Fabrication of Bamboo-Based Activated Carbon for Low-Level CO2 Adsorption toward Sustainable Indoor Air
Abstract
:1. Introduction
2. Experimental Method
2.1. Materials
2.2. Methods
2.2.1. Preparation of the Activated Cabon
2.2.2. Surface Modification of AC
2.2.3. Characterization of the Activated Carbon Adsorbents
2.2.4. CO2 Adsorption Test
3. Results
3.1. Preparing the AC Adsorbents
3.1.1. Morphology
3.1.2. Textural Analysis
3.2. Characterization of Chemical Structure
3.2.1. Surface Chemical Composition
3.2.2. Surface Functionalities
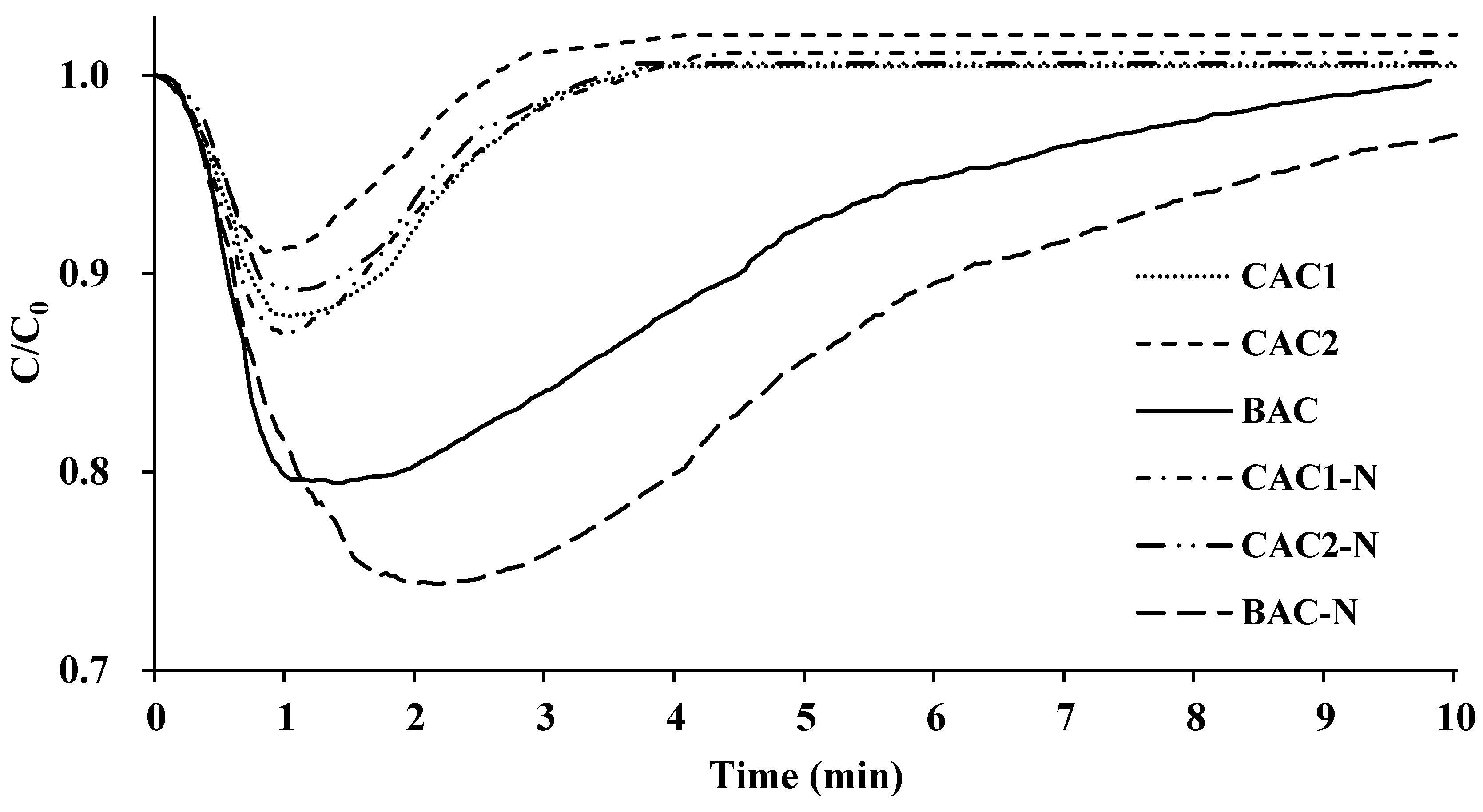
3.3. Evaluating the Low-Level CO2 Adsorption Capacity
3.4. Evaluating the CO2/N2 Adsorption Selectivity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Karimi, H.; Adibhesami, M.A.; Bazzazzadah, H.; Movafagh, S. Green buildings: Human-centered and energy efficiency optimization strategy. Energies 2023, 16, 3681. [Google Scholar] [CrossRef]
- Mannan, M.; Al-Ghamdi, S.G. Indoor air quality in buildings: A comprehensive review on the factors influencing air pollution in residential and commercial structure. Int. J. Environ. Res. Public Health 2021, 18, 3276. [Google Scholar] [CrossRef]
- Burridge, H.C.; Bhagat, R.K.; Stettler, M.E.J.; Kumar, P.; De Mel, I.; Demis, P.; Hart, A.; Johnson-Llambias, Y.; King, M.F.; Klymenko, O.; et al. The ventilation of buildings and other mitigating measures for COVID-19: A focus on wintertime. Proc. R. Soc. A 2021, 477, 20200855. [Google Scholar] [CrossRef]
- Moreno-Rangel, A.; Sharpe, T.; Musau, F.; McGill, G. Field evaluation of a low-cost indoor air quality monitor to quantify exposure to pollutants in residential environments. J. Sens. Sens. Syst. 2018, 7, 373. [Google Scholar] [CrossRef]
- Kumar, P.; Hama, S.; Abbass, R.A.; Nogueira, T.; Brand, V.S.; Wu, H.-W. CO2 exposure, ventilation, thermal comfort and health risks in low-income home kitchens of twelve global cities. J. Build. Eng. 2022, 61, 105254. [Google Scholar] [CrossRef]
- Gawande, S.; Tiwari, R.R.; Narayanan, P.; Bhadri, A. Indoor air quality and sick building syndrome: Are green buildings better than conventional buildings? Indian J. Occup. Environ. Med. 2020, 24, 30–32. [Google Scholar]
