Combination of Photo-Fenton and Granular Activated Carbon for the Removal of Microcontaminants from Municipal Wastewater via an Acidic Dye
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Analytical Determinations
2.3. Matrix Characterisation
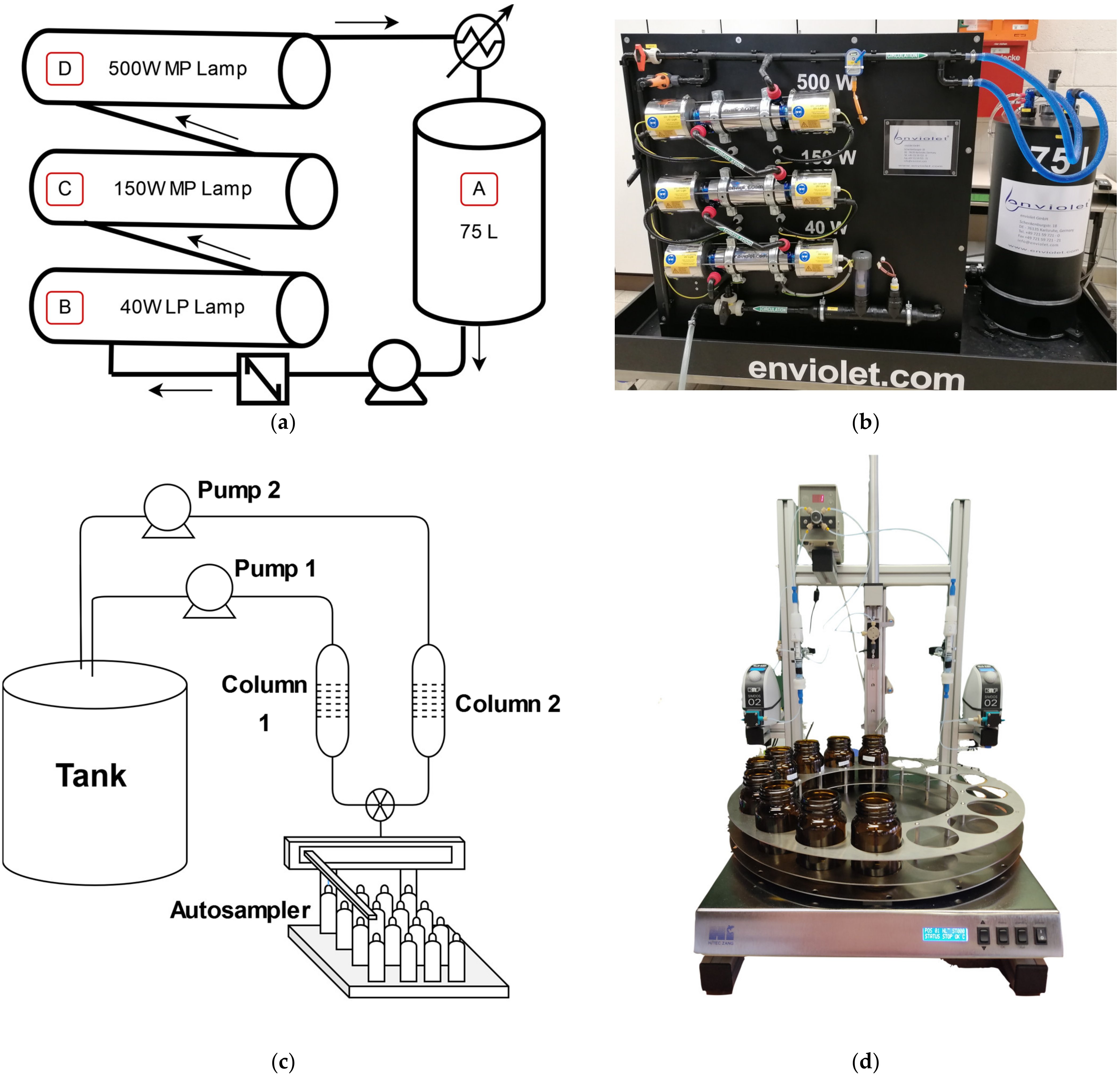
2.4. Experimental Setup
2.4.1. Photo-Fenton System
2.4.2. Granular Activated Carbon Behaviour (Adsorption Determination)
2.4.3. Granular Activated Carbon Rapid Small-Scale Columns Test
2.4.4. Combination of Photo-Fenton Process and Granular Activated Carbon
2.5. Methods
2.5.1. Optimization by Response Surface Methodology
2.5.2. Curve Fitting of the Adsorption Breakthrough
3. Results and Discussion
3.1. Photo-Fenton
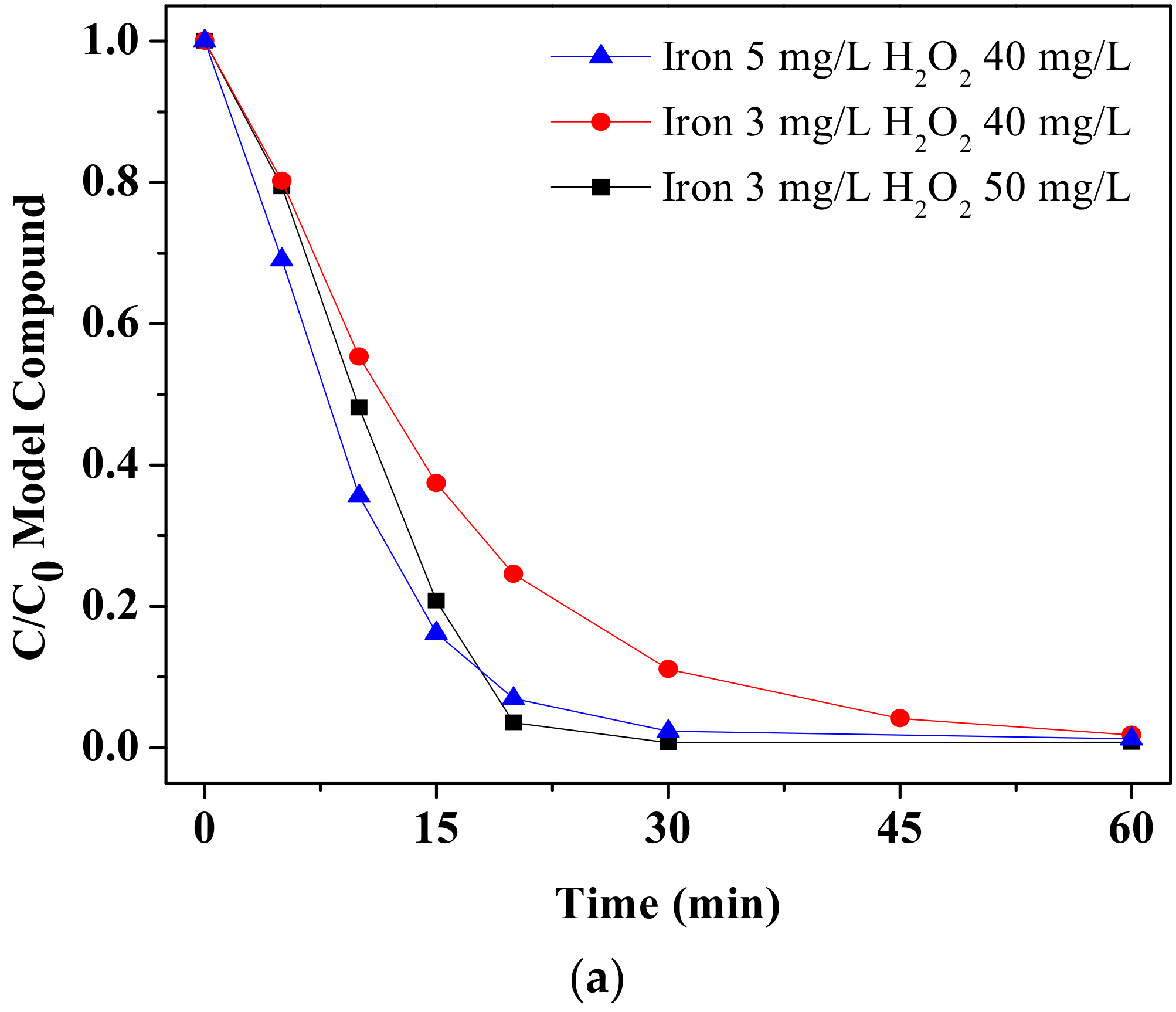
3.1.1. Determination of the Optimised Operational Conditions
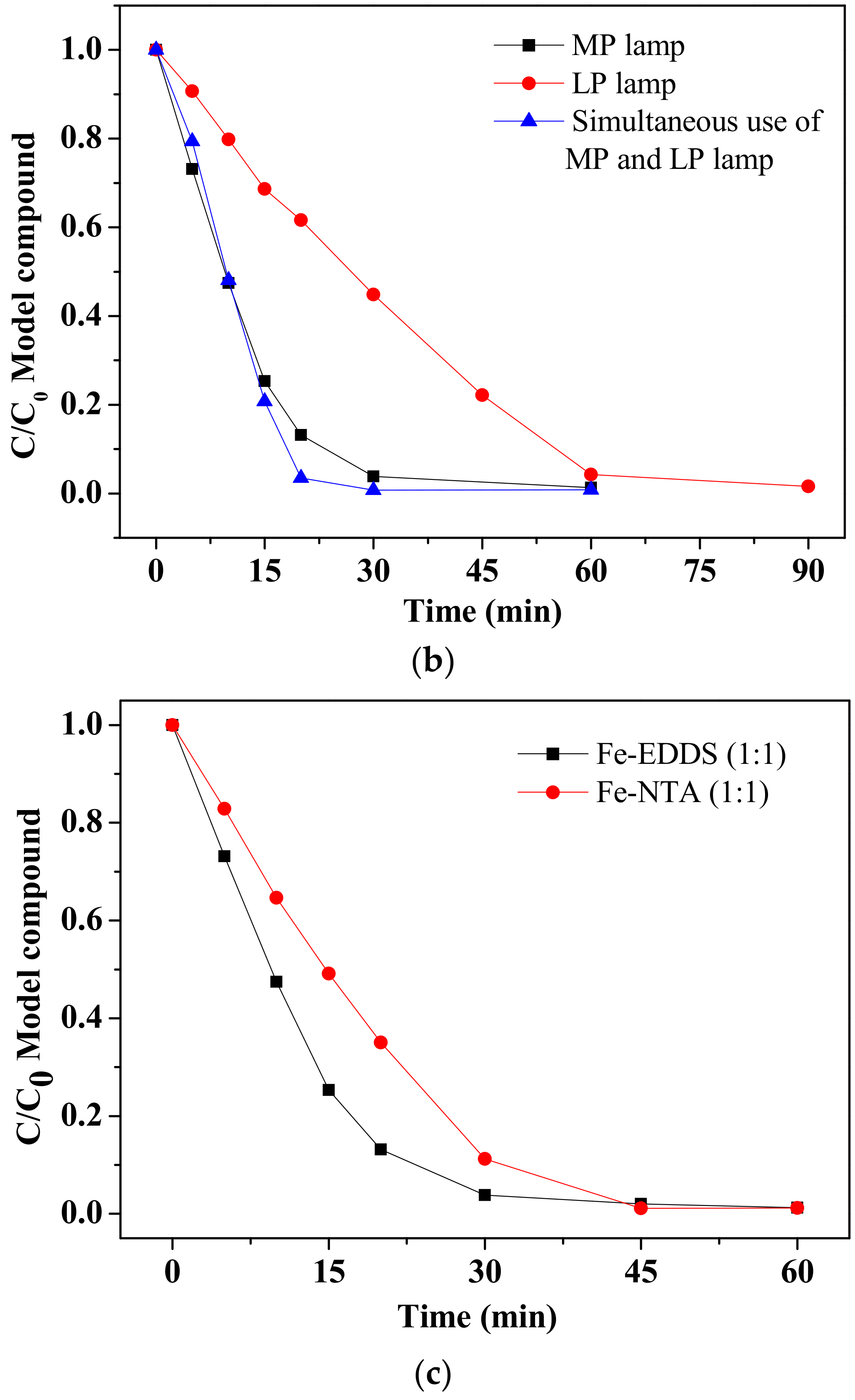
3.1.2. Simultaneous Application of MP and LP Lamps
3.1.3. Comparison of the Photo-Fenton Process: LP lamp, MP Lamp and Simultaneous Application of MP and LP Lamps
3.1.4. Chelating Agents’ Assessment
3.2. Granular Activated Carbon
3.2.1. GAC Behaviour (Adsorption Determination)
3.2.2. Rapid Small-Scale Columns
3.3. Real Wastewater
3.3.1. Photo-Fenton
3.3.2. Rapid Small-Scale Columns
3.4. Economical Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Júnior, O.G.; Batista, L.L.; Ueira-Vieira, C.; Sousa, R.M.; Starling, M.C.V.; Trovó, A.G. Degradation mechanism of fipronil and its transformation products, matrix effects and toxicity during the solar/photo-Fenton process using ferric citrate complex. J. Environ. Manag. 2020, 269, 110756. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, R.; Tian, T.; Shang, X.; Du, X.; He, Y.; Matsuura, N.; Luo, T.; Wang, Y.; Chen, J.; et al. Screening and ecological risk of 1200 organic micropollutants in Yangtze Estuary water. Water Res. 2021, 201, 117341. [Google Scholar] [CrossRef] [PubMed]
- Grandclément, C.; Seyssiecq, I.; Piram, A.; Wong-Wah-Chung, P.; Vanot, G.; Tiliacos, N.; Roche, N.; Doumenq, P. From the conventional biological wastewater treatment to hybrid processes, the evaluation of organic micropollutant removal: A review. Water Res. 2017, 111, 297–317. [Google Scholar] [CrossRef] [PubMed]
- Di Marcantonio, C.; Chiavola, A.; Dossi, S.; Cecchini, G.; Leoni, S.; Frugis, A.; Spizzirri, M.; Boni, M.R. Occurrence, seasonal variations and removal of Organic Micropollutants in 76 Wastewater Treatment Plants. Process. Saf. Environ. Prot. 2020, 141, 61–72. [Google Scholar] [CrossRef]
