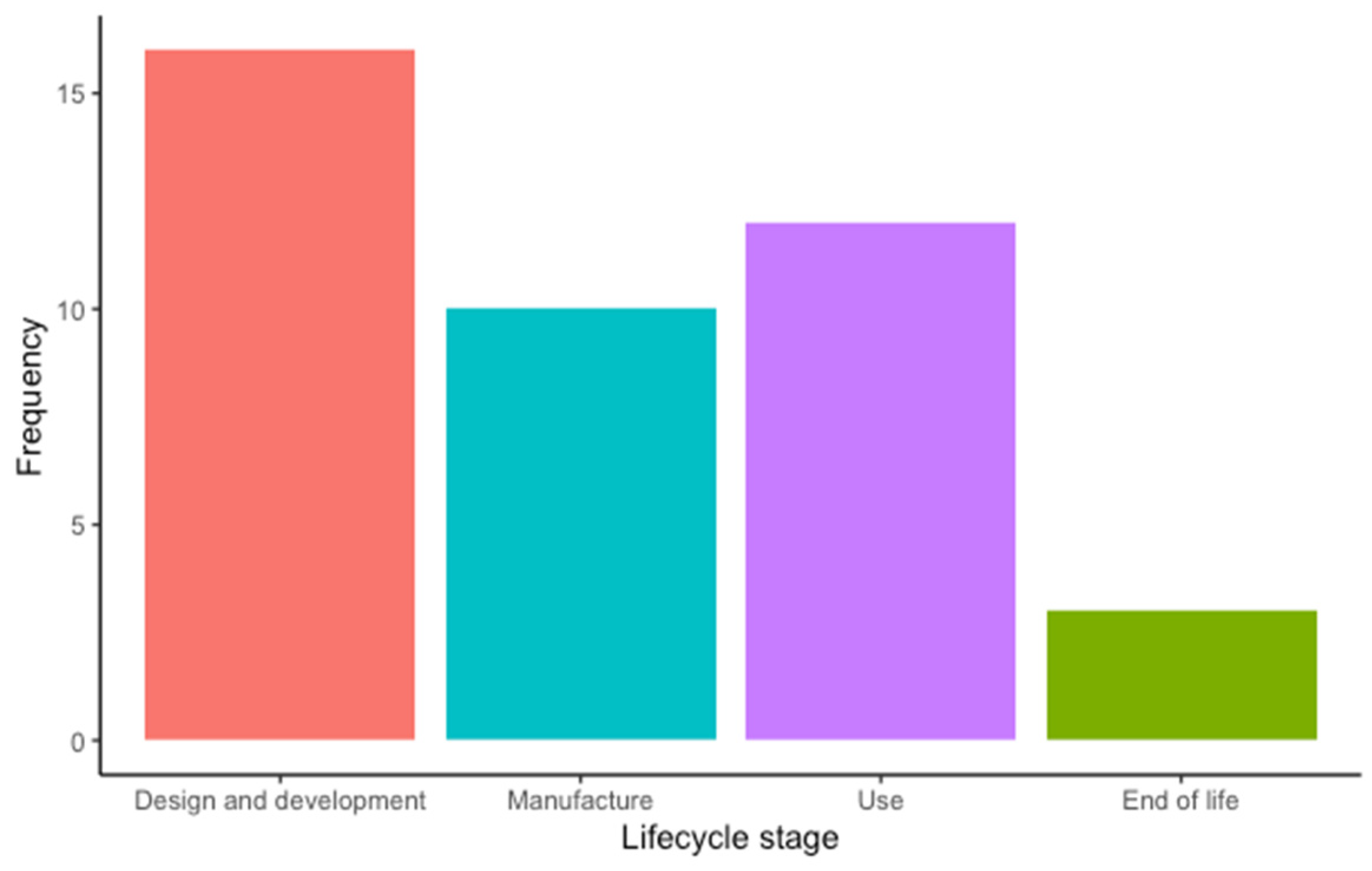
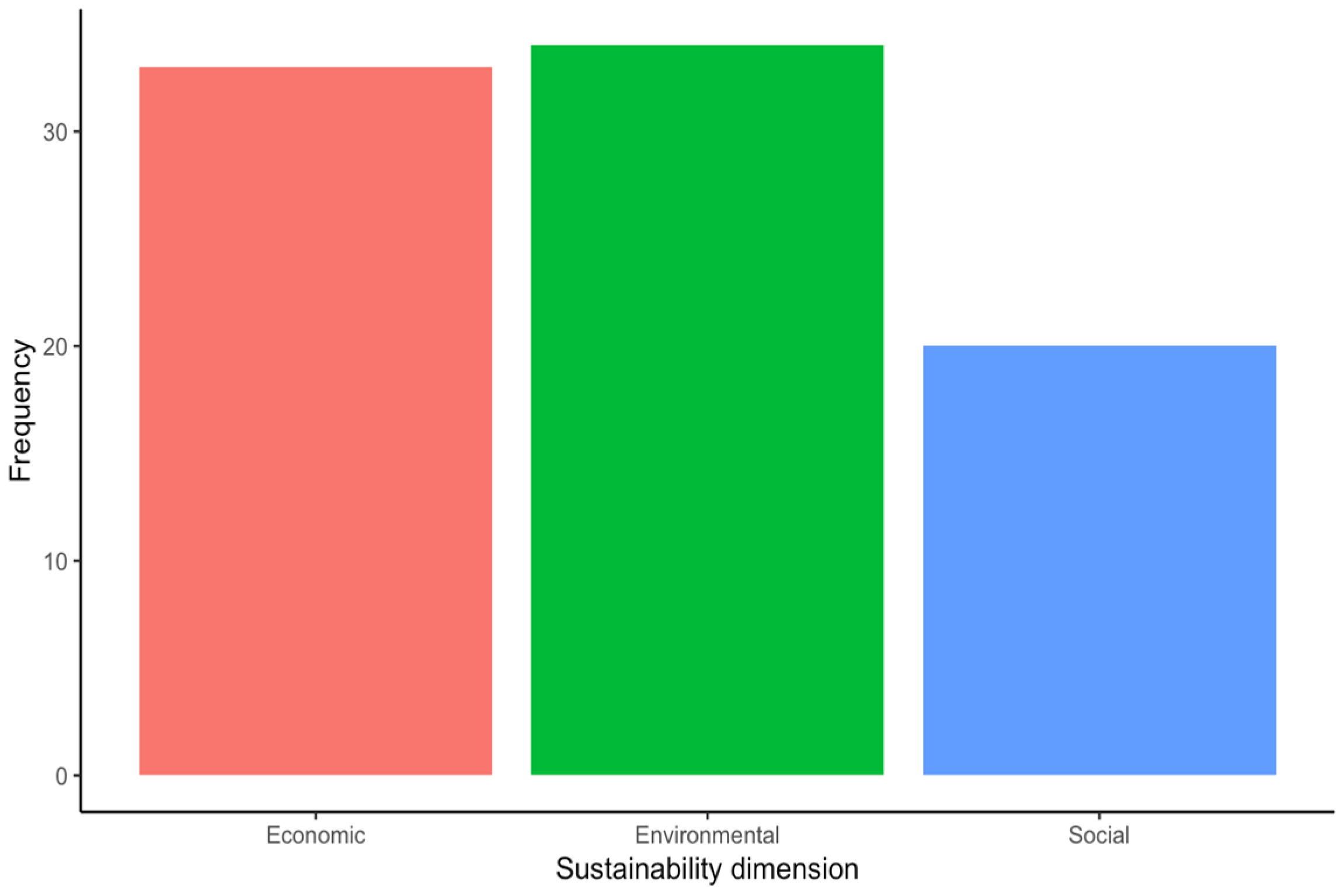
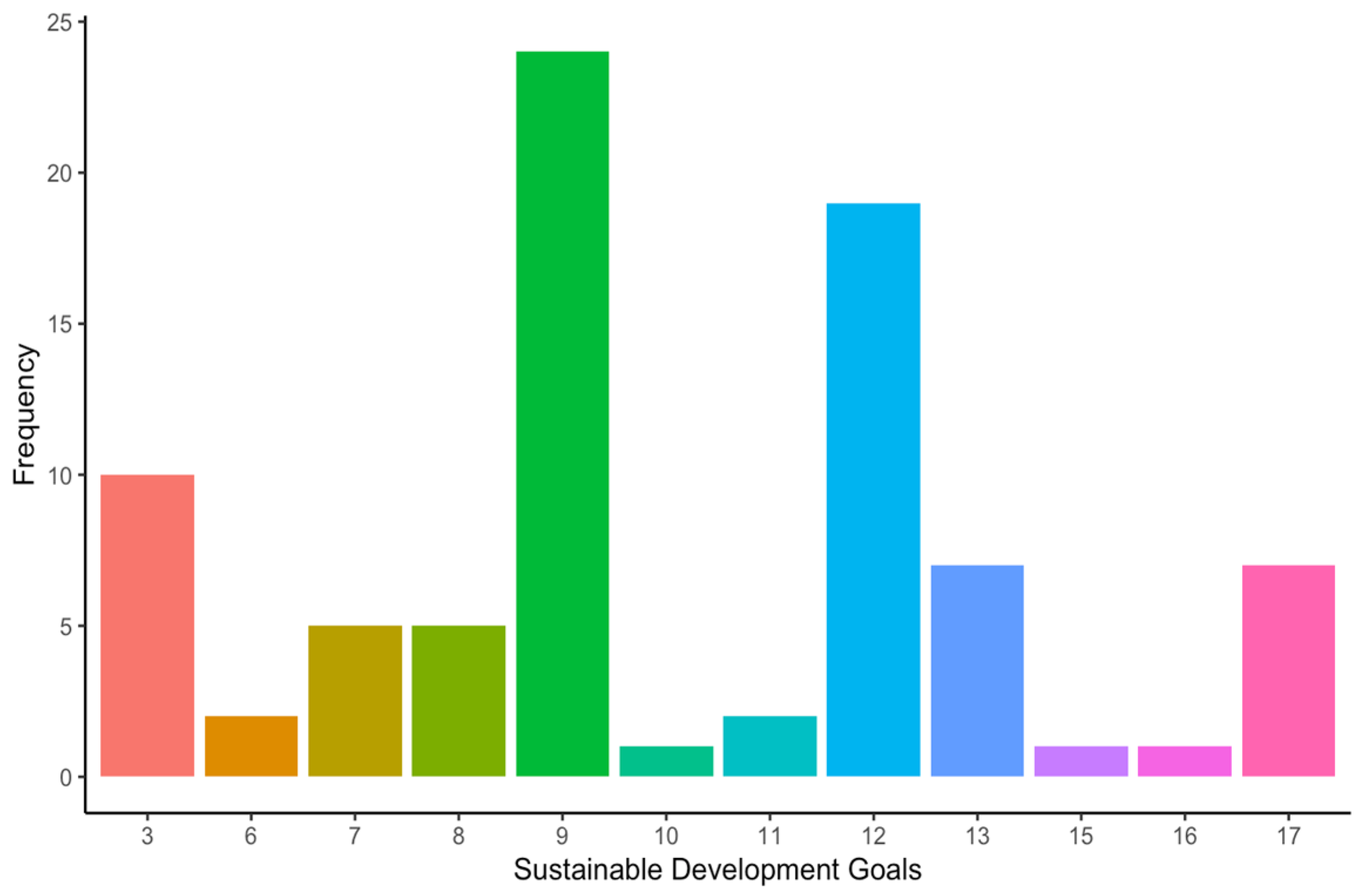
Most of the included articles focused on the “Design and development” stage (16 articles, 39%), followed by the “Use” (12 articles, 29.3%), “Manufacture” (10 articles, 24.4%), and the “End-of-life” stages (3 articles, 7.3%) (Figure 4). Among the included studies, the environment was the sustainability dimension most addressed by the authors (39.1% of the occurrences), closely followed by the economic (37.9% of the occurrences) and the social dimension (23% of the occurrences) (Figure 5). Regarding the SDGs, SDG#9: industry, innovation, and infrastructure; SDG#12: responsible consumption and production; and SDG#3: good health and well-being were the three most addressed by the studies (28.6%, 22.6%, and 11.9% of the occurrences, respectively) (Figure 6). A summary of the extracted information is presented in Table 2 (please refer to the Supplementary Materials for the full version). A narrative synthesis of the results organised by lifecycle stage is provided below.