- Jacobson, T.A.; Kler, J.S.; Hernke, M.T.; Braun, R.K.; Meyer, K.C.; Funk, W.E. Direct human health risks of increased atmospheric carbon dioxide. Nat. Sustain. 2019, 2, 691–701. [Google Scholar] [CrossRef]
- Lowther, S.D.; Dimitroulopoulou, S.; Foxall, K.; Shrubsole, C.; Cheek, E.; Gadeberg, E.; Sepai, O. Low level carbon dioxide indoor–A pollution indicator or a pollutant? A health-based perspective. Environments 2021, 8, 125. [Google Scholar] [CrossRef]
- Lee, M.; Ham, J.; Lee, J.-W.; Cho, H. Analysis of thermal comfort, energy consumption, and CO2 reduction of indoor space according to the type of local heating under winter rest conditions. Energy 2023, 268, 126722. [Google Scholar] [CrossRef]
- Hattori, S.; Iwamatsu, T.; Miura, T.; Tsutsumi, F.; Tanaka, N. Investigation of indoor air quality in residential buildings by measuring CO2 concentration and a questionnaire survey. Sensors 2022, 22, 7331. [Google Scholar] [CrossRef]
- Raupach, M.R.; Davis, S.J.; Peters, G.P.; Andrew, R.M.; Canadell, J.G.; Ciais, P.; Le Quere, C. Sharing a quota on cumulative carbon emissions. Nat. Clim. Chang. 2014, 4, 873–879. [Google Scholar] [CrossRef]
- Huston, N.D. Structural effects on the high-temperature adsorption of CO2 on a synthetic hydrotalcite. Chem. Mater. 2004, 16, 4135–4143. [Google Scholar] [CrossRef]
- Kim, Y.E.; Choi, J.H.; Yun, S.H.; Nam, S.C.; Yoon, Y.I. CO2 capture using aqueous solution of K2CO3-methylpiperazine and monoethanolamine: Specific heat capacity and heat of absorption. Korean J. Chem. Eng. 2016, 33, 3465–3472. [Google Scholar] [CrossRef]
- Pintola, T.; Tontiwachwuthikul, P.; Meisen, A. Simulation of pilot plant and industrial CO2 MEA absorbers. Gas Sep. Purif. 1993, 7, 47–52. [Google Scholar] [CrossRef]
- Strazisar, B.R. Degradation pathways for monoethanolamine in a CO2 capture facility. Energy Fuels 2003, 17, 1034–1039. [Google Scholar] [CrossRef]
- Herzog, H. An Introduction to CO2 Separation and Capture Technologies; Massachusetts Institute of Technology: Cambridge, MA, USA, 1999; pp. 1–8. [Google Scholar]
