Abstract
A decline in pollinators is a real concern for the biodiversity and pollination of insect-dependent plants in landscapes and agriculture. Turfgrass is often presumed to be an ecological desert, as it is maintained at a low height with no floral resources for pollinators. Weeds are common on low-maintenance lawns in the southeastern USA and have rarely been studied as resources for pollinators. Thus, this study aimed to determine the abundance and diversity of bees on weed-infested lawns. Bees were sampled using yellow, white, and blue bowls and by bagging bees foraging on flowering weeds during the growing season from 2021 to 2023. Over three years, 539 bees from 16 genera were collected from weed-infested turfgrass lawns. Weeds were present throughout the growing seasons, and bees were also collected from them. Bombus, Apis, and Lasioglossum bees were the dominant genera collected. Large-sized bees, such as Bombus, were mostly collected from white clover (Trifolium repens L.), whereas small-sized bees, such as Lasioglossum, were mostly collected from common dandelion (Taraxacum officinale Weber). Other bee genera collected were Agapostemon, Augochlora, Augochlorella, Calliopsis, Ceratina, Epeolus, Halictus, Melissodes, Osmia, Panurginus, Ptilothrix, Svastra, and Xylocopa. This showed that a diverse group of bees utilized lawns infested with weeds.
1. Introduction
The diversity and richness of wild bee species have declined over the last 50 years [1]. Human land use and ecological interventions, such as destroying natural habitats and urbanization, are leading causes of the dramatic decline in pollinators [2,3]. According to the US Census Bureau, approximately 80% of the population lives in urban areas [4]. As more natural lands are converted to support the continued expansion of suburban and urban areas, fewer resources are available to help bee communities thrive. Thus, efforts for pollinator host conservation to support bee populations need to be enhanced.
Turfgrass is an important landscape component that dominates the southeastern US and is one of the largest agricultural commodities commercially produced on farms in Georgia. In 2010, the turfgrass industry of Georgia (USA) was valued at 7.8 billion USD [5]. Turfgrass, an aesthetically appealing vegetative cover, is planted extensively on surfaces ranging from athletic fields to home lawns, providing recreational and social attributes [6]. In addition to its physical benefits, turfgrass positively supports the human environment by moderating temperatures, stabilizing soil, reducing erosion, absorbing pollutants, and improving the overall quality of human life [7]. These green covers are managed with regular mowing and pesticide sprays to achieve a clean and appealing surface according to human preference. Such intensively managed lawns offer limited foraging resources for pollinators [8]. The turfgrass system is generally considered an ecological desert for pollinators and is rarely perceived as a potential food source for them. However, recent studies showed that different bees visit turfgrass environments [9]. Bees forage on the spikelets of centipedegrass [10,11] and bahiagrass lawns [12].
When allowed to grow out, turfgrass naturally hosts a diverse group of flowering plants, which are mostly referred to as weeds, such as white clover (Trifolium repens L.) and common dandelion (Taraxacum officinale Weber) in the southeastern US [13,14]. Generally, these weeds are considered pests, as they are not deliberately planted and sometimes outcompete the turfgrass cover, causing aesthetically unpleasant situations. Regardless, weed plants are prevalent in residential, commercial, and public lawns in the southeastern US. Tolerance of weed plants in lawns varies among people, as their maintenance can be challenging and cost-prohibitive for some communities.
Previous studies have shown that many common weeds in lawns attract the bee community [15,16]. Larson et al. (2014) showed that common weeds, such as white clover and common dandelion, harbored bees in Kentucky. This further confirms that flowering weeds can provide supplemental resources, such as pollen and nectar, to visiting pollinators. Turfgrass lawns can be managed to intentionally benefit pollinators, which is often overlooked by managers [3]. However, limited studies evaluated the season-long abundance of pollinators in weedy lawns in the southeastern US. Documenting the diversity and temporal distribution of bees will inform some landscape managers on the importance of weeds and how they can be utilized as a tool to intentionally benefit pollinators and conserve the bee community. In addition, such data might increase awareness and cause an attitudinal shift toward acceptance of flowering weeds in commercial and residential lawns to promote pollinator activity. Thus, the objective of the current study was to document the temporal foraging patterns of the bee community in central Georgia (USA) during the warm season, specifically on flowering weeds that naturally grow on turfgrass lawns. The hypothesis is that a diverse group of bees transit through and utilize weed-infested lawns.
2. Materials and Methods
2.1. Study Sites
This study was conducted at the University of Georgia Griffin Campus, Griffin, Georgia (USA) in 2021, 2022, and 2023. In 2021 and 2022, four lawn sites (referred to as plots) were randomly selected. In 2023, four new lawn sites were randomly selected. The GPS coordinates of all plots are listed in Table 1. Each lawn plot was 200 m2 (10 × 20 m; length × width). These lawns were planted with ‘Tifway’ bermudagrass. Throughout the sampling periods, 0–30% of the lawn plot area (200 m2) was covered with weeds. The plots were at least 30 m apart from each other and at least 3 m from any human-made structures, such as buildings, storage sheds, etc. Other vegetation, such as trees or shrubs, was at least 3 m away if present in some lawn plots. No pesticides or fertilizers were applied to the selected lawn plots before (at least 6 months) or during the study. The lawn plots were not irrigated. These lawn plots were part of a larger green cover in the University of Georgia Griffin Campus. All lawn plots were rarely used by pedestrian traffic. The green cover areas around the selected plots were mowed at 14–16 d intervals depending on rain events and maintained at ~6 cm tall.

Table 1.
The GPS coordinates showing the exact locations of the plots during 2021–2023.
2.2. Experimental Design
The experiment was arranged in a completely randomized design with four replicates. The lawn plots were replicates. The four lawn plots were not mowed for 14 d following an initial mowing. The non-mowing interval allowed sufficient time for flowering weeds naturally growing in the plots to re-flush, grow, and produce flowers. When mowed, the lawn within the plots was maintained at ~6 cm tall. Bees were sampled using the colored-bowl and bagging methods. For the bowl method, bee bowls were deployed on the 13th day within the plots after initial mowing for 24 h in 2021, 2022, and 2023. Three 354.8 mL bowls were placed in a triangular pattern at 1 m from each other at the center of each plot (Figure 1A). These bowls were 4 × 17.5 × 8.5 cm [depth × top diameter × bottom diameter] and were yellow (“yellow sunshine” Amscan, Elmsford, NY, USA), blue (“bright royal blue”; Amscan), and white (“classic white” PLASTICPRO, Sibiu, Romania). To secure the bowls to the lawn surface, an initial bowl was placed on the grassy area. A 15 cm nail was hammered through the center of the bowl into the ground; then, another bowl of the same color and size was placed on the first bowl, and they were secured together using a large binder clip (Figure 1B). A soap solution was prepared by adding ~3 mL of dish soap (Dawn, The Procter & Gamble Company, Cincinnati, OH, USA) to 3.78 L of water, and ~250 mL of the soap solution was poured into each bowl trap. After 24 h (14th day after initial mowing), the contents of every bowl trap were strained from the soap water and emptied into individually labeled plastic bags. The samples were transported to the laboratory and stored in a freezer (−18 °C) until they were prepared for identification. In the field, the bowls, including the nailed bowls, were removed from the plots after sampling and redeployed after 13 d post-mowing. Although the bowl method was used in 2021, the bowls were only deployed for two dates and, therefore, not included in the study.

Figure 1.
Bee samples were collected using (A,B) colored bowls placed on the turfgrass surface and (C) the bagging method, where a clear plastic bag was placed on a foraging bee on a flower.
