The Effect of CuO on the Thermal Behavior and Combustion Features of Pyrotechnic Compositions with AN/MgAl
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
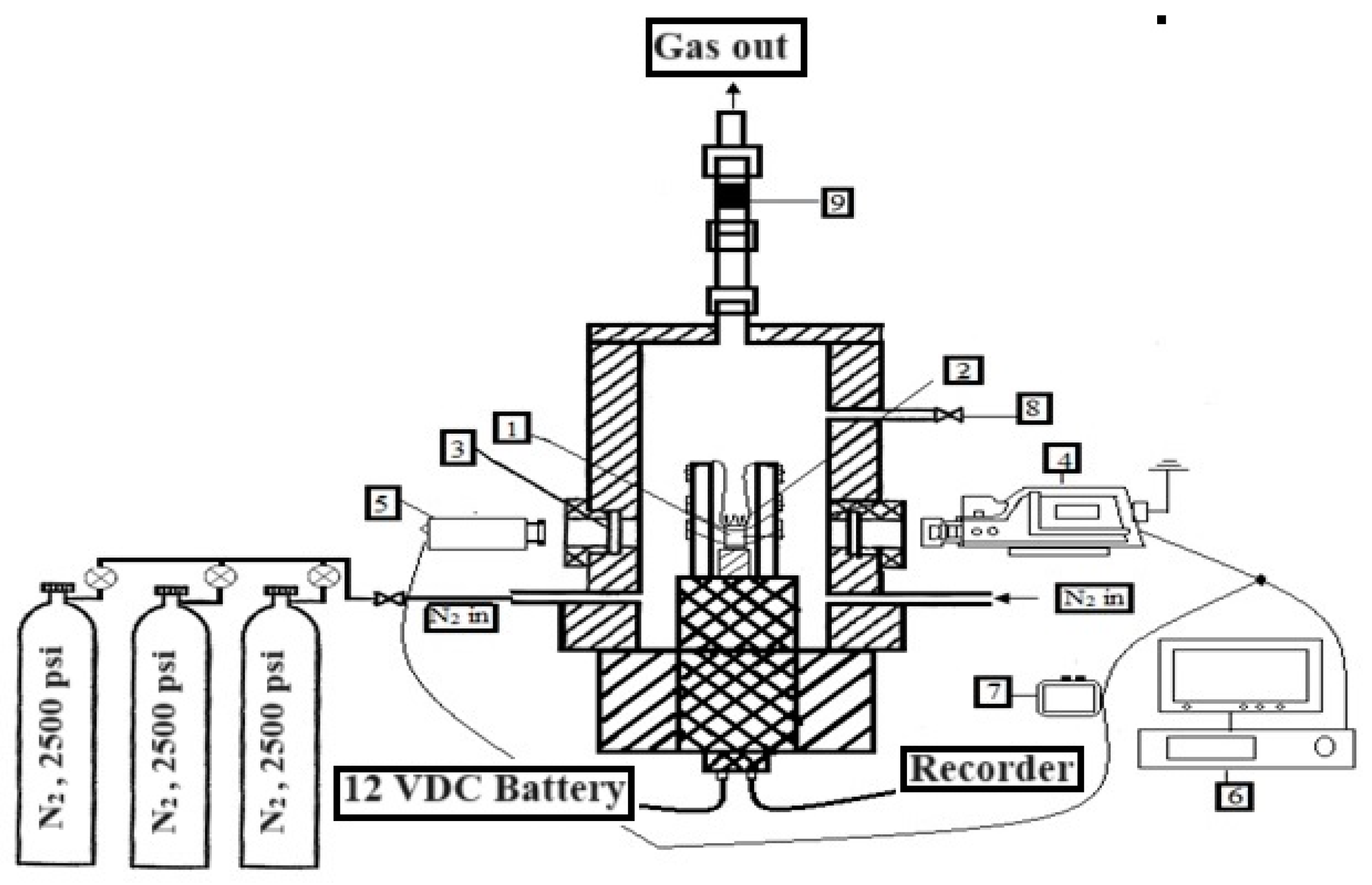
2.2. Preparation of Samples
2.3. Evaluation of Combustion Properties
2.4. Assessment of Thermal Characteristics of Pyrotechnic Mixtures
2.5. Determination of Composition and Structure of Materials
2.5.1. X-ray Diffraction Analysis
2.5.2. Methods for Studying Structure
3. Results
3.1. Physicochemical Characteristics of MgAl Alloy
3.2. Thermal Properties of Pyrotechnic Mixtures
3.3. Thermal Decomposition Kinetics
3.4. Burning Characteristics of AN/MgA/CuO Pyrotechnic Mixtures
4. Discussion
4.1. Physicochemical Characteristics of MgAl Alloy
4.2. Characteristics of Thermal Decomposition
4.3. Thermal Decomposition Kinetics
4.4. Burning Behaviour of AN/MgAl Pyrotechnic Mixtures with and without CuO
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yuan, J.; Liu, J.; Zhou, Y.; Zhang, Y.; Cen, K. Thermal decomposition and combustion characteristics of Al/AP/HTPB propellant. J. Therm. Anal. Calorim. 2020, 143, 3935–3944. [Google Scholar] [CrossRef]
- Mallick, L.; Kumar, S.; Chowdhury, A. Thermal decomposition of ammonium perchlorate—A TGA–FTIR–MS study: Part I. Thermochim. Acta 2017, 653, 83–96. [Google Scholar] [CrossRef]