- Rougerol, F.; Rougerol, J.; Sing, K. Adsorption by Powders and Porous Solids; Academic Press: London, UK, 2012. [Google Scholar] [CrossRef]
- Gamal, M.; Mousa, H.A. Bio regeneration of activated carbon: A comprehensive review. Fuel Processing Technology. Sep. Purif. Technol. 2018, 197, 345–359. [Google Scholar] [CrossRef]
- Bai, J.; Huang, J.; Yu, Q.; Demir, M.; Akgul, E.; Altay, B.N.; Hu, X.; Wang, L. Fabrication of coconut shell-derived porous carbons for CO2 adsorption application. Front. Chem. Sci. Eng. 2023, 17, 1122–1130. [Google Scholar] [CrossRef]
- Duan, C.; Meng, M.; Huang, H.; Wang, H. Performance and characterization of bamboo-based activated carbon prepared by boric acid activation. Mater. Chem. Phys. 2023, 295, 127130. [Google Scholar] [CrossRef]
- Sarkisov, L. Accessible surface area of porous materials: Understanding theoretical limits. Adv. Mater. 2012, 24, 3130–3133. [Google Scholar] [CrossRef]
- Park, J.-W.; Ly, H.V.; Oh, C.; Kim, S.-S. Preparation and characterization of bamboo-based activated carbon by phosphoric acid and steam activation. Clean Technol. 2019, 25, 129–139. [Google Scholar] [CrossRef]
- Shao, Y.; Li, J.; Fang, X.; Yang, Z. Chemical modification of bamboo activated carbon surface and its adsorption property of simultaneous removal of phosphate and nitrate. Chemosphere 2022, 287, 132118. [Google Scholar] [CrossRef]
- Li, S.; Zhou, S.; Zhao, G. Tuning the morphology of micro and nanospheres from bamboo shoot shell cytosol lignin. Ind. Corps Prod. 2021, 171, 113860. [Google Scholar] [CrossRef]
- Wei, H.; Deng, S.; Hu, B.; Chen, Z.; Wang, B.; Huang, J.; Yu, G. Granular bamboo-derived activated carbon for high CO2 adsorption: The dominant role of narrow micropores. ChemSusChem 2012, 5, 2354–2360. [Google Scholar] [CrossRef]
- Wu, W.; Wu, C.; Zhang, G.; Liu, J.; Li, Y.; Li, G. Synthesis and characterization of magnetic K2CO3-activated carbon produced from bamboo shoot for the adsorption of Rhodamine b and CO2 capture. Fuel 2023, 332, 126107. [Google Scholar] [CrossRef]
- Adelodun, A.A.; Lim, Y.H.; Jo, Y.M. Stabilization of potassium-doped activated carbon by amination for improved CO2 selective capture. J. Anal. Appl. Pyrolysis 2014, 108, 151–159. [Google Scholar] [CrossRef]