On the sample day at 14 d post-mowing, bees visiting the flowers of weeds were sampled using the bagging method. When a bee was on a flower for at least 2 s, a clear 45.75 × 10.25 × 20.75 cm plastic bag was gently placed on the flower. The bag was vertically expanded to provide more space for the trapped bee to move upward. Once the trapped bee moved to the upper region of the bag (Figure 1C), the bag was gently lifted from the flower, and the open end was secured. The sampling was conducted between 08:30 and 14:30 h. At each site, sampling was conducted by a person for 30 min. On the sampling days, the air temperatures ranged from 15.5 to 29.5 °C, and the days were mostly clear or had scattered clouds. The wind speeds were <15 km per h. The bagging method was adopted because it reduced the escape of bees during the collection process, as the trapped bees naturally flew up into the bag. The trapped bees were kept in the same bags and transported to the entomology laboratory. Sampled bees were temporarily stored at −18 °C. The weed species from where the bees were collected using the bagging method were identified and recorded. In all three years, weed species naturally growing on the plots from which bees were collected while foraging were recorded on each bee sampling day. In addition, the percentages of these weed species found on the plots were visually assessed and recorded.
The frozen bee samples were processed within the same year. First, the bees sampled with the bowl and bagging methods were sorted. The sorted bees were then washed for 10 min and then dried with hot air using a hair dryer for 20 min. The dried bees were immediately pinned, labeled (location, date, and sampling method), and stored in cardboard boxes in the laboratory at ~21 °C and ~40% relative humidity. The bees were later identified to the genus and species level.
2.3. Identification
The bees collected with the colored-bowl and bagging methods were identified to the genus and species level [17,18] with an online identification guide [19]. The weeds were identified using an identification guide [20]. The bees were grouped by size. The small bee groups included Lasioglossum, Halictus, Agapostemon, Augochora, Augochlorella, Ceratina, Panurginus, Epeolus, Osmia, and Calliopsis. The large bee groups included Apis, Bombus, Melissodes, Ptilothrix, Svastra, and Xylocopa. Not all bee genera were collected each year or with both sampling methods.
2.4. Statistical Analyses
All statistical analyses were conducted using the Statistical Analysis System (SAS) software (version 9.4) [21]. The data on the numbers of small- and large-sized bees were analyzed by sampling date and year. Bee group data were subjected to one-way analysis of variance (ANOVA) in a generalized linear mixed model using the PROC GLIMMIX procedure in SAS with the log link function and Poisson distribution, since the data were discrete counts and were not normally distributed. The estimation method used was that of Laplace to accommodate for the Poisson distribution. The sampling date was the treatment with four plot replicates. The treatments and replications were fixed and random effects, respectively. One was added to all data points for the small-sized bees in 2021 and the large-sized bees for both the bowl and bagging methods in 2022 and 2023 before the analysis to counter the zero-inflation issue in the data.
Both small- and large-sized bees collected with both the bowl and bagging methods were re-organized according to the bee genus for each year. The treatment was that of the bee genus, and the four plots were the replications. Bee abundances were subjected to one-way analysis of variance (ANOVA) in a generalized linear mixed model using the PROC GLIMMIX procedure in SAS with the log link function and Poisson distribution, since the data were discrete counts were and not normally distributed. The method used was that of Laplace estimation. The treatments and replications were fixed and random effects, respectively. A separate analysis was performed for each year’s data. One was added to the data points from 2022 before analysis. The percentages of weed species data was not analyzed, as they were used to show the prevalence of weed infestations in the plots at any given time.
To determine the associations of the bee groups with weed species, the numbers of bees in each bee group (small or large) associated with the weed species were subjected to one-way analysis of variance (ANOVA) in the generalized linear mixed model using the PROC GLIMMIX procedure in SAS with the log link function and Poisson distribution, since the data were discrete counts and were not normally distributed. The method used was that of Laplace estimation. Only the bees collected using the bagging method were utilized for this analysis. The sampling dates when no bees were collected (small or large) were omitted from the analysis, and the data from all three years were combined. The bee groups and sampling dates were the treatments and replications, respectively. If the weed species–bee group association events were four or fewer, they were not included in the analysis.
Means and standard errors were calculated for temporal weed species and the bee group, bee genus by year, and weed species–bee group data using the PROC MEANS procedure and were separated with the Tukey HSD (honestly significant difference) test (p < 0.05).
3. Results
Over three years, 539 bees from 16 different genera were collected across four different weed-infested turfgrass lawns (Table 2). In 2021, 127 bees in 8 genera were collected, followed by 208 bees in 12 genera in 2022 and 204 bees in 8 genera in 2023. Bombus was the dominant large-sized bee group collected from the weeds on turfgrass in all years. Approximately 48%, 16%, and 18% of the total bee captures were Bombus in 2021, 2022, and 2023, respectively. Apis bees were the second most dominant large-sized bee genus, accounting for 32% of the total bees captured in 2021. All 39 Apis bees were bagged from white clover in 2021. In 2022, no Apis bees were collected, regardless of the collection method. In 2023, approximately 28% of the total bees captured were Apis bees, with 53 Apis bees being bagged from white clover and none being directly bagged from common dandelion. Among the small-sized bees, the dominant foraging genus collected was Lasioglossum. Approximately 69% and 36% of the total bee captures in 2022 and 2023, respectively, were Lasioglossum bees.

Table 2.
Bee species captured from weed-infested lawn plots in Griffin, Georgia.
3.1. Sampling in 2021
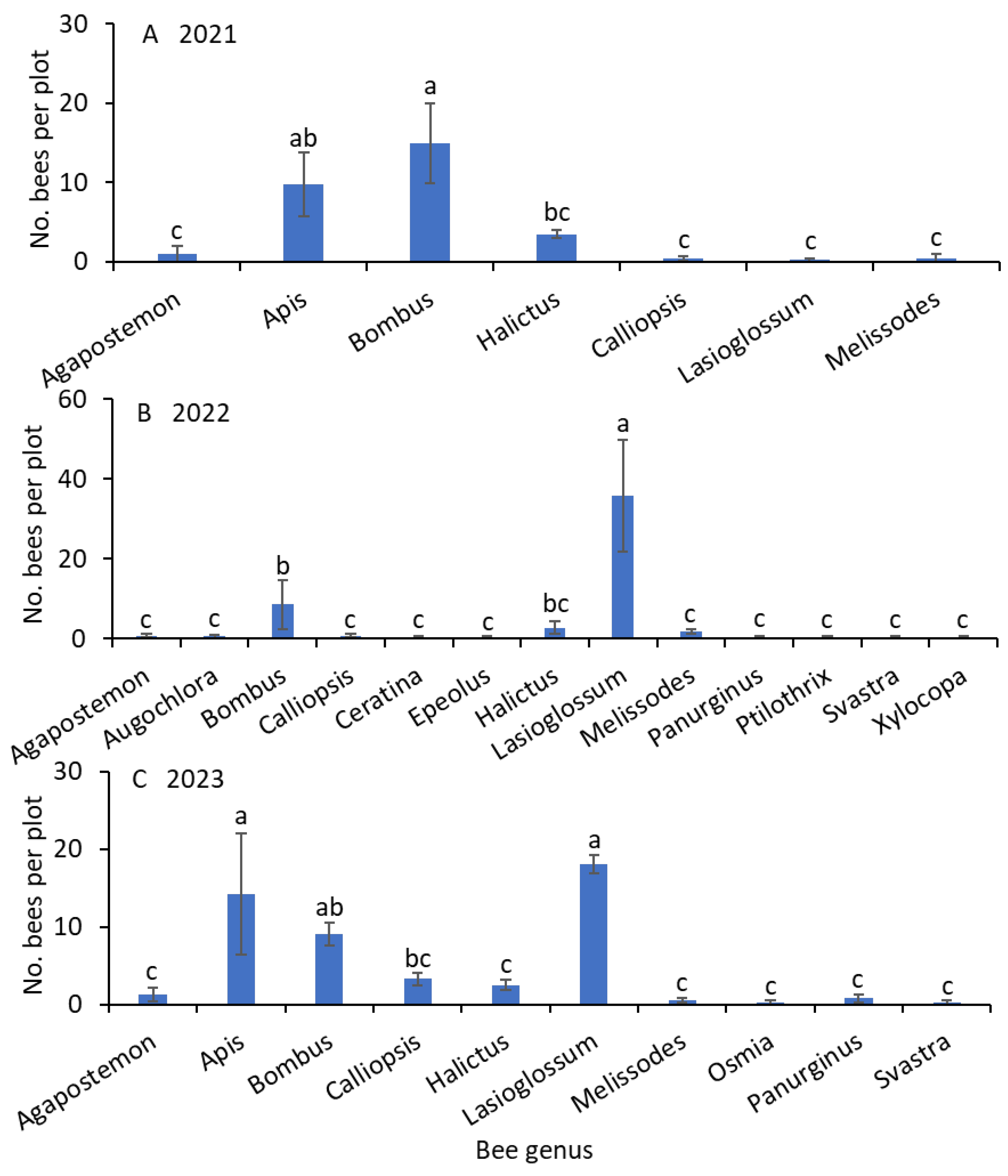
Significantly greater numbers of Bombus and Apis bees than Agapostemon, Calliopsis, Lasioglossum, and Melissodes bees were captured from weed-infested turfgrass (F = 15.7; df = 6.18; p < 0.001; Figure 2A). The numbers of Halictus bee captures were not significantly different from the numbers of captures of Apis bees (Figure 2).