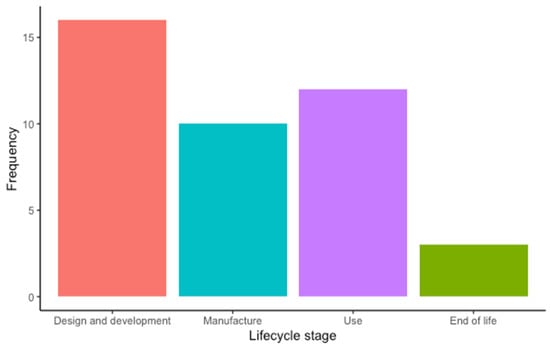
Figure 4.
Frequency of lifecycle stage addressed by the included studies: design and development (16), manufacture (12), use (10), and end of life (3).
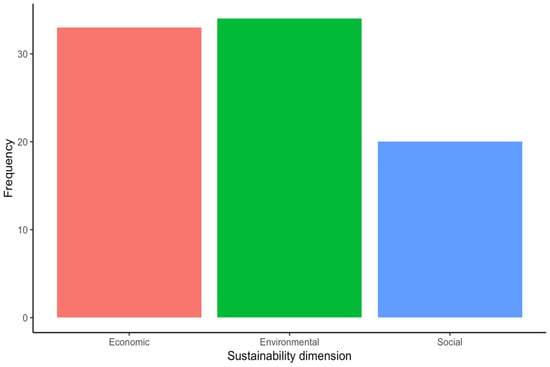
Figure 5.
Frequency of sustainability dimension addressed by the included studies.
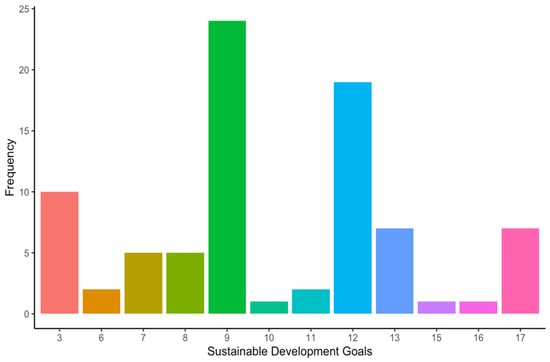
Figure 6.
Frequency of sustainable development goals addressed by the included studies. SDG#3: good health and well-being; SDG#6: clean water and sanitation; SDG#7: affordable and clean energy; SDG#8: decent work and economic growth; SDG#9: industry, innovation, and infrastructure; SDG#10: reduced inequalities; SDG#11: sustainable cities and communities; SDG#12: responsible consumption and production; SDG#13: climate action; SDG#15: life on land; SDG#16: peace, justice, and strong institutions; and SDG#17: partnerships for the goals.

Table 2.
Summary table. This table presents a summary of the extraction table (see the Supplementary Materials for a full version).
3.1. Design and Development
Among the studies included, the authors employed two overarching strategies to promote sustainability in the design and development of MDs. Firstly, they adopted a more comprehensive methodological framework encompassing various practices and techniques to integrate sustainability considerations into product design and development. Secondly, they adopted a more targeted approach that centred around specific features of the device, such as energy consumption and the materials used, with sustainability as a critical consideration. A summary of the studies included in the design and development stage, categorised by approach, is presented below.
3.1.1. Ecodesign, Sustainable Design, and Frugal Engineering
Among the studies included in this research, two proposed ecodesign practices to integrate environmental considerations into the design and development process. The objective was to minimise the ecological impact of MDs throughout their lifecycle. The first study, conducted by Barbero et al., employed an interdisciplinary systemic approach to design a haemodialysis machine [38]. The primary aim was to improve the haemodialysis treatment’s environmental sustainability while meeting the needs of technicians, healthcare staff, and patients. The authors then tested, revised, and adapted their methodology to apply it to other types of MDs. The second study, conducted by Moultrie et al., developed a series of ecodesign maturity grids to support the environmentally conscious design of MDs [36]. Each grid contains a list of ecodesign issues relevant to a specific product lifecycle stage. The problems include raw material sourcing, manufacture and assembly, packaging and distribution, product use, and end-of-life considerations.
Additionally, the grids describe five levels of maturity or achievement for each issue. For example, the maturity grid for product packaging and distribution guides designers and product marketers in assessing the maturity level of five related topics: the space efficiency of packaging; structure of packaging; recycled, reused, or remanufactured content of packaging; recyclability, reusability, or manufacturability of packaging; and polyvinyl chloride content of the packaging. These grids were developed based on semistructured interviews with eight key opinion leaders in healthcare design and were subsequently validated based on interviews with five MD companies. Overall, the grids provide valuable insights to designers and product marketers on improving the design of MDs at different lifecycle stages.
Expanding upon ecodesign, Hede et al. have put forth a comprehensive and multifaceted conceptual framework that delves into a broader scope of sustainable design [30]. This framework aims to integrate the three dimensions of sustainability (namely, economic, environmental, and social) into the design and development of MDs. The proposed framework includes the Multicriteria Hierarchical Model, an extensive revision of the Analytical Hierarchy Process, and comprises a subcategory of the Multicriteria Decision Analysis methodology. The primary objective of this conceptual framework is to provide a thorough evaluation and a workable roadmap for the development of sustainable MDs. The proposed multifaceted framework was created to optimise the design while considering technical parameters and the stakeholders’ perspectives, such as the creation of employment opportunities and investment in community welfare. Four European companies involved in developing class I and II MD validated the proposed framework.
In other studies, a frugal innovation or frugal engineering perspective was adopted in the design of MDs by various researchers [29,34,56,68]. Frugal engineering emphasises cost reduction, concentration on core functionalities, and optimised performance levels to develop simple and affordable solutions to address the needs of underserved populations, often in resource-constrained settings, without compromising functionality or quality [70]. Thus, frugal engineering primarily addresses sustainability’s economic and social dimensions, although some works also consider environmental issues. To innovate global health technologies, Gupta et al. designed the Embrace Nest, a family of MDs that aims to address neonatal hypothermia, a condition affecting primarily newborns with low birth weight, in resource-limited settings [34]. Although the authors followed the traditional principles and stages of design theory (i.e., empathising, defining, ideating, prototyping, and testing), their frugal perspective allowed them to manufacture and commercialise those devices in India, Uganda, Afghanistan, Zambia, and Haiti, benefiting an estimated number of 87,000 newborns with low birth weight.
