Abstract
Energy utilization in wastewater degradation has important implications for sustainability; however, efficient multiphase Fenton-like catalysts are still needed. In this study, a heterogeneous Fe2O3/coconut shell activated carbon (CSAC) Fenton-like catalyst was prepared and evaluated with respect to degradation performance and exothermic reaction for the treatment of organic wastewater. Fe2O3@CSAC retained the porous morphology of CSAS, and Fe2O3 was uniformly loaded on the surface of CSAS. In the reaction system, the degradation rate of wastewater was higher and a large amount of heat was released; therefore, it could be applied to the energy recovery from wastewater source heat pump technology. The degradation rate of 300 mL of o-phenylenediamine solution with a concentration of 0.04 mol·L−1 was 89.0% under 0.25 mol·L−1 H2O2, 532 g·L−1 Fe2O3@CSAC, pH 7.1, and an initial reaction temperature of 30 °C, elevated to 7.9 °C. These findings clearly demonstrate the degradation performance and exothermic laws of the Fe2O3@CSAC/H2O2 multiphase Fenton-like system.
1. Introduction
Human activities and industrial processes produce a variety of wastes or emissions, and energy recovery is an important strategy for minimizing waste and the adverse impact on the environment. Various technologies, such as waste incineration for power generation, biogas fermentation, and flue gas recovery, are commonly used; however, the energy converted during wastewater treatment has rarely been studied and utilized. Wastewater source heat pump technology is a green energy technology that utilizes a small amount of high-grade electrical energy to enhance the low-grade heat energy in wastewater that cannot be directly utilized for enterprise production or winter heating [1,2]. The temperature drop of wastewater after heat extraction is generally controlled within 5 °C due to the wastewater treatment process. Therefore, analyses of the wastewater treatment process focused on wastewater temperature increases to enhance the external heat supply of wastewater source heat pumps and improve energy utilization are of great significance.
o-Phenylenediamine (OPD) is a raw material for the preparation of pesticides, fungicides, medical drugs, and various chemical products, and is easily discharged into the environment with industrial wastewater, agricultural runoff water, and domestic wastewater; it is potentially hazardous to aquatic organisms and human health [3,4]. Advanced oxidation technology is currently used to treat difficult-to-degrade wastewater. This technology mainly involves Fenton- [5], ozone- [6], electrochemical- [7] and microwave-assisted [8], as well as photocatalytic, oxidations [9]. Of these, the Fenton process is the most commonly utilized wastewater oxidation technology owing to its convenient dosing and high oxidizing capacity [10,11]. The Fenton reaction is a Fenton reagent composed of ferrous ions and oxidant H2O2, which produces a short but extremely active substance, namely hydroxyl radical (·OH), which has a significant oxidizing ability to convert pollutants into non-toxic products, including CO2 and H2O. However, a homogeneous Fenton reaction is only feasible when the pH value is lower than 4. When the pH value exceeds 4, iron ions are converted into ferric hydroxide sludge, and some catalysts are lost, thus decreasing the efficiency of the Fenton reaction; furthermore, the treatment and disposal of solid sludge also produces additional costs, which limits its application to a certain extent [12,13,14]. Multiphase Fenton technology is an advanced oxidation technology that replaces the iron ions in traditional Fenton technology with multiphase Fenton catalysts to catalyze the production of free radicals in hydrogen peroxide; it reduces some process limitations, such as sludge production and chemical inputs, thereby reducing costs [12]. For example, Pelalak et al. investigated the reaction mechanism of multimetallic catalysts in the Fenton process, describing effective methods to enhance the activity, electron transfer rate, and generation of hydroxyl radicals [15]. Dong et al. investigated Fe/Al2O3-loaded catalysts for the degradation of wastewater and proposed an efficient and low-cost solution for Fenton treatment with loaded catalysts [16]. Liu et al. investigated the performance of elemental iron-loaded catalysts for the degradation and treatment of sulfamethoxazole wastewater and measured the contributions of adsorption and Fenton oxidation to the degradation rate [17]. Zhang et al. prepared a non-homogeneous Fenton catalyst containing iron resin, which showed high degradation rates for organic wastewater with multiple reuses [18]. The selection of catalyst materials that overcome the disadvantages of existing materials, such as high costs and complicated preparation processes, has become a major area of research.
The application of biomass feedstock as a sustainable resource in wastewater treatment processes has increased worldwide. Coconut shell activated carbon (CSAC) is a biomass activated carbon prepared from coconut shell, an agricultural waste and sustainable resource. CSAC is a commonly used catalyst due to its large surface area and porosity [19]. For example, Li et al. synthesized CuFe2O4@CSAC for the degradation of tetrabromobisphenol A (TBBPA) and demonstrated that the surface active sites are the key factors affecting the degradation performance [20]. Pang et al. prepared coconut shell composites with high photocatalytic properties for methyl orange [21]; the metal oxide particles were uniformly loaded onto the CSAC, which improved the thermal stability and specific surface area of the material. Sun et al. developed a CSAC catalyst for the synthesis of H2/CO, obtaining a conversion rate greater than 90%, and showed that CH4/CO2 content has a great influence on the synthesis of H2/CO [22].
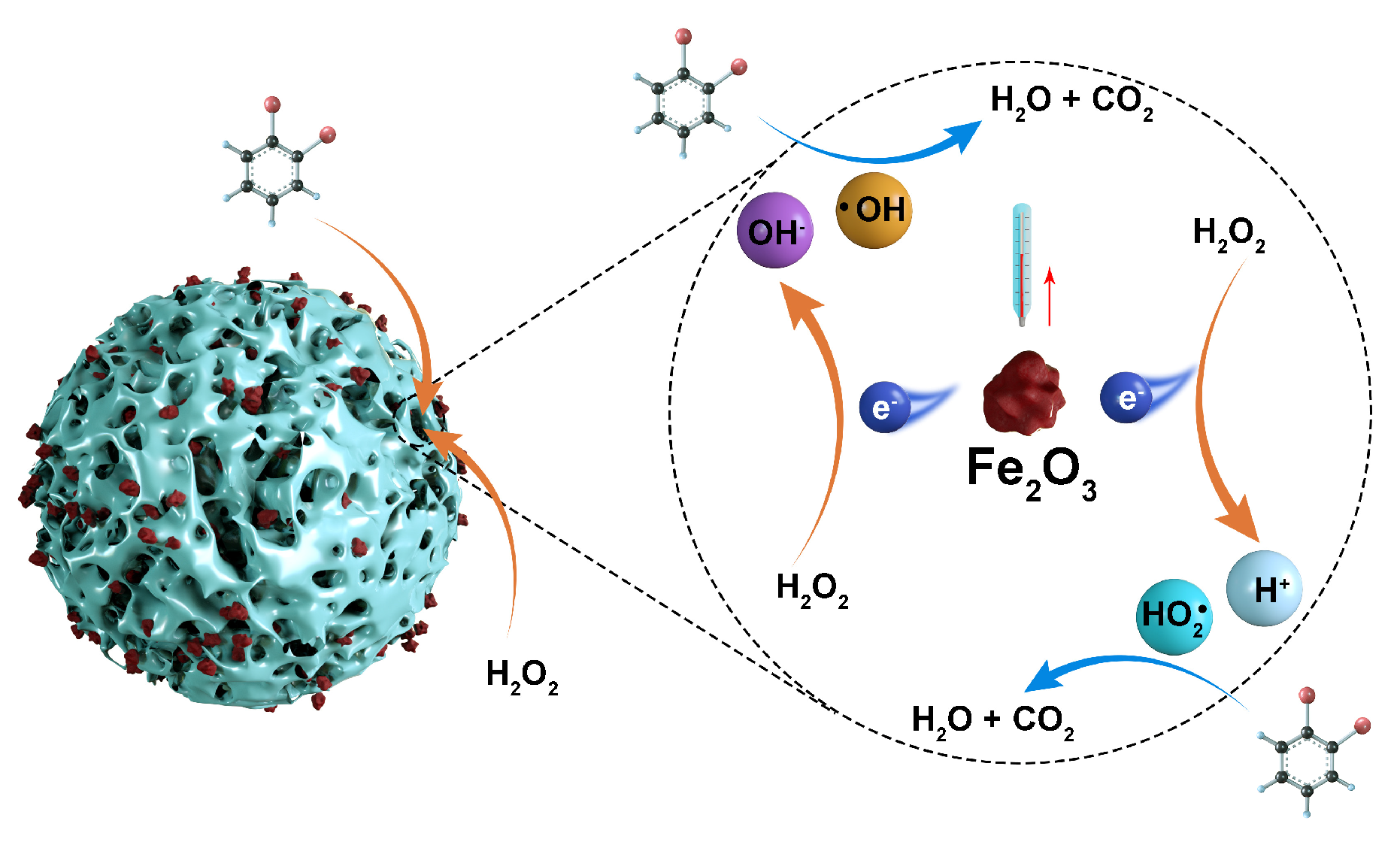
Previous studies have mainly been concerned with the degradation of wastewater using catalysts and conversion of organic matter, and studies on energy utilization in wastewater degradation processes are lacking. In this study, a CSAC-loaded Fe2O3 multiphase Fenton-like catalyst was prepared using 2–4 mm industrial-grade granular CSAC as the carrier, and the degradation performance of the Fe2O3@CSAC/H2O2 multiphase Fenton-like system and exothermic pattern of the degradation of OPD simulated wastewater were investigated. The newly developed catalyst had good degradation performance for OPD wastewater, and the degradation of wastewater increased the temperature of the system so that it contained more recoverable thermal energy, providing a theoretical basis for the energy utilization of wastewater. The study schematic is shown in Figure 1.
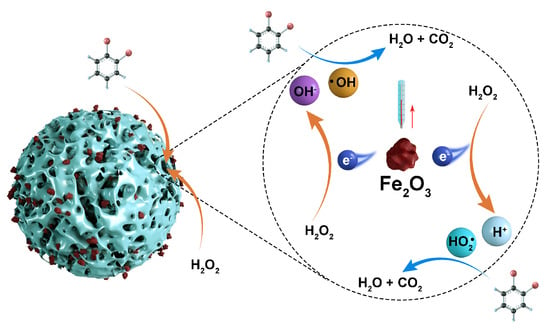
Figure 1.