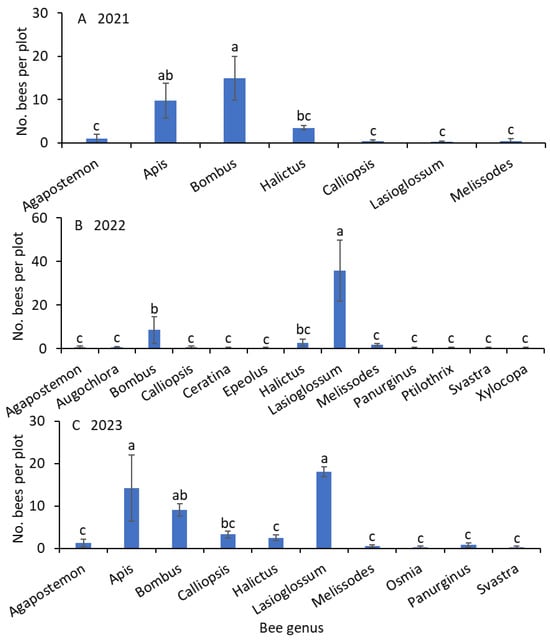
Figure 2.
Mean (±SE) of the bee genera collected using the colored-bowl and bagging methods in (A) 2021, (B) 2022, and (C) 2023. Bars with the same letters within a figure are not significantly different at α = 0.05 according to the Tukey–Kramer test.
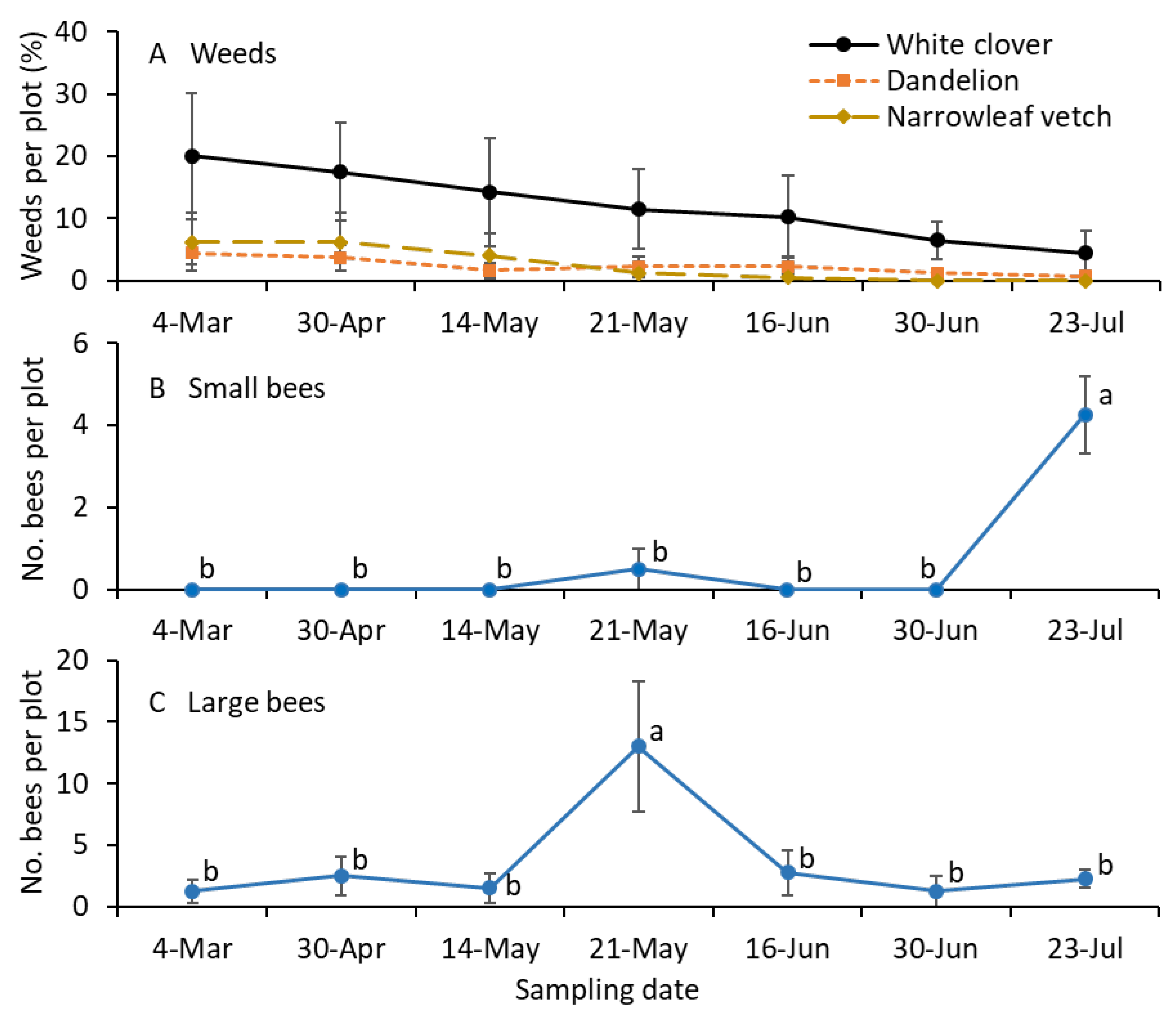
Weeds, including white clover and common dandelion inflorescences, were prevalent in the turfgrass plots throughout the growing season (Figure 3A). The numbers of small bees were significantly greater for the mid-July sample than for the remaining sampling dates with the bagging method (F = 4.9; df = 6.18; p = 0.004; Figure 3B). The numbers of large-sized bees were significantly greater for the early June sample than for the remaining sampling dates with the bagging method (F = 14.9; df = 6.18; p < 0.001; Figure 3C).