Similarly, Hamner et al. describe the development of ReMotion Knee, an affordable polycentric prosthetic knee joint designed for low-income countries whose performance competes with other devices commercialised in more industrialised regions [29]. This device considered criteria highly relevant in low-resource settings, such as affordability (with a projected final cost below USD 80) and durability (3–5 years of use in rugged terrains and wet environments). As of September 2012, the initial version of the ReMotion Knee had benefited over 4200 amputees. Additionally, Piaggio et al. present the initial results of their study, which aims to design, prototype, and validate a smartphone-based pupillometer following the European MD Regulation and relevant standards [56]. The design of this MD was performed by considering the constraints often found in low-resource contexts, including the need for specialised clinicians, funds, spare parts and consumables, poor maintenance, and harsh environmental conditions. Lastly, Williams et al. present the redesign of the inlet filter of an oxygen concentrator, which is often out of use and difficult to replace due to the limited supply chain in low-resource settings [68]. The redesign was based on a reverse engineering approach and the use of 3D printing and activated charcoal as a filtering agent. Ultimately, the authors aimed to empower and encourage local communities to produce simple spare parts for MDs leveraging 3D printing, prototyping, and locally produced activated charcoal.
3.1.2. Energy Harvesting and Efficiency Management
Several implantable MDs, such as cardiac pacemakers and defibrillators, operate on batteries. However, the limited lifespan of conventional batteries results in additional surgeries to replace the battery and the device itself as they are airtight for safety reasons. This causes inconvenience for patients and generates unnecessary electronic waste that could otherwise be avoided as the device may still function correctly. To address this issue, several studies propose the concept of body energy harvesting, which could enable a new generation of self-powered implantable MDs [31,46,49,53]. For instance, MacVittie et al. developed enzyme-based biofuel cells to power electronic devices and tested their concept in two ways. Initially, they implanted biofuel cells into two lobsters [31]. They connected them in series, generating an open circuit voltage (Voc) of up to 1.2 V, sufficient to power a digital watch. Later, they created a fluidic system that consisted of five cells filled with a human serum solution and connected them in series, generating a Voc of 3 V, which was used to power a pacemaker. Therefore, they demonstrated the feasibility of future electronic implantable medical devices powered by electricity harvested from the human body.
Furthermore, Xu et al. presented a novel cardiac energy harvester composed of composite microfilms utilising piezoelectric materials [49]. These microfilms were ingeniously fashioned to create a multibeam cylindrical structure positioned on a pacemaker lead, which effectively harnesses energy from the motion of the lead propelled by the heartbeat. The feasibility of their prototype was evaluated in three various testing environments, namely air, phosphate-buffered saline, and simulated blood, resulting in electrical output of 0.78 ± 0.05, 0.73 ± 0.03, and 0.71 ± 0.03 V, respectively. Subsequently, the rectified electrical output obtained from the multibeam structure was utilised to charge two commercial capacitors with a capacitance of 47 μF and 120 μF, respectively. Xu et al. [49] reported that the 47 μF capacitor attained 0.34 V in 14.1 s, and the 120 μF capacitor reached 0.33 V in 34.6 s.
Similarly, Hanani et al. devised a bioflexible piezoelectric nanogenerator by using lead-free biocompatible nanoparticles [53]. The researchers reported that this nanogenerator yielded an open-circuit voltage of 14.4 V and a short-circuit current of 0.55 μA when subjected to gentle finger tapping. Furthermore, they demonstrated the nanogenerator’s efficacy by activating electronic components commercially available, such as charging a 1 μF capacitor to store 3.92 μJ of energy within 115 s under gentle finger tapping and illuminating an LED.
Differently, Magno et al. proposed a novel approach to extend the lifetime of wearable devices by combining solar energy harvesting with low-power design and energy-efficient processing [44]. Specifically, the authors developed a self-powered wearable pulse oximeter assembled in a 3D ring-like geometry, leveraging solar energy harvesting, efficient power management, and ultralow power processing in a multicore microcontroller. Their experimental results on the designed and developed prototype demonstrated that the accurate measurement of blood oxygenation could be achieved once every minute at a daily energy consumption of 28 J, which includes hourly Bluetooth transmissions. The authors attributed the system’s self-sustainability to the low-power design, which required only 64 min of sunlight or 12 h of indoor home light daily.
3.1.3. Material Selection and Reduction
One critical decision that can significantly impact sustainability during the design and development process of MDs is selecting materials and reducing waste. Several studies have addressed this issue, focusing on using biopolymers as an alternative to petroleum-based plastics. For example, Unger et al. conducted a comparative lifecycle assessment on single-use disposable medical products used in hysterectomies, comparing those made with biopolymers to those made with conventional plastic [39]. The study found that while biopolymers can reduce the environmental impact of MDs in terms of carcinogenic and respiratory effects, the significant agricultural inputs required for their manufacture can exacerbate ecological impacts. Thus, carefully considering material selection is crucial for achieving MD design and development sustainability goals.
On the other hand, Mann et al. (2018) conducted a case study that examined the heightened functionality and decreased disposability of a commercial external-fracture-fixation apparatus, along with the impact of these product features on its commercial acceptance [42]. Specifically, the authors systematically compiled and analysed the available literature on the product, such as sales brochures, medical reports, white papers, patents filed, user manuals, web tutorial videos, and spare parts catalogues, to discern the product design decisions that influenced its functionality and sustainability. The researchers determined that using stainless steel, aluminium, and carbon fibre as materials enhanced the durability and increased reusability by facilitating sterilisation, thereby lessening the device’s environmental impact. Furthermore, the ease of obtaining raw materials locally due to reduced transportation also contributed to reducing this impact according to the authors.
Riutord-Sbert et al. proposed a potential remedy for the shortage of Personal Protective Equipment (PPE) amidst the COVID-19 pandemic, resulting in high costs and a depletion of global mask stocks [48]. The authors suggested the conversion of conventional surgical masks into PPE masks by affixing a face seal made of polylactic acid (corn starch) thermoplastic resin. The basis for selecting polylactic acid was its ability to withstand disinfection with 0.1% sodium hypochlorite, which allows for its reuse and biodegradability. The overarching objective of this proposition was to ensure the clinical efficacy of healthcare professionals and organisations cost-efficiently while enhancing clinical safety.
Finally, Alrashoudi et al. developed a lateral flow immunoassay to facilitate rapid COVID-19 testing while considering the environmental impact of the disposable medical device (MD) [50]. Specifically, the authors utilised a material extrusion-based bioprinting setup with the aid of a robotic arm to construct the testing strips. Moreover, 3D printing technology was used to create a strip housing unit. Employing finite element analysis, the authors simulated the physical strains on the designed housing unit, i.e., the cassette, to determine its minimal thickness while ensuring usability and durability when conducting the test. The authors posit that reducing the amount of material required to fabricate the cassette minimises the amount of wasted and discarded material from each cassette.
3.2. Manufacture
Among the included studies, the authors followed two approaches to foster sustainability in MD manufacturing. First, one study focused on the production of the MD. Also, some other studies focused on the supply chain associated with the production of some MDs. An overview of the included studies in the manufacture stage grouped by approach is provided below.
3.2.1. Production
Cosgrove et al. presented a tool to achieve energy savings in the production processes [40]. Namely, the authors propose the application of a normalised coefficient to view production and energy data and the development of a rolling energy performance coefficient to provide alerts to ‘out-of-control’ production operations. This proposal is motivated by evidence showing that, whilst companies have focused on reducing energy at a facilities level, specific production processes generate significant environmental impact through energy consumption and greenhouse gas emissions. Finally, the authors provide an example of implementing this approach in a large MD manufacturing facility, which led to significant energy savings.
3.2.2. Supply Chain
Studies addressing the supply chain associated with the manufacture of MDs focus on its management or configuration (three and two articles, respectively).
Within supply chain management, Abad et al. proposed a hybrid chance-constrained programming and cost function for a medical ventilator supply chain by defining several parameters for each impact type, highlighting cost, customer demand, and CO2 released into the atmosphere [69]. This innovation has resulted in substantial improvements to social responsibility (SR), as it was demonstrated that at specific points throughout the demand escalation, an enhancement in “several parameters of the SR index, such as fixed and variable job opportunities” occurred. Nonetheless, the authors acknowledged that applying the hybrid-chance constrained programming and cost function produces more significant cost variability when stricter environmental policies are implemented, thus creating possible financial insecurity within the company.
Moreover, Izadikhah and Farzipoor Saen proposed a new data envelopment analysis (DEA) model for assessing sustainability in economic, social, and environmental criteria [35]. This model includes two stages: the first stage (supplier) feedback is the input for the second one (manufacturer). DEA analyses decision-making units (DMUs), treated as independent black boxes, whose main feature is their inputs and outputs being considered harmful. Throughout the conduction of the case study in 29 Iranian supply chains dedicated to the production of disposable MDs, it was concluded that the importance and relevance of the manufacturing stage (76%) played a more significant role than the supplier (24%), with only 7 out of the 29 companies being overall efficient in both sections. Unfortunately, the authors did not propose any specific solution to the 29 companies examined, as their purpose was to assess their current efficiency and establish comparisons between both stages: the supplier and manufacturer.
Finally, Fargnoli et al. (2022) suggested implementing a product–service system (PSS) approach in the medical equipment sector based on its functional matrix, Screening Lifecycle Modelling (SLCM), and stock management theory [61]. Its reliability was proved through a case study by displaying improvements in some areas. Firstly, the equipment’s lifecycle was increased from four to five years while maintaining its correct functionalities. This improvement was possible due to an optimisation of the spare parts from production, reusing them in the maintenance department. Moreover, it offered a better understanding of estimating quantity orders, reducing waste and saving financial resources. Therefore, it can be gathered that there were economic and environmental benefits thanks to applying this approach.