Heterogeneous Fenton system degrades OPD and releases heat.
2. Materials and Methods
2.1. Instruments
Analytically pure C6H8N2(OPD), 30% H2O2, Fe2(SO4)3, H2SO4, NaOH, Ag2SO4, (NH4)2Fe(SO4)2·6H2O, and K2Cr2O7 were obtained from Sinopharm Chemical Reagent Co. (Shanghai, China), and coconut shell activated carbon (CSAC) was obtained from Zhengzhou Wastewater Treatment Materials Co. (Zhengzhou, China). Additional instruments included a tube furnace (XL-R; Yangzhou Xingliu Electric Co., Ltd., Yangzhou, China), drying oven (DZF; Beijing Kewei Yongxing Instrument Co., Ltd., Beijing, China) infrared spectrometer (ALPHAII; Bruker), field emission scanning electron microscope (Tescan Mira4; Regulus 8100, Hitachi, Tokyo, Japan), and an X-ray diffractometer (D8 Advance; Bruker, Billerica, MA, USA).
2.2. Preparation of Fe2O3@CSAC
CSAC particles with a particle size of 2–4 mm were selected and soaked in 1.5 mol·L−1 NaOH solution and 1.0 mol·L−1 H2SO4 solution for 24 h, respectively. After drying at 200 °C for 12 h, the modified CSAC was obtained. In order to ensure that the ratio between Fe3+ and CSAC in the preparation process was 1.2 mol·L−1: 500 g, 500 g modified CSAC was placed in 500 mL Fe2(SO4)3 solution with a concentration of 0.6 mol·L−1 and fully stirred for 24 h. The filtered samples were immersed in 500 mL of 0.6 mol·L−1 NaOH solution for 2 h, and then filtered and washed with deionized water until the solution was neutral. Fe2O3@CSAC was obtained by heating the filtered and washed material in a tube furnace at 500 °C for 2 h.
2.3. Degradation Experiment
A certain mass of OPD agent was accurately weighed with a precision balance and used to prepare a certain concentration of OPD simulated wastewater in a 2000 mL volumetric flask with deionized water. The pH of the simulated wastewater was adjusted with 0.05 mol·L−1 H2SO4 and 0.1 mol·L−1 NaOH.
The reaction vessel was a 500 mL round-bottomed flask with a temperature probe and a stirring rotary head inside the vessel. The temperature probe was connected to an external temperature recorder, the stirring rotary head was connected to an external mixer, and the reaction vessel was wrapped with a heat preservation layer. The water temperature of the constant temperature water bath was set at 10 °C above the reaction temperature at the beginning of the experiment for heating the simulated wastewater backup. The weighed Fe2O3@CSAC catalyst and 300 mL of heated OPD solution were poured into the round-bottomed flask successively, followed by stirring at 600 r·min−1. When the temperatures of the OPD solution and the Fe2O3@CSAC catalyst in the flask dropped to the set initial reaction temperature, the H2O2 solution was injected into the OPD solution, and a temperature recorder was activated at the same time. The sample solution was pipetted at 10 min intervals after the start of the reaction, and the reaction solution was filtered with a 102-type medium-speed qualitative filter paper and then used for COD (Chemical Oxygen Demand) testing.
2.4. Data Processing
The default reaction conditions were as follows: The temperature at the beginning of the reaction was 30 °C. The concentration of OPD was 0.04 mol·L−1, and H2O2 was added at 0.25 mol·L−1. Fe2O3@CSAC was added at 532 g·L−1, and the pH was adjusted to neutral. The number of experimental repetitions was 3, and the data were averaged across all experiments. The COD degradation rate was expressed as η. The amount of degradation was expressed as ΔC, and the amount of solution temperature increase during the reaction process was expressed as ΔT, calculated as shown in Equations (1)–(3):
where C0 is the COD value at the beginning of the reaction, C is the COD value during the reaction process, T is the temperature of the solution after the reaction, and T0 is the temperature of the reaction at the beginning of the reaction.
η (%) = ΔC/C0 × 100
ΔC = C0 − C
ΔT = T − T0
3. Results and Discussion
3.1. X-ray Diffraction (XRD) Analysis
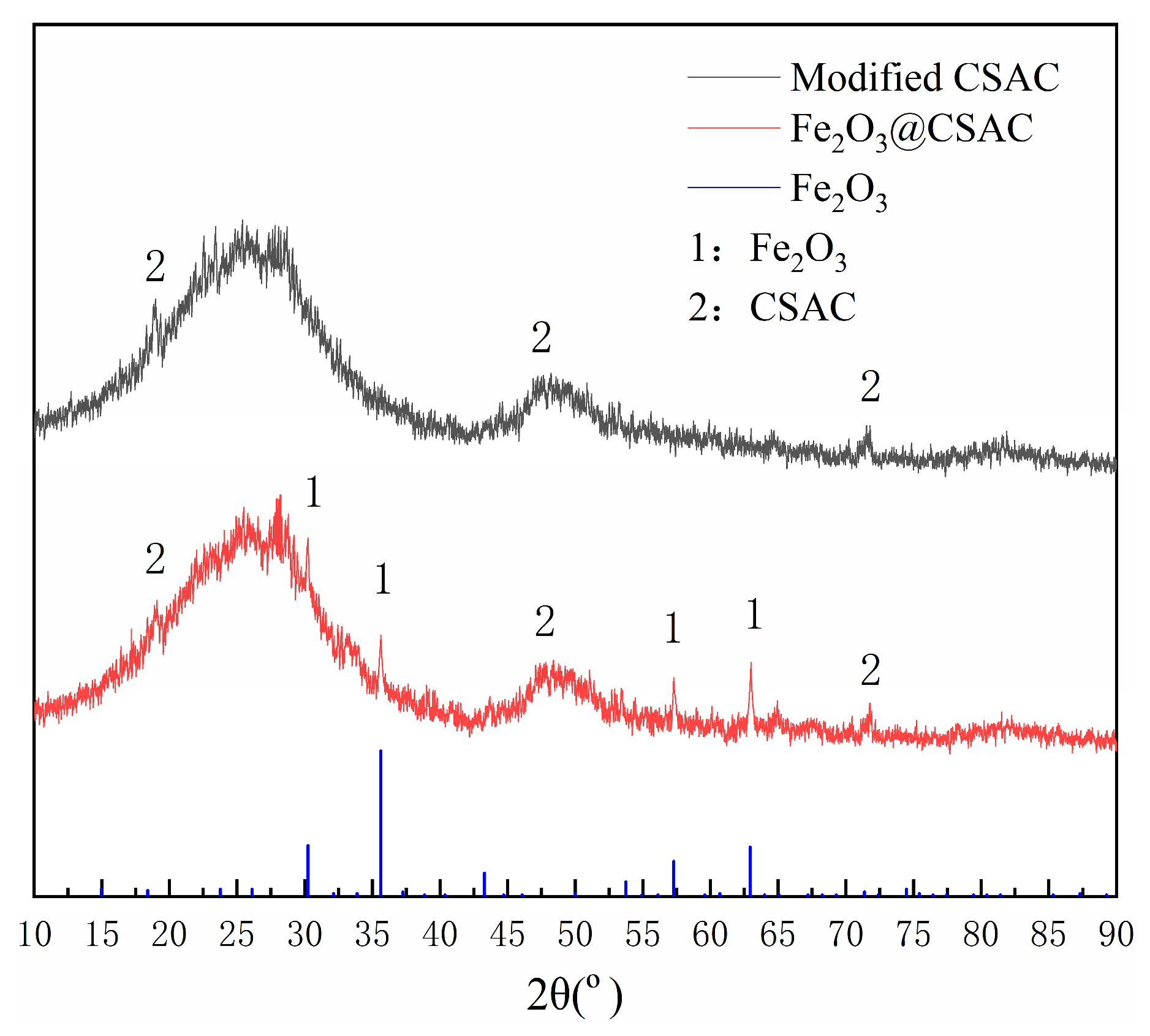
Figure 2 presents the XRD results of the modified CSAC and Fe2O3@CSAC. New characteristic peaks appeared for Fe2O3@CSAC, and the diffraction peaks at 30.2°, 35.6°, 57.3° and 63.0° corresponded to the (220), (311), (511), and (440) crystal planes, respectively, which is in general agreement with the standard card of maghemite γ-Fe2O3 (PDF#39-1346) [23]. These results indicate that Fe2O3 is the main active form and was successfully loaded onto the surface of the activated carbon. The Fe2O3@CSAC diffraction peaks were clear and sharp, suggesting that the backbone structure of CSAC did not change after loading Fe2O3.

Figure 2.
XRD patterns of modified CSAC and Fe2O3@CSAC.