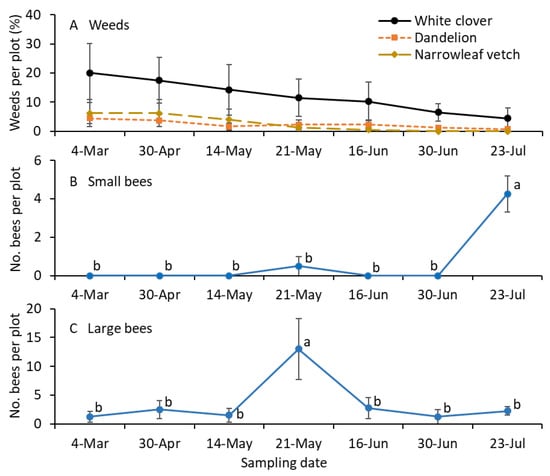
Figure 3.
Mean (±SE) of the (A) percentage of weed species infestation and (B) small and (C) large-sized bees collected per plot in 2021. Small-sized bees, including Lasioglossum and Halictus, and large-sized bees, including Melissodes, Bombus, and Apis, were collected using the bagging method. (A) The percentage of weed species was not statistically analyzed. For the bee sample data (B,C), the same letters within the figure are not significantly different at α = 0.05 according to the Tukey–Kramer test. Where no differences were observed among samples, no letters are given.
3.2. Sampling in 2022
The numbers of Lasioglossum bees were significantly greater than those of the Bombus bees, followed by the remaining genera from weed-infested turfgrass (F = 38.4; df = 12.36; p < 0.001; Figure 2B). The numbers of Halictus bee captures were not significantly different from the numbers of captures of Bombus bees. Apis bees were not collected in 2022.
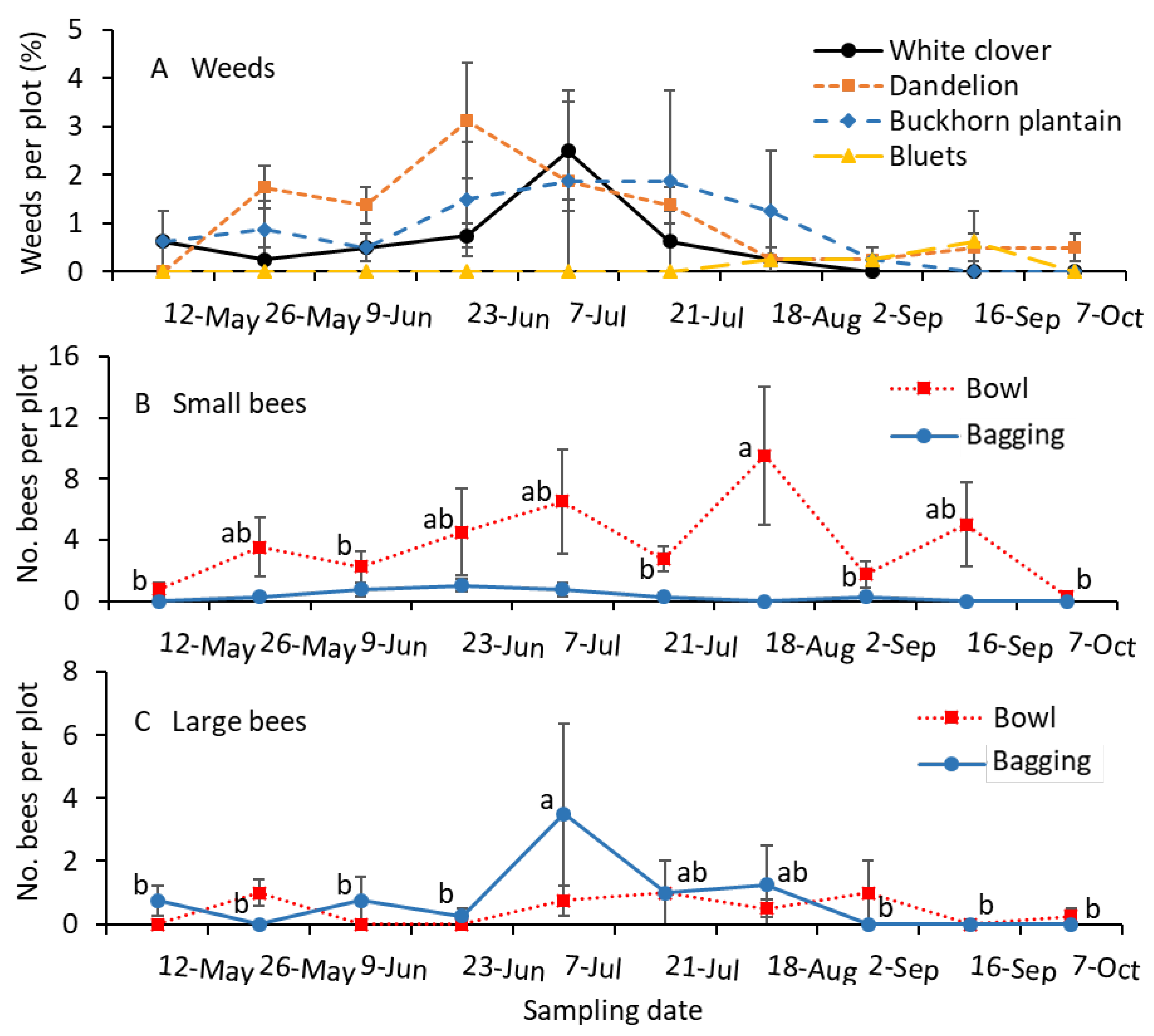
Inflorescences of white clover and common dandelion were prevalent in May. From early June to early August, buckhorn plantain inflorescences were also found, along with white clover and common dandelion inflorescences in the turfgrass plots (Figure 4A). The numbers of small-sized bees collected in bowls were significantly greater for the mid-August sample than for the early June, mid-July, late August, and October samples (F = 6.3; df = 6.27; p < 0.001; Figure 4B). The small bee captures in bowls during mid-May, late June, early July, mid-August, and mid-September were not significantly different from each other. There were no significant differences among the captures of small-sized bees using the bagging method during the entire growing period (F = 0.3; df = 7.21; p = 0.925; Figure 4B).
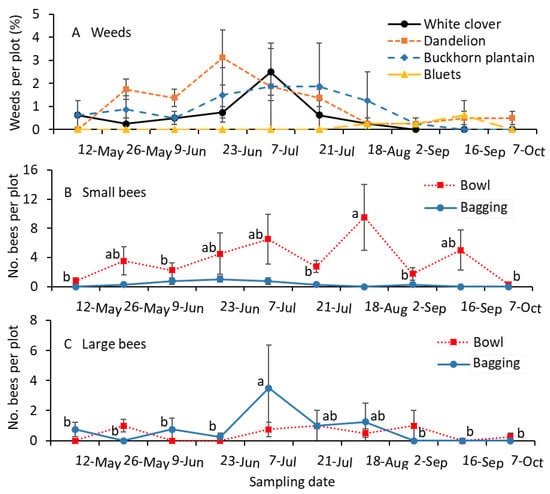
Figure 4.
Mean (±SE) of the (A) percentage of weed species infestation (B) small and (C) large-sized bees collected per plot in 2022. Small-sized bees, including Lasioglossum and Halictus, and large-sized bees, including Melissodes and Bombus, were collected using the colored-bowl and bagging methods. (A) The percentage of weed species was not statistically analyzed. For the bee sample data (B,C), the same letters within the figure and sampling method are not significantly different at α = 0.05 according to the Tukey–Kramer test. Where no differences were observed among samples, no letters are given.
The numbers of large-sized bees collected in bowls were not significantly different among the sampling dates throughout the growing season (F = 0.5; df = 9.27; p = 0.870; Figure 4C). The captures of large-sized bees were significantly greater for the early July sample than for the May, June, Late August, September, and October samples (F = 2.7; df = 7.21; p = 0.039). There were no significant differences between the July and mid-August samples (Figure 4C).