- Babinszki, B.; Sebestyén, Z.; Jakab, E.; Kőhalmi, L.; Bozi, J.; Várhegyi, G.; Wang, L.; Skreiberg, Ø.; Czégény, Z. Effect of slow pyrolysis conditions on biocarbon yield and properties: Characterization of the volatiles. Bioresour. Technol. 2021, 338, 125567. [Google Scholar] [CrossRef]
- Laine, J.; Yunes, S. Effect of the preparation method on the pore size distribution of activated carbon from coconut shell. Carbon 1992, 30, 601–604. [Google Scholar] [CrossRef]
- Goel, C.; Dinesha, S. CO2 capture by adsorption on biomass-derived activated char: A review. Sci. Total Environ. 2021, 798, 149296. [Google Scholar] [CrossRef]
- Mochizuki, Y.; Bud, J.; Byambajav, E.; Tsubouchi, N. Influence of ammonia treatment on the CO2 adsorption of activated carbon. J. Environ. Chem. Eng. 2022, 10, 107273. [Google Scholar] [CrossRef]
- El-Nemr, M.; El Nemr, A.; Hassaan, M.A.; Ragab, S.; Tedone, L.; De Mastro, G.; Pantaleo, A. Microporous activated carbon from Pisum sativum pods using various activated methods and tested for adsorption of acid orange 7 dye from water. Molecules 2022, 27, 4840. [Google Scholar] [CrossRef]
- Kim, T.K.; Suh, M.P. Selective CO2 adsorption in a flexible non-interpenetrated metal-organic framework. Chem. Commun. 2011, 14, 4258–4260. [Google Scholar] [CrossRef] [PubMed]
- Correa, C.R.; Otto, T.; Kruse, A. Influence of the biomass components on the pore formation of activated carbon. Biomass Bioenergy 2016, 97, 53–64. [Google Scholar] [CrossRef]
- Araujo, T.; Parnell, A.J.; Bernardo, G.; Mendes, A. Cellulose-based carbon membranes for gas separations—Unravelling structural parameters and surface chemistry for superior separation performance. Carbon 2023, 204, 398–410. [Google Scholar] [CrossRef]
- Altintig, E.; Sarici, B.; Karataş, S. Prepared activated carbon from hazelnut shell where coated nanocomposite with Ag+, used for antibacterial and adsorption properties. Emviron. Sci. Pollut. Res. Int. 2022, 30, 13671–13687. [Google Scholar] [CrossRef]
- Patawat, C.; Silakate, K.; Chuan-Udom, S.; Supanchaiyamat, N.; Hunt, A.J.; Ngernyen, Y. Preparation of activated carbon from Dipterocarpus alatus fruit and its application for methylene blue adsorption. RSC Adv. 2020, 36, 21082. [Google Scholar] [CrossRef] [PubMed]