3.2. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
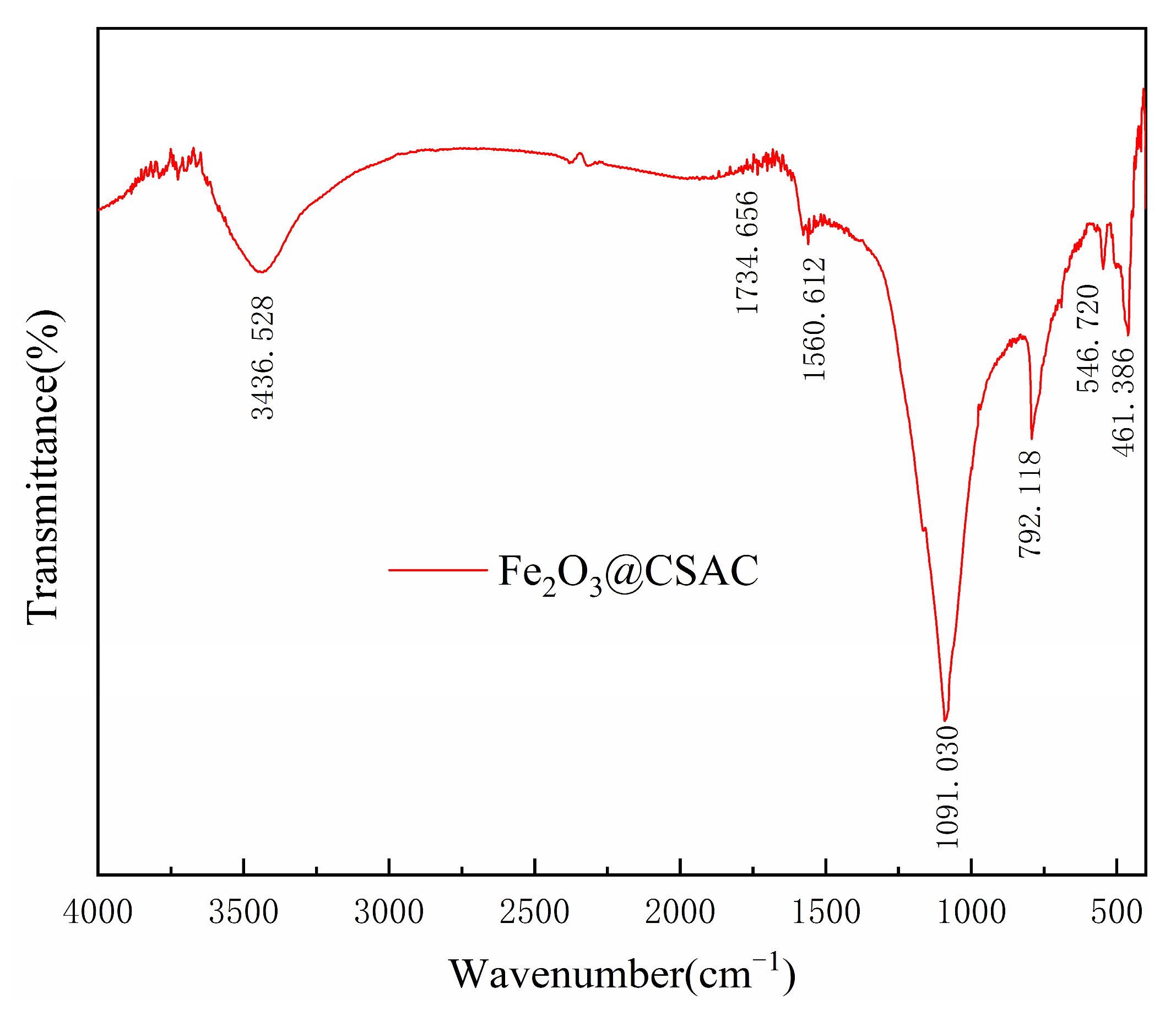
Figure 3 presents the FTIR results for Fe2O3@CSAC. The modified CSAC particles contained a large number of functional groups that are easily adsorbed and are good carrier materials for Fenton catalysts. The peaks near 3436.528 cm−1 were mainly due to the stretching vibration of O-H bonds in hydroxyl and carboxyl groups [24,25]. The absorption peaks at 1734.656 cm−1 and 1560.612 cm−1 were generated by the stretching vibration of C=O and aryl ring skeleton [24,26]. The peaks at 1091.030 cm−1 corresponded to C-O telescopic vibration peaks [24]. The 792.118 cm−1 absorption band indicates the presence of aromatic C-H bonds in CSAS [21,26]. Two telescopic vibration peaks, 546.720 cm−1 and 461.386 cm−1, were also present on Fe2O3@CSAC, consistent with Fe-O vibrations in γ-Fe2O3 [27], further demonstrating that Fe2O3 was successfully loaded onto the modified CSAC.
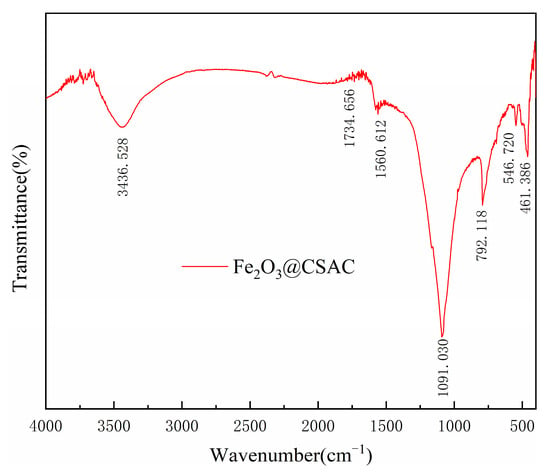
Figure 3.
FTIR images of Fe2O3@CSAC.
3.3. Scanning Electron Microscope (SEM) Analysis
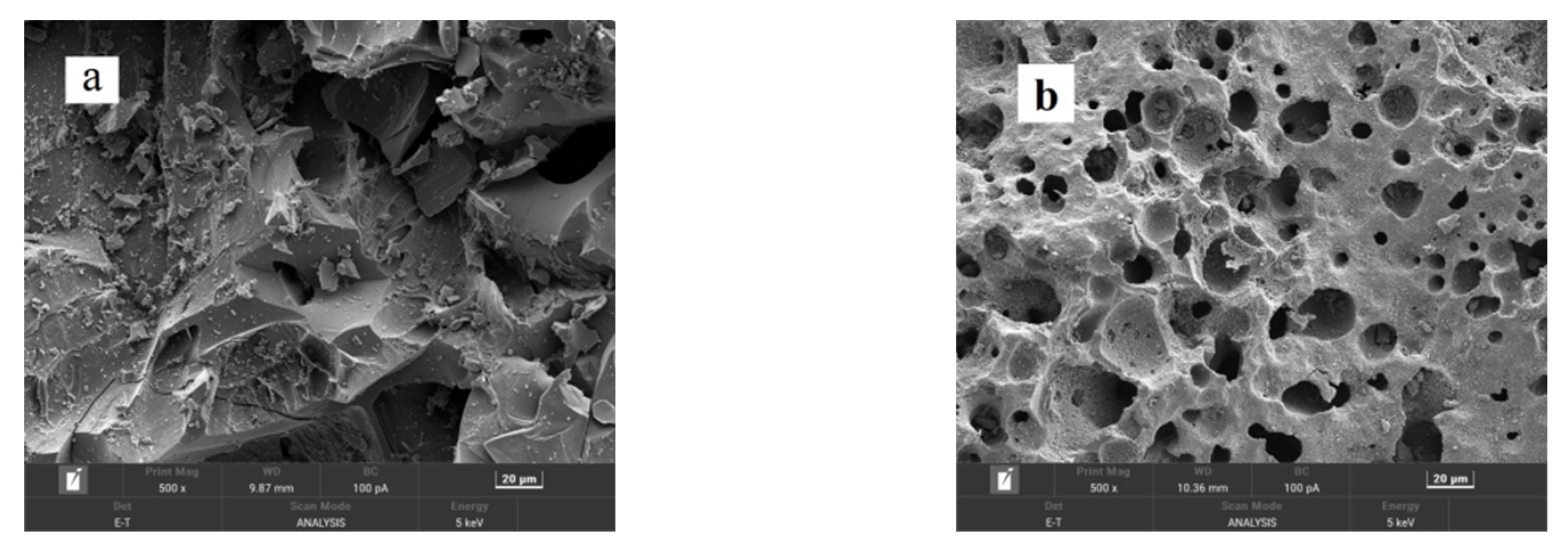
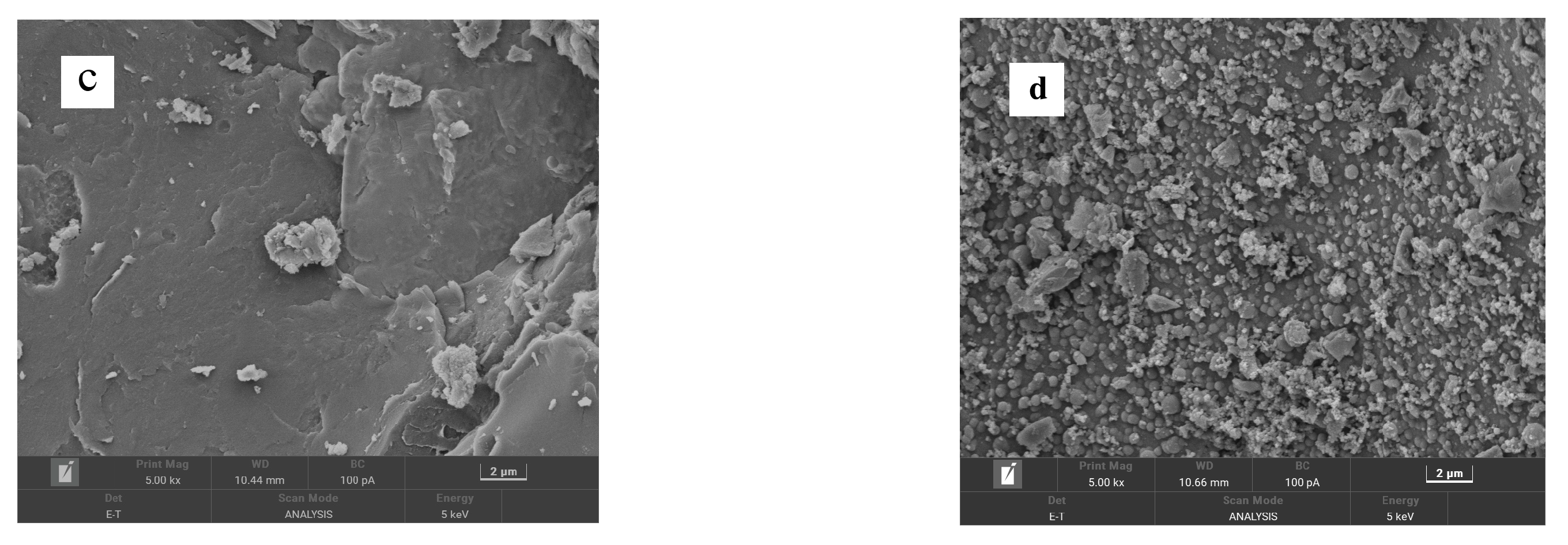
Figure 4 presents the SEM images of CSAC, modified CSAC (20 μm), modified CSAC (2 μm), and Fe2O3@CSAC. Modified CSAC pores were more abundant than those for CSAC. The porous structure of modified CSAC has a relatively large specific surface area that can be used for adsorption sites, making it an effective adsorbent. Adsorption performance is one of the main factors affecting the degradation performance of a heterogeneous Fenton system [12]. As shown in Figure 4c,d, Fe2O3 nanoparticles were uniformly dispersed on the modified CSAC without agglomeration, indicating that the Fe2O3 nanoparticles can expose more active sites, thereby increasing the catalytic activity of Fe2O3@CSAC.