3.3. Sampling in 2023
Significantly greater numbers of Lasioglossum and Apis bees than those of Calliopsis bees were captured from weed-infested turfgrass, followed by Agapostemon, Halictus, Melissodes, Osmia, Panurginus, and Svastra bees (F = 17.2; df = 9.27; p < 0.001; Figure 2C). The numbers of Bombus bee captured were not significantly different from those of Lasioglossum and Apis bees. Similarly, the numbers of Bombus bees were not significantly different from those of Calliopsis bees.
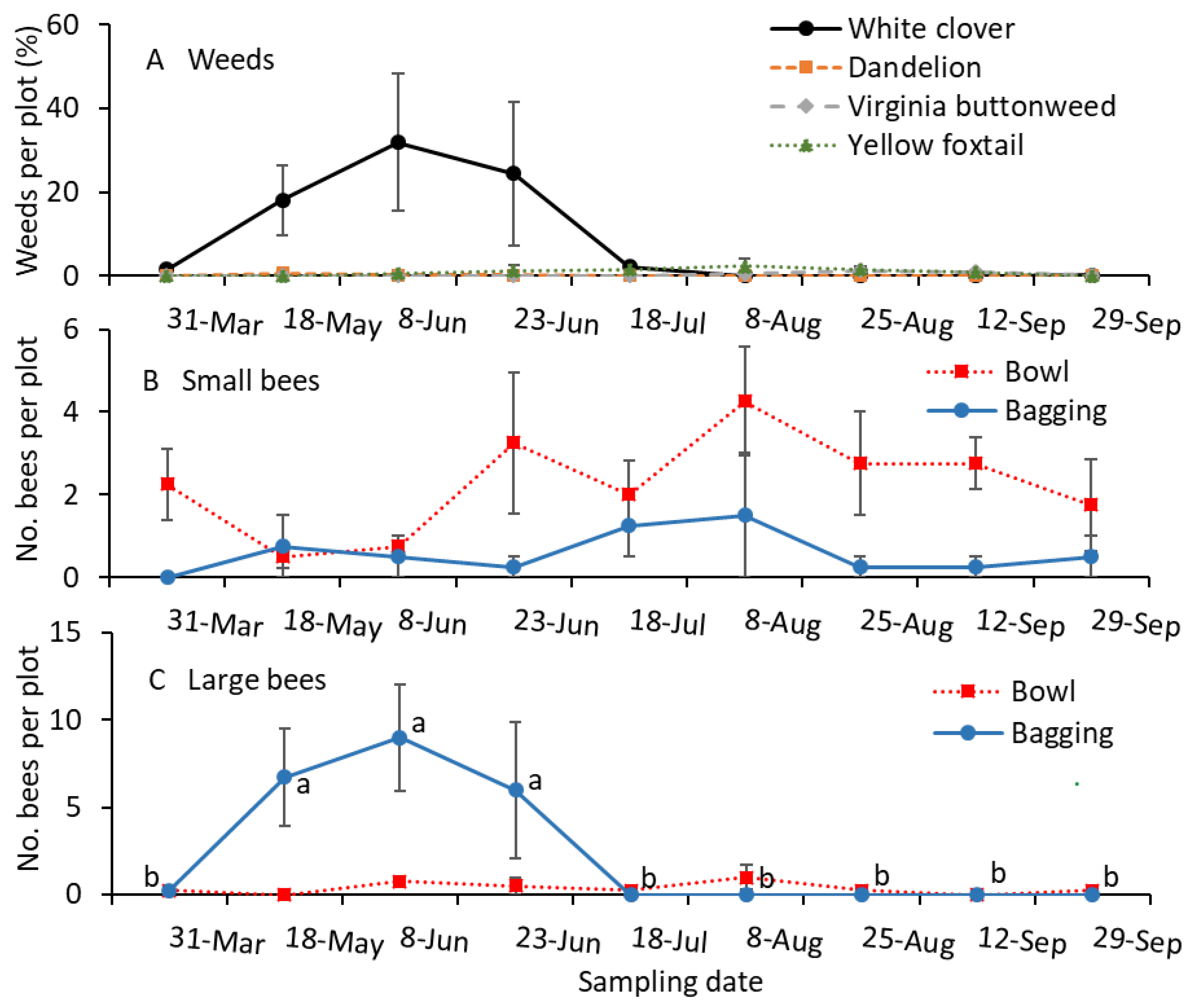
White clover was prevalent from mid-May to mid-July in the turfgrass plots (Figure 5A). There were no significant differences among the sampling dates for the small-sized bees collected in bowls (F = 2.1; df = 8.24; p = 0.078; Figure 5B) or bags (F = 1.0; df = 8.24; p = 0.435). The numbers of large-sized bees collected with the bagging method were significantly greater for the May and June samples than for the remaining samples (F = 11.1; df = 8.24; p < 0.001; Figure 5C). However, the numbers of large-sized bees collected in bowls were not significantly different among the sample dates (F = 0.3; df = 8.24; p = 0.951; Figure 5C).
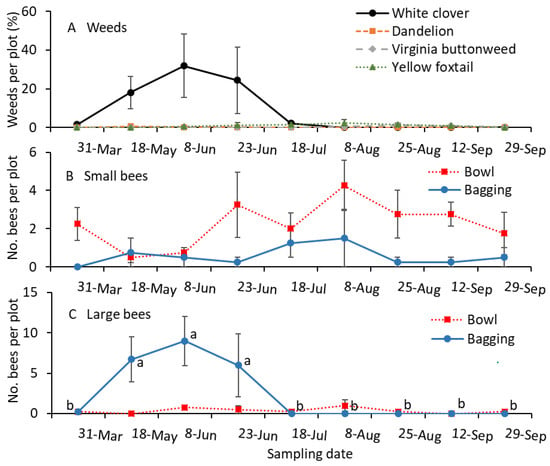
Figure 5.
Mean (±SE) of the (A) percentage of weed species infestation and (B) small and (C) large-sized bees collected per plot in 2023. Small-sized bees, including Lasioglossum and Halictus, and large-sized bees, including Melissodes, Bombus, and Apis, were collected using the colored-bowl and bagging methods. (A) The percentage of weed species was not statistically analyzed. For the bee sample data (B,C), the same letters within a figure and sample method are not significantly different at α = 0.05 according to the Tukey–Kramer test. Where no differences were observed among samples, no letters are given.