- Lyu, J.; Yang, S.; Wu, S.; Tang, G.; Yang, W.; Yan, Q. Burning rate modulation for composite propellants by interfacial control of Al@AP with precise catalysis of CuO. Combust. Flame 2022, 240, 112029. [Google Scholar] [CrossRef]
- Oommen, C.; Jain, S. Ammonium nitrate: A promising rocket propellant oxidizer. J. Hazard. Mater. 1999, 67, 253–281. [Google Scholar] [CrossRef] [PubMed]
- Sinditskii, V.P.; Egorshev, V.Y.; Levshenkov, A.I.; Serushkin, V.V. Ammonium nitrate: Combustion mechanism and the role of additives. Propellants Explos. Pyrotech. 2005, 30, 269–280. [Google Scholar] [CrossRef]
- Dîrloman, F.M.; Rotariu, A.N.; Rotariu, T.; Noja, G.F.; Ginghină, R.E.; Zvîncu, N.D. Ballistic and thermal characterisation of greener composite solid propellants based on phase stabilized ammonium nitrate. Case Stud. Therm. Eng. 2024, 54, 103987. [Google Scholar] [CrossRef]
- Robbins, D.L.; Anderson, E.K.; Anderson, M.U.; Jackson, S.I.; Short, M. Cylinder Test Characterization of an Ammonium Nitrate and Aluminum Powder Explosive. In Proceedings of the 15th International Detonation Symposium, San Francisco, CA, USA, 13–18 July 2014; Office of Naval Research: Arlington, VA, USA, 2015; pp. 797–803. Available online: https://public.lanl.gov/sjackson/papers/2015-AndersonAmmonal.pdf (accessed on 7 October 2023).