On the other hand, within supply chain configuration, Hasani et al. presented a multiobjective optimisation model to design a green and resilient global supply chain network (GSCN) [54]. This model considers competitiveness, resiliency, and environmental issues. Namely, the model aims to maximise the expected profit (economic target) while minimising CO2 emissions produced via material shipment within the GSCN (environmental target) under disruption scenarios (for instance, the one caused by the COVID-19 pandemic). Moreover, the authors present a case study of applying the proposed model to a newcomer global MD manufacturing company (whose name they do not disclose for confidentiality reasons). Overall, profitability was reduced under disruption scenarios. However, the countermeasures proposed by the authors positively impacted other indicators. For instance, by decentralising factories, any disruption in one factory does not imply the stoppage of manufacturing other parts or products, maintaining the flow within the system, an indicator of resiliency. Furthermore, the geographical relocation of material suppliers to closer locations helps reduce the environmental impact of the supply chain. Finally, the authors proposed planning an uncertainty budget to aid in managing and planning more extreme measures when facing disruptions from the company or global recessions.
Similarly, Nayeri et al. assessed the sustainability, resiliency, and responsiveness of the supply chain network (SCN) associated with blood bank refrigerators during the global disruption generated by the COVID-19 pandemic [65]. Namely, they proposed a multiobjective programming model (MOPM) to minimise the environmental impacts and the total cost and maximise the social impacts, resiliency, and responsiveness of the SCN while considering the global SC factors. Then, to cope with the uncertainty in the SCN design problem, the Modified Fuzzy Robust Stochastic (MFRS) optimisation method was developed. The authors’ findings suggest that an increase in the responsiveness level of the supply chain can lead to increasing sustainability dimensions, including job opportunities, safety, carbon emission, and economic aspects. Moreover, increased demands harm the economic, environmental, and responsiveness targets.
3.2.3. Supplier Selection
Ghadimi et al. showed, by implementing a multiagent system (MAS), that the financial performance of manufacturing companies adopting environmental and social sustainability in their operations strategy enhances their competitive advantage, which can lead to long-term sourcing relationships for the buyer–supplier dyad [41]. Better communication was emphasised in the literature as a critical factor in establishing a long-term supply chain partnership among suppliers and buyers. In a literature review conducted by Jain et al., it was highlighted that integrating supply chain functions regarding buyer–supplier relationships could be possible by using advanced communication technology that allows for the real-time flow of information among the participating members [71]. In the case study by Ghadimi et al., three suppliers were assessed for their social, environmental, and financial sustainability. Supplier #3 recorded the best overall sustainability, whilst the other two lacked efficiency in social and financial aspects, respectively. The approach was generally successful as it can be implemented in real-life situations, aiding the relationships between manufacturers and suppliers. Expressly, ameliorations in cost efficiency, time consumption, and information accuracy can be guaranteed via an optimal MAS.
In the research conducted by Ghadimi et al., the MAS approach was implemented directly into 4.0 SCs, where different features were studied, such as distribution, autonomy, mobility, intelligence, and self-learning [43]. These characteristics were used as measurements of the issues regarding communicability and information automation. Also, Ghadimi et al. proposed using a fuzzy inference system, which is effective due to the ambiguity regarding the sustainability topic, as well as reducing human interaction along the process, minimising misinterpretations. By applying this methodology, manufacturers mark the path regarding sustainability principles, and suppliers would have to keep evolving this fuzzy technique to meet those imposed requirements. Moreover, a new concept, viable SC (VSC), has surfaced recently. SCs need to consider other factors (leagile, sustainability, and digitalisation) for the system to meet the current optimal specifications. VSCs must comply with these new attributes as they should be adaptive and time-responsive, demonstrating resiliency and polyvalency [72].
Finally, Rostami et al. recently applied this to a Supplier Selection Problem (SSP) of an oxygen concentrator device [66]. Their paper determined that the aspects above are essential and permit the analysis of economic, social, and environmental sustainability indicators, validating the procedure.
3.2.4. Distribution
Szmelter-Jarosz et al. expose an uncertain closed-loop SC, where a fuzzy approach is considered to deal with the uncertainty caused by the COVID-19 pandemic [57]. Distribution comprises the location of potential facilities for the delivery and return of goods. The objective is to allow strategic and tactical conclusions concomitantly, the first one referring to the location of the different facilities needed throughout the SC, such as production centres or recycling points, while the tactical section alludes to the medical equipment transported and its flow between these premises. This case study was developed during the outbreak of the COVID-19 pandemic, so predefined timetables, as well as the minimal use of time and resources, were primordial for this approach to be successful. Ultimately, it is demonstrated that a more optimal SC, in all three terms of sustainability, can be achieved by implementing this fuzzy technique.
3.3. Use
Among the included studies, the authors followed three similar approaches to foster sustainability in the MD use stage. The first approach focuses on the assessment through tools that describe the impact of products in the various stages of the product lifecycle. Unger and Landis evaluated the disparities in the environmental impacts of disposable and reusable dental burs through a comparative lifecycle assessment (LCA) [33]. According to this research, reusable burs had 40% less environmental impact than disposable burs when the ultrasonic and autoclave were loaded optimally. Conversely, when the autoclave and ultrasonic were loaded to approximately one-third capacity, reusable dental burs posed more negative environmental impacts in eight of nine ecological impact categories when compared to disposable burs. Again, S. Unger and Landis proposed a model based on MD supply chains’ environmental, human health, and economic impacts when varying levels of reprocessed devices are used by using LCA and a lifecycle cost assessment (LCCA), and the results showed that the impact on global warming was marginally lower in reprocessing scenarios when compared to scenarios that employed no reprocessing, and the human health impact results marginally favoured that of no reprocessing when compared to reprocessing scenarios [37].
Van Straten et al. used the LCA tool to estimate the performance and impact of the proposed solutions in each stage of this tool [58]. Face masks showed approximately the same results after sterilising and applying a reusing process up to five times. Epelle et al. assessed the impact of ultraviolet-C (UVC) and gaseous ozone on decontaminating different materials from different agents [60]. The combination of both systems overcomes the single-system limits. The efficacy of this combined method depends on the type of material. This novel method could be a sustainable alternative for MD disinfection instead of carcinogenic ethylene oxide. Likewise, Rouvière et al. assessed the ecological and economic impacts of sustainable actions targeting MDs [67]. Integrating sustainable measures in operating rooms leads to environmental benefits and generates savings.
The second approach focuses on harnessing resources to reduce the negative impact on the environment. Haber and Fargnoli performed this by proposing solutions tailored to the consumer’s demands and adapting existing solutions to suit the needs of customers better [52]. This approach enables manufacturers to improve their environmental performance by optimising their product’s lifecycle and fostering remanufacturing, reusing, and recycling activities. Bayrak and Soylu described the importance of regulations allowing for the reprocessing of single-use devices to take better advantage of device features and to be targeted for specific use cases [51]. Likewise, Liao et al. used decision-making models to repair medical equipment [55]. Finally, Benedettini advised looking into the trend towards green servitisation in the single-use MD market and how it is assisting the sector in moving towards a more sustainable economic model through the green servitisation of Original Equipment Manufacturers (OEMs) [59].
The third approach emphasises the value of remotely operating and managing equipment, such as photovoltaic (PV) generators, to power MDs in remote locations [32]. Solar PV systems can be an alternative technology for remote rural areas where grid electric power is unavailable.
Each approach impacts the economic and environmental pillars, although the contribution of the social pillar is indirect because the approaches seek the benefit of the users who use the products. However, the impact on the evaluation is limited.
3.4. End of Life
This stage takes advantage of the residual resources employed for other functions. According to the literature, three principal approaches expand the functions of products that have completed their cycle of use. The first approach was proposed by Goli and Sadeghi, whose contribution is to reuse the polymers contained in single-use face masks that can be used to improve the properties and performance of hot mix asphalt [62]. Adding such elements improved the tensile strength, the aggregate–binder adhesion, and the resistance to moisture damage. This is extremely useful in the current settings where tons of masks are being produced, used, and abandoned daily.
The second approach focused on the Medical Waste Chain system that supports the waste transactions between the medical centre and waste centre, recycling plant, and the sorting factory, and can easily manage and query the related data. This aids the reuse of medical equipment, prolonging their life and accelerating the waste management and treatment process [63].
Finally, Mallick et al. suggested contributing to a more circular economy for disposable MDs by designing and implementing a take-back system for single-use devices [64]. This is based on the company return, whose goal is to reuse the postconsumer recycled materials in its production. The main objective is to promote the return of used insulin pens to recycle and reuse some of their components.
Each of these approaches positively impact the economic and environmental dimensions thanks to the reuse of the products whose end life has been completed, as these products become raw materials to create novel alternative solutions for resolving other types of issues.
4. Discussion
This scoping review was performed with the aim of analysing the state-of-the-art research on the sustainability of MDs across their lifecycle and proposing an evidence-based way forward.
From our analysis, it was clear that there is an unequal distribution of research efforts to incorporate sustainability across different lifecycle stages. The “Design and development”, “Manufacture”, and “Use” stages have received acceptable attention, with 39%, 24.4%, and 29.3% of the included studies, respectively, addressing that stage. Contrastingly, the “End-of-life” stage has received minimal attention, with only 7.3% of the studies addressing this stage, highlighting the urgent need for further research in this area. Indeed, it is crucial to develop innovative approaches to handle MDs at their current end of life sustainably (e.g., safely using the discarded device, its parts, or materials in a new product with a different function).
While not as dramatic as the above, an unequal distribution of research efforts was also observed regarding the three dimensions of sustainability (i.e., environmental, economic, and social). Namely, the environmental dimension was the most common, accounting for 39.1% of the occurrences, closely followed by the economic dimension with 37.9% of the occurrences. In comparison, the social dimension was addressed in 23% of the occurrences. Interestingly, while MDs focus naturally on an essential element of sustainability (i.e., health and well-being), the research efforts to improve it across the MD lifecycle have paid more attention to fostering environmental and economic sustainability. This highlights the need for novel approaches to increase the social impact of MDs. For instance, increasing the access of a broader population to healthcare by following frugal engineering principles during the design and development process of MDs.