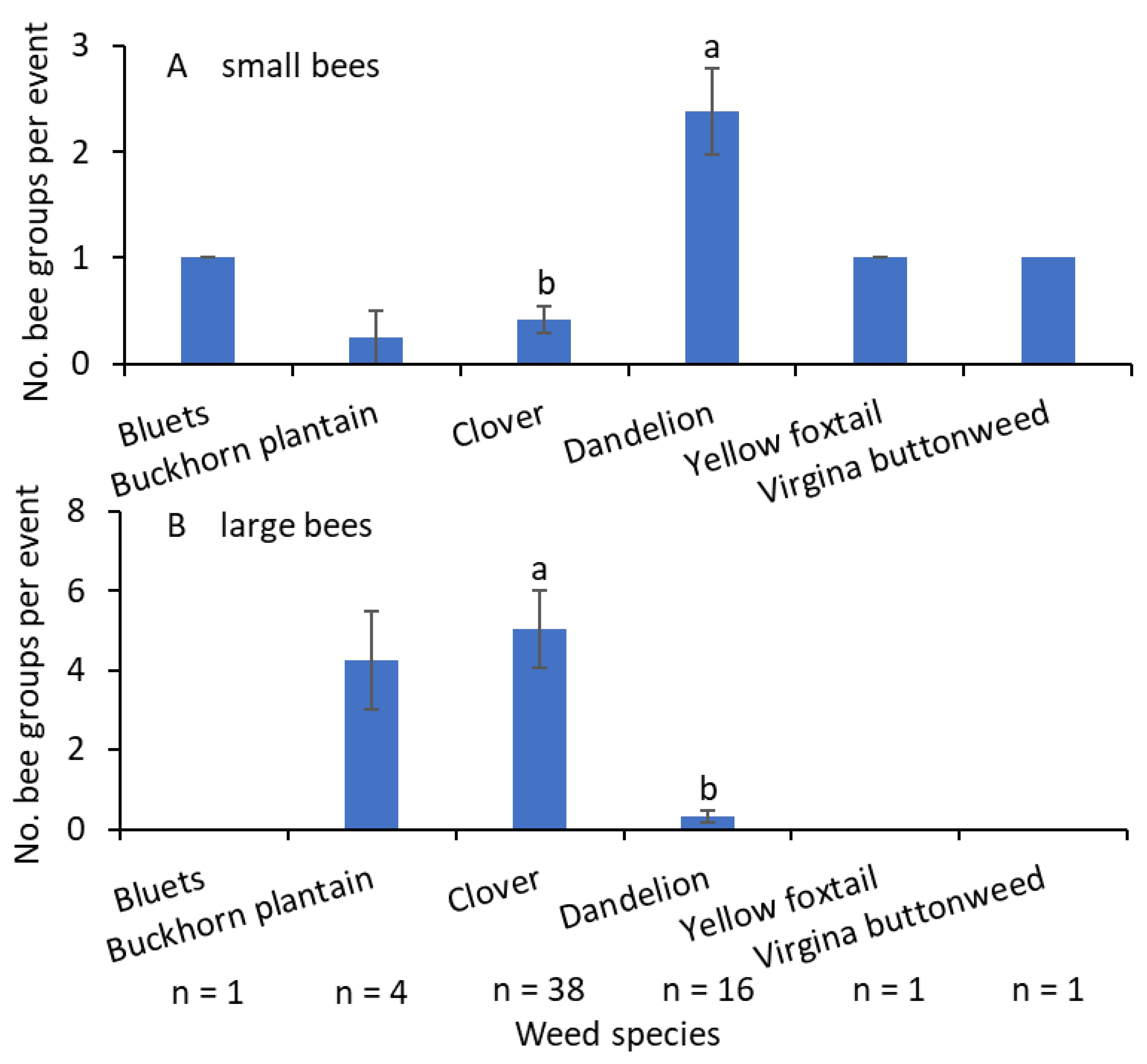
3.4. Bees and Weed Species
More small-sized bees were collected from the common dandelion than from the white clover (F = 30.9; df = 1.14; p < 0.001; Figure 6A). Small-sized bees were collected from bluets (Houstonia caerulea L.), buckhorn plantain (Plantago lanceolate L.), yellow foxtail [Setaria pumila (Poir.) Roem. et Schult.], and Virginia buttonweed (Diodia virginiana L.). The numbers of large-sized bees were significantly greater on white clover than on common dandelion (F = 37.9; df = 1.14; p < 0.001; Figure 6B). The only other weed species from which large-sized bees were collected was buckhorn plantain.
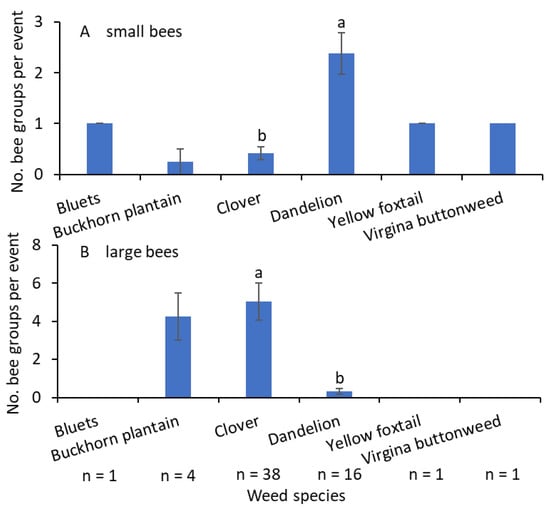
Figure 6.
Mean (±SE) of the bee group–weed species association when data from 2021–2023 were combined, (A) small- and (B) large-sized bees. Only bees collected using the bagging method were included. n ≤ 4—not included in the analysis. Bars with the same letters within a figure are not significantly different at α = 0.05 according to the Tukey–Kramer test.
4. Discussion
Turfgrass management continues to reinforce the need for intensive maintenance of private and public lawns. Lawn habitats can host a variety of flowering weeds. These plants are often viewed as pests and are removed using herbicides or regular mowing [8]. However, multiple studies have shown that weeds found on turfgrass lawns serve as a supplementary resource for pollinators in urban and suburban areas. Considering the rapid decline of pollinators, all possible sources of conservation must be evaluated [1]. Promoting the benefits of flowering weeds for the bee community may increase their foraging resources in the landscape. The current study is the first to examine the temporal incidence and abundance of bees in Georgia lawns, confirming the value of weeds for pollinators in turfgrass. It is important to note that the fields grew naturally and had different levels of weed coverage. The natural weed density varied by year as the growing conditions, such as rainfall and temperature, varied. These factors might have impacted the abundance of bees. Bombus and Apis were the most abundant large-sized bees captured during the spring and early to mid-summer periods. Bombus pensylvanicus De Geer, a rare bumblebee, was only collected once [18]. Similarly, small-sized bees, such as Lasioglossum, were abundant during mid-summer. These trends followed the natural temporal abundance of the dominant weeds within turfgrass plots. Other bees, such as those of Calliopsis, Agapostemon, Halictus, Melissodes, Osmia, Panurginus, and Svastra, were rarely collected when foraging on flowers of weeds relative to the Bombus, Apis, or Lasioglossum bees. It is unclear why Apis bees were not collected in 2022. Perhaps honeybees were not maintained by the beekeepers in the area, or they had access to more resourceful floral hosts in the vicinity during 2022. Regardless, weedy lawns support the biodiversity of bees and deliver vital ecosystem services.
Flowering weeds, such as white clover and common dandelion, support bumblebee and honeybee foraging activity. White clover is better suited for bee conservation than common dandelion [14] because white clover covers extensive lawn areas with high floral abundance throughout the growing season and produces a stable source of pollen and nectar. Longer blooming periods are valuable to bee communities [22]. Additionally, white clover has been described as one of the few vital nectar providers in urban areas [23]. This is especially critical in the late summer and fall, when most of the pollen and nectar resources decline in the landscape. White clover naturally grows on turfgrass plots in the southeastern USA, and it not only fixes nitrogen but also transfers it to turfgrass [24]. The common dandelion, however, supported small-sized bees, such as Lasioglossum. The role of the common dandelion as a pollen and nectar source is poorly studied and warrants more research. Buckhorn plantain was another weed of interest that grows from late spring through summer in central Georgia. In 2022, about 20 bees were collected directly from buckhorn plantain inflorescence. Although bee captures were lower on buckhorn plantain than on white clover or common dandelion, the specific supporting role of buckhorn plantain for the bee community has not been adequately documented.