- Rotariu, T.; Pulpea, B.-G.; Dîrloman, F.-M.; Diacon, A.; Rusen, E.; Toader, G.; Zvîncu, N.-D.; Iordache, T.-V.; Botiș, R.H. The Influence of Potassium Salts Phase Stabilizers and Binder Matrix on the Properties of Novel Composite Rocket Propellants Based on Ammonium Nitrate. Materials 2022, 15, 8960. [Google Scholar] [CrossRef] [PubMed]
- Jos, J.; Mathew, S. Ammonium nitrate as an eco-friendly oxidizer for composite solid propellants: Promises and challenges. Crit. Rev. Solid State Mater. Sci. 2017, 42, 470–498. [Google Scholar] [CrossRef]
- Kohga, M.; Togo, S. Influence of iron oxide on thermal decomposition behavior and burning characteristics of ammonium nitrate/ammonium perchlorate-based composite propellants. Combust. Flame 2018, 192, 10–24. [Google Scholar] [CrossRef]
- Bogdan, Z. Detonation Parameters of Mixtures Containing Ammonium Nitrate and Aluminium. Cent. Eur. J. Energetic Mater. 2009, 6, 57–66. Available online: https://www.researchgate.net/publication/267996645_Detonation_Parameters_of_Mixtures_Containing_Ammonium_Nitrate_and_Aluminium (accessed on 7 October 2023).
- Shen, C.; Yan, S.; Yao, J.; Li, S.; Guo, X.; Nie, J.; Ou, Y.; Jiao, Q. Combustion behavior of composite solid propellant reinforced with Al-based alloy fuel. Mater. Lett. 2021, 304, 130608. [Google Scholar] [CrossRef]
- Ao, W.; Liu, X.; Rezaiguia, H.; Liu, H.; Wang, Z.; Liu, P. Aluminum agglomeration involving the second mergence of agglomerates on the solid propellants burning surface: Experiments and modeling. Acta Astronaut. 2017, 136, 219–229. [Google Scholar] [CrossRef]
- Xin, H.; Wang, K.; Ren, H.; Jiao, Q. Comparative Study on Combustion Behavior of Aluminum-Based Alloy Fuels and Aluminum Powder in Solid Propellants. Metals 2023, 13, 1492. [Google Scholar] [CrossRef]
- Liu, B.; Yang, J.; Zhang, X.; Yang, Q.; Zhang, J.; Li, X. Development and application of magnesium alloy parts for automotive OEMs: A review. J. Magnes. Alloy. 2023, 11, 15–47. [Google Scholar] [CrossRef]
- Palma, A.S.; Iturbe-García, J.L.; López-Muñoz, B.E.; Jiménez, A.S. MgAl alloy synthesis, characterization and its use in hydrogen storage. Int. J. Hydrogen Energy 2010, 35, 12120–12124. [Google Scholar] [CrossRef]
- Rambabu, P.; Eswara, P.N.; Kutumbarao, V.V.; Wanhill, R.J.H. Aluminium Alloys for Aerospace Applications. In Aerospace Materials and Material Technologies; Springer: Berlin/Heidelberg, Germany, 2016; pp. 29–52. [Google Scholar] [CrossRef]
- Starke, E.A., Jr.; Staley, J.T. Application of modern aluminum alloys to aircraft. Prog. Aerosp. Sci. 1996, 32, 131–172. [Google Scholar] [CrossRef]
- Yao, S.; Li, Y.F. Review on the development and application of magnesium alloys. In Design, Manufacturing and Mechatronics; World Scientific Publishing Co., Inc.: Hackensack, NJ, USA, 2015; pp. 1014–1020. [Google Scholar] [CrossRef]
- Yang, M.; Chen, X.; Wang, Y.; Yuan, B.; Niu, Y.; Zhang, Y.; Liao, R.; Zhang, Z. Comparative evaluation of thermal decomposition behavior and thermal stability of powdered ammonium nitrate under different atmosphere conditions. J. Hazard. Mater. 2017, 337, 10–19. [Google Scholar] [CrossRef]
- Miyake, A.; Izato, Y.-I. Thermal Decomposition Behaviors of Ammonium Nitrate and Carbon Mixtures. Int. J. Energetic Mater. Chem. Propuls. 2010, 9, 523–531. [Google Scholar] [CrossRef]
- Zheng, D.; Wang, J.; Duo, Y.; Liu, J. Pyrolysis mechanisms of commonly used oxidizers on the 5-amino-1H-tetrazole. Propellants Explos. Pyrotech. 2023, 48, e202300024. [Google Scholar] [CrossRef]
- Shih, T.-S.; Wang, J.-H.; Chong, K.-Z. Combustion of magnesium alloys in air. Mater. Chem. Phys. 2004, 85, 302–309. [Google Scholar] [CrossRef]
- Habu, H.; Hori, K. The burning rate characteristics of magnalium (Mg/Al)-AP based solid propellant. J. Sci. Technol. Energetic Mater. 2006, 67, 187–192. Available online: https://www.researchgate.net/publication/286291434_The_burning_rate_characteristics_of_magnalium_MgAl-AP_based_solid_propellant (accessed on 7 October 2023).