When it comes to the sustainable development goals (SDGs), most of them (13 out of 17) were addressed by at least one study (Figure 6). The three most addressed SDGs were:
- SDG#9: industry, innovation, and infrastructure (28.6% of the occurrences).
- SDG#12: responsible consumption and production (22.6% of the occurrences).
- SDG#3: good health and well-being (11.9% of the occurrences).
SDGs #9 and #12 align with the findings above, which revealed the strong attention that research studies have paid to improving the sustainability of MDs during the design and development, manufacture, and use stages. Furthermore, SDG #3 aligns with the intention of MDs to treat, cure, prevent, mitigate, and diagnose disease in humans. Nevertheless, there are still areas of opportunity to improve the environmental impact of MDs by reducing energy and water consumption during their manufacture and use.
This review also allowed for the collection, evaluation, and reporting of the flagship sustainability principles in the MD industry per lifecycle stage, which is aligned with the green supply chain method [73]. These are presented below, organised by lifecycle stage and matched to the 9R framework whenever possible. This framework promotes a circular economy approach by examining how products and materials can be used and reused at their highest value while minimising waste and environmental impact [74]. Namely, the 9Rs are rethink, reduce, reuse, repair, refurbish, remanufacture, repurpose, recycle, and recover (from R1 to R9). Furthermore, Table 3 presents the included studies addressing each R, if applicable.

Table 3.
Summary of included studies addressing each R from the 9R framework.
- (a)
- Design and development
- (i)
- Context-aware, lifecycle-aware design and development. This principle refers to incorporating sustainability considerations during the product design and development process using eco-, sustainable, and frugal design approaches. This principle loosely matches R1, which refers to rethinking a product to make it more sustainable by design.
- (ii)
- Energy considerations include eliminating the need for external supplies to power MDs by harvesting energy from the body or optimising energy usage through electronics design and microprogramming. This principle matches R2, which includes increasing efficiency in product manufacturing and use by consuming fewer resources.
- (iii)
- Material reduction and selection, including optimising the amount of materials in MDs and using more recyclable materials (e.g., less single-use plastics). The latter matches R8, which refers to processing materials to obtain high- or lower-grade materials, while the former matches R2, which also includes consuming fewer materials to manufacture a product.
- (b)
- Manufacture
- (i)
- Energy savings in production processes. While the included studies refer specifically to reducing energy usage during production, this principle can be extended to lowering all the required resources (e.g., energy and water). Hence, this principle aligns with R2, which includes increasing the efficiency of producing MDs by consuming fewer resources.
- (ii)
- Supply chain management, which considers optimising the entire supply chain to reduce the resources involved in product manufacturing, from supplying raw materials to distributing finished products. This principle also aligns with R2.
- (c)
- Use
- (i)
- Reusable vs. disposable. This principle refers to reusing MDs after proper sterilisation procedures whenever possible, which directly matches R3. Needless to say, scientific and clinical evidence must support the decision to reuse specific MDs to meet applicable safety standards.
- (ii)
- Adapting existing solutions to current needs and customer demands. This principle can align with R1, which considers using the same product for different functions, thus eliminating the need for a new product, or with R7, which considers using a discarded product (or its parts) in a new product with a different function.
- (iii)
- Repairing MDs based on decision-making tools, which extends the service life of MDs, reducing the need for new products. This principle aligns with R4 repair and R5 refurbish.
- (d)
- End of life
- (i)
- Repurposing. This principle aligns directly with R7 repurposing, which considers using a discarded product or its parts in a new product with a different function.
- (ii)
- Designing and implementing a take-back system for single-use MDs. Take-back systems aim to reduce MD manufacturers’ environmental impacts and increase efficiency and economic value by recycling or remanufacturing products or their materials. This principle aligns with R6 to R8, which includes remanufacturing, repurposing, and recycling.
- (iii)
- Improving waste management and communication between medical centres and waste centres. This principle aligns with R8 and R9 as it focuses on recovering the waste generated by MDs to recycle some of their materials or recovering energy from their incineration.
Other than being aligned with the 9Rs presented above, the proposed points are also well aligned with three core pillars of frugal engineering, defined as “achieving more with fewer resources”, namely cost reduction, core functionalities, and optimised performance levels [30]. Piaggio et al. built on this concept to create a framework for designing MDs resilient to low-resource settings [28]. The main areas they pinpointed, namely cost, lifetime, health technology management, design, user type, materials, and reliance on external factors, are again well aligned with the abovementioned principles.
Sustainable development is a global objective guided by the sustainable development goals (SDGs) proposed by the United Nations [21,22]. Therefore, finding new ways and setting new guidelines for reducing our environmental impact on the planet (e.g., less emissions, less plastic and pollution, etc.) is crucial. Estimates report that if the world switched to sustainable practices, USD 26 trillion would be saved by 2030 [75]; beyond academic study, there is a tangible momentum to bring sustainability to the real economy. This is exemplified in recent industrial developments in automotive manufacturing, software, and fashion [76,77].
Conversely, the MD industry is still very carbon-intensive and needs to be more active in implementing environmentally conscious practices. Nearly 60% of healthcare professionals agree that more advancements must be made towards the sustainability goals. As discussed above, MDs contribute substantially to the emissions attributed to the healthcare sector, given their widespread use and very short lifecycles [13]. In fact, a disproportionate share of their emissions could be credited to a preference for single-use, often plastic, disposable devices over reusable ones, especially in high-income countries [15].
This is due to the strict regulations governing the production of MDs, favouring homogenous sterilised manufacturing processes often accompanied by certifications assuring fit-for-use status [78]. Beyond the engineering and material science questions holding the decarbonisation of MD production, the issue is further complicated by a net of regulatory constraints, once built to safeguard patients and ensure high-quality standards and now locking a portion of global emission behind red tape. This is exemplified by the ubiquitous exemption of the healthcare industry to create tangible plans to be more sustainable.
Current MD regulations (e.g., 2017/745) essentially do not consider sustainability—although some are moving in this direction (e.g., the new UK regulation on MDs). In this regard, there is only one international standard relative to sustainability and MDs, IEC 60601-1-9, amended in 2020, which broadly asks medical electrical equipment manufacturers to consider their potential adverse impacts on the environment. This, however, needs to implement a full decarbonisation of the supply chain of MDs as well as a drastic reduction in the use of single-use plastics both in packaging and the end products. Beyond the regulatory hurdle, promoting sustainability in MDs requires a detailed yet holistic understanding of the sources of emissions throughout the design, manufacture, use, and end-use stages discussed.
From an ethical perspective, it is noteworthy to mention that sustainability is not only a matter of environmental impact and ecological significance but also has numerous ethical implications [79], spanning from environmental to medical ones. In fact, MDs are the main instruments that mediate the doctor–patient relation, with the related acceptability and paternalism versus self-determination themes. Other ethical themes that should not be forgotten encompass distributive justice and intergenerational equity.
MDs represent an ethical contradiction. While they provide short-term health benefits to patients by contributing to their care, in the long run, they pose a risk to patient’s health due to the high environmental impact of their lifecycle. MDs, therefore, can negatively impact people’s health and quality of life. Despite many advancements at the MD design stage, one of the most underestimated areas, as mentioned above, is that of their disposal at the end of their lives. This is a global problem that affects all kinds of settings, including low-resource ones, in which the lack of complete guidelines for MD donations, for the receivers (enquiring, for example, about their current needs and local expertise), could be used as a loophole for using MD donations as a sugar-coated way for one country to dispose of their obsolete MDs. This was proved to be an economic and environmental burden, as such donated MDs are prone to breaking down easily or never getting installed due to the lack of expertise, limited supply chains, poor infrastructures, and harsh environmental conditions [80,81]. The reusable vs. single-use MD debate is strictly related to the end-of-life concept. In the past couple of decades, there was a significant shift towards the use of single-use MDs, ignited by the concerns raised by the HIV and Hepatitis B epidemic of the 1980s and propelled by other pathogens for which sterilisation was trickier (e.g., bovine spongiform encephalopathy or mad cow disease) [82]. The study by Donahue et al. showed how reusable vaginal specula were associated with reduced greenhouse gas equivalents compared to single-use ones with no significant clinical difference [45]. The improvement in MD companies could also be obtained by re-evaluating the advantages of reusable MDs in healthcare, considering the possible clinical implications linked to a rise in iatrogenic infections. It is advised that reusable MDs could be preferred in clinical situations where the risk of infection transmission, despite sterilisation, is low and performed along with promoting more environment-friendly sterilisation options (e.g., autoclaves).
Moreover, sustainability calls into question another ethical principle, the one of responsibility. Among the various stakeholders involved, it would be essential to identify who is responsible for the sustainability or, in the case of the specific challenge highlighted above related to the end of life, for the proper disposal of MDs: the designer, the manufacturer, the user, the distributor, or the hospital? This responsibility concept strictly relates to liability, where the moral obligation leaves the place to the legal duty associated with sustainable practices for safeguarding the planet’s future [83]. Indeed, the MD industry faces liability concerns, such as environmental impact, single-use plastics, and end-of-life disposal. Apart from the theoretical and ethical responsibility of acting correctly or not, the responsible risks being prosecuted civilly and criminally for the damage that their actions or omissions cause to the users or the environment. The polluter-pays principle could be a way forward and requires holding manufacturers responsible for their products’ lifecycle, advocating for extended producer responsibility schemes [84]. There should be a stronger emphasis on the link between sustainability, liability, and responsibility and discussions about shared and global sustainability, collective yet diversified, because it varies at each stage. It is true that the economy also plays a significant role in this reasoning, where the cost and value of things are substantial, and cost savings are essential in such processes. Therefore, an attempt should be made to rewrite the paradigm of priorities, where health and sustainability should be considered before cost savings and other economic issues.