- He, J.; Yang, X.; Men, B.; Wang, D. Interfacial mechanisms of heterogeneous Fenton reactions catalyzed by iron-based materials: A review. J. Environ. Sci. 2016, 39, 97–109. [Google Scholar] [CrossRef]
- López-Vinent, N.; Cruz-Alcalde, A.; Gutiérrez, C.; Marco, P.; Giménez, J.; Esplugas, S. Micropollutant removal in real WW by photo-Fenton (circumneutral and acid pH) with BLB and LED lamps. Chem. Eng. J. 2020, 379, 122416. [Google Scholar] [CrossRef]
- Díaz-Angulo, J.; Cotillas, S.; Gomes, A.I.; Miranda, S.M.; Mueses, M.; Machuca-Martínez, F.; Rodrigo, M.A.; Boaventura, R.A.; Vilar, V.J. A tube-in-tube membrane microreactor for tertiary treatment of urban wastewaters by photo-Fenton at neutral pH: A proof of concept. Chemosphere 2020, 263, 128049. [Google Scholar] [CrossRef] [PubMed]
- Clarizia, L.; Russo, D.; Di Somma, I.; Marotta, R.; Andreozzi, R. Homogeneous photo-Fenton processes at near neutral pH: A review. Appl. Catal. B Environ. 2017, 209, 358–371. [Google Scholar] [CrossRef]
- Arzate, S.; Campos-Mañas, M.; Miralles-Cuevas, S.; Agüera, A.; Sánchez, J.G.; Pérez, J.S. Removal of contaminants of emerging concern by continuous flow solar photo-Fenton process at neutral pH in open reactors. J. Environ. Manag. 2020, 261, 110265. [Google Scholar] [CrossRef]
- Lin, H.H.-H.; Lin, A.Y.-C. Solar photo-Fenton oxidation of cytostatic drugs via Fe(III)-EDDS at circumneutral pH in an aqueous environment. J. Water Process. Eng. 2021, 41, 102066. [Google Scholar] [CrossRef]
- Dong, W.Y. Jin, K. Zhou, S.P. Sun, Y. Li, X.D. Chen, Efficient degradation of pharmaceutical micropollutants in water and wastewater by FeIII-NTA-catalyzed neutral photo-Fenton process. Sci. Total Environ. 2019, 688, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Oller, I.; Malato, S. Photo-Fenton applied to the removal of pharmaceutical and other pollutants of emerging concern. Curr. Opin. Green Sustain. Chem. 2021, 29, 100458. [Google Scholar] [CrossRef]
- García, L.; Leyva-Díaz, J.C.; Díaz, E.; Ordóñez, S. A review of the adsorption-biological hybrid processes for the abatement of emerging pollutants: Removal efficiencies, physicochemical analysis, and economic evaluation. Sci. Total. Environ. 2021, 780, 146554. [Google Scholar] [CrossRef]
- Telgmann, U.; Borowska, E.; Felmeden, J.; Frechen, F.-B. The locally resolved filtration process for removal of phosphorus and micropollutants with GAC. J. Water Process. Eng. 2020, 35, 101236. [Google Scholar] [CrossRef]
- Paredes, L.; Alfonsin, C.; Allegue, T.; Omil, F.; Carballa, M. Integrating granular activated carbon in the post-treatment of membrane and settler effluents to improve organic micropollutants removal. Chem. Eng. J. 2018, 345, 79–86. [Google Scholar] [CrossRef]