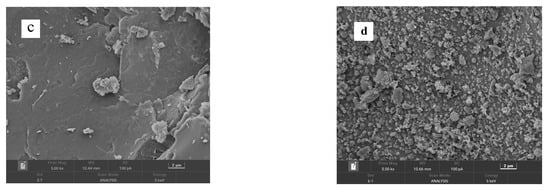
Figure 4.
SEM images of CSAC (a), modified CSAC (20 μm) (b), modified CSAC (2 μm) (c), and Fe2O3@CSAC (d).
3.4. Effect of Catalyst Dosage
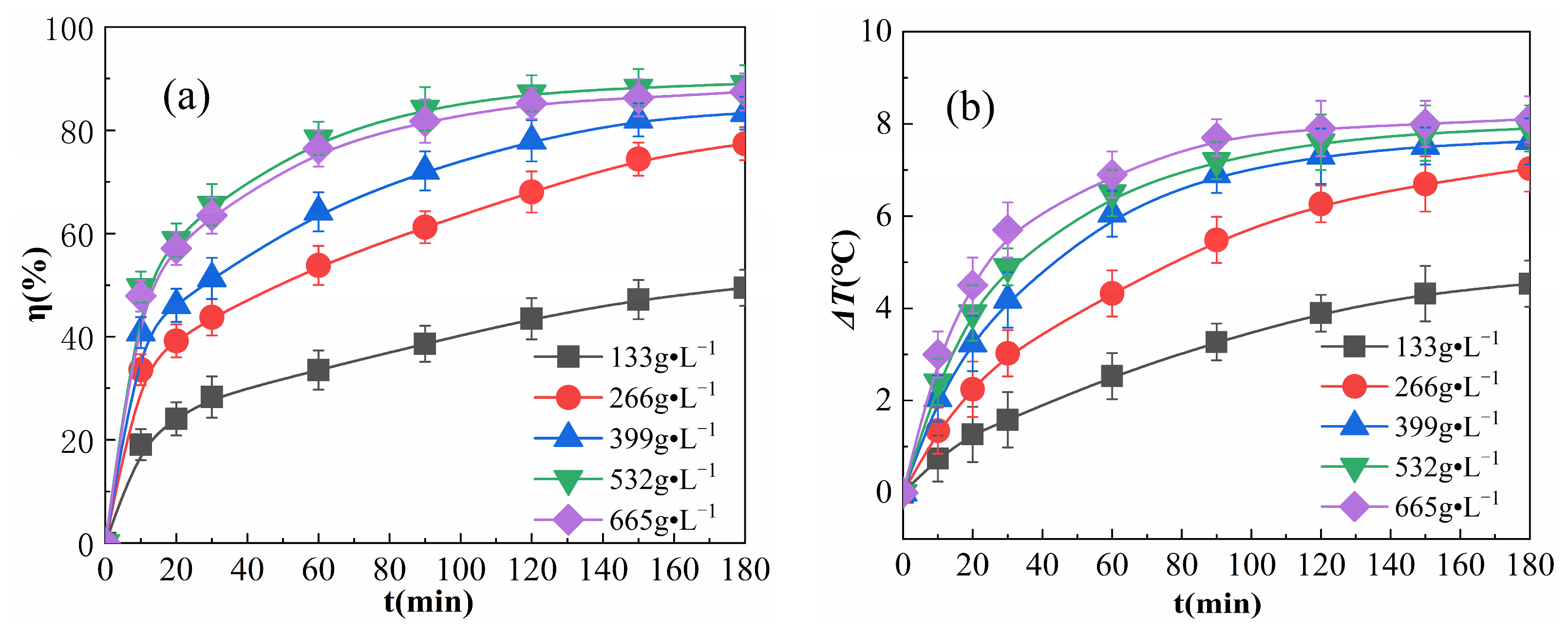
The effects of various concentrations of Fe2O3@CSAC in the reaction are summarized in Figure 5.
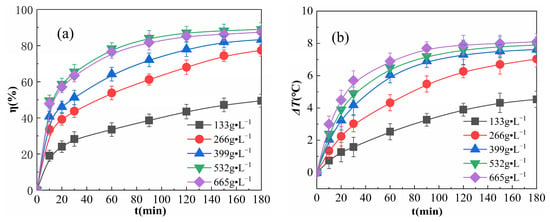
Figure 5.
Effect of catalyst addition on η (a) and ΔT (b).
As shown in Figure 5a, η increased rapidly in the first 20 min of the reaction. After the reaction proceeded for 20 min, η showed an initial increase, followed by a decrease in the rate of increase. Fe2O3@CSAC increased and eventually reached a plateau at 665 g·L−1. This is because as Fe2O3@CSAC increased, the active points increased and the number of radicals, such as ·OH and HO2·, produced from catalyzed H2O2 per unit time increased, prompting a rise in η. Further increasing Fe2O3@CSAC led to the rapid decomposition of H2O2 according to Reaction (4) below, followed by rapid decomposition via Reaction (5), while accelerating Reactions (6)–(9) and depleting free radicals, such as ·OH, were used to degrade OPD molecules [28]:
Fe3+ + H2O2 → Fe2+ + HO2· + H+
Fe2+ + H2O2 → Fe3+ + ·OH + HO−
Fe2+ + ·OH →Fe3+ + HO−
HO2· → O2— + H+
·OH + HO2· → H2O + O2
Fe3+ + O2− → Fe2+ + O2
Furthermore, the growth rates of η for different Fe2O3@CSAC concentrations all slowed gradually as the reaction proceeded. This is because as the reaction continues and H2O2 is consumed, the amount of radicals, such as ·OH, decomposed by H2O2 decreases and the degradation rate slows down.
As shown in Figure 5b, the solution temperature rose gradually as the reaction proceeded, indicating that heat was released during the reaction, and the more Fe2O3@CSAC was added, the larger ΔT reached. At each time point, the increase in ΔT after the addition of Fe2O3@CSAC over 399 g·L−1 was small. This is due to the fact that the total exothermic capacity in the multiphase Fenton-like system is related to the total reaction volume in the system, mainly derived from the amount of free radicals, such as ·OH, for the catalytic decomposition of H2O2, which is affected by two factors, the amount of active points of Fe2O3@CSAC and the amount of H2O2 adsorbed. A gradual increase in the amount of Fe2O3@CSAC increased the amount of active points and adsorbed H2O2 in the system, and more H2O2 was catalyzed to decompose ·OH and other radicals, resulting in an increase in the total reaction volume and the total exothermic reaction, as well as a larger increase in ΔT. Continuing to increase the addition of Fe2O3@CSAC, the amount of active points in the system increased; however, limited by the amount of H2O2 in the system, the amount of H2O2 adsorbed and the amount of catalytic decomposition of radicals, such as ·OH, did not increase further, and the increase in ΔT slowed. As shown in Figure 5b, the reaction proceeded to a certain point, and as Fe2O3@CSAC increased, the ΔT increased faster. This is because the active points in the system rise with the addition of Fe2O3@CSAC, and a greater number of radicals, such as ·OH, can be catalytically decomposed per unit time to react with OPD.
As seen in Figure 5a,b, when Fe2O3@CSAC is added at 665 g·L−1, η decreases while ΔT increases, compared to the addition of 532 g·L−1. This is because there are several reactions in the system, such as free radical production from H2O2 catalyzed by Fe2O3@CSAC, free radical degradation of OPD, free radical self-consumption, and the decomposition of H2O2 itself. The free radical degradation of OPD and free radical self-consumption are exothermic, and the exothermic degradation reaction only accounts for a part of the total exothermic reactions [29]. When the addition of Fe2O3@CSAC increases, the number of free radicals, such as ·OH, produced by catalytic H2O2 increases; accordingly, the total reaction volume increases, resulting in a continued increase in ΔT.
3.5. Effect of the H2O2 Concentration
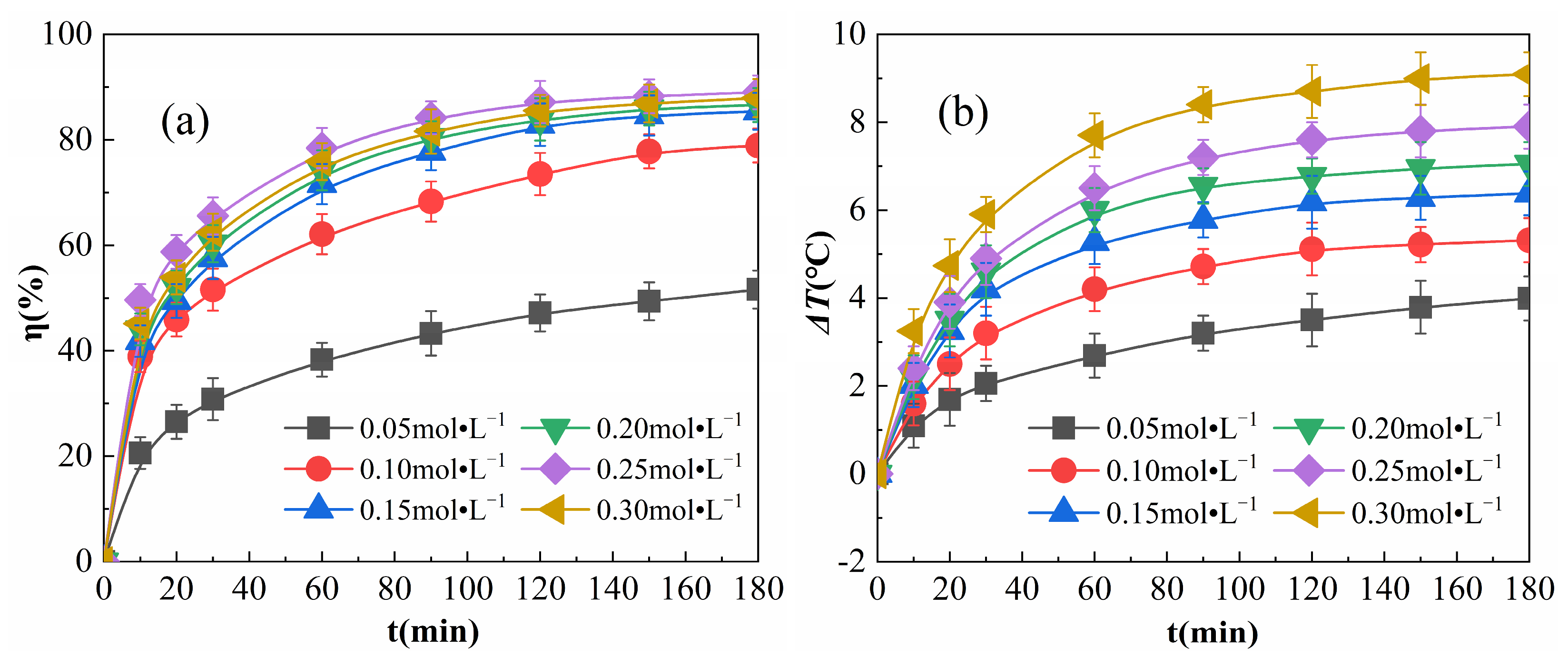
The results obtained when various amounts of H2O2 were added to the reaction conditions are shown in Figure 6.