This means understanding sustainability in terms of climate justice and solidarity, achievable through a novel consideration of MDs and their lifecycles. Sustainable healthcare, in fact, means healthcare capable of addressing the present and future challenges of the environment, such as climate change. In this sense, sustainable healthcare minimises healthcare delivery’s impact on the environment while providing the best quality care and promoting healthy living. Hence, there is a need for the establishment of ethical guidelines that feature equity, responsibility, and sustainability as fundamental principles for the provision of global healthcare. These principles can provide an ethical framework to guide decisions and actions in the healthcare sector, ensuring equitable access to care, that resources are used responsibly, and that healthcare practices respect the environment. The definition and implementation of such an ethical framework represent a crucial step towards achieving a more sustainable and fair global healthcare system [85].
In undertaking this scoping literature review, it is paramount to acknowledge and address certain limitations inherent to the nature of the study. In fact, transparency regarding the scope, criteria, and potential constraints is crucial. In terms of time constraints, the search period, while spanning a considerable time frame, may not capture the most recent developments in the field. In fact, the latest articles published in this domain in 2023 are not included. Regarding language, the criterion of only including studies in English may have introduced some selection bias. Finally, despite our best efforts at trying to mitigate the publication bias through a systematic search, the tendency for studies with statistically significant results to be more readily published may skew the overall representation of the literature [86].
5. Conclusions
This work underscores the critical importance of integrating sustainability principles into the entire lifecycle of MDs. While the global movement towards sustainability is imminent, the MD industry faces significant challenges in aligning its practices with environmentally conscious approaches.
Our scoping review reveals a notable disparity in research efforts across the different lifecycle stages of MDs. Considerable attention has been given to their design, development, manufacture, and use, which represents an advancement in achieving more sustainable MDs. In contrast, the end-of-life stage still needs to be further explored, which represents an opportunity for researchers, the industry, and healthcare providers. The distribution of research across the three dimensions of sustainability (environmental, economic, and social) also highlights the need for increased focus on the social dimension, given the inherent impact of MDs on health and well-being.
Regulatory constraints, particularly the need to consider sustainability in current MD regulations, pose a substantial barrier. Despite a recent shift in UK regulation and the existence of international standards like IEC 60601-1-9, more comprehensive measures are required to drive sustainability in medical device production, supply chains, and end-of-life management.
The identified flagship sustainability principles, aligned with the 9R framework and frugal engineering principles, offer a structured approach to addressing MDs’ environmental impact. These principles span design and development, manufacturing, use, and end-of-life stages, emphasising the importance of energy savings, material reduction, and a circular economy approach.
Ethical considerations further complicate the sustainability discourse, emphasising the need for responsible practices throughout the MDs’ lifecycle. The ethical implications extend from environmental concerns to issues of distributive justice, intergenerational equity, and the doctor–patient relationship. The debate between reusable and single-use MDs, influenced by historical events and infection control, adds another layer of complexity.
Moreover, the concept of responsibility and liability emerges as a critical aspect, necessitating a shift in the paradigm of priorities where health and sustainability precede economic considerations. The polluter-pays principle and extended producer responsibility schemes are proposed as avenues to hold manufacturers accountable for the environmental impact of their products.
Integrating ethical guidelines grounded in equity, responsibility, and sustainability is fundamental to achieving a more sustainable and fair global healthcare system. This ethical framework can guide decision making in the healthcare sector, ensuring equitable access to care, responsible resource use, and environmentally conscious practices. The call for climate justice and solidarity underscores the urgency of reshaping the healthcare paradigm to align with the broader goals of sustainability and planetary well-being.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/su16041433/s1: Table S1: Data extraction table.
Author Contributions
L.M.: conceptualisation, methodology, software, validation, formal analysis, investigation, resources, data curation, writing—original draft, writing—review and editing, visualisation, and funding acquisition. P.C.R.: validation, formal analysis, investigation, data curation, and writing—original draft. M.R.F.: formal analysis, investigation, and writing—original draft. J.M.-R.: formal analysis, investigation, and writing—original draft. S.C.: methodology, validation, and writing—review and editing. A.M.: formal analysis, investigation, resources, writing—original draft, and funding acquisition. D.P.: conceptualisation, methodology, software, validation, formal analysis, investigation, resources, writing—original draft, writing—review and editing, supervision, project administration, and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Health Global Research Priorities (GRP) and Innovative Manufacturing and Future Materials GRP of the University of Warwick and by the EPSRC Impact Accelerator Award (EP/K503848/1 and EP/R511808/1). Pedro Checa Rifá received further financial support from the School of Engineering, University of Warwick.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest. Luis Montesinos was employed by Tecnologico de Monterrey; Mireya Rifá Fabregat was employed by the European Network of Safety and Health Professional Organizations; Lübeck; Javier Maldonado-Romo was employed by Tecnologico de Monterrey; Stefano Capacci was employed by the company Longevity Partners. The views presented in this paper do not represent those of Longevity Partners.
References
- Erb, T.; Perciasepe, B.; Radulovic, V.; Niland, M. Corporate Climate Commitments: The Trend Towards Net Zero. In Handbook of Climate Change Mitigation and Adaptation; Lackner, M., Sajjadi, B., Chen, W.-Y., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 2985–3018. ISBN 978-3-030-72578-5. [Google Scholar]
- Savaresi, A. The Paris Agreement: A New Beginning? J. Energy Nat. Resour. Law 2016, 34, 16–26. [Google Scholar] [CrossRef]
- Smil, V. Energy and Civilization: A History; The MIT Press: Cambridge, MA, USA, 2017; ISBN 978-0-262-03577-4. [Google Scholar]
- Della Vigna, M.; Stavrinou, Z.; Bhandari, N.; Neil, M. Brian Singer Carbonomics the Future of Energy in the Age of Climate Change; The Goldman Sachs Group, Inc.: New York, NY, USA, 2019. [Google Scholar]
- Hagens, N. Economics for the future—Beyond the superorganism. Ecol. Econ. 2020, 169, 106520. [Google Scholar] [CrossRef]
- Alvis, S.; Murphy, L.; Martínez, L.C.; Emden, J.; Jung, C. The End of Greenwashing—Driving Decarbonisation in the Real Economy; Institute for Public Policy Research: London, UK, 2023. [Google Scholar]
- Cui, R.Y.; Hultman, N.; Edwards, M.R.; He, L.; Sen, A.; Surana, K.; McJeon, H.; Iyer, G.; Patel, P.; Yu, S.; et al. Quantifying Operational Lifetimes for Coal Power Plants under the Paris Goals. Nat. Commun. 2019, 10, 4759. [Google Scholar] [CrossRef] [PubMed]
- Calvin, K.; Dasgupta, D.; Krinner, G.; Mukherji, A.; Thorne, P.W.; Trisos, C.; Romero, J.; Aldunce, P.; Barrett, K.; Blanco, G.; et al. Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Lee, H., Romero, J., Eds.; Intergovernmental Panel on Climate Change (IPCC): Geneva, Switzerland, 2023. [Google Scholar]
- Statista Global CO2 Emissions by Year 1940–2023. Available online: https://www.statista.com/statistics/276629/global-co2-emissions/ (accessed on 31 January 2024).
- Lindsey, R. Climate Change: Atmospheric Carbon Dioxide. Available online: https://www.climate.gov/news-features/understanding-climate/climate-change-atmospheric-carbon-dioxide (accessed on 31 January 2024).
- Ritchie, H.; Roser, M. CO₂ Emissions; Our World in Data: Oxford, UK, 2024. [Google Scholar]
- Kent, C. How Hospitals and the Medtech Sector Are Tackling the Climate Crisis. Medical Device Network 2021. Available online: https://www.medicaldevice-network.com/features/hospitals-medtech-environment/ (accessed on 15 January 2024).