- Mo, W.; Cornejo, P.K.; Malley, J.P.; Kane, T.E.; Collins, M.R. Life cycle environmental and economic implications of small drinking water system upgrades to reduce disinfection byproducts. Water Res. 2018, 143, 155–164. [Google Scholar] [CrossRef]
- Durna, E.; Erkişi, E.; Genç, N. Regeneration of diclofenac-spent granular activated carbon by sulphate radical based methods: Multi-response optimisation of adsorptive capacity and operating cost. Int. J. Environ. Anal. Chem. 2020, 102, 4695–4709. [Google Scholar] [CrossRef]
- Larasati, A.; Fowler, G.D.; Graham, N.J. Extending granular activated carbon (GAC) bed life: A column study of in-situ chemical regeneration of pesticide loaded activated carbon for water treatment. Chemosphere 2021, 286, 131888. [Google Scholar] [CrossRef]
- Genç, N.; Durna, E.; Erkişi, E. Optimization of the adsorption of diclofenac by activated carbon and the acidic regeneration of spent activated carbon. Water Sci. Technol. 2020, 83, 396–408. [Google Scholar] [CrossRef]
- Michael, S.G.; Michael-Kordatou, I.; Beretsou, V.G.; Jäger, T.; Michael, C.; Schwartz, T.; Fatta-Kassinos, D. Solar photo-Fenton oxidation followed by adsorption on activated carbon for the minimisation of antibiotic resistance determinants and toxicity present in urban wastewater. Appl. Catal. B Environ. 2019, 244, 871–880. [Google Scholar] [CrossRef]
- Della-Flora, A.; Wilde, M.L.; Thue, P.S.; Lima, D.; Lima, E.C.; Sirtori, C. Combination of solar photo-Fenton and adsorption process for removal of the anticancer drug Flutamide and its transformation products from hospital wastewater. J. Hazard. Mater. 2020, 396, 122699. [Google Scholar] [CrossRef]
- Khalil, A.; Nasser, W.S.; Osman, T.; Toprak, M.S.; Muhammed, M.; Uheida, A. Surface modified of polyacrylonitrile nanofibers by TiO2/MWCNT for photodegradation of organic dyes and pharmaceutical drugs under visible light irradiation. Environ. Res. 2019, 179, 108788. [Google Scholar] [CrossRef]
- Gemeay, A.H.; El-Halwagy, M.E.; El-Sharkawy, R.G.; Zaki, A.B. Chelation mode impact of copper(II)-aminosilane complexes immobilized onto graphene oxide as an oxidative catalyst. J. Environ. Chem. Eng. 2017, 5, 2761–2772. [Google Scholar] [CrossRef]
- Liao, H.; Stenman, D.; Jonsson, M. Study of Indigo carmine as radical probe in photocatalysis. J. Photochem. Photobiol. A Chem. 2009, 202, 86–91. [Google Scholar] [CrossRef]
- Azam, K.; Shezad, N.; Shafiq, I.; Akhter, P.; Akhtar, F.; Jamil, F.; Shafique, S.; Park, Y.-K.; Hussain, M. A review on activated carbon modifications for the treatment of wastewater containing anionic dyes. Chemosphere 2022, 306, 135566. [Google Scholar] [CrossRef]