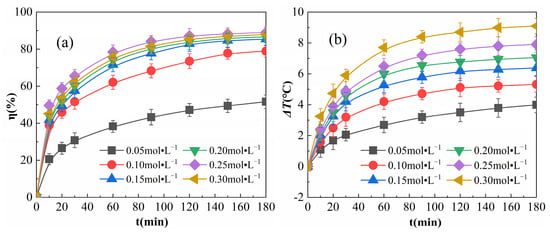
Figure 6.
Effects of H2O2 addition on η (a) and ΔT (b).
As shown in Figure 6a, the growth rate of η decreased gradually as the reaction proceeded. When H2O2 addition was increased from 0.05 to 0.25 mol·L−1, η increased, and from 0.25 to 0.3 mol·L−1, η decreased. This pattern of η increasing and then decreasing with the increase of H2O2 is similar to the results of Forouzesh et al. [30]. This is due to the fact that the number of free radicals, such as ·OH and HO2, in the reaction system increases with increasing H2O2 concentrations, which promotes the degradation of organic matter and leads to an increase in η. When increasing the amount of H2O2 added, the excess H2O2 may convert the highly reactive ·OH to other low-activity and highly selective radicals [31], as in Equation (10):
·OH + H2O2 → H2O + HO2
The depletion of radicals increases and the frequency of collisions between radicals and OPD molecules decreases, resulting in a decrease in η of the OPD solution [32,33]. As the reaction proceeds, the depletion of ·OH in the system results in a reduced rate of η growth. Additionally, η was found to be lower when the amount of H2O2 added was 0.05 mol·L−1, and still tended to continue to rise when the reaction reached 180 min; this is because when the concentration of H2O2 was too low, the surface of Fe2O3@CSAC was unable to adsorb a sufficient amount of H2O2, and in turn, the catalytic decomposition of free radicals such as ·OH was also low, which delayed the degradation of OPD.
As shown in Figure 6b, in the initiation phase of the reaction, as more H2O2 was added, ΔT rose faster. This is because the greater the amount of H2O2 added, the more easily H2O2 can be adsorbed by Fe2O3@CSAC and decompose ·OH, thereby increasing the total reaction volume and heat release in the system. The rate of increase of ΔT decreased as the reaction proceeded because as the H2O2 in the system was consumed, both the adsorption and decomposition of H2O2 slowed down, resulting in a reduction in the total reaction volume and exothermic amount. At any time point, ΔT showed a similar increase to that for the H2O2 addition, indicating that the surface of Fe2O3@CSAC is very rich in active sites with superior catalytic performance. As a result, the catalytic decomposition of a higher concentration of H2O2 is still efficient, and an increase in H2O2 can effectively increase the exothermic reaction in the system.
3.6. Influence of the Initial Reaction Temperature
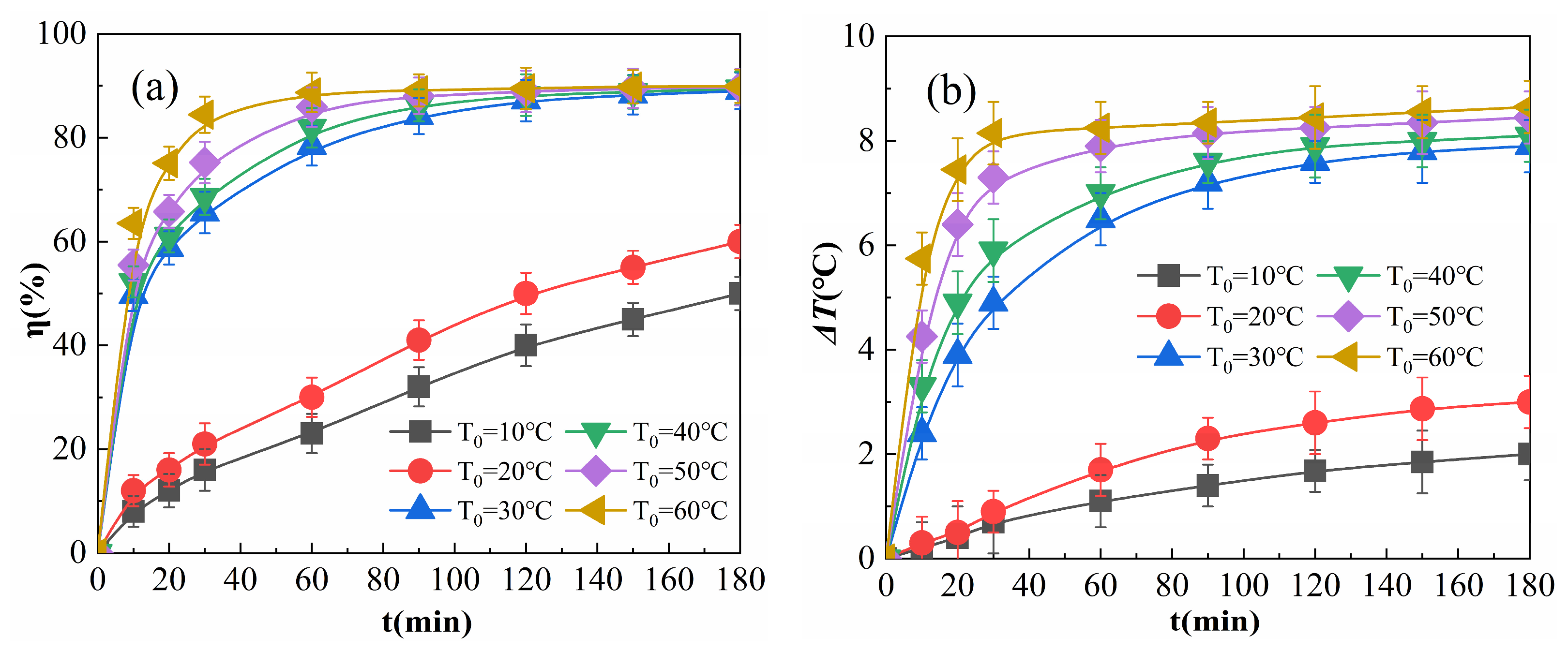
The results for various starting temperatures (T0) of the solution are shown in Figure 7.
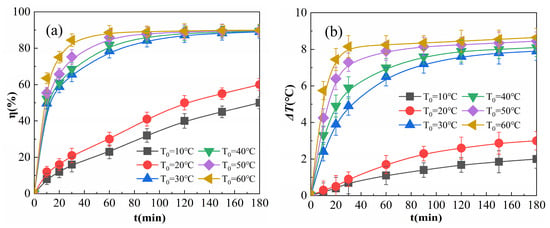
Figure 7.
Effects of the initial reaction temperature on η (a) and ΔT (b).
As shown in Figure 7a, when T0 is greater than 30 °C, at the initial stage of the reaction, η increases with T0, consistent with the reports of Wang et al. [34] and Liu et al. [35]. This is due to the fact that an increase in T0 in the initial stage of the reaction improves molecular activity, accelerates the adsorption and catalytic decomposition of H2O2, increases the production of free radicals, such as ·OH, per unit time, and increases degraded organic matter. When T0 is greater than 30 °C and the reaction proceeds to 180 min, η is very close. This is due to the fact that when the amount of H2O2 and OPD in the system is constant, the total amount of ·OH catalyzed by H2O2 as well as the number of OPD molecules degraded are nearly constant, even though the temperature increases the rate of the reaction. Although the temperature increases the reaction rate, it has a small effect on the percentage of collisions between radicals and OPD molecules or between the radicals, and therefore, the higher T0 is, the faster the rate of degradation. When the reaction time is sufficiently long, the η of the solution is almost constant. When T0 values were 10 °C and 20 °C, η was lower and the growth rate was slower, and η tended to increase when the reaction was carried out to 180 min. This is because the low T0 affects the speed of Reactions (4)–(9); when the temperature is less than 20 °C, only some of the active H2O2 molecules have gained enough activation energy for the reaction, and as the reaction proceeds, the temperature of the system gradually increases and the H2O2 molecules gradually react, so that η and ΔT rise more gradually below 20 °C.
As shown in Figure 7b, the higher T0 was at the initial stage of the reaction, the faster ΔT increased as the reaction proceeded. When T0 was raised to 60 °C from 30 °C with 180 min of reaction, ΔT increased slightly. A higher T0 is associated with faster adsorption of Fe2O3@CSAC, which improves the rates of catalytic decomposition and degradation, and more heat is generated per unit time of the reaction, resulting in a higher temperature on the surface of Fe2O3@CSAC. Because higher temperatures lead to faster diffusion of heat, ΔT rises faster in the initiation phase of the reaction, and the reaction continues to the same moment at a higher ΔT. When T0 is 10 °C or 20 °C, ΔT is smaller and ΔT continues to increase as the reaction proceeds. This is because the low temperature inhibits the adsorption and catalytic decomposition of H2O2 and reduces the reaction rate, resulting in a lower rate of heat production in the system; the heat output is not sufficient to rapidly increase molecular activity in the system, and thus, the reaction rate and ΔT are maintained at a low level. Therefore, for values of 10 °C and 20 °C, T0 has a substantial influence on the exothermic rate of the reaction; when T0 is greater than 30 °C, it has weaker effect on the total exothermic reaction.