Small-sized bees were notable visitors on the weedy plots. Lasioglossum bees were the most abundant small-sized bee genus sampled from the turfgrass environment. Halictus rubicundus Christ. preferred edges of the pebbled surface over bare ground for nesting [25]. Other reports also noted similar behavior, where H. rubicundus and other halictids preferred pebbled or stoned areas along unpaved surfaces, such as roadways, for nesting [26,27], as they became warmer than bare ground [25], while bees used them as a landmark to guide their flights back to their nests [28]. Such areas are preferred by solitary bees as a nesting site. Although distinct edges similar to pebbles may not be available for bee nesting in turfgrass, early weed infestations leave open areas within the turfgrass, which eventually cause heavy thinning of the turfgrass or expose thatch surfaces. However, it is unclear if these edges in turfgrass created after weed infestation are used by solitary ground-nesting bees, such as Lasioglossum. These small-sized bees foraged the flowers of weeds in turfgrass plots but were not collected in high numbers using the bagging method because of their quick foraging behavior and small size. This could be one reason why very few Lasioglossum bees were collected in 2021, as we did not use the bowl sampling method then, which is when most of the Lasioglossum bees were collected. Thus, understanding the role of weeds in the activity of solitary bees will be critical in developing a conservation plan for turfgrass.
More than 530 bees in 16 genera were collected from weed-infested turfgrass lawns (Table 2). These showed that diverse bees utilized the flowers of weeds growing in turfgrass lawns. This was consistent with the results of a previous study, where 173 bees were collected from mowed and non-mowed centipedegrass lawns in central Georgia [9]. Thus, a diverse group of bees utilize turfgrass as a pollen and nectar resource or a space to transit between habitats. Calls for the conservation of weeds have been described across the USA to improve the conservation of bee populations [3,14], including white clover and common dandelion. These weedy plots effectively attract and support bee communities. Members of Asteraceae attracted not only bees but other types of pollinators [29]. Native wildflowers attract pollinators to crops growing in close proximity [30].
For homeowners and landscape managers who are open to weed conservation, the first step is to start minimizing the use of pesticides and to be selective with any products that they use. Careful practice when following the precautionary statements highlighted on pesticide labels is important to prevent the contamination of nearby flowering weeds or any harmful residues [31]. Chlorantraniliprole, an effective insecticide for soil pests, such as white grubs, is relatively less toxic to foraging bees [31]. Granular formulations of insecticides, including neonicotinoids, are likely much less disruptive to foraging bees than liquid formulations are [32,33]. Practices such as removing flowers by mowing before insecticide application may reduce bee activity and exposure [33]. However, it can be challenging for certain homeowners or property managers to coordinate mowing with pest management companies for timely service [14]. Bumblebees and honeybees are active foragers of weeds in May and June (Figure 3, Figure 4 and Figure 5). This is critical because new queens need this vital resource to start and sustain new colonies in the spring, especially in years when late frost events reduce the floral resources from trees. A reduced or inconsistent mowing routine may generate floral resources and value for foraging bees. The current study showed that mowing operations with at least a 14-day interval were sufficient to support fresh floral resources, including white clover, and to attract pollinators. On centipedegrass and bahiagrass, a 14 d mowing gap allowed enough time for the emergence of new spikelets to be available for foraging bees [11,12]. White clover is known to disappear from grassy weed areas over several years [34]; thus, reseeding with white clover is recommended [35]. Bombus, Apis, and Lasioglossum bees dominated the bee community on weeds in turfgrass. The contribution of weed-pollen to their biology is still unknown, especially for developing larvae in their colonies. The pollen of white clover is composed of 35.4% protein, which is considered moderately high [36]. Similarly, a supply of nectar resources is critical for sustaining energy needs and enabling commuting flights [37] between habitats. Weedy lawns could supplement nectar resources during the foraging activity of bees. However, the specific contributions of weeds to the health of developing bee colonies are not well understood, which warrants future research.
The current study showed that weedy turfgrass lawns could provide foragers with more sustainable pollen and nectar resources. Once a turfgrass lawn is infested with flowering weeds, especially white clover and common dandelion, they can sustain a continuous supply of resources. The results showed that these weeds were present throughout the growing season. Because any level of flowering weeds on lawns can sustain the bee community, practitioners, such as homeowners or hired landscape maintenance companies, can modify the density of flowering weeds that they can tolerate. This will be especially important in the fall, when most pollen and nectar resources dry up in landscapes. This study was conducted with no additional inputs, such as nutrients and irrigation, in the experimental plots. This suggests that managing these flowering weeds in lawns requires minimal effort from practitioners to sustain them.
5. Conclusions
Low-maintenance weedy lawns spread throughout urban and suburban areas can be a refuge for pollinators [38]. The current study indicated that a diversity of bees exists with flowering weeds in lawns. Thus, weedy turfgrass lawns can serve as a critical resource for foraging bees and contribute to the health, wellbeing, and success of colonies. As floral resources are dwindling in landscapes, weedy turfgrass lawns can supplement foraging bees with pollen and nectar, and these resources should be conserved. Although current and past studies showed the value of weedy areas in turfgrass lawns [35], homeowners and lawn maintenance managers still strive for well-maintained, manicured turfgrass lawns. Raising awareness of weedy turfgrass may encourage more pollinator-benefitting practices within urban and suburban areas in the future.
Author Contributions
A.J.: Writing—original draft, Methodology, Project administration; S.V.J.: Writing—review and editing, Conceptualization, Methodology, Project administration, Formal Analysis. All authors have read and agreed to the published version of the manuscript.
Funding
We appreciate the UGA Hatch project for funding this project.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be available upon request.
Acknowledgments
We thank C. Hardin for assisting with sample collection in 2021 and obtaining research materials. Additionally, we thank B.W. Fields, C. Daughtery, D. Nordstrom, R.W. Hodgson, and all other University of Georgia Griffin Campus groundskeeping and maintenance personnel for mowing the lawn sites as necessary.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lander, T. Network modelling, citizen science and targeted interventions to predict, monitor and reverse bee decline. Plants People Planet 2019, 2, 111–120. [Google Scholar] [CrossRef]
- Cameron, S.A.; Lozier, J.D.; Strange, J.P.; Koch, J.B.; Cordes, N.; Solter, L.F.; Griswold, T.L. Patterns of widespread decline in North American bumble bees. Proc. Natl. Acad. Sci. USA 2011, 108, 662–667. [Google Scholar] [CrossRef]
- Wolfen, J.; Watkins, E.; Lane, I.; Portman, Z.M.; Spivak, M. Floral enhancement of turfgrass lawns benefits wild bees and honey bees (Apis mellifera). Urban Ecosyst. 2023, 26, 361–375. [Google Scholar] [CrossRef]
- US Census. 2020 Census Urban Areas Facts. United States Census Bureau. 2023. Available online: https://www.census.gov/programs-surveys/geography/guidance/geo-areas/urban-rural/2020-ua-facts.html (accessed on 11 February 2024).
- Kane, S.P.; Wolfe, K.L. Economic Contribution of Turfgrass Production, Ornamental Horticulture, Landscape Services, and Related Industry in the Georgia Economy, 2010. University of Georgia Center for Agribusiness & Economic Development. 2012. Available online: https://esploro.libs.uga.edu/esploro/outputs/report/Economic-contribution-of-turfgrass-production-ornamental/9949316486902959?institution=01GALI_UGA (accessed on 11 February 2024).
- Chawla, S.L.; Agnihotri, R.; Patel, M.A.; Patil, S.; Shah, H.P. Turfgrass: A Billion Dollar Industry. National Conference on Floriculture for Rural and Urban Prosperity in the Scenerio of Climate Change-2018. Available online: https://www.researchgate.net/profile/Roshni-Agnihotri-2/publication/324483293_Turfgrass_A_Billion_Dollar_Industry/links/5acf88c5aca2723a33454f73/Turfgrass-A-Billion-Dollar-Industry.pdf (accessed on 11 February 2024).