- Chowdhury, M.F.; Khandaker, S.; Sarker, F.; Islam, A.; Rahman, M.T.; Awual, M.R. Current treatment technologies and mechanisms for removal of indigo carmine dyes from wastewater: A review. J. Mol. Liq. 2020, 318, 114061. [Google Scholar] [CrossRef]
- El-Kammah, M.; Elkhatib, E.; Gouveia, S.; Cameselle, C.; Aboukila, E. Enhanced removal of Indigo Carmine dye from textile effluent using green cost-efficient nanomaterial: Adsorption, kinetics, thermodynamics and mechanisms. Sustain. Chem. Pharm. 2022, 29, 100753. [Google Scholar] [CrossRef]
- Tien, H.N.; Bui, D.N.; Manh, T.D.; Tram, N.T.; Ngo, V.D.; Mwazighe, F.M.; Hoang, H.Y.; Le, V.T. Electrochemical degradation of indigo carmine, P-nitrosodimethylaniline and clothianidin on a fabricated Ti/SnO2–Sb/Co-βPbO2 electrode: Roles of radicals, water matrices effects and performance. Chemosphere 2023, 313, 137352. [Google Scholar] [CrossRef] [PubMed]
- Lekshmi, K.V.; Yesodharan, S.; Yesodharan, E. MnO2 efficiently removes indigo carmine dyes from polluted water. Heliyon 2018, 4, e00897. [Google Scholar] [CrossRef] [PubMed]
- Ghanmi, I.; Sassi, W.; Oulego, P.; Collado, S.; Ghorbal, A.; Díaz, M. Optimization and comparison study of adsorption and photosorption processes of mesoporous nano-TiO2 during discoloration of Indigo Carmine dye. Microporous Mesoporous Mater. 2022, 342, 112138. [Google Scholar] [CrossRef]
- Saikam, L.; Arthi, P.; Jayram, N.D.; Sykam, N. Rapid removal of organic dyes from aqueous solutions using mesoporous exfoliated graphite. Diam. Relat. Mater. 2022, 130, 109480. [Google Scholar] [CrossRef]
- De la Cruz, N.; Esquius, L.; Grandjean, D.; Magnet, A.; Tungler, A.; de Alencastro, L.; Pulgarín, C. Degradation of emergent contaminants by UV, UV/H2O2 and neutral photo-Fenton at pilot scale in a domestic wastewater treatment plant. Water Res. 2013, 47, 5836–5845. [Google Scholar] [CrossRef]
- Miklos, D.B.; Hartl, R.; Michel, P.; Linden, K.G.; Drewes, J.E.; Hübner, U. UV/H2O2 process stability and pilot-scale validation for trace organic chemical removal from wastewater treatment plant effluents. Water Res. 2018, 136, 169–179. [Google Scholar] [CrossRef]
- Zietzschmann, F.; Müller, J.; Sperlich, A.; Ruhl, A.S.; Meinel, F.; Altmann, J.; Jekel, M. Rapid small-scale column testing of granular activated carbon for organic micro-pollutant removal in treated domestic wastewater. Water Sci. Technol. 2014, 70, 1271–1278. [Google Scholar] [CrossRef]