- Sousa, A.C.; Veiga, A.; Maurício, A.C.; Lopes, M.A.; Santos, J.D.; Neto, B. Assessment of the environmental impacts of medical devices: A review. Environ. Dev. Sustain. 2021, 23, 9641–9666. [Google Scholar] [CrossRef]
- Karliner, J.; Slotterback, S.; Boyd, R.; Ashby, B.; Steele, K.; Wang, J. Health Care’s Climate Footprint: The Health Sector Contribution and Opportunities for Action; Health Care Without Harm: Washington, DC, USA, 2019. [Google Scholar]
- MacNeill, A.J.; Hopf, H.; Khanuja, A.; Alizamir, S.; Bilec, M.; Eckelman, M.J.; Hernandez, L.; McGain, F.; Simonsen, K.; Thiel, C.; et al. Transforming the Medical Device Industry: Road Map to a Circular Economy. Health Aff. 2020, 39, 2088–2097. [Google Scholar] [CrossRef]
- Belkhir, L.; Elmeligi, A. Carbon Footprint of the Global Pharmaceutical Industry and Relative Impact of Its Major Players. J. Clean. Prod. 2019, 214, 185–194. [Google Scholar] [CrossRef]
- Gavurova, B.; Rigelsky, M.; Ivankova, V. Greenhouse Gas Emissions and Health in the Countries of the European Union. Front. Public Health 2021, 9, 756652. [Google Scholar] [CrossRef]
- McAlister, S.; Morton, R.L.; Barratt, A. Incorporating Carbon into Health Care: Adding Carbon Emissions to Health Technology Assessments. Lancet Planet. Health 2022, 6, e993–e999. [Google Scholar] [CrossRef] [PubMed]
- Roostaie, S.; Nawari, N.; Kibert, C. Sustainability and Resilience: A Review of Definitions, Relationships, and Their Integration into a Combined Building Assessment Framework. Build. Environ. 2019, 154, 132–144. [Google Scholar] [CrossRef]
- Warde, P. The Invention of Sustainability: Nature and Destiny, c.1500–1870, 1st ed.; Cambridge University Press: Cambridge, UK, 2018; ISBN 978-1-316-58476-7. [Google Scholar]
- Brundtland, G.H. Our Common Future; World Commission on Environment and Development: London, UK, 1987. [Google Scholar]
- Jonas, H. The Imperative of Responsibility. In Search of an Ethics for the Technological Age; University of Chicago Press: Chicago, IL, USA, 1985; ISBN 978-0-226-40597-1. [Google Scholar]
- Elkington, J. Towards the Sustainable Corporation: Win-Win-Win Business Strategies for Sustainable Development. Calif. Manag. Rev. 1994, 36, 90–100. [Google Scholar] [CrossRef]
- Fankhauser, S.; Smith, S.M.; Allen, M.; Axelsson, K.; Hale, T.; Hepburn, C.; Kendall, J.M.; Khosla, R.; Lezaun, J.; Mitchell-Larson, E.; et al. The Meaning of Net Zero and How to Get It Right. Nat. Clim. Chang. 2022, 12, 15–21. [Google Scholar] [CrossRef]
- Lockwood, C.; dos Santos, K.B.; Pap, R. Practical Guidance for Knowledge Synthesis: Scoping Review Methods. Asian Nurs. Res. 2019, 13, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Booth, A.; Sutton, A.; Papaioannou, D. Systematic Approaches to a Successful Literature Review, 2nd ed.; Sage: Los Angeles, LA, USA, 2016; ISBN 978-1-4739-1245-8. [Google Scholar]
- Popay, J.; Roberts, H.; Sowden, A.; Petticrew, M.; Arai, L.; Rodgers, M.; Britten, N. Guidance on the Conduct of Narrative Synthesis in Systematic Reviews. A Product from the ESRC Methods Programme Version. 2006. Available online: https://citeseerx.ist.psu.edu/document?repid=rep1&type=pdf&doi=ed8b23836338f6fdea0cc55e161b0fc5805f9e27 (accessed on 15 January 2024).
- Hamner, S.R.; Narayan, V.G.; Donaldson, K.M. Designing for Scale: Development of the ReMotion Knee for Global Emerging Markets. Ann. Biomed. Eng. 2013, 41, 1851–1859. [Google Scholar] [CrossRef] [PubMed]
- Hede, S.; Nunes, M.J.L.; Ferreira, P.F.V.; Rocha, L.A. Incorporating Sustainability in Decision-Making for Medical Device Development. Technol. Soc. 2013, 35, 276–293. [Google Scholar] [CrossRef]
- MacVittie, K.; Halámek, J.; Halámková, L.; Southcott, M.; Jemison, W.D.; Lobel, R.; Katz, E. From “Cyborg” Lobsters to a Pacemaker Powered by Implantable Biofuel Cells. Energy Environ. Sci. 2013, 6, 81–86. [Google Scholar] [CrossRef]
- Hamza, A.O.; Garma, F.B.B.; Eisa, B.I.B.; Almahdi, O.A.B.; Awadalla, A.M.B.; Dafaallah, A.S.B. Application of Solar Energy in Medical Instrumentation: Microscope. J. Clin. Eng. 2014, 39, 132–135. [Google Scholar] [CrossRef]
- Unger, S.R.; Landis, A.E. Comparative Life Cycle Assessment of Reused Versus Disposable Dental Burs. Int. J. Life Cycle Assess. 2014, 19, 1623–1631. [Google Scholar] [CrossRef]
- Gupta, R.; Patel, R.; Murty, N.; Panicker, R.; Chen, J. Developing Sustainable Global Health Technologies: Insight from an Initiative to Address Neonatal Hypothermia. J. Public Health Policy 2015, 36, 24–40. [Google Scholar] [CrossRef]
- Izadikhah, M.; Saen, R.F. Evaluating Sustainability of Supply Chains by Two-Stage Range Directional Measure in the Presence of Negative Data. Transp. Res. Part D Transp. Environ. 2016, 49, 110–126. [Google Scholar] [CrossRef]
- Moultrie, J.; Sutcliffe, L.; Maier, A. A Maturity Grid Assessment Tool for Environmentally Conscious Design in the Medical Device Industry. J. Clean. Prod. 2016, 122, 252–265. [Google Scholar] [CrossRef]
- Unger, S.; Landis, A. Assessing the Environmental, Human Health, and Economic Impacts of Reprocessed Medical Devices in a Phoenix Hospital’s Supply Chain. J. Clean. Prod. 2016, 112, 1995–2003. [Google Scholar] [CrossRef]
- Barbero, S.; Pereno, A.; Tamborrini, P. Systemic Innovation in Sustainable Design of Medical Devices. Des. J. 2017, 20, S2486–S2497. [Google Scholar] [CrossRef]
- Unger, S.R.; Hottle, T.A.; Hobbs, S.R.; Thiel, C.L.; Campion, N.; Bilec, M.M.; Landis, A.E. Do Single-Use Medical Devices Containing Biopolymers Reduce the Environmental Impacts of Surgical Procedures Compared with Their Plastic Equivalents? J. Health Serv. Res. Policy 2017, 22, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, J.; Littlewood, J.; Wilgeroth, P. Development of a Framework of Key Performance Indicators to Identify Reductions in Energy Consumption in a Medical Devices Production Facility. Int. J. Ambient. Energy 2018, 39, 202–210. [Google Scholar] [CrossRef]
- Ghadimi, P.; Ghassemi Toosi, F.; Heavey, C. A Multi-Agent Systems Approach for Sustainable Supplier Selection and Order Allocation in a Partnership Supply Chain. Eur. J. Oper. Res. 2018, 269, 286–301. [Google Scholar] [CrossRef]
- Mann, H.; Mann, I.; Gullaiya, N. A Case in Medical Equipment Design for Strategic Sustainability. South Asian J. Bus. Manag. Cases 2018, 7, 111–119. [Google Scholar] [CrossRef]
- Ghadimi, P.; Wang, C.; Lim, M.K.; Heavey, C. Intelligent Sustainable Supplier Selection Using Multi-Agent Technology: Theory and Application for Industry 4.0 Supply Chains. Comput. Ind. Eng. 2019, 127, 588–600. [Google Scholar] [CrossRef]
- Magno, M.; Salvatore, G.A.; Jokic, P.; Benini, L. Self-Sustainable Smart Ring for Long-Term Monitoring of Blood Oxygenation. IEEE Access 2019, 7, 115400–115408. [Google Scholar] [CrossRef]
- Donahue, L.M.; Hilton, S.; Bell, S.G.; Williams, B.C.; Keoleian, G.A. A Comparative Carbon Footprint Analysis of Disposable and Reusable Vaginal Specula. Am. J. Obstet. Gynecol. 2020, 223, 225.e1–225.e7. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Xu, S.; Liu, Y.; Gao, Y.; Tong, T.; Qi, Y.; Zhang, C. Flexible Drug Release Device Powered by Triboelectric Nanogenerator. Adv. Funct. Mater. 2020, 30, 1909886. [Google Scholar] [CrossRef]
- Lyne, A.; Ashley, P.; Saget, S.; Costa, M.P.; Underwood, B.; Duane, B. Combining Evidence-Based Healthcare with Environmental Sustainability: Using the Toothbrush as a Model. Br. Dent. J. 2020, 229, 303–309. [Google Scholar] [CrossRef]
- Riutord-Sbert, P.; De Pedro Gómez, J.E.; Pereira, T.C.; López-Safont, N.; García-Mosquera, I.; Jiménez-Recaredo, J.; Alomar-Velasco, P.J.; Paublini-Oliveira, H.J.; Dominguez-Pérez, J.; González-Carrasco, D.; et al. An Innovative, Reusable and Sustainable Face-Seal Device to Improve Protection Efficacy of Surgical Masks against COVID-19. Med. Balear. 2020, 35, 74–77. [Google Scholar] [CrossRef]
- Xu, Z.; Jin, C.; Cabe, A.; Escobedo, D.; Hao, N.; Trase, I.; Closson, A.B.; Dong, L.; Nie, Y.; Elliott, J.; et al. Flexible Energy Harvester on a Pacemaker Lead Using Multibeam Piezoelectric Composite Thin Films. ACS Appl. Mater. Interfaces 2020, 12, 34170–34179. [Google Scholar] [CrossRef]
- Alrashoudi, A.A.; Albalawi, H.I.; Aldoukhi, A.H.; Moretti, M.; Bilalis, P.; Abedalthagafi, M.; Hauser, C.A.E. Fabrication of a Lateral Flow Assay for Rapid In-Field Detection of COVID-19 Antibodies Using Additive Manufacturing Printing Technologies. Int. J. Bioprint. 2021, 7, 399. [Google Scholar] [CrossRef]