- Brosnan, J.T.; Chandra, A.; Gaussoin, R.E.; Kowalewski, A.; Leinauer, B.; Rossi, F.S.; Soldat, D.J.; Stier, J.C.; Unruh, B.J. A justification for continued management of turfgrass during economic contraction. Agri. Environ. Lett. 2020, 5, e20033. [Google Scholar] [CrossRef]
- Tonietto, R.; Fant, J.; Ascher, J.; Ellis, K.; Larkin, D. A comparison of bee communities of Chicago green roofs, parks and prairies. Landsc. Urban Plan. 2011, 103, 102–108. [Google Scholar] [CrossRef]
- Joseph, S.V.; Harris-Shultz, K.; Jespersen, D.; Vermeer, B.; Julian, C. Incidence of bees and wasps in centipedegrass lawns in Georgia. J. Entomol. Sci. 2020, 55, 547–559. [Google Scholar] [CrossRef]
- Jones, T. Why is the lawn buzzing? Biodivers. Data J. 2014, e1101. [Google Scholar] [CrossRef] [PubMed]
- Joseph, S.V.; Harris-Shultz, K.; Jespersen, D. Evidence of pollinators foraging on centipedegrass inflorescences. Insects 2020, 11, 795. [Google Scholar] [CrossRef]
- Joseph, S.V.; Hardin, C.B. Bees forage on bahiagrass spikelets. Fla. Entomol. 2022, 105, 95–98. [Google Scholar] [CrossRef]
- Potter, D.A.; Braman, S.K. Ecology and management of turfgrass insects. Annu. Rev. Entomol. 1991, 36, 383–406. [Google Scholar] [CrossRef]
- Larson, J.L.; Kesheimer, A.J.; Potter, D.A. Pollinator assemblages on dandelions and white clover in urban and suburban lawns. J. Insect Conserv. 2014, 18, 863–873. [Google Scholar] [CrossRef]
- Lerman, S.B.; Milam, J. Bee fauna and flora abundance within lawn-dominated suburban yards in Springfield, MA. Ann. Entomol. Soc. Am. 2016, 109, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Godara, N.; Williamson, R.C.; Koo, D.; Askew, S.D. Effect of herbicides on pollinator foraging behavior and flower morphology in white clover (Trifolium repens L.)—Infested turfgrass. Weed Technol. 2023, 37, 221–225. [Google Scholar] [CrossRef]
- Wilson, J.S.; Carril, O.M. Common Bees of Eastern North America (Princeton Field Guides); Princeton University Press: Princeton, NJ, USA, 2021. [Google Scholar]
- Colla, S.; Richardson, L.; Williams, P. Bumble Bees of the Eastern United States. FS-972. 2011. Available online: https://www.xerces.org/publications/identification-and-monitoring-guides/bumble-bees-of-eastern-united-states (accessed on 11 February 2024).
- Discover Life. Sam Houston State University, Texas. 2023. Available online: https://www.discoverlife.org/ (accessed on 11 February 2024).
- Murphy, T.R. Weeds of Southern Turfgrasses (Golf Courses, Lawns, Roadsides, Recreational Areas, Commercial Sod); University of Florida IFAS Extension: Gainesville, FL, USA, 2004. [Google Scholar]
- SAS Institute. Statistical Analysis System, Version 9.4; SAS Institute Inc.: Cary, NC, USA, 2016. [Google Scholar]
- Pleasants, J.M. Bumblebee response to variation in nectar availability. Ecology 1981, 62, 1648–1661. [Google Scholar] [CrossRef]
- Tew, N.E.; Memmott, J.; Vaughan, I.P.; Bird, S.; Stone, G.N.; Potts, S.G.; Baldock, C.R. Quantifying nectar production by flowering plants in urban and rural landscapes. J. Ecol. 2021, 109, 1747–1757. [Google Scholar] [CrossRef]
- Sincik, M.; Acikgoz, E. Effects of white clover inclusion on turf characteristics, nitrogen fixation, and nitrogen transfer from white clover to grass species in turf mixtures. Commun. Soil Sci. Plant Anal. 2007, 38, 1861–1877. [Google Scholar] [CrossRef]
- Cane, J.H. Landscaping pebbles attract nesting by the native ground-nesting bee Halictus rubicundus (Hymenoptera: Halictidae). Apidologie 2015, 46, 728–734. [Google Scholar] [CrossRef]
- Packer, L.; Sampson, B.J.; Lockerbie, C.; Jessome, V. Nest architecture and brood mortality in four species of sweat bee (Hymenoptera, Halictidae) from Cape Breton Island Nova Scotia, Canada. Can. J. Zool. 1989, 67, 2864–2870. [Google Scholar] [CrossRef]
- Soucy, S.L. Nesting biology and socially polymorphic behavior of the sweat bee Halictus rubicundus (Hymenoptera: Halictidae). Ann. Entomol. Soc. Am. 2002, 95, 57–65. [Google Scholar] [CrossRef]
- Brünnert, U.; Kelber, A.; Zeil, J. Ground-nesting bees determine the location of their nest relative to a landmark by other than angular size cues. J. Comp. Physiol. A 1994, 175, 363–369. [Google Scholar] [CrossRef]
- Deeksha, M.G.; Khan, M.S.; Kumar, G.; Udikeri, A. Pollination interaction with selected ‘weeds’ flora, Asteraceae, in the context of land use. Orient. Insects 2023, 57, 935–950. [Google Scholar] [CrossRef]
- Kleiman, B. Weeds enhance insect diversity and abundance and may improve soil conditions in mango cultivation of south Florida. Insects 2023, 14, 65–83. [Google Scholar] [CrossRef]
- Larson, J.L.; Redmond, C.T.; Potter, D.A. Impacts of a neonicotinoid, neonicotinoid-pyrethroid premix, and anthranilic diamide insecticide on four species of turf-inhabiting beneficial insects. Ecotoxicology 2014, 23, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Gels, J.A.; Held, D.W.; Potter, D.A. Hazards of insecticides to the bumble bees Bombus impatiens (Hymenoptera: Apidae) foraging on flowering white clover in turf. J. Econ. Entomol. 2002, 95, 722–728. [Google Scholar] [CrossRef]
- Larson, J.L.; Redmond, C.T.; Potter, D.A. Assessing insecticide hazard to bumble bees foraging on flowering weeds in treated lawns. PLoS ONE 2013, 8, e66375. [Google Scholar] [CrossRef]
- NTEP. Mean Turfgrass Quality and Other Ratings of Cool-Season Cultivars in the 2015 National Low Input Cool-Season Test at Columbia, MO. National Turfgrass Evaluation Program. 2019. Available online: https://ntep.org/data/cs15l/cs15l_20-8/cs15lmo119t.txt (accessed on 11 February 2024).
- Potter, D.A.; Redmond, C.T.; McNamara, T.D.; Munshaw, G.C. Dwarf white clover supports pollinators, augments nitrogen in clover–turfgrass lawns, and suppresses root-feeding grubs in monoculture but not in mixed swards. Sustainability 2021, 13, 11801. [Google Scholar] [CrossRef]
- Roulston, T.A.H.; Cane, J.H.; Buchmann, S.L. What governs protein content of pollen Pollinator preferences, pollen-pistil interactions, or phylogeny? Ecol. Monogr. 2000, 70, 617–643. [Google Scholar] [CrossRef]
- Inouye, D.W. The effect of proboscis and corolla tube lengths on patterns and rates of flower visitation by bumblebees. Oecologia 1980, 45, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Baldock, K.C.R.; Goddard, M.A.; Hicks, D.M.; Kunin, W.E.; Mitschunas, N.; Osgathorpe, L.M.; Potts, S.G.; Robertson, K.M.; Scott, A.V.; Stone, G.N.; et al. Where is the UK’s pollinator biodiversity? The importance of urban areas for flower-visiting insects. Proc. R. Soc. B 2015, 282, 20142849. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).