Carbon Nanotube Composites with Bimetallic Transition Metal Selenides as Efficient Electrocatalysts for Oxygen Evolution Reaction
Abstract
1. Introduction
2. Experimental
2.1. Reagents and Materials
2.2. Synthesis of NiCoSe@CNTs and MnCoSe@CNTs over the Ni-Foam
2.3. Material Characterization
2.4. Electrochemical Assessment
3. Results and Discussions
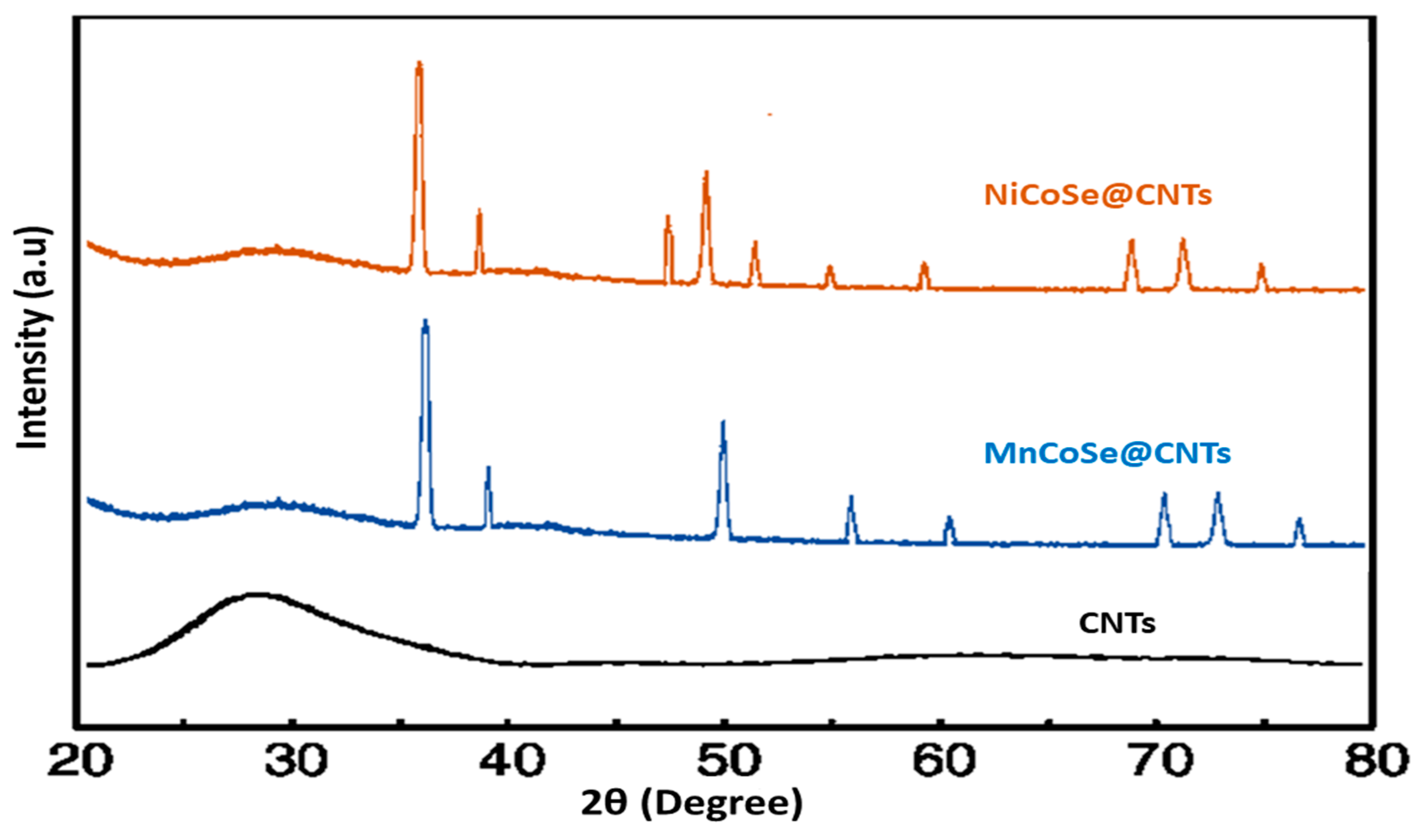
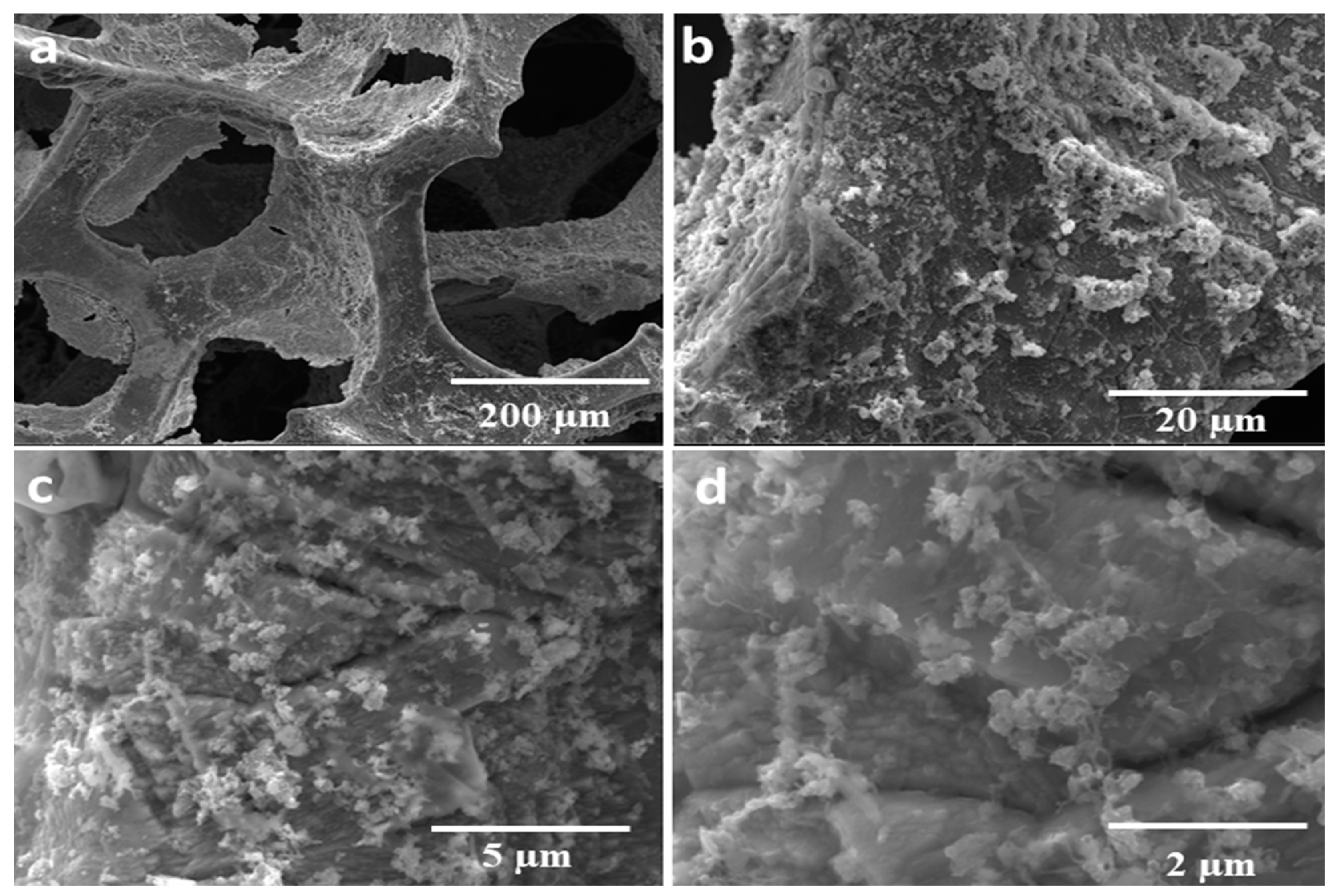
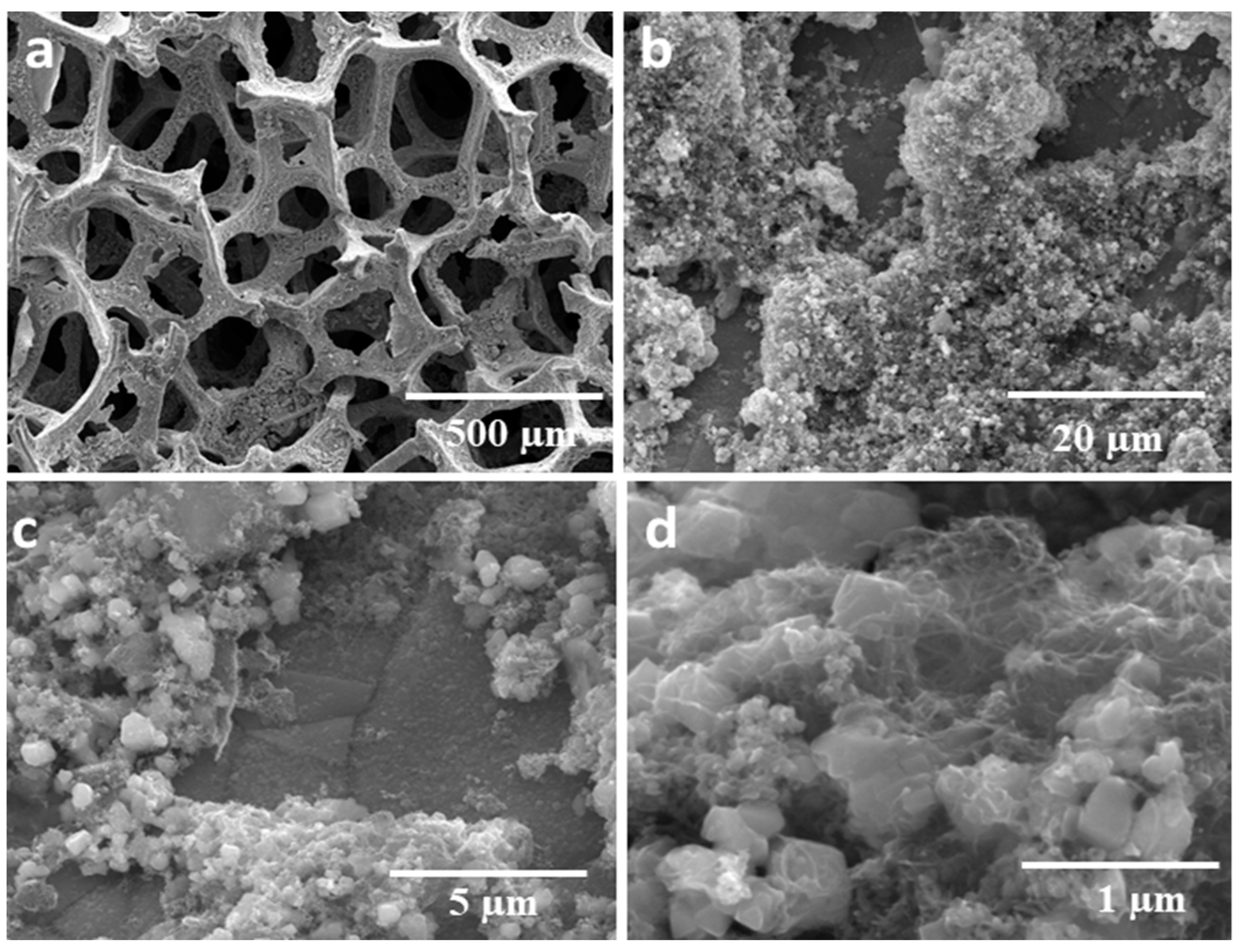
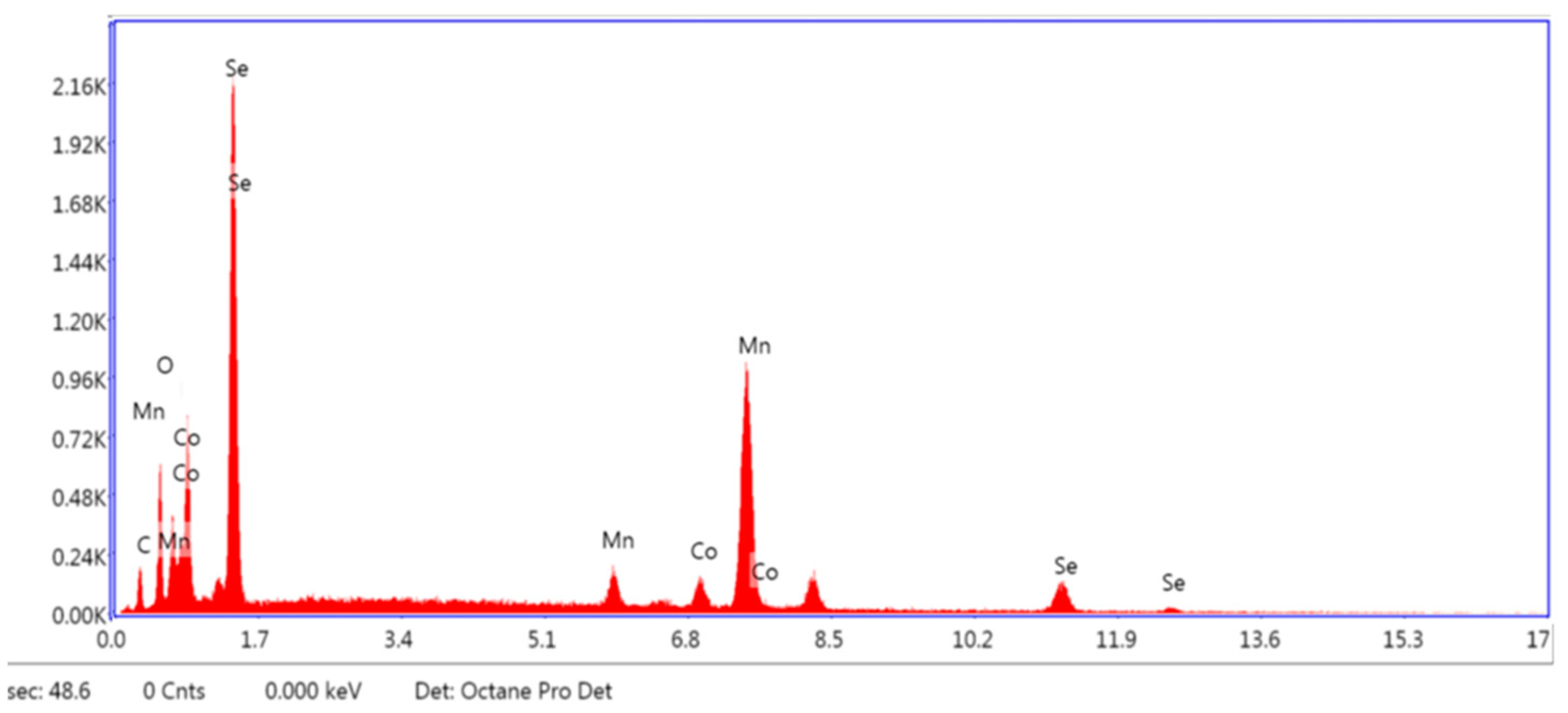
3.1. Structure, Morphology, and Composition of the As-Prepared Catalyst
SEM and EDX Analysis
3.2. Electrochemical Studies for OER
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cao, X.; Johnson, E.; Nath, M. Expanding multinary selenide based high-efficiency oxygen evolution electrocatalysts through combinatorial electrodeposition: Case study with Fe–Cu–Co selenides. ACS Sustain. Chem. Eng. 2019, 7, 9588–9600. [Google Scholar] [CrossRef]
- Sivanantham, A.; Shanmugam, S. Nickel selenide supported on nickel foam as an efficient and durable non-precious electrocatalyst for the alkaline water electrolysis. Appl. Catal. B Environ. 2017, 203, 485–493. [Google Scholar] [CrossRef]
- Srinivas, K.; Chen, Y.; Wang, B.; Yu, B.; Wang, X.; Hu, Y.; Lu, Y.; Li, W.; Zhang, W.; Yang, D. Metal–organic framework-derived NiS/Fe3O4 heterostructure-decorated carbon nanotubes as highly efficient and durable electrocatalysts for oxygen evolution reaction. ACS Appl. Mater. Interfaces 2020, 12, 31552–31563. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Tan, Y. Hierarchical CoNiSe2 nano-architecture as a high-performance electrocatalyst for water splitting. Nano Res. 2018, 11, 1331–1344. [Google Scholar] [CrossRef]
- Oyetade, O.A.; Kriek, R.J. NiSe-Ni3Se2/Multiwalled Carbon Nanotube Composites as Efficient Electrocatalysts for the Oxygen Evolution Reaction in Alkaline Media. Electrocatalysis 2020, 11, 35–45. [Google Scholar] [CrossRef]