- Yao, M.; Chen, L.; Yu, J.; Peng, J. Thermoanalytical investigation on pyrotechnic mixtures containing Mg-Al alloy powder and barium nitrate. Procedia Eng. 2012, 45, 567–573. [Google Scholar] [CrossRef]
- Kamunur, K.; Jandosov, J.; Abdulkarimova, R.; Hori, K.; Yelemessova, Z. Combustion Study of Different Transitional Metal Oxide based on AN/MgAl Composites Gas Generators. Eurasian Chem. J. 2017, 19, 341–346. [Google Scholar] [CrossRef]
- Kang, X.; Yang, F.; Luo, J.; Tang, Y. Thermal-Ignition and Combustion Behavior of Pyrotechnic Composition Containing Mechanically Activated Commercial Mg-Al Alloy. Combust. Sci. Technol. 2015, 187, 963–975. [Google Scholar] [CrossRef]
- Ouyang, D.; Pan, G.; Guan, H.; Zhu, C.; Chen, X. Effect of different additives on the thermal properties and combustion characteristics of pyrotechnic mixtures containing the KClO4/Mg–Al alloy. Thermochim. Acta 2011, 513, 119–123. [Google Scholar] [CrossRef]
- Aly, Y.; Schoenitz, M.; Dreizin, E.L. Ignition and combustion of mechanically alloyed Al–Mg powders with customized particle sizes. Combust. Flame 2013, 160, 835–842. [Google Scholar] [CrossRef]
- Murata, H.; Azuma, Y.; Tohara, T. The effect of magnalium(Mg-Al alloy) on combustion characteristics of ammonium nitrate-based solid propellant. Sci. Technol. Energetic Mater. 2000, 61, 58–66. Available online: https://www.researchgate.net/publication/279540562_Effect_of_magnalium_Mg-Al_alloy_on_combustion_characteristics_of_ammonium_nitrate-based_solid_propellant (accessed on 7 October 2023).
- Shoshin, Y.L.; Mudryy, R.S.; Dreizin, E.L. Preparation and characterization of energetic Al-Mg mechanical alloy powders. Combust. Flame 2002, 128, 259–269. [Google Scholar] [CrossRef]
- Elbasuney, S.; Yehia, M. Thermal decomposition of ammonium perchlorate catalyzed with CuO nanoparticles. Def. Technol. 2019, 15, 868–874. [Google Scholar] [CrossRef]
- Vargeese, A.A.; Muralidharan, K. Anatase–brookite mixed phase nano TiO2 catalyzed homolytic decomposition of ammonium nitrate. J. Hazard. Mater. 2011, 192, 1314–1320. [Google Scholar] [CrossRef] [PubMed]
- Hagihara, Y.; Ichikawa, T.; Shinpo, H.; Suzuki, M. Effects of chromium and cobalt compounds on burning rate of ammonium nitrate/hydroxyl-terminated polybutadiene composite propellants. Sci. Technol. Energetic Mater. 1991, 52, 390–395. Available online: https://www.jes.or.jp/mag_eng/stem/Vol.52/No.6.03.html (accessed on 7 October 2023).