- Bayrak, T.; Soylu, S.I. Reprocessing of Single Use Medical Devices: A New Proposal for a Regulation. Health Policy Technol. 2021, 10, 100553. [Google Scholar] [CrossRef]
- Haber, N.; Fargnoli, M. Sustainable Product-Service Systems Customization: A Case Study Research in the Medical Equipment Sector. Sustainability 2021, 13, 6624. [Google Scholar] [CrossRef]
- Hanani, Z.; Izanzar, I.; Amjoud, M.; Mezzane, D.; Lahcini, M.; Uršič, H.; Prah, U.; Saadoune, I.; El Marssi, M.; Luk’Yanchuk, I.A.; et al. Lead-Free Nanocomposite Piezoelectric Nanogenerator Film for Biomechanical Energy Harvesting. Nano Energy 2021, 81, 105661. [Google Scholar] [CrossRef]
- Hasani, A.; Mokhtari, H.; Fattahi, M. A Multi-Objective Optimization Approach for Green and Resilient Supply Chain Network Design: A Real-Life Case Study. J. Clean. Prod. 2021, 278, 123199. [Google Scholar] [CrossRef]
- Liao, H.-Y.; Cade, W.; Behdad, S. Markov Chain Optimization of Repair and Replacement Decisions of Medical Equipment. Resour. Conserv. Recycl. 2021, 171, 105609. [Google Scholar] [CrossRef]
- Piaggio, D.; Namm, G.; Melillo, P.; Simonelli, F.; Iadanza, E.; Pecchia, L. Pupillometry via smartphone for low-resource settings. Biocybern. Biomed. Eng. 2021, 41, 891–902. [Google Scholar] [CrossRef]
- Szmelter-Jarosz, A.; Ghahremani-Nahr, J.; Nozari, H. A Neutrosophic Fuzzy Optimisation Model for Optimal Sustainable Closed-Loop Supply Chain Network during COVID-19. J. Risk Financ. Manag. 2021, 14, 519. [Google Scholar] [CrossRef]
- van Straten, B.; Ligtelijn, S.; Droog, L.; Putman, E.; Dankelman, J.; Weiland, N.H.S.; Horeman, T. A Life Cycle Assessment of Reprocessing Face Masks during the COVID-19 Pandemic. Sci. Rep. 2021, 11, 17680. [Google Scholar] [CrossRef]
- Benedettini, O. Green Servitization in the Single-Use Medical Device Industry: How Device OEMs Create Supply Chain Circularity through Reprocessing. Sustainability 2022, 14, 12670. [Google Scholar] [CrossRef]
- Epelle, E.I.; Macfarlane, A.; Cusack, M.; Burns, A.; Mackay, W.G.; Rateb, M.E.; Yaseen, M. Application of Ultraviolet-C Radiation and Gaseous Ozone for Microbial Inactivation on Different Materials. ACS Omega 2022, 7, 43006–43021. [Google Scholar] [CrossRef] [PubMed]
- Fargnoli, M.; Haber, N.; Tronci, M. Case Study Research to Foster the Optimization of Supply Chain Management through the PSS Approach. Sustainability 2022, 14, 2235. [Google Scholar] [CrossRef]
- Goli, A.; Sadeghi, P. Evaluation on the Use of COVID-19 Single-Use Face Masks to Improve the Properties of Hot Mix Asphalt. Road Mater. Pavement Des. 2022, 24, 1371–1388. [Google Scholar] [CrossRef]
- Le, H.T.; Le Quoc, K.; Nguyen, T.A.; Dang, K.T.; Vo, H.K.; Luong, H.H.; Le Van, H.; Gia, K.H.; Phu, L.V.C.; Quoc, D.N.T.; et al. Medical-Waste Chain: A Medical Waste Collection, Classification and Treatment Management by Blockchain Technology. Computers 2022, 11, 113. [Google Scholar] [CrossRef]
- Mallick, P.K.; Salling, K.B.; Pigosso, D.C.A.; McAloone, T.C. Designing Take-Back for Single Use Medical Devices: The Case of ReturpenTM. J. Diabetes Sci. Technol. 2022, 16, 1363–1369. [Google Scholar] [CrossRef]
- Nayeri, S.; Sazvar, Z.; Heydari, J. A Global-Responsive Supply Chain Considering Sustainability and Resiliency: Application in the Medical Devices Industry. Socio-Econ. Plan. Sci. 2022, 82, 101303. [Google Scholar] [CrossRef]
- Rostami, O.; Tavakoli, M.; Tajally, A.; GhanavatiNejad, M. A Goal Programming-Based Fuzzy Best–Worst Method for the Viable Supplier Selection Problem: A Case Study. Soft Comput. 2022, 27, 2827–2852. [Google Scholar] [CrossRef]
- Rouvière, N.; Chkair, S.; Auger, F.; Alovisetti, C.; Bernard, M.; Cuvillon, P.; Kinowski, J.-M.; Leguelinel-Blache, G.; Chasseigne, V. Ecoresponsible actions in Operating Rooms: A Health Ecological and Economic Evaluation. Int. J. Surg. 2022, 101, 106637. [Google Scholar] [CrossRef]
- Williams, E.; Piaggio, D.; Andellini, M.; Pecchia, L. 3D-Printed Activated Charcoal Inlet Filters for Oxygen Concentrators: A Circular Economy Approach. Dev. Eng. 2022, 7, 100094. [Google Scholar] [CrossRef]
- Abad, A.R.K.K.; Barzinpour, F.; Pasandideh, S.H.R. A Novel Separate Chance-Constrained Programming Model to Design a Sustainable Medical Ventilator Supply Chain Network during the COVID-19 Pandemic. J. Ind. Manag. Optim. 2023, 19, 1395. [Google Scholar] [CrossRef]
- Weyrauch, T.; Herstatt, C. What is Frugal Innovation? Three Defining Criteria. J. Frugal Innov. 2017, 2, 1. [Google Scholar] [CrossRef]
- Jain, V.; Wadhwa, S.; Deshmukh, S. Select Supplier-Related Issues in Modelling a Dynamic Supply Chain: Potential, Challenges and Direction for Future Research. Int. J. Prod. Res. 2009, 47, 3013–3039. [Google Scholar] [CrossRef]
- Ivanov, D.; Dolgui, A. Viability of Intertwined Supply Networks: Extending the Supply Chain Resilience Angles Towards Survivability. A Position Paper Motivated by COVID-19 Outbreak. Int. J. Prod. Res. 2020, 58, 2904–2915. [Google Scholar] [CrossRef]
- Singh, A.; Singh, A.; Trivedi, A.; Trivedi, A. Sustainable Green Supply Chain Management: Trends and Current Practices. Compet. Rev. Int. Bus. J. 2016, 26, 265–288. [Google Scholar] [CrossRef]
- Henry, M.; van Ardenne-van der Hoeven, A.; Demmers, M.; Dykstra, E.; Frissen, L.; de Graeff, J.J.; Hooimeijer, P.; Koeman, N.; van Lier Lels, M.; Meester, G. Circular Economy. From Wish to Practice. 2015. Available online: https://en.rli.nl/sites/default/files/advice_rli_circular_economy_interactive_def.pdf (accessed on 15 January 2024).
- United Nations Climate Action Fast Facts. Available online: https://www.un.org/en/climatechange/science/key-findings (accessed on 31 January 2024).
- Busalim, A.; Fox, G.; Lynn, T. Consumer Behavior in Sustainable Fashion: A Systematic Literature Review and Future Research Agenda. Int. J. Consum. Stud. 2022, 46, 1804–1828. [Google Scholar] [CrossRef]
- Puspita, H.; Chae, H. An explorative Study and Comparison between Companies’ and Customers’ Perspectives in the Sustainable Fashion Industry. J. Glob. Fash. Mark. 2021, 12, 133–145. [Google Scholar] [CrossRef]
- Sherman, J.D.; Thiel, C.; MacNeill, A.; Eckelman, M.J.; Dubrow, R.; Hopf, H.; Lagasse, R.; Bialowitz, J.; Costello, A.; Forbes, M.; et al. The Green Print: Advancement of Environmental Sustainability in Healthcare. Resour. Conserv. Recycl. 2020, 161, 104882. [Google Scholar] [CrossRef]
- Brightman, A.O.; Beever, J.; Hiles, M.C. Next-Generation Ethical Development of Medical Devices: Considering Harms, Benefits, Fairness, and Freedom. In Next-Generation Ethics; Abbas, A.E., Ed.; Cambridge University Press: Cambridge, UK, 2019; pp. 387–410. ISBN 978-1-108-61618-8. [Google Scholar]
- Di Pietro, L.; Piaggio, D.; Oronti, I.; Maccaro, A.; Houessouvo, R.C.; Medenou, D.; De Maria, C.; Pecchia, L.; Ahluwalia, A. A Framework for Assessing Healthcare Facilities in Low-Resource Settings: Field Studies in Benin and Uganda. J. Med. Biol. Eng. 2020, 40, 526–534. [Google Scholar] [CrossRef]
- Piaggio, D.; Castaldo, R.; Cinelli, M.; Cinelli, S.; Maccaro, A.; Pecchia, L. A Framework for Designing Medical Devices Resilient to Low-Resource Settings. Glob. Health 2021, 17, 64. [Google Scholar] [CrossRef] [PubMed]
- Das, A.K.; Okita, T.; Enzo, A.; Asai, A. The Ethics of the Reuse of Disposable Medical Supplies. Asian Bioeth. Rev. 2020, 12, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Cornock, M. Legal definitions of Responsibility, Accountability and Liability: Marc Cornock Clarifies the Use of Terms That Are Sometimes Used Interchangeably but Have Distinct Ramifications in Law. Nurs. Child. Young- People 2011, 23, 25–26. [Google Scholar] [CrossRef]
- Khan, M. Polluter-Pays-Principle: The Cardinal Instrument for Addressing Climate Change. Laws 2015, 4, 638–653. [Google Scholar] [CrossRef]
- Pratt, B. Sustainable Global Health Practice: An Ethical Imperative? Bioethics 2022, 36, 874–882. [Google Scholar] [CrossRef]
- Sun, J.; Freeman, B.D.; Natanson, C. Meta-Analysis of Clinical Trials. In Principles and Practice of Clinical Research; Elsevier: Amsterdam, The Netherlands, 2018; pp. 317–327. ISBN 978-0-12-849905-4. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).