3.7. Effect of Solution pH
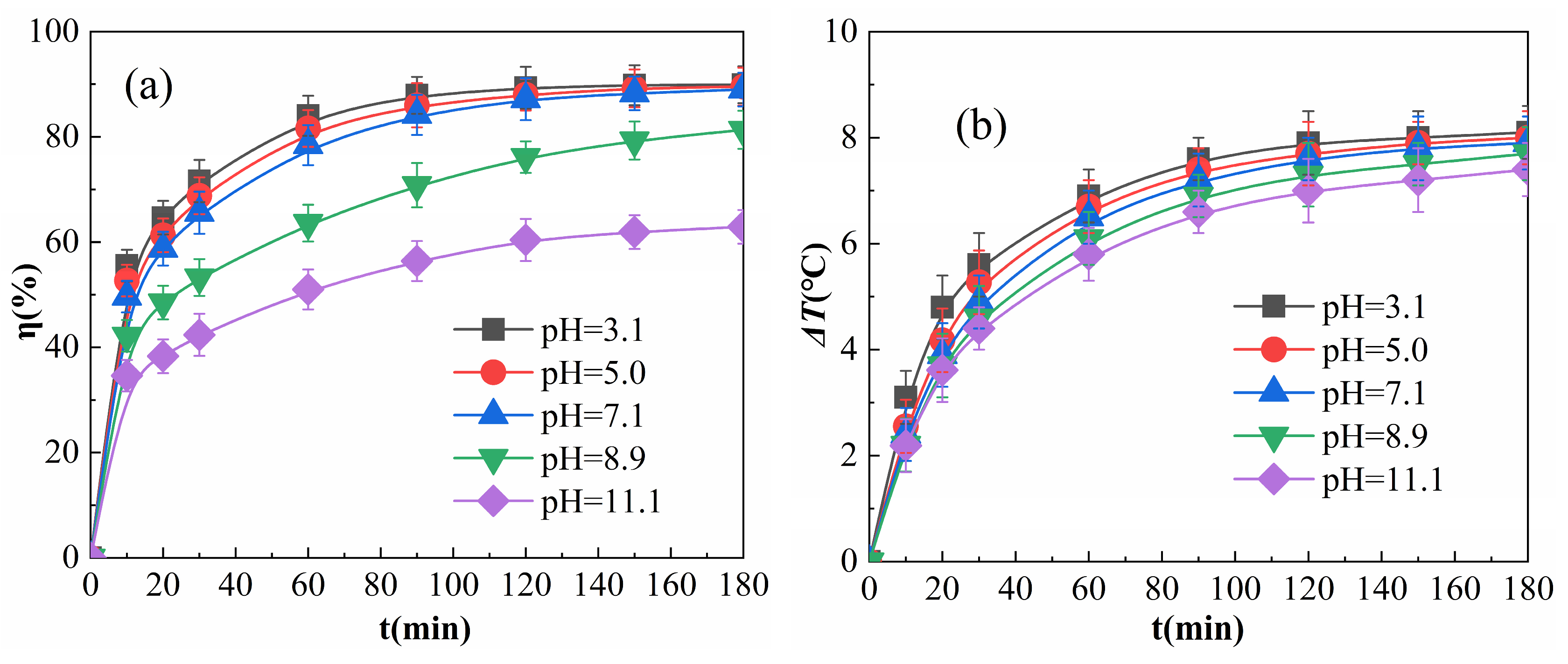
The results obtained when the initial pH of the reaction solution was adjusted are shown in Figure 8.
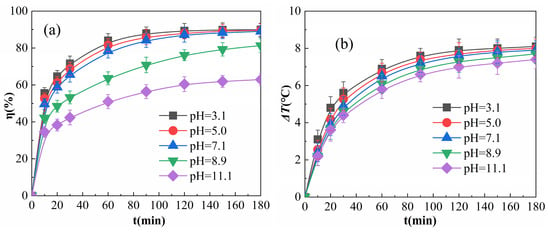
Figure 8.
Effects of pH on η (a) and ΔT (b).
As shown in Figure 8a, η tended to decrease with an increase in the initial pH from 3.1 to 11.1. η was very similar within the range of pH 3.1 to 7.1, decreasing slightly when the pH was increased to 8.9, and decreasing drastically when the pH was increased to 11.1. This is because Equations (4)–(5) are the key processes in the formation of ·OH in the multiphase Fenton-like system [36]. Since Fe3+ undergoes hydrolysis reaction to generate Fe(OH)3 precipitate at pH greater than 3 [37,38], Fe3+ has a strong binding ability with -OH (hydroxide ions). When the reaction system is in an alkaline environment, the concentration of -OH in the system is high, and the force between Fe3+ and -OH exposed in the active site of Fe2O3@CSAC influences the collisional binding between Fe3+ and H2O2; it also reduces the catalytic efficiency of H2O2 and fails to produce a sufficient amount of -OH, resulting in a lower η. Under the alkaline environment of pH 8.9, the rate of η increase slowed down as the reaction proceeded; however, there was still a gradual increase after 180 min of the reaction, indicating that Fe2O3@CSAC still had catalytic activity under the weak alkaline environment. When the pH was 11.1, the concentration of -OH in the solution was higher and the active sites of Fe2O3@CSAC were largely inhibited, resulting in a lower η than those for other pH values and little increase in η after 180 min of reaction.
As shown in Figure 8b, in the pre-reaction period, both ΔT and its rate of increase decreased slightly as the initial pH increased when the reaction proceeded. This is due to the fact that -OH binds to Fe3+ and inhibits the activity at the Fe2O3@CSAC active site. As pH increases, the higher the concentration of -OH, the more easily the Fe3+ of the Fe2O3@CSAC active site is seized by -OH, resulting in a lower efficiency of catalytic decomposition of H2O2 and a decrease in the exothermic reaction. An alkaline environment had a weaker effect than those of other pH values on ΔT of the system. Although the active site of Fe2O3@CSAC is inhibited by -OH, some of the H2O2 molecules are still catalytically decomposed when adsorbed onto the surface of Fe2O3@CSAC; however, the amount of free radicals catalyzed is small as radical quenching [39] occurs before diffusion. Due to the quenching reaction, free radicals themselves also release heat [40], resulting in a local temperature increase on the Fe2O3@CSAC surface and the pores within, and this increase improves the molecular activity and diffusion rate. Although a higher -OH concentration reduces the probability of collision between H2O2 molecules and the active site, the increase in molecular activity can also increase the number of collisions between the active site and H2O2 molecules per unit of time. The ΔT of the system is induced by the quenching reaction rather than the degradation reaction, resulting in a high ΔT.
3.8. Effect of the Initial Concentration of OPD
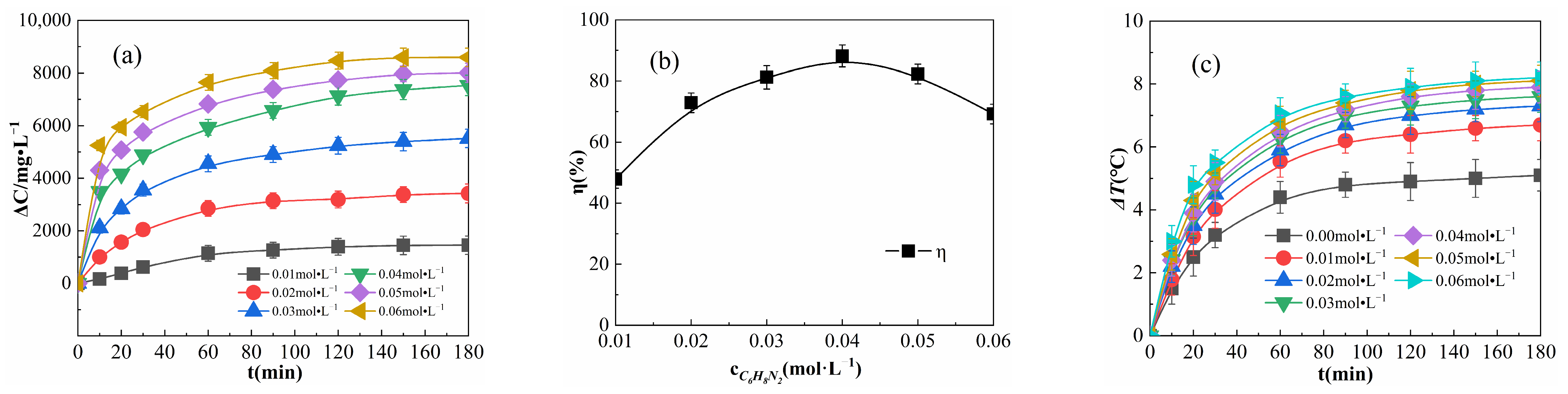
The results obtained for various OPD concentrations in the reaction system are shown in Figure 9.
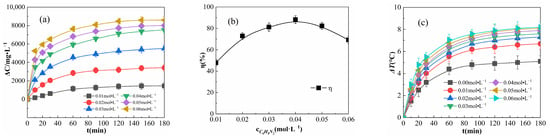
Figure 9.
Effects of the OPD concentration on ΔC (a), η (b), and ΔT (c).
As shown in Figure 9a, ΔC increases with the increase in OPD concentration; when the concentration of OPD is greater than 0.04 mol·L−1 and the reaction time is greater than 60 min, the difference between the ΔC values decreases. For example, when the reaction was carried out for 180 min, the ΔC difference between 0.03 and 0.04 mol·L−1 was 2014.362 mg·L−1, and the ΔC difference between 0.04 and 0.05 mol·L−1 was 477.288 mg·L−1. As shown in Figure 9a, at any time point, ΔC increased with an increase in the OPD concentration, and the increase in ΔC became smaller when the addition of OPD exceeded 0.04 mol·L−1. This is due to the fact that increasing OPD concentration results in increasing the chances of effective collision between OPD molecules and free radicals in the solution as well as an increase in the number of degraded OPD molecules. As the number of OPD molecules increases, limited by the amount of Fe2O3@CSAC and H2O2 in the system, H2O2 is rapidly consumed and a sufficient amount of ·OH cannot be produced, resulting in a decrease in the rate of ∆C increase. Figure 9b shows the η of the reaction for 180 min under different OPD concentrations, revealing that η first increased and then decreased. Limited by the Fe2O3@CSAC and H2O2 injection, when the OPD concentration is lower than 0.04 mol·L−1, the excess catalyst and H2O2 in the solution can produce enough free radicals to degrade OPD, and when the addition of OPD exceeds 0.04 mol·L−1, the increase in OPD exceeds the degradation of OPD and η decreases.