- Naya, T.; Kohga, M. Burning characteristics of ammonium nitrate-based composite propellants supplemented with Fe2O3. Propellants Explos. Pyrotech. 2013, 38, 547–554. [Google Scholar] [CrossRef]
- Kohga, M.; Naya, T. Thermal Decomposition Behaviors and Burning Characteristics of AN/RDX-Based Composite Propellants Supplemented with MnO2 and Fe2O3. J. Energetic Mater. 2015, 33, 288–304. [Google Scholar] [CrossRef]
- Deng, P.; Fang, H.; Liu, R.; Guo, X.; Chen, P. One-pot hydrothermal synthesis of flower-like MnO2 nanostructure with rich oxygen vacancy for catalysis thermal-induced pyrolysis of energetic molecular perovskite. Vacuum 2022, 203, 111234. [Google Scholar] [CrossRef]
- Deng, P.; Chen, P.; Fang, H.; Liu, R.; Guo, X. The combustion behavior of boron particles by using molecular perovskite energetic materials as high-energy oxidants. Combust. Flame 2022, 241, 112118. [Google Scholar] [CrossRef]
- Atamanov, M.; Yelemessova, Z.; Imangazy, A.; Kamunur, K.; Lesbayev, B.; Mansurov, Z.; Yue, T.; Shen, R.; Yan, Q.-L. The Catalytic Effect of CuO-Doped Activated Carbon on Thermal Decomposition and Combustion of AN/Mg/NC Composite. J. Phys. Chem. C 2019, 37, 22941–22948. [Google Scholar] [CrossRef]
- Akhinzhanova, A.; Sultahan, S.; Tauanov, Z.; Mansurov, Z.; Capobianachi, A.; Amrousse, R.; Atamanov, M.; Yan, Q.-L. Preparation and Evaluation of Effective Thermal Decomposition of Tetraamminecopper (II) Nitrate Carried by Graphene Oxide. Combust. Flame 2023, 250, 112672. [Google Scholar] [CrossRef]
- Kajiyama, K.; Izato, Y.-I.; Miyake, A. Thermal characteristics of ammonium nitrate, carbon, and copper(II) oxide mixtures. J. Therm. Anal. Calorim. 2013, 113, 1475–1480. [Google Scholar] [CrossRef]
- Popok, V.N.; Khmelev, V.N. Influence of metal oxides and chlorides on energy release parameters in energy materials based on ammonium nitrate. Polzunovsky Bull. 2009, 3, 253–255. Available online: https://cyberleninka.ru/article/n/vliyanie-oksidov-i-hloridov-metallov-na-parametry-energovydeleniya-v-energeticheskih-materialah-na-osnove-nitrata-ammoniya/viewer (accessed on 7 October 2023).
- Arkhipov, V.A.; Gorbenko, T.I.; Pevchenko, B.V.; Savelieva, L.A. Influence of silicon dioxide on the combustion characteristics of mixed compositions. Chem. Phys. Mesoscopy 2014, 16, 177–183. Available online: https://cyberleninka.ru/article/n/vliyanie-dvuokisi-kremniya-na-harakteristiki-goreniya-smesevyh-kompozitsiy/viewer (accessed on 7 October 2023).
- Glazkova, A.P. Catalysis of Combustion of Explosives. Russsia, 1976, 234 p. Available online: https://urss.ru/cgi-bin/db.pl?lang=Ru&blang=ru&page=Book&id=162894 (accessed on 7 October 2023).
- Lee, J.-K.; Kim, S.K. Effect of CaO Addition on the Ignition Resistance of Mg-Al Alloys. Mater. Trans. 2011, 7, 1483–1488. [Google Scholar] [CrossRef]
- IPC C01C 1/18, C05C 1/02. Patent RU, No. 2227121 C1, 20 April 2004. Available online: http://allpatents.ru/patent/2227121.html (accessed on 7 October 2023).
- Mărmureanu, M.I. Solid rocket motor internal ballistics simulation using different burning rate models. U.P.B. Sci. Bull. Series D 2014, 76, 1454–2358. Available online: https://core.ac.uk/download/pdf/4823473.pdf (accessed on 7 October 2023).