- Tay, T.; Ucar, S.; Karagoz, S. Preparation and characterization of activated carbon from waste biomass. J. Hazard. Mater. 2009, 165, 481–485. [Google Scholar] [CrossRef]
- Zuo, S.-L.; Gao, S.-Y.; Yuan, X.-G.; Xu, B.-S. Carbonization mechanism of bamboo (Phyllostachys) by means of Fourier Transform Infrared and elemental analysis. J. For. Res. 2003, 14, 75–79. [Google Scholar] [CrossRef]
- Zhu, Y.; Huang, J.; Wang, K.; Wang, B.; Sun, S.; Lin, X.; Song, L.; Wu, A.; Li, H. Characterization of Lignin Structures in Phyllostachys edulis (Moso Bamboo) at Different Ages. Polymers 2020, 12, 187. [Google Scholar] [CrossRef]
- Czernik, C.; Bridgwater, V. Overview of applications of biomass fast pyrolysis oil. Energy Fuels 2006, 18, 590–598. [Google Scholar] [CrossRef]
- Zhang, D.; Wei, W.; Lu, L.; Jin, H.; Guo, L. Variation of pore structure in Zhundong coal particle with stepped K2CO3 loading during supercritical water gasification. Fuel 2021, 305, 121457. [Google Scholar] [CrossRef]
- Adelodun, A.A.; Jo, Y.M. Integrated basic treatment of activated carbon for enhanced CO2 selectivity. Appl. Surf. Sci. 2013, 286, 306–313. [Google Scholar] [CrossRef]
- Bai, J.; Huang, J.; Yu, Q.; Demir, M.; Kilic, M.; Altay, B.N.; Hu, X.; Wang, L. N-doped porous carbon derived from macadamia nut shell for CO2 adsorption. Fuel Process. Technol. 2023, 249, 107854. [Google Scholar] [CrossRef]
- Lee, K.-M.; Lim, Y.-H.; Park, C.-J.; Jo, Y.-M. Adsorption of low-level CO2 using modified zeolites and activated carbon. Ind. Eng. Chem. Res. 2012, 51, 1355–1363. [Google Scholar] [CrossRef]
- Jo, Y.-M.; Hong, H.-E.; Adelodun, A.A. Preparation of KOH impregnated AC pellets for selective CO2 capture. J. Korean Soc. Odor Res. Eng. 2013, 12, 203–209. [Google Scholar]
- Adelodun, A.A.; Lim, Y.-H.; Jo, Y.-M. Effect of UV-C on pre-oxidation prior amination for preparation of a selective CO2 adsorbent. J. Anal. Appl. Pyrolysis 2014, 105, 191–198. [Google Scholar] [CrossRef]
- Wang, S.; Lee, Y.-R.; Won, Y.; Kim, H.; Jeong, S.-E.; Hwang, B.W.; Cho, A.R.; Kim, J.-Y.; Park, Y.C.; Nam, H.; et al. Development of high-performance adsorbent using KOH-impregnated rice husk-based activated carbon for indoor CO2 adsorption. Chem. Eng. J. 2022, 437, 135378. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. CO2 adsorption by activated templated carbons. J. Colloid Interface Sci. 2012, 366, 147–154. [Google Scholar] [CrossRef]
- Swietlik, U.; Grzyb, B.; Torchala, K.; Gryglewicz, G.; Machnikowski, J. High-temperature ammonia treatment of pitch particulates and fibers for nitro-gen enriched microporous carbons. Fuel Process. Technol. 2014, 119, 211–217. [Google Scholar] [CrossRef]
- Dilokekunakul, W.; Teerachawanwong, P.; Klomkliang, N.; Supasitmongkol, S.; Chaemchuen, S. Effects of nitrogen and oxygen functional groups and pore width of activated carbon on carbon dioxide capture: Temperature dependence. Chem. Eng. J. 2020, 389, 124413. [Google Scholar] [CrossRef]
- Petrovic, B.; Gorbounov, M.; Soltani, S.M. Influence of surface modification on selective CO2 adsorption: A technical review on mechanisms and methods. Microporous Mesoporous Mater. 2021, 312, 110751. [Google Scholar] [CrossRef]
- Hui, T.S.; Zaini, M.A.A. Potassium hydroxide activation of activated carbon: A commentary. Carbon Lett. 2015, 16, 275–280. [Google Scholar] [CrossRef]
- Azuma, K.; Kagi, N.; Yanagi, U.; Osawa, H. Effects of low-level inhalation exposure to carbon dioxide in indoor environments: A short review on human health and psychomotor performance. Environ. Int. 2018, 121, 51–56. [Google Scholar] [CrossRef]
- Kuga, K.; Ito, K.; Wargocki, P. The effects of warmth and CO2 concentration, with and without bioeffluents, on the emission of CO2 by occupants and physiological responses. Indoor Air 2021, 31, 2176–2187. [Google Scholar] [CrossRef] [PubMed]
- Snow, S.; Boyson, A.S.; Paas, K.H.W.; Gough, H.; King, M.-F.; Barlow, J.; Noakes, C.J.; Schraefel, M.C. Exploring the physiological, neurophysiological and cognitive performance effects of elevated carbon dioxide concentrations indoors. Build. Environ. 2019, 156, 243–252. [Google Scholar] [CrossRef]