As shown in Figure 9c, ΔT was still greater than 0 at an OPD concentration of 0 mol·L−1, indicating that the Fe2O3@CSAC/H2O2 multiphase Fenton-like system itself generates heat. The increase in ΔT is greater with increasing OPD concentration, which is due to the fact that free radicals, such as -OH, react with OPD molecules in a more exothermic manner than quenching themselves [29]. Therefore, in the degradation of OPD by the Fe2O3@CSAC/H2O2 multiphase Fenton-like system, increasing the concentration of OPD can improve the exothermic reaction and rate of temperature rise.
3.9. Kinetics
The solution ΔT is very closely related to the degradation of OPD; accordingly, analyzing the kinetics of the degradation process can provide insight into the exothermic process of the system. The first-order kinetic equation is shown in Equation (11):
where t denotes the reaction time, Ct is the amount of reactants at time t, and k is the first-order reaction rate constant. The solution of the kinetic equation expressed in terms of η is as described in Equation (12):
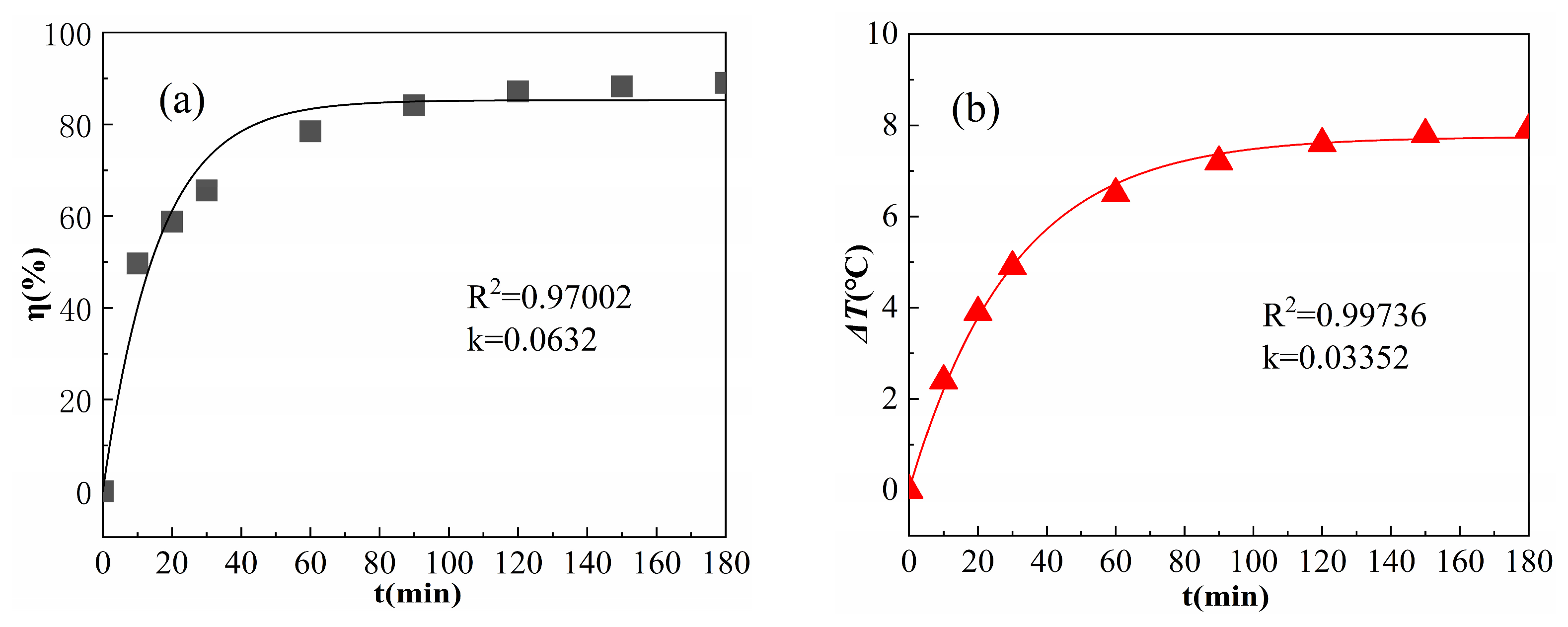
The kinetics of η under the fitted default reaction conditions are shown in Figure 10a.

Figure 10.
First-order kinetic equation fitting of η (a) and ΔT (b).
As shown in Figure 10a, the R2 value obtained after η fitting was 0.97002, and k was 0.0632 min−1, indicating a good linear correlation and conformation to the characteristics of the proposed first-order kinetics. Because ΔT was mainly caused by the degradation of OPD and the quenching of free radicals, such as OH, ΔT and η were expected to have a similar influence on the change rule. Equation (12) was modified to obtain Equation (13), and an attempt was made to model the law of ΔT versus reaction time using Equation (13).
The results of the simulation of ΔT with the first-order kinetic equation are shown in Figure 10b. The R2 value obtained after fitting was 0.99736 and the value of k was 0.03352 min−1, which shows that the pattern of ΔT versus reaction time is similar to the first-order kinetic characteristics. This is because the heat released from the degradation of OPD is higher than that of the quenching reaction [28], and ΔT is more closely related to the degradation of OPD; accordingly, ΔT has a kinetic law similar to that of η. Since the kinetic equation mainly represents the reaction rate relationship, the law of ΔT and reaction time expressed in Equation (13) only suggests a simple prediction of ΔT in actual industrial production.
4. Conclusions
Through analyses of the degradation performance and exothermic law of the Fe2O3@CSAC/H2O2 multiphase Fenton-like system, the following conclusions were drawn.
- (1)
- Fe2O3 was uniformly dispersed on the CSAC carrier and had a better catalytic effect in the Fe2O3@CSAC/H2O2 multiphase-like Fenton system. The OPD degradation process releases a large amount of heat, which is in line with the characteristics of a first-order reaction and can be used as a theoretical basis for the use of energy in wastewater treatment. A 300 mL solution of 0.04 mol·L−1 OPD had an η of 89.0% and a ΔT of 7.9 °C under an H2O2 concentration of 0.25 mol·L−1, Fe2O3@CSAC concentration of 532g·L−1, pH of 7.1, and T0 of 30 °C.
- (2)
- In the Fe2O3@CSAC/H2O2 multiphase Fenton-like system, the additions of H2O2 and Fe2O3@CSAC had a large influence on the exothermic reaction and degradation rate. Increases in H2O2 and Fe2O3@CSAC within a certain range could accelerate the reaction rate, exothermic reaction, and degradation rate of the system.
- (3)
- The Fe2O3@CSAC/H2O2 multiphase Fenton-like system had a wide initial pH adaptability and good degradation performance in the range of pH 3.1–8.9. Variation in the initial pH had almost no effect on the exothermic reaction.
- (4)
- The higher the temperature at the beginning of the reaction, the faster the degradation of pollutants and the faster the temperature rise of the system. Under the same conditions, the exothermic reaction is enhanced by a concentration increase.
This research studied the degradation performance and exothermic rules of the Fe2O3@CSAC/H2O2 multiphase Fenton-like system, which has important implications for energy recovery during wastewater treatment. In practical industrial applications, the amount of H2O2 and Fe2O3@CSAC added can be adjusted according to various factors including energy recovery and cost of chemicals to obtain higher economic benefits. Further, utilizing the Fe2O3@CSAC/H2O2 multiphase Fenton-like system to treat wastewater while controlling the pH of wastewater in the range of 3.1–8.9 can improve degradation; concentrating the wastewater and increasing the initial temperature can further improve the degradation of pollutants and the speed of heat recovery. However, analyses of the exothermic reaction in each process in the system are still lacking and this will be the focus of subsequent research.