- Schoenitz, M.; Dreizin, E.L. Structure and properties of Al–Mg mechanical alloys. J. Mater. Res. 2003, 18, 1827–1836. [Google Scholar] [CrossRef]
- Vargeese, A.A.; Joshi, S.S.; Krishnamurthy, V. Effect of method of crystallization on the IV–III and IV–II polymorphic transitions of ammonium nitrate. J. Hazard. Mater. 2009, 161, 373–379. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Dave, P.N. Review on thermal decomposition of ammonium nitrate. J. Energetic Mater. 2013, 31, 1–26. [Google Scholar] [CrossRef]
- Babrauskas, V.; Leggett, D. Thermal decomposition of ammonium nitrate. Fire Mater. 2019, 44, 250–268. [Google Scholar] [CrossRef]










| Sample | β, °C/min | Thermal Decomposition | |||||
|---|---|---|---|---|---|---|---|
| 1st Step | 2nd Step | ||||||
| Tonset | Toffset | Tmax | Tonset | Toffset | Tmax | ||
| AN/CuO | (200–300 °C) | (250–310 °C) | |||||
| 2.5 | 207.63 | 250.77 | 245.38 | 251.24 | 252.24 | 251.67 | |
| 5 | 224.09 | 267.89 | 261.41 | 268.51 | 271.29 | 269.33 | |
| 10 | 239.26 | 279.96 | 274.25 | 280.16 | 284.42 | 281.36 | |
| 20 | 243.59 | 297.99 | 291.01 | 297.75 | 302.53 | 299.52 | |
| AN/MgAl | (140–220 °C) | (230–310 °C) | |||||
| 2.5 | 146.70 | 164.64 | 153.22 | 230.89 | 246.23 | 239.20 | |
| 5 | 153.39 | 174.41 | 166.64 | 245.62 | 278.75 | 260.98 | |
| 10 | 159.11 | 205.76 | 172.59 | 269.17 | 292.78 | 282.34 | |
| 20 | 169.05 | 216.34 | 184.44 | 281.36 | 303.54 | 298.62 | |
| AN/MgAl/CuO | (130–180 °C) | (230–300 °C) | |||||
| 2.5 | 130.30 | 169.35 | 152.21 | 235.31 | 245.83 | 239.75 | |
| 5 | 153.60 | 172.96 | 161.34 | 256.79 | 267.89 | 268.43 | |
| 10 | 160.03 | 182.91 | 170.68 | 268.09 | 277.90 | 271.94 | |
| 20 | 163.71 | 197.25 | 177.75 | 280.61 | 291.74 | 284.73 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ketegenov, T.; Nadirov, R.; Teltayev, B.; Milikhat, B.; Kalmuratova, B.; Keiichi, H.; Kamunur, K. The Effect of CuO on the Thermal Behavior and Combustion Features of Pyrotechnic Compositions with AN/MgAl. Sustainability 2024, 16, 1488. https://doi.org/10.3390/su16041488
Ketegenov T, Nadirov R, Teltayev B, Milikhat B, Kalmuratova B, Keiichi H, Kamunur K. The Effect of CuO on the Thermal Behavior and Combustion Features of Pyrotechnic Compositions with AN/MgAl. Sustainability. 2024; 16(4):1488. https://doi.org/10.3390/su16041488
Chicago/Turabian StyleKetegenov, Tlek, Rashid Nadirov, Bagdat Teltayev, Bagdatgul Milikhat, Bakhyt Kalmuratova, Hori Keiichi, and Kaster Kamunur. 2024. "The Effect of CuO on the Thermal Behavior and Combustion Features of Pyrotechnic Compositions with AN/MgAl" Sustainability 16, no. 4: 1488. https://doi.org/10.3390/su16041488