- Ji, X.; Liu, B.; Ren, X.; Shi, X.; Asiri, A.M.; Sun, X. P-doped Ag nanoparticles embedded in N-doped carbon nanoflake: An efficient electrocatalyst for the hydrogen evolution reaction. ACS Sustain. Chem. Eng. 2018, 6, 4499–4503. [Google Scholar] [CrossRef]
- Lu, B.; Wang, Y.; Li, W.; Song, S.; Tian, P.; Li, R.; Tian, X.; Liu, X.; Zang, J. Ni–P alloy@carbon nanotubes immobilized on the framework of Ni foam as a 3D hierarchical porous self-supporting electrode for hydrogen evolution reaction. Int. J. Hydrogen Energy 2021, 46, 23245–23253. [Google Scholar] [CrossRef]
- Cheng, L.; Huang, W.; Gong, Q.; Liu, C.; Liu, Z.; Li, Y.; Dai, H. Ultrathin WS2 nanoflakes as a high-performance electrocatalyst for the hydrogen evolution reaction. Angew. Chem. Int. Ed. 2014, 53, 7860–7863. [Google Scholar] [CrossRef]
- Liu, Q.; Xie, L.; Qu, F.; Liu, Z.; Du, G.; Asiri, A.M.; Sun, X. A porous Ni3N nanosheet array as a high-performance non-noble-metal catalyst for urea-assisted electrochemical hydrogen production. Inorg. Chem. Front. 2017, 4, 1120–1124. [Google Scholar] [CrossRef]
- Gong, Y.; Pan, H.; Xu, Z.; Yang, Z.; Lin, Y.; Zhang, M. ACo2O4 (A = Ni, Zn, Mn) nanostructure arrays grown on nickel foam as efficient electrocatalysts for oxygen evolution reaction. Int. J. Hydrogen Energy 2018, 43, 14360–14368. [Google Scholar] [CrossRef]
- Zhang, C.; Xie, Y.; Deng, H.; Zhang, C.; Su, J.-W.; Dong, Y.; Lin, J. Ternary nickel iron phosphide supported on nickel foam as a high-efficiency electrocatalyst for overall water splitting. Int. J. Hydrogen Energy 2018, 43, 7299–7306. [Google Scholar] [CrossRef]
- Li, F.L.; Shao, Q.; Huang, X.; Lang, J.P. Nanoscale trimetallic metal–organic frameworks enable efficient oxygen evolution electrocatalysis. Angew. Chem. 2018, 130, 1906–1910. [Google Scholar] [CrossRef]
- Zhang, F.; Pei, Y.; Ge, Y.; Chu, H.; Craig, S.; Dong, P.; Cao, J.; Ajayan, P.M.; Ye, M.; Shen, J. Controlled synthesis of eutectic NiSe/Ni3Se2 self-supported on Ni foam: An excellent bifunctional electrocatalyst for overall water splitting. Adv. Mater. Interfaces 2018, 5, 1701507. [Google Scholar] [CrossRef]
- Sivanantham, A.; Ganesan, P.; Shanmugam, S. Hierarchical NiCo2S4 nanowire arrays supported on Ni foam: An efficient and durable bifunctional electrocatalyst for oxygen and hydrogen evolution reactions. Adv. Funct. Mater. 2016, 26, 4661–4672. [Google Scholar] [CrossRef]
- Smith, R.D.; Prévot, M.S.; Fagan, R.D.; Zhang, Z.; Sedach, P.A.; Siu, M.K.J.; Trudel, S.; Berlinguette, C.P. Photochemical route for accessing amorphous metal oxide materials for water oxidation catalysis. Science 2013, 340, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Zhou, W.; Kenney, M.J.; Kapusta, R.; Cowley, S.; Wu, Y.; Lu, B.; Lin, M.C.; Wang, D.Y.; Yang, J. Blending Cr2O3 into a NiO–Ni electrocatalyst for sustained water splitting. Angew. Chem. Int. Ed. 2015, 54, 11989–11993. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Cheng, N.; Pu, Z.; Xing, W.; Sun, X. NiSe nanowire film supported on nickel foam: An efficient and stable 3D bifunctional electrode for full water splitting. Angew. Chem. 2015, 127, 9483–9487. [Google Scholar] [CrossRef]
- Cao, X.; Johnson, E.; Nath, M. Identifying high-efficiency oxygen evolution electrocatalysts from Co–Ni–Cu based selenides through combinatorial electrodeposition. J. Mater. Chem. A 2019, 7, 9877–9889. [Google Scholar] [CrossRef]
- Zare, A.; Bayat, A.; Saievar-Iranizad, E.; Naffakh-Moosavy, H. One step preparation of Fe doped CoSe2 supported on nickel foam by facile electrodeposition method as a highly efficient oxygen evolution reaction electrocatalyst. J. Electroanal. Chem. 2020, 878, 114595. [Google Scholar] [CrossRef]
- Chen, T.-Y.; Vedhanarayanan, B.; Lin, S.-Y.; Shao, L.-D.; Sofer, Z.; Lin, J.-Y.; Lin, T.-W. Electrodeposited NiSe on a forest of carbon nanotubes as a free-standing electrode for hybrid supercapacitors and overall water splitting. J. Colloid Interface Sci. 2020, 574, 300–311. [Google Scholar] [CrossRef]