Author Contributions
Project administration, Y.Y; funding acquisition, Y.Y.; writing—original draft preparation, K.Z.; writing—review and editing, Y.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Key Research and Development Project of Shandong (No. 2022CXGC021002-1).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Cheng, Y.; Wu, Y.; Bai, S. A smart community waste heat recovery system based on air source-sewage source compound heat pump. Int. J. Heat Technol. 2021, 39, 503–511. [Google Scholar] [CrossRef]
- Yu, Y.; Li, H.; Wang, L. Applications and future development of sewage source heat pump. In Proceedings of the 2016 International Conference on Biological Engineering and Pharmacy (BEP 2016), Shanghai, China, 9–11 December 2016; Volume 3, pp. 332–334. [Google Scholar]
- Yang, B.; Wang, Y.; Liu, Z.; Liu, J.; Cai, J. Optimum removal conditions of aniline compounds in simulated wastewater by laccase from white-rot fungi. J. Environ. Health Sci. Eng. 2019, 17, 135–140. [Google Scholar] [CrossRef]
- Hou, C.; Fu, L.; Wang, Y.; Chen, W.; Chen, F.; Zhang, S.; Wang, J. Core-shell magnetic Fe3O4/CNC@MOF composites with peroxidase-like activity for colorimetric detection of phenol. Cellulose 2021, 28, 9253–9268. [Google Scholar] [CrossRef]
- Yu, Q.; Feng, L.; Chai, X.; Qiu, X.; Ouyang, H.; Deng, G. Enhanced surface Fenton degradation of BPA in soil with a high pH. Chemosphere 2019, 220, 335–343. [Google Scholar] [CrossRef]
- Li, X.; Chen, W.; Ma, L.; Wang, H.; Fan, J. Industrial wastewater advanced treatment via catalytic ozonation with an Fe-based catalyst. Chemosphere 2018, 195, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Alazaiza, M.Y.D.; Albahnasawi, A.; Eyvaz, M.; Nassani, D.E.; Abu Amr, S.S.; Abujazar, M.S.S.; Al-Maskari, O. Electrochemical-based advanced oxidation for hospital wastewater treatment. Desalin. Water Treat. 2023, 300, 44–56. [Google Scholar] [CrossRef]
- Sun, Y.; Yu, I.K.M.; Tsang, D.C.W.; Fan, J.; Clark, J.H.; Luo, G.; Zhang, S.; Khan, E.; Graham, N.J.D. Tailored design of graphitic biochar for high-efficiency and chemical-free microwave-assisted removal of refractory organic contaminants. Chem. Eng. J. 2020, 398, 125505. [Google Scholar] [CrossRef]
- Sun, Y.; Cho, D.; Graham, N.J.D.; Hou, D.; Yip, A.C.K.; Khan, E.; Song, H.; Li, Y.; Tsang, D.C.W. Degradation of antibiotics by modified vacuum-UV based processes: Mechanistic consequences of H2O2 and K2S2O8 in the presence of halide ions. Sci. Total Environ. 2019, 664, 312–321. [Google Scholar] [CrossRef]
- Cüce, H.; Aydın Temel, F.A. Efficient removal performance of COD in real laundry wastewater via conventional and photo-Fenton degradation systems: A comparative study on oxidants and operating time by H2O2/Fe2+. Arab. J. Sci. Eng. 2023, 48, 15823–15835. [Google Scholar] [CrossRef]
- Wang, R.; Liu, X.; Wang, Y.; Shao, Z. Research progress of Fenton oxidation treatment refractory organic wastewater. Ind. Water Treat. 2022, 42, 58–66. [Google Scholar]
- Hussain, S.; Aneggi, E.; Goi, D. Catalytic activity of metals in heterogeneous Fenton-like oxidation of wastewater contaminants: A review. Environ. Chem. Lett. 2021, 19, 2405–2424. [Google Scholar] [CrossRef]
- Liu, Y.; Jin, W.; Zhao, Y.; Zhang, G.; Zhang, W. Enhanced catalytic degradation of methylene blue by α-Fe2O3/graphene oxide via heterogeneous photo-Fenton reactions. Appl. Catal. B 2017, 206, 642–652. [Google Scholar] [CrossRef]
- Zhang, M.H.; Dong, H.; Zhao, L.; Wang, D.X.; Meng, D. A review on Fenton process for organic wastewater treatment based on optimization perspective. Sci. Total Environ. 2019, 670, 110–121. [Google Scholar] [CrossRef]
- Pelalak, R.; Hassani, A.; Heidari, Z.; Zhou, M. State-of-the-art recent applications of layered double hydroxides (LDHs) material in Fenton-based oxidation processes for water and wastewater treatment. Chem. Eng. J. 2023, 474, 145511. [Google Scholar] [CrossRef]
- Dong, P.; Shan, P.; Wang, S.; Ge, B.; Zhao, C. Heterogeneous Fenton treatment of shale gas fracturing flow-back wastewater by spherical Fe/Al2O3 catalyst. Environ. Sci. Pollut. Res. Int. 2023, 30, 105685–105699. [Google Scholar] [CrossRef]
- Liu, L.; Yu, R.; Zhao, S.; Cao, X.; Zhang, X.; Bai, S. Heterogeneous Fenton system driven by iron-loaded sludge biochar for sulfamethoxazole-containing wastewater treatment. J. Environ. Manag. 2023, 335, 117576. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, Y.; Hong, M.; Peng, B.; Bao, C.; Xu, X.L.; Li, D.; Chen, J.; Wang, B.; Zhang, Q. Ferrocene-based resin as heterogeneous Fenton-like catalyst for efficient treatment of high salinity wastewater at acidic, neutral, and basic pH. J. Haz. Mater. 2023, 459, 132258. [Google Scholar] [CrossRef]
- Ahmad, R.K.; Sulaiman, S.A.; Yusup, S.; Dol, S.S.; Inayat, M.; Umar, H.A. Exploring the potential of coconut shell biomass for charcoal production. Ain Shams Eng. J. 2022, 13, 101499. [Google Scholar] [CrossRef]
- Li, X.; Li, X.; Song, C.; Yang, X.; Liu, Y.; Zhu, J. Efficient degradation of tetrabromobisphenol A using peroxymonosulfate oxidation activated by a novel Nano-CuFe2O4@coconut shell biochar catalyst. Environ. Pollut. 2023, 337, 122488. [Google Scholar] [CrossRef]
- Pang, Y.L.; Law, Z.X.; Lim, S.; Chan, Y.Y.; Shuit, S.H.; Chong, W.C.; Lai, C.W. Enhanced photocatalytic degradation of methyl orange by coconut shell–derived biochar composites under visible LED light irradiation. Environ. Sci. Pollut. Res. Int. 2021, 28, 27457–27473. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.H.; Zhang, G.J.; Liu, J.W.; Zhao, P.Y.; Hou, P.; Xu, Y.; Zhang, R.G. Effect of different activated carbon support on CH4-CO2 reforming over Co-based catalysts. Int. J. Hydrogen Energy 2017, 43, 1497–1507. [Google Scholar] [CrossRef]
- Shiyuan, H.; Jian, D.; Hanqin, Y.; Guohua, W.; Xingliang, W.U. Experimental study on activation of peroxymonosulfate by cobalt-enhanced ferromagnet. CIESC J. 2022, 73, 3045–3056. [Google Scholar]
- Li, N.; Dai, W.H.; Kang, H.B.; Lv, B.; Jiang, P.; Wang, W. Study on the adsorption performance and adsorption mechanism of graphene oxide by red sandstone in aqueous solution. Adsorpt. Sci. Technol. 2022, 2022, 2557107. [Google Scholar] [CrossRef]
- Feng, L.; Cheng, J.; Ding, S.; Zhang, H.; Zhang, H. Dynamic adsorption performance of activated carbon for advanced treatment on phenolic chemical wastewater. J. Saf. Environ. 2023, 23, 2447–2456. [Google Scholar]
- Jiayu, L.; Yue, Z.; Yuan, L.; Jingjing, X.; Shitang, T. Structure and properties of biochar under different materials and carbonization temperatures. Chin. J. Environ. Eng. 2016, 10, 3200–3206. [Google Scholar]
- Jian, J.I.N.; Zhihong, Z.; Hongfan, B.; Shitang, T. Preparation and performance of γ- Fe2O3/AC desulfurizer at low temperature for CS2 removal. Chem. Ind. Eng. Prog. 2018, 37, 4397–4404. [Google Scholar] [CrossRef]
- Wang, N.; Zheng, T.; Zhang, G.; Wang, P. A review on Fenton-like processes for organic wastewater treatment. J. Environ. Chem. Eng. 2016, 4, 762–787. [Google Scholar] [CrossRef]
- Yan, Y.; Mao, Y.; Dong, Y.; Zhang, K.; Sun, X.; Ma, C. Exothermic laws applicable to the degradation of o-phenylenediamine in wastewater via a Fe3+/H2O2 homogeneous quasi-Fenton system. RSC Adv. 2019, 9, 26283–26290. [Google Scholar] [CrossRef]
- Forouzesh, M.; Ebadi, A.; Aghaeinejad-Meybodi, A. Degradation of metronidazole antibiotic in aqueous medium using activated carbon as a persulfate activator. Sep. Purif. Technol. 2019, 210, 145–151. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, Z.; Yang, Z.; Yang, Q.; Li, B.; Bai, Z. Heterogeneous Fenton-like catalytic degradation of 2,4-dichlorophenoxyacetic acid in water with FeS. Chem. Eng. J. 2015, 273, 481–489. [Google Scholar] [CrossRef]
- Ding, M.; Chen, W.; Xu, H.; Lu, C.; Lin, T.; Shen, Z.; Tao, H.; Zhang, K. Synergistic features of superoxide molecule anchoring and charge transfer on two-dimensional Ti3C2Tx MXene for efficient peroxymonosulfate activation. ACS Appl. Mater. Interfaces 2020, 12, 9209–9218. [Google Scholar] [CrossRef]
- Huang, R.; Yang, J.; Cao, Y.; Dionysiou, D.D.; Wang, C. Peroxymonosulfate catalytic degradation of persistent organic pollutants by engineered catalyst of self-doped iron/carbon nanocomposite derived from waste toner powder. Sep. Purif. Technol. 2022, 291, 120963. [Google Scholar] [CrossRef]
- Wang, C.; Huang, R.; Sun, R.; Yang, J.; Dionysiou, D.D. Microplastics separation and subsequent carbonization: Synthesis, characterization, and catalytic performance of iron/carbon nanocomposite. J. Clean. Prod. 2022, 330, 129901. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, R.; Li, Y.; Qi, J.; Wang, C.; Li, J.; Wang, L. Sandwich-like Co3O4/MXene composite with enhanced catalytic performance for bisphenol A degradation. Chem. Eng. 2018, 347, 731–740. [Google Scholar] [CrossRef]
- Jin, X.; Su, J.; Yang, Q. A comparison study of Fenton-like and Fenton reactions in dichloromethane removal. Environ. Technol. 2022, 43, 4504–4514. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.L.; Mao, J.L.; Luo, G.W.; Lu, S.J.; Zhang, P.; Ma, Y.T.; Chen, S.L.; Du, Z.J.; Bowen, L.; Qiao, J.X. The formation mechanism of the third phase in nickel electrolyte. In Materials Engineering—From Ideas to Practice: An EPD Symposium in Honor of Jiann-Yang Hwang; Springer International Publishing: Cham, Switzerland, 2021; pp. 129–138. [Google Scholar] [CrossRef]
- Chen, A.L.; Mao, J.L.; Luo, G.W.; Lu, S.J.; Zhang, P.; Ma, Y.T.; Chen, S.L.; Du, Z.J.; Bowen, L.; Qiao, J.X. Iron(III) hydrolysis and solubility at 25 °C. Environ. Sci. Technol. 2007, 41, 6117–6123. [Google Scholar] [CrossRef]
- Zhou, P.; Meng, S.; Sun, M.L.; Hu, K.S.; Yang, Y.Y.; Lai, B.; Wang, S.B.; Duan, X.G. Insights into boron accelerated Fenton-like chemistry: Sustainable and fast FeIII/FeII circulation. Separation Purif. Technol. 2023, 317, 123860. [Google Scholar] [CrossRef]
- Yan, Y.T.; Zhang, K.; Mao, Y.P.; Dong, Y. Study on the exothermic characteristics of Fe2+/H2O2 homogeneous Fenton degradation of o-phenylenediamine wastewater. Desal. Water Treat. 2021, 228, 335–342. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).