- Mishra, A.K.; Schiavon, S.; Wargocki, P.; Tham, K.W. Respiratory performance of humans exposed to moderate levels of carbon dioxide. Indoor Air 2021, 31, 1540–1552. [Google Scholar] [CrossRef] [PubMed]
- Tu, Z.; Geng, S.; Zhou, K.; Wang, R.; Dong, X. Human responses to high levels of carbon dioxide and air temperature. Indoor Air 2020, 31, 872–886. [Google Scholar] [CrossRef]
- Chen, D.; Huebner, G.; Bagkeris, E.; Ucci, M.; Mumovic, D. Effects of short-term exposure to moderate pure carbon dioxide levels on cognitive performance, health symptoms and perceived indoor environment quality. Build. Environ. 2023, 245, 110967. [Google Scholar] [CrossRef]
- Park, S.; Song, D. CO2 concentration as an indicator of indoor ventilation performance to control airborne transmission of SARS-CoV-2. J. Infect. Public Health 2023, 16, 1037–1044. [Google Scholar] [CrossRef]
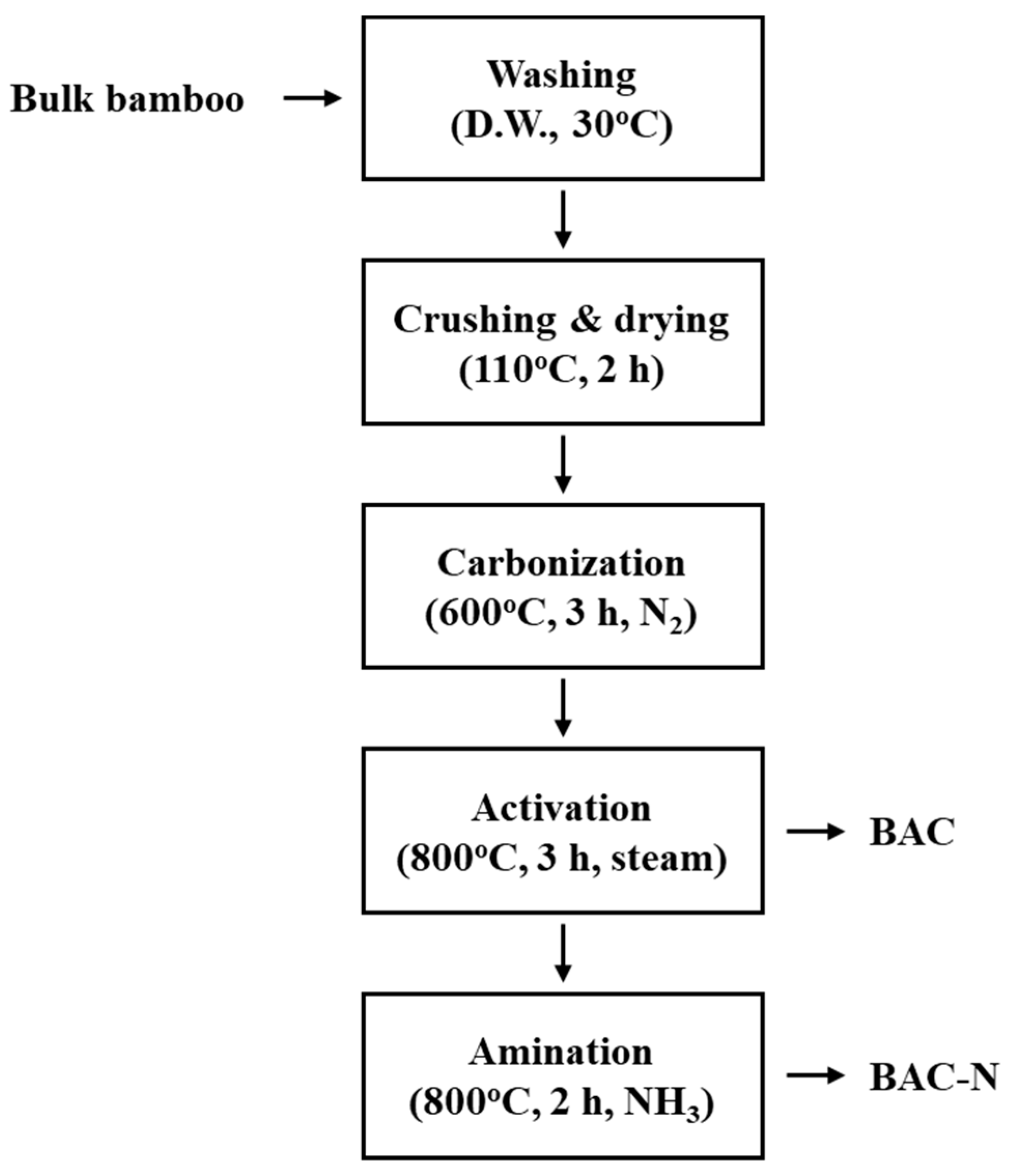
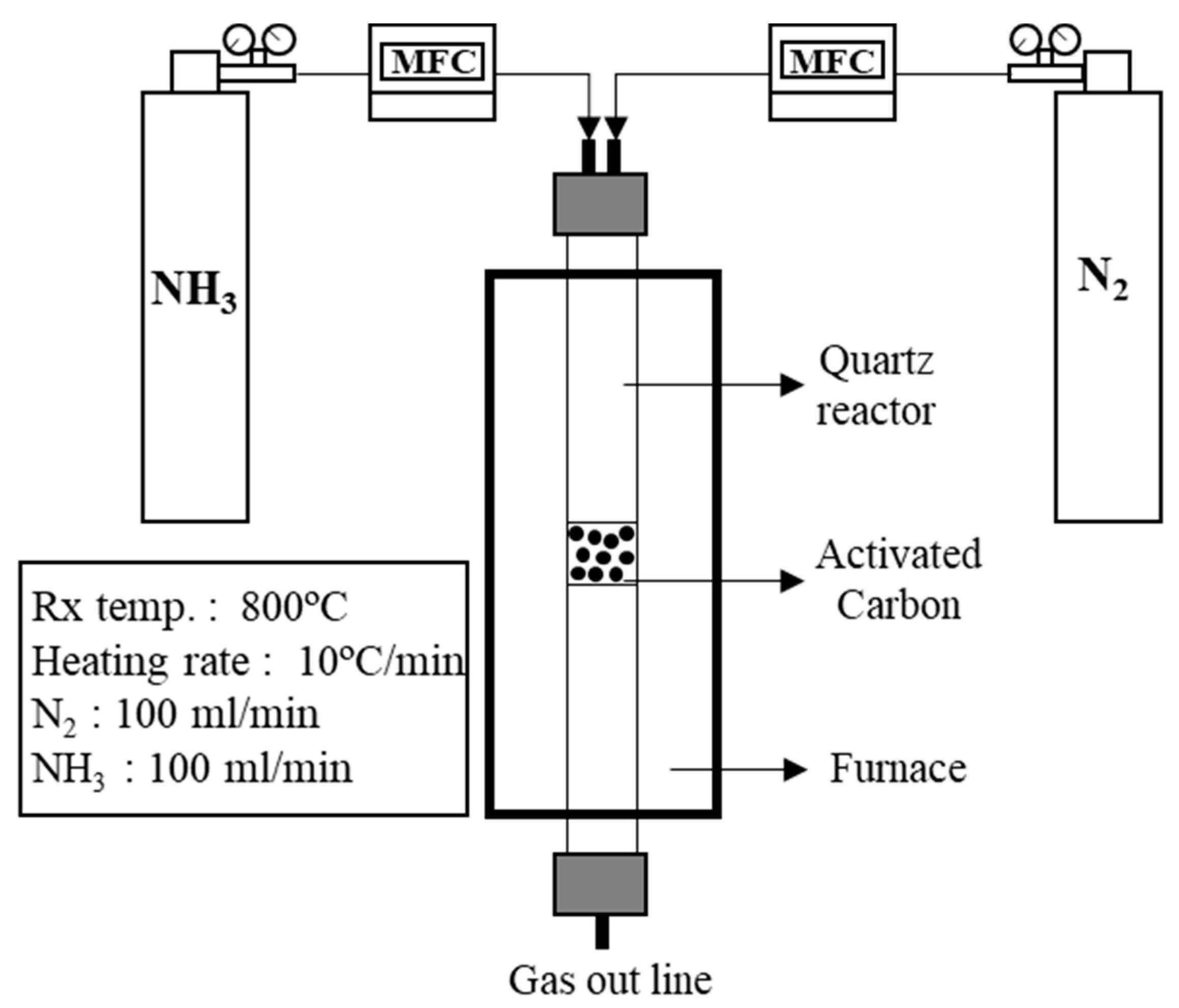



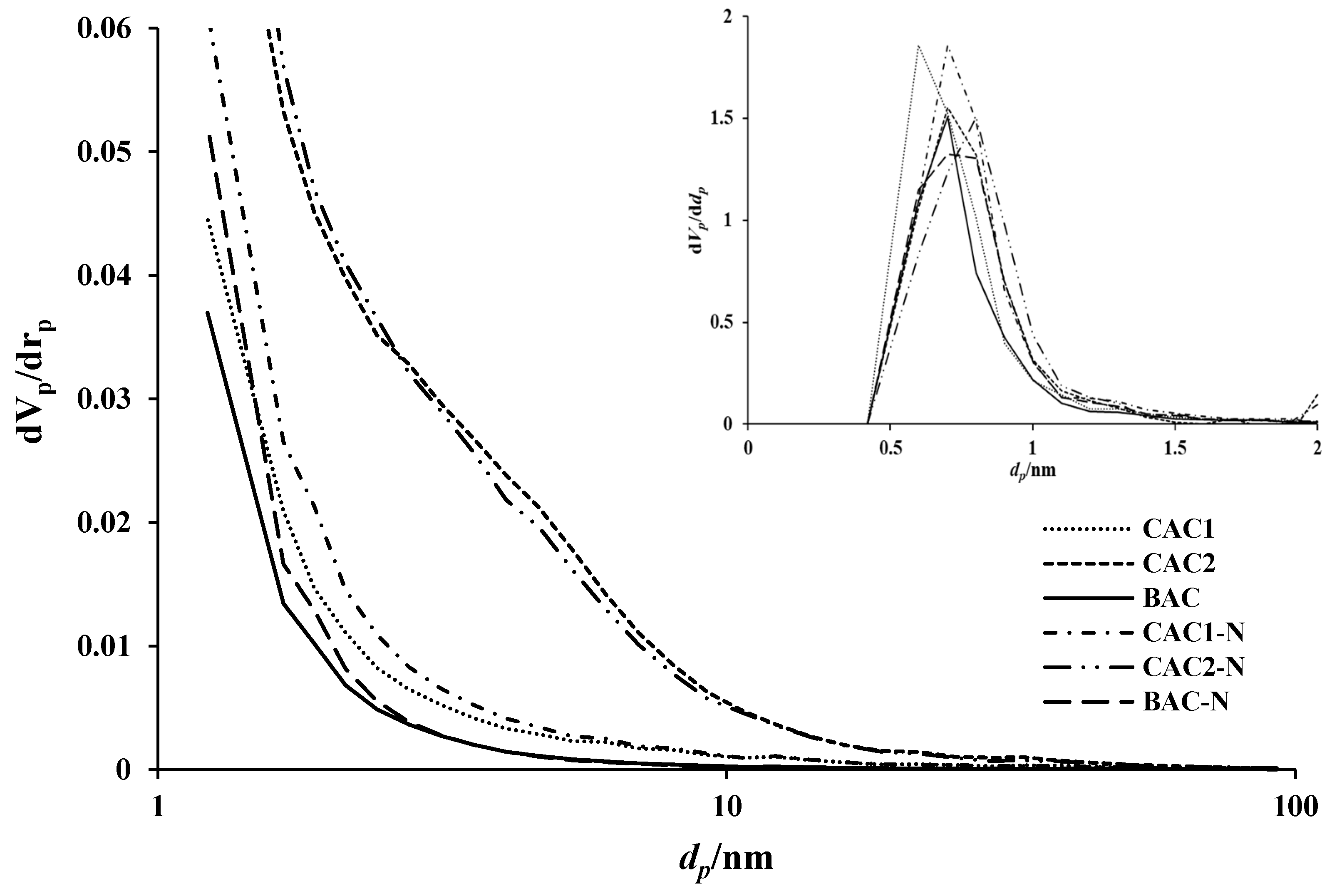
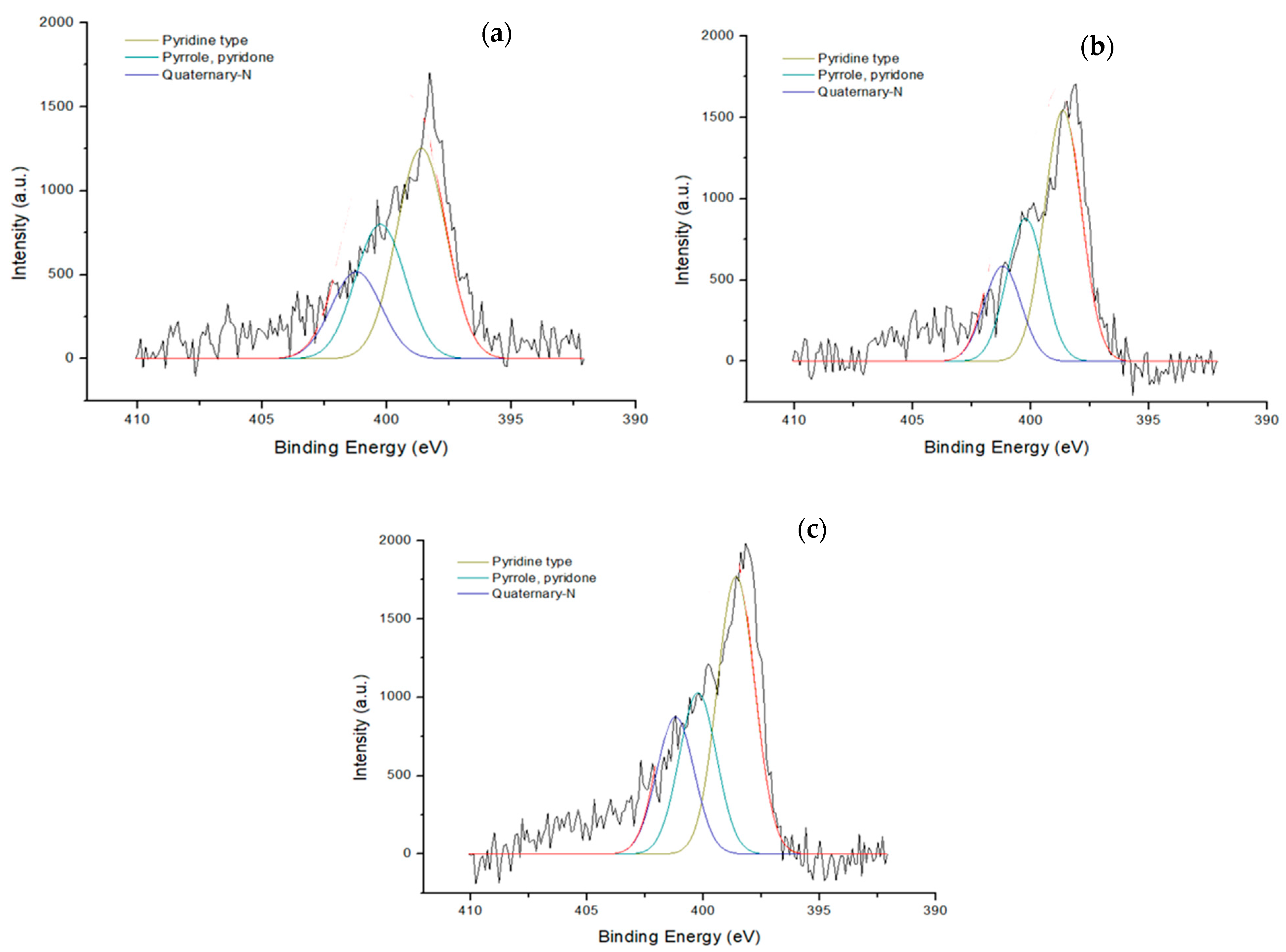

| Sample | Density (g‧cm−3) | Specific Surface Area (m2‧g−1) | Iodine Adsorption (mg‧g−1) |
|---|---|---|---|
| CAC1 (Coconut shell-based AC from Kurary) | 2.0 | >1000 | >900 |
| CAC2 (Coconut shell-based AC from Wi-Fine Tech., Chungju, Republic of Korea) | 2.1 | >1100 | >1000 |
| Sample | a SBET (m2‧g−1) | b Vp (cm3‧g−1) | c Dp (nm) | d Vmicro (cm3‧g−1) | e Dmicro (nm) |
|---|---|---|---|---|---|
| BAC | 984 | 0.42 | 1.8 | 0.42 | 0.7 |
| CAC1 | 1247 | 0.56 | 1.8 | 0.55 | 0.6 |
| CAC2 | 1336 | 0.77 | 2.3 | 0.54 | 0.7 |
| BAC-N | 1175 | 0.51 | 1.7 | 0.51 | 0.7 |
| CAC1-N | 1358 | 0.62 | 1.8 | 0.60 | 0.7 |
| CAC2-N | 1351 | 0.77 | 2.3 | 0.57 | 0.8 |
| Sample | 1 XRF Elemental Data (wt%) | 2 EA (CHNO) Elemental Data (wt%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Al | Si | P | S | Cl | K | Ca | Fe | Cu | C | H | N | O | |
| CAC1 | 0.097 | 0.785 | 0.304 | 0.568 | - | 1.366 | 1.343 | 2.417 | - | 89.2 | 2.03 | 0.33 | 1.56 |
| CAC2 | 0.191 | 0.637 | 0.350 | 1.300 | 3.430 | 1.145 | 0.710 | 1.635 | - | 86.2 | 2.51 | 0.15 | 1.71 |
| BAC | 0.019 | 0.344 | 0.056 | 0.048 | 0.021 | 3.800 | 0.527 | 0.775 | - | 85.3 | 1.50 | 0.30 | 7.86 |
| CAC1-N | 0.131 | 0.870 | 1.931 | - | - | 4.810 | 2.875 | 2.233 | - | 81.2 | 1.41 | 1.86 | 2.65 |