- Wang, D.; Xu, Y.; Guo, X.; Fu, Z.; Yang, Z.; Sun, W. Nickel foam as conductive substrate enhanced low-crystallinity two-dimensional iron hydrogen phosphate for oxygen evolution reaction. J. Alloys Compd. 2021, 870, 159472. [Google Scholar] [CrossRef]
- Zhang, M.; Hu, A.; Liu, Z.; Xu, Y.; Fan, B.; Tang, Q.; Zhang, S.; Deng, W.; Chen, X. Synergistic effect of three-dimensional cobalt diselenide/carbon nanotube arrays composites for enhanced hydrogen evolution reaction. Electrochim. Acta 2018, 285, 254–261. [Google Scholar] [CrossRef]
- Over, H. Surface chemistry of ruthenium dioxide in heterogeneous catalysis and electrocatalysis: From fundamental to applied research. Chem. Rev. 2012, 112, 3356–3426. [Google Scholar] [CrossRef]
- Reier, T.; Teschner, D.; Lunkenbein, T.; Bergmann, A.; Selve, S.; Kraehnert, R.; Schlögl, R.; Strasser, P. Electrocatalytic oxygen evolution on iridium oxide: Uncovering catalyst-substrate interactions and active iridium oxide species. J. Electrochem. Soc. 2014, 161, F876. [Google Scholar] [CrossRef]
- Chi, J.-Q.; Yan, K.-L.; Xiao, Z.; Dong, B.; Shang, X.; Gao, W.-K.; Li, X.; Chai, Y.-M.; Liu, C.-G. Trimetallic NiFeCo selenides nanoparticles supported on carbon fiber cloth as efficient electrocatalyst for oxygen evolution reaction. Int. J. Hydrogen Energy 2017, 42, 20599–20607. [Google Scholar] [CrossRef]
- Luo, Q.; Peng, M.; Sun, X.; Luo, Y.; Asiri, A.M. Efficient electrochemical water splitting catalyzed by electrodeposited NiFe nanosheets film. Int. J. Hydrogen Energy 2016, 41, 8785–8792. [Google Scholar] [CrossRef]
- Zhao, M.; Huang, J.; Zhou, Y.; Pan, X.; He, H.; Ye, Z.; Pan, X. Controlled synthesis of spinel ZnFe2O4 decorated ZnO heterostructures as peroxidase mimetics for enhanced colorimetric biosensing. Chem. Commun. 2013, 49, 7656–7658. [Google Scholar] [CrossRef]
- Li, D.; Baydoun, H.; Verani, C.N.; Brock, S.L. Efficient water oxidation using CoMnP nanoparticles. J. Am. Chem. Soc. 2016, 138, 4006–4009. [Google Scholar] [CrossRef] [PubMed]
- Pu, Z.; Luo, Y.; Asiri, A.M.; Sun, X. Efficient electrochemical water splitting catalyzed by electrodeposited nickel diselenide nanoparticles based film. ACS Appl. Mater. Interfaces 2016, 8, 4718–4723. [Google Scholar] [CrossRef]
- Ganesan, P.; Prabu, M.; Sanetuntikul, J.; Shanmugam, S. Cobalt sulfide nanoparticles grown on nitrogen and sulfur codoped graphene oxide: An efficient electrocatalyst for oxygen reduction and evolution reactions. ACS Catal. 2015, 5, 3625–3637. [Google Scholar] [CrossRef]
- Cabán-Acevedo, M.; Stone, M.L.; Schmidt, J.; Thomas, J.G.; Ding, Q.; Chang, H.-C.; Tsai, M.-L.; He, J.-H.; Jin, S. Efficient hydrogen evolution catalysis using ternary pyrite-type cobalt phosphosulphide. Nat. Mater. 2015, 14, 1245–1251. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, Y.; He, R.; Yu, F.; Sun, J.; Wang, F.; Lan, Y.; Ren, Z.; Chen, S. One-step synthesis of self-supported porous NiSe2/Ni hybrid foam: An efficient 3D electrode for hydrogen evolution reaction. Nano Energy 2016, 20, 29–36. [Google Scholar] [CrossRef]
- Zhuang, L.; Jia, Y.; He, T.; Du, A.; Yan, X.; Ge, L.; Zhu, Z.; Yao, X. Tuning oxygen vacancies in two-dimensional iron-cobalt oxide nanosheets through hydrogenation for enhanced oxygen evolution activity. Nano Res. 2018, 11, 3509–3518. [Google Scholar] [CrossRef]
- Xu, K.; Ding, H.; Lv, H.; Tao, S.; Chen, P.; Wu, X.; Chu, W.; Wu, C.; Xie, Y. Understanding structure-dependent catalytic performance of nickel selenides for electrochemical water oxidation. ACS Catal. 2017, 7, 310–315. [Google Scholar] [CrossRef]
- Cao, X.; Hong, Y.; Zhang, N.; Chen, Q.; Masud, J.; Zaeem, M.A.; Nath, M. Phase exploration and identification of multinary transition-metal selenides as high-efficiency oxygen evolution electrocatalysts through combinatorial electrodeposition. ACS Catal. 2018, 8, 8273–8289. [Google Scholar] [CrossRef]