- Ramos, R.O.; Albuquerque, M.V.; Lopes, W.S.; Sousa, J.T.; Leite, V.D. Degradation of indigo carmine by photo-Fenton, Fenton, H2O2/UV-C and direct UV-C: Comparison of pathways, products and kinetics. J. Water Process. Eng. 2020, 37, 101535. [Google Scholar] [CrossRef]
- Wroch, E. Adsorption Technology in Water Treatment: Fundamentals, Processes and Modeling, 2nd ed.; De Gruyter: Berlin, Germany, 2021. [Google Scholar]
- Kumar, J.A.; Kumar, P.S.; Krithiga, T.; Prabu, D.; Amarnath, D.J.; Sathish, S.; Venkatesan, D.; Hosseini-Bandegharaei, A.; Prashant, P. Acenaphthene adsorption onto ultrasonic assisted fatty acid mediated porous activated carbon-characterization, isotherm and kinetic studies. Chemosphere 2021, 284, 131249. [Google Scholar] [CrossRef]
- Katsigiannis, A.; Noutsopoulos, C.; Mantziaras, J.; Gioldasi, M. Removal of emerging pollutants through Granular Activated Carbon. Chem. Eng. J. 2015, 280, 49–57. [Google Scholar] [CrossRef]
- Sun, Y.; Pignatello, J.J. Photochemical reactions involved in the total mineralization of 2,4-D by iron(3+)/hydrogen peroxide/UV. Environ. Sci. Technol. 1993, 27, 304–310. [Google Scholar] [CrossRef]
- Arslan-Alaton, I.; Tureli, G.; Olmez-Hanci, T. Treatment of azo dye production wastewaters using Photo-Fenton-like advanced oxidation processes: Optimization by response surface methodology. J. Photochem. Photobiol. A Chem. 2009, 202, 142–153. [Google Scholar] [CrossRef]
- Mitsika, E.E.; Christophoridis, C.; Kouinoglou, N.; Lazaridis, N.; Zacharis, C.K.; Fytianos, K. Optimized Photo-Fenton degradation of psychoactive pharmaceuticals alprazolam and diazepam using a chemometric approach—Structure and toxicity of transformation products. J. Hazard. Mater. 2020, 403, 123819. [Google Scholar] [CrossRef] [PubMed]
- Palma-Goyes, R.E.; Silva-Agredo, J.; González, I.; Torres-Palma, R.A. Comparative degradation of indigo carmine by electrochemical oxidation and advanced oxidation processes. Electrochimica Acta 2014, 140, 427–433. [Google Scholar] [CrossRef]
- Lumbaque, E.C.; Araújo, D.S.; Klein, T.M.; Tiburtius, E.R.L.; Argüello, J.; Sirtori, C. Solar photo-Fenton-like process at neutral pH: Fe(III)-EDDS complex formation and optimization of experimental conditions for degradation of pharmaceuticals. Catal. Today 2019, 328, 259–266. [Google Scholar] [CrossRef]
- Land Brandenburg. Strategischen Gesamtplan zur Senkung der Bergbaubedingten Stoffeinträge in die Spree und deren Zuflüsse in der Lausitz. 2015. Available online: https://mluk.brandenburg.de/mluk/de/umwelt/wasser/bergbaufolgen-fuer-den-wasserhaushalt/# (accessed on 9 February 2024).
- Nippes, R.P.; Macruz, P.D.; Scaliante, M.H.N.O. Toxicity reduction of persistent pollutants through the photo-fenton process and radiation/H2O2 using different sources of radiation and neutral pH. J. Environ. Manag. 2021, 289, 112500. [Google Scholar] [CrossRef] [PubMed]
- Mejri, A.; Soriano-Molina, P.; Miralles-Cuevas, S.; Pérez, J.A.S. Fe3+-NTA as iron source for solar photo-Fenton at neutral pH in raceway pond reactors. Sci. Total. Environ. 2020, 736, 139617. [Google Scholar] [CrossRef] [PubMed]
- Soriano-Molina, P.; De la Obra, I.; Miralles-Cuevas, S.; Gualda-Alonso, E.; López, J.C.; Pérez, J.S. Assessment of different iron sources for continuous flow solar photo-Fenton at neutral pH for sulfamethoxazole removal in actual MWWTP effluents. J. Water Process. Eng. 2021, 42, 102109. [Google Scholar] [CrossRef]
- Nunes, K.G.P.; Sfreddo, L.W.; Rosset, M.; Féris, L.A. Efficiency evaluation of thermal, ultrasound and solvent techniques in activated carbon regeneration. Environ. Technol. 2020, 42, 4189–4200. [Google Scholar] [CrossRef]
- Kempisty, D.M.; Arevalo, E.; Spinelli, A.M.; Edeback, V.; Dickenson, E.R.V.; Husted, C.; Higgins, C.P.; Summers, R.S.; Knappe, D.R.U. Granular activated carbon adsorption of perfluoroalkyl acids from ground and surface water. AWWA Water Sci. 2022, 4, e1269. [Google Scholar] [CrossRef]
- Dutta, T.; Kim, T.; Vellingiri, K.; Tsang, D.C.; Shon, J.; Kim, K.-H.; Kumar, S. Recycling and regeneration of carbonaceous and porous materials through thermal or solvent treatment. Chem. Eng. J. 2019, 364, 514–529. [Google Scholar] [CrossRef]
- Han, C.-H.; Park, H.-D.; Kim, S.-B.; Yargeau, V.; Choi, J.-W.; Lee, S.-H.; Park, J.-A. Oxidation of tetracycline and oxytetracycline for the photo-Fenton process: Their transformation products and toxicity assessment. Water Res. 2020, 172, 115514. [Google Scholar] [CrossRef]
- Lumbaque, E.C.; Becker, R.W.; Araújo, D.S.; Dallegrave, A.; Ost Fracari, T.; Lavayen, V.; Sirtori, C. Degradation of pharmaceuticals in different water matrices by a solar homo/heterogeneous photo-Fenton process over modified alginate spheres. Environ. Sci. Pollut. Res. 2019, 26, 6532–6544. [Google Scholar] [CrossRef]
- Belalcázar-Saldarriaga, A.; Prato-Garcia, D.; Vasquez-Medrano, R. Photo-Fenton processes in raceway reactors: Technical, economic, and environmental implications during treatment of colored wastewaters. J. Clean. Prod. 2018, 182, 818–829. [Google Scholar] [CrossRef]
- Miralles-Cuevas, S.; Oller, I.; Agüera, A.; Pérez, J.A.S.; Sánchez-Moreno, R.; Malato, S. Is the combination of nanofiltration membranes and AOPs for removing microcontaminants cost effective in real municipal wastewater effluents? Environ. Sci. Water Res. Technol. 2016, 2, 511–520. [Google Scholar] [CrossRef]

 0.75 g L−1 GAC, ★ 1 g L−1 GAC.
0.75 g L−1 GAC, ★ 1 g L−1 GAC.
 0.75 g L−1 GAC, ★ 1 g L−1 GAC.