| CAC2-N | 0.694 | 3.000 | 1.474 | 1.037 | - | 3.126 | 1.596 | 5.280 | 0.787 | 77.5 | 1.53 | 0.55 | 3.18 |
| BAC-N | - | - | 0.327 | - | - | 10.62 | 1.465 | 0.220 | - | 74.2 | 0.50 | 2.50 | 9.60 |
| Sample | N-Atomic (%) | N 1s Area | Main Peak |
|---|---|---|---|
| CAC1 | 1.55 | 86.6 | Quaternary |
| CAC2 | 0.61 | 615 | Pyrrole |
| BAC | 0.52 | 512 | Pyrrole |
| CAC1-N | 3.74 | 4108 | Pyridine |
| CAC2-N | 3.46 | 4311 | Pyridine |
| BAC-N | 4.33 | 5672 | Pyridine |
| Sample | 0.3% CO2 q (mmol‧g−1) | Breakthrough Time (tb) | CO2/N2 (αs,g) | (min−1) |
|---|---|---|---|---|
| CAC1 | 0.14 | 3.66 | 1.52 | 5.15 |
| CAC2 | 0.08 | 2.93 | 1.16 | 4.70 |
| BAC | 0.62 | 10.7 | 8.60 | 8.48 |
| CAC1-N | 0.14 | 3.64 | 1.50 | 5.10 |
| CAC2-N | 0.12 | 3.63 | 1.38 | 5.05 |
| BAC-N | 0.98 | 12.7 | 13.4 | 18.2 |
| S/N | Adsorbent | Fabrication/Modification Method | q (mmol‧g−1) | Ref. |
|---|---|---|---|---|
| 1 | Coconut shell-based 1 AC |
| 2.230 | [26] |
| 0.280 | [43] | ||
| 0.310 | [43] | ||
| 0.410 | [45] | ||
| 0.260 | [47] | ||
| 2 | Rice husk-based AC |
| 2.100 | [48] |
| 3 | Zeolite |
| 1.87 | [45] |
| 4 | Bamboo-based AC |
| 0.980 | 3 C.S. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heo, S.; Kim, W.; Jo, Y.; Adelodun, A.A. Fabrication of Bamboo-Based Activated Carbon for Low-Level CO2 Adsorption toward Sustainable Indoor Air. Sustainability 2024, 16, 1634. https://doi.org/10.3390/su16041634
Heo S, Kim W, Jo Y, Adelodun AA. Fabrication of Bamboo-Based Activated Carbon for Low-Level CO2 Adsorption toward Sustainable Indoor Air. Sustainability. 2024; 16(4):1634. https://doi.org/10.3390/su16041634
Chicago/Turabian StyleHeo, Sujeong, Wooram Kim, Youngmin Jo, and Adedeji Adebukola Adelodun. 2024. "Fabrication of Bamboo-Based Activated Carbon for Low-Level CO2 Adsorption toward Sustainable Indoor Air" Sustainability 16, no. 4: 1634. https://doi.org/10.3390/su16041634
APA StyleHeo, S., Kim, W., Jo, Y., & Adelodun, A. A. (2024). Fabrication of Bamboo-Based Activated Carbon for Low-Level CO2 Adsorption toward Sustainable Indoor Air. Sustainability, 16(4), 1634. https://doi.org/10.3390/su16041634