- Zhao, X.; Li, X.; Yan, Y.; Xing, Y.; Lu, S.; Zhao, L.; Zhou, S.; Peng, Z.; Zeng, J. Electrical and structural engineering of cobalt selenide nanosheets by Mn modulation for efficient oxygen evolution. Appl. Catal. B Environ. 2018, 236, 569–575. [Google Scholar] [CrossRef]
- Zhang, B.; Zheng, X.; Voznyy, O.; Comin, R.; Bajdich, M.; García-Melchor, M.; Han, L.; Xu, J.; Liu, M.; Zheng, L. Homogeneously dispersed multimetal oxygen-evolving catalysts. Science 2016, 352, 333–337. [Google Scholar] [CrossRef]
- Li, P.; Jin, Z.; Yang, J.; Jin, Y.; Xiao, D. Highly active 3D-nanoarray-supported oxygen-evolving electrode generated from cobalt-phytate nanoplates. Chem. Mater. 2016, 28, 153–161. [Google Scholar] [CrossRef]
- Huang, C.; Zhang, B.; Luo, Y.; Xiao, D.; Tang, K.; Ruan, Q.; Yang, Y.; Gao, B.; Chu, P.K. A hybrid Co NPs@CNT nanocomposite as highly efficient electrocatalyst for oxygen evolution reaction. Appl. Surf. Sci. 2020, 507, 145155. [Google Scholar] [CrossRef]
- Xu, X.; Pan, Y.; Ge, L.; Chen, Y.; Mao, X.; Guan, D.; Li, M.; Zhong, Y.; Hu, Z.; Peterson, V.K.; et al. High-Performance Perovskite Composite Electrocatalysts Enabled by Controllable Interface Engineering. Small 2021, 17, 2101573. [Google Scholar] [CrossRef]
- Cheng, H.; Su, C.-Y.; Tan, Z.-Y.; Tai, S.-Z.; Liu, Z.-Q. Interacting ZnCo2O4 and Au nanodots on carbon nanotubes as highly efficient water oxidation electrocatalyst. J. Power Sources 2017, 357, 1–10. [Google Scholar] [CrossRef]
- Chao, S.; Bai, Z.; Cui, Q.; Yan, H.; Wang, K.; Yang, L. Hollowed-out octahedral Co/N-codoped carbon as a highly efficient non-precious metal catalyst for oxygen reduction reaction. Carbon 2015, 82, 77–86. [Google Scholar] [CrossRef]
- Huang, L.; Wu, H.; Zhang, Y. One-step synthesis of CoPSe–CoSe2/CNTs as efficient electrocatalyst for oxygen evolution reaction. Electrochim. Acta 2020, 331, 135362. [Google Scholar] [CrossRef]