0.75 g L−1 GAC, ★ 1 g L−1 GAC.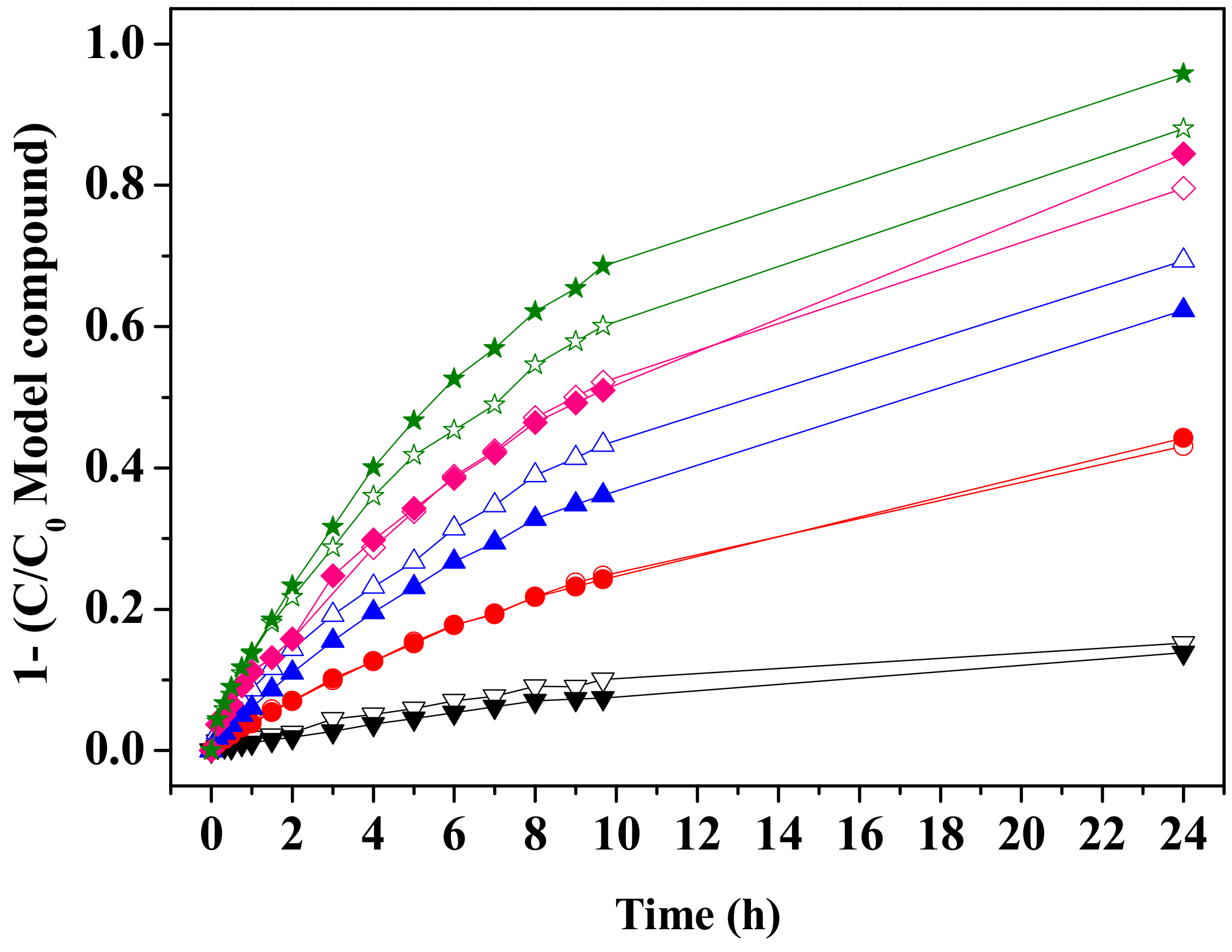

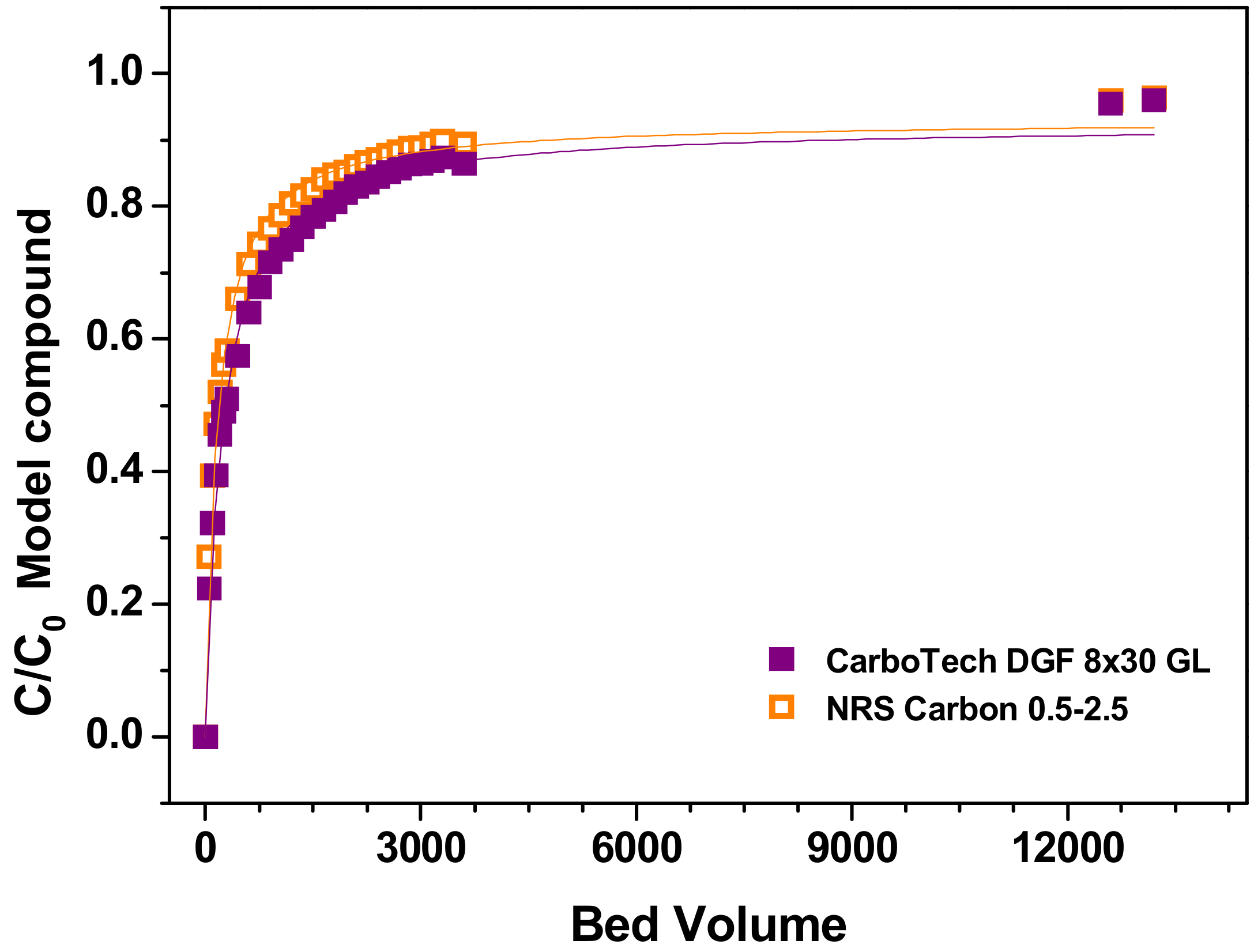

| Source | Sum of Square | Degree of Freedom | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| 500 W MP Lamp | ||||||
| Quadratic model | 1581.88 | 5 | 316.38 | 96.68 | 0.003 | Significant |
| A-Iron | 870.92 | 1 | 870.92 | 266.14 | <0.0001 | |
| B-H2O2 | 333.48 | 1 | 333.48 | 101.91 | 0.0005 | |
| AB | 5.64 | 1 | 5.64 | 1.72 | 0.2595 | |
| A2 | 330.88 | 1 | 330.88 | 101.11 | 0.0006 | |
| B2 | 97.71 | 197.71 | 29.86 | 0.0055 | ||
| Residual | 13.09 | 4 | 3.27 | Not significant | ||
| Lack of fit | 8.94 | 3 | 2.98 | |||
| Pure error | 4.15 | 1 | 4.15 | |||
| 40 W LP Lamp | ||||||
| Lineal model | 944.07 | 2 | 472.04 | 12.54 | 0.0048 | Significant |
| A-Iron | 681.81 | 1 | 681.81 | 18.11 | 0.0038 | |
| B-H2O2 | 262.26 | 1 | 262.26 | 6.96 | 0.0335 | |
| Residual | 2.63.59 | 7 | 37.66 | |||
| Lack of fit | 261.55 | 6 | 43.59 | 21.37 | 0.1641 | Not significant |
| Pure error | 2.04 | 1 | 2.04 | |||
| CarboTech DGF 8x30 GL | NRS Carbon 0.5–2.5 | ||
|---|---|---|---|
| First-order kinetic | q | 86.38 | 73.21 |
| K | 5.601 x 10-4 | 6.069 x 10-4 | |
| R2 | 0.992 | 0.999 | |
| Second-order kinetic | A | 0.477 | 0.479 |
| B | 0.016 | 0.018 | |
| R2 | 0.884 | 0.897 |