- Sun, H.; Xu, X.; Kim, H.; Shao, Z.; Jung, W.C. Advanced Electrocatalysts with Unusual Active Sites for Electrochemical Water Splitting. InfoMat 2024, 6, e12494. [Google Scholar] [CrossRef]
- Yuan, M.; Wang, M.; Lu, P.; Sun, Y.; Dipazir, S.; Zhang, J.; Li, S.; Zhang, G. Tuning carbon nanotube-grafted core-shell-structured cobalt selenide@carbon hybrids for efficient oxygen evolution reaction. J. Colloid Interface Sci. 2019, 533, 503–512. [Google Scholar] [CrossRef]
- Yang, L.; Ru, F.; Shi, J.; Yang, T.; Guo, C.; Chen, Y.; Wang, E.; Du, Z.; Chou, K.C.; Hou, X. Trifunctional Electrocatalysts Based on Feather-like NiCoP 3D Architecture for Hydrogen Evolution, Oxygen Evolution, and Urea Oxidation Reactions. Ceram. Int. 2023, 49, 659–668. [Google Scholar] [CrossRef]
- Jamesh, M.-I.; Akila, A.; Sudha, D.; Gnana Priya, K.; Sivaprakash, V.; Revathi, A. Fabrication of Earth-Abundant Electrocatalysts Based on Green-Chemistry Approaches to Achieve Efficient Alkaline Water Splitting—A Review. Sustainability 2022, 14, 16359. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, W.; Li, T.; Zhu, H.; Zheng, Y. In situ growth of tetrametallic FeCoMnNi-MOF-74 on nickel foam as efficient bifunctional electrocatalysts for the evolution reaction of oxygen and hydrogen. Inorg. Chem. 2020, 59, 15467–15477. [Google Scholar] [CrossRef]
- Vedhanarayanan, B.; Huang, T.-H.; Lin, T.-W. Fabrication of 3D hierarchically structured carbon electrode for supercapacitors by carbonization of polyaniline/carbon nanotube/graphene composites. Inorg. Chim. Acta 2019, 489, 217–223. [Google Scholar] [CrossRef]
- Swanson, H.E.; McMurdie, H.F.; Morris, M.C.; Evans, E.H. Marlene Standard X-ray Powder Patterns. 1985. Available online: https://www.google.com.hk/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwi88ODs9sqEAxXWjq8BHTTOBokQFnoECBUQAQ&url=https%3A%2F%2Fnvlpubs.nist.gov%2Fnistpubs%2FLegacy%2FMONO%2Fnbsmonograph25-7.pdf&usg=AOvVaw2cGmDy_pShMNiC1frayel-&opi=89978449 (accessed on 10 December 2023).
- Zhang, J.; Sun, X.P.; Wei, P.; Lu, G.; Sun, S.X.; Xu, Y.; Fang, C.; Li, Q.; Han, J.T. Bimetallic Co/Mo2C Nanoparticles Embedded in 3D Hierarchical N-doped Carbon Heterostructures as Highly Efficient Electrocatalysts for Water Splitting. ChemCatChem 2020, 12, 3737–3745. [Google Scholar] [CrossRef]

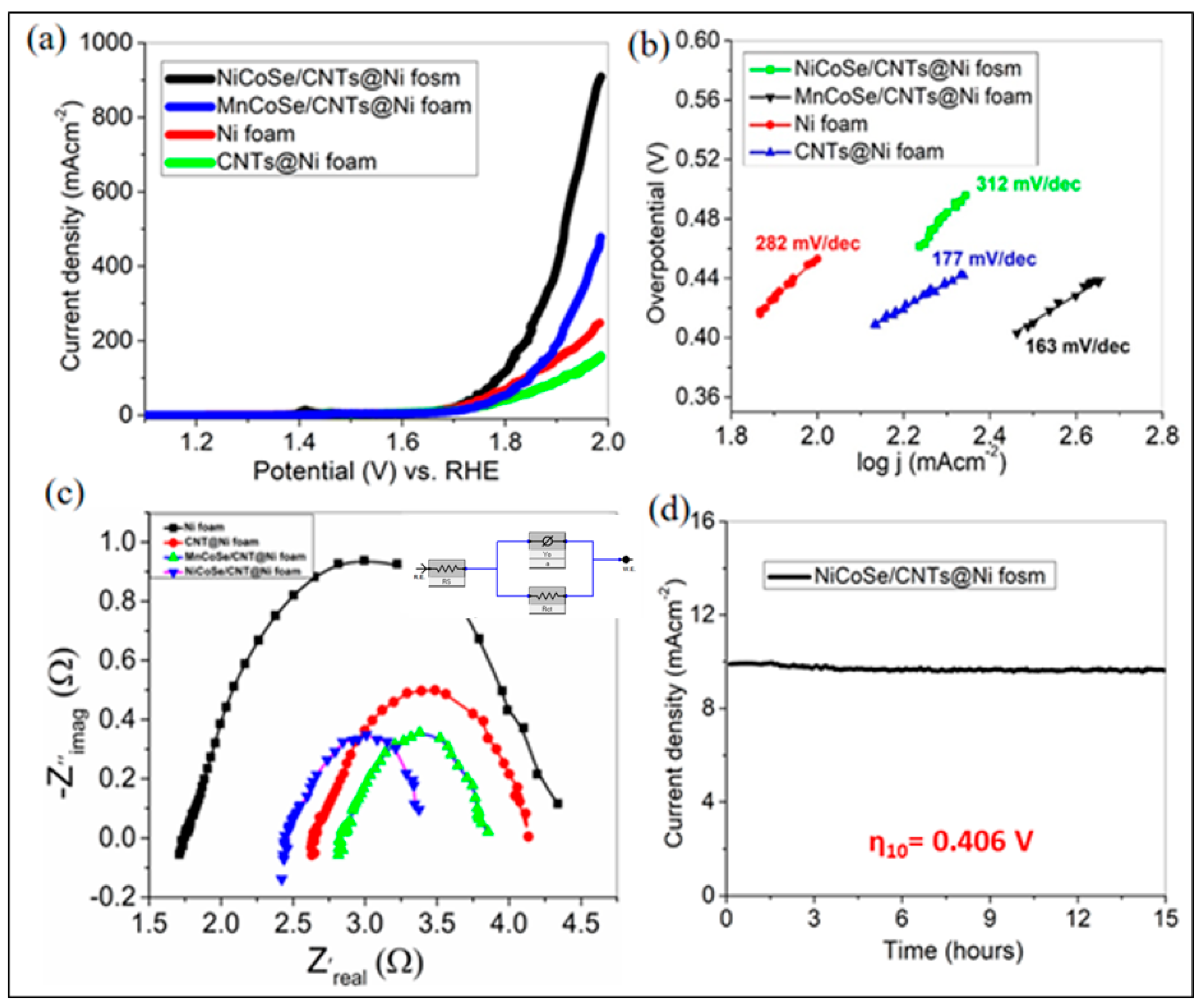