| Photo-Fenton | GAC Columns | Combination of Processes | |||
|---|---|---|---|---|---|
| CarboTech DGF 8x30 GL | NRS Carbon GA 0.5–2.5 | CarboTech DGF 8x30 GL | NRS Carbon GA 0.5–2.5 | ||
| Energy (€/m3) | 0.059 | -- | -- | 0.010 | 0.010 |
| GAC cost (€/m3) | -- | 0.060 | 0.055 | 0.008 | 0.015 |
| Reagents (€/m3) | 0.144 | -- | -- | 0.144 | 0.144 |
| Total (€/m3) | 0.203 | 0.060 | 0.055 | 0.161 | 0.169 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Núñez-Tafalla, P.; Salmerón, I.; Venditti, S.; Hansen, J. Combination of Photo-Fenton and Granular Activated Carbon for the Removal of Microcontaminants from Municipal Wastewater via an Acidic Dye. Sustainability 2024, 16, 1605. https://doi.org/10.3390/su16041605
Núñez-Tafalla P, Salmerón I, Venditti S, Hansen J. Combination of Photo-Fenton and Granular Activated Carbon for the Removal of Microcontaminants from Municipal Wastewater via an Acidic Dye. Sustainability. 2024; 16(4):1605. https://doi.org/10.3390/su16041605
Chicago/Turabian StyleNúñez-Tafalla, Paula, Irene Salmerón, Silvia Venditti, and Joachim Hansen. 2024. "Combination of Photo-Fenton and Granular Activated Carbon for the Removal of Microcontaminants from Municipal Wastewater via an Acidic Dye" Sustainability 16, no. 4: 1605. https://doi.org/10.3390/su16041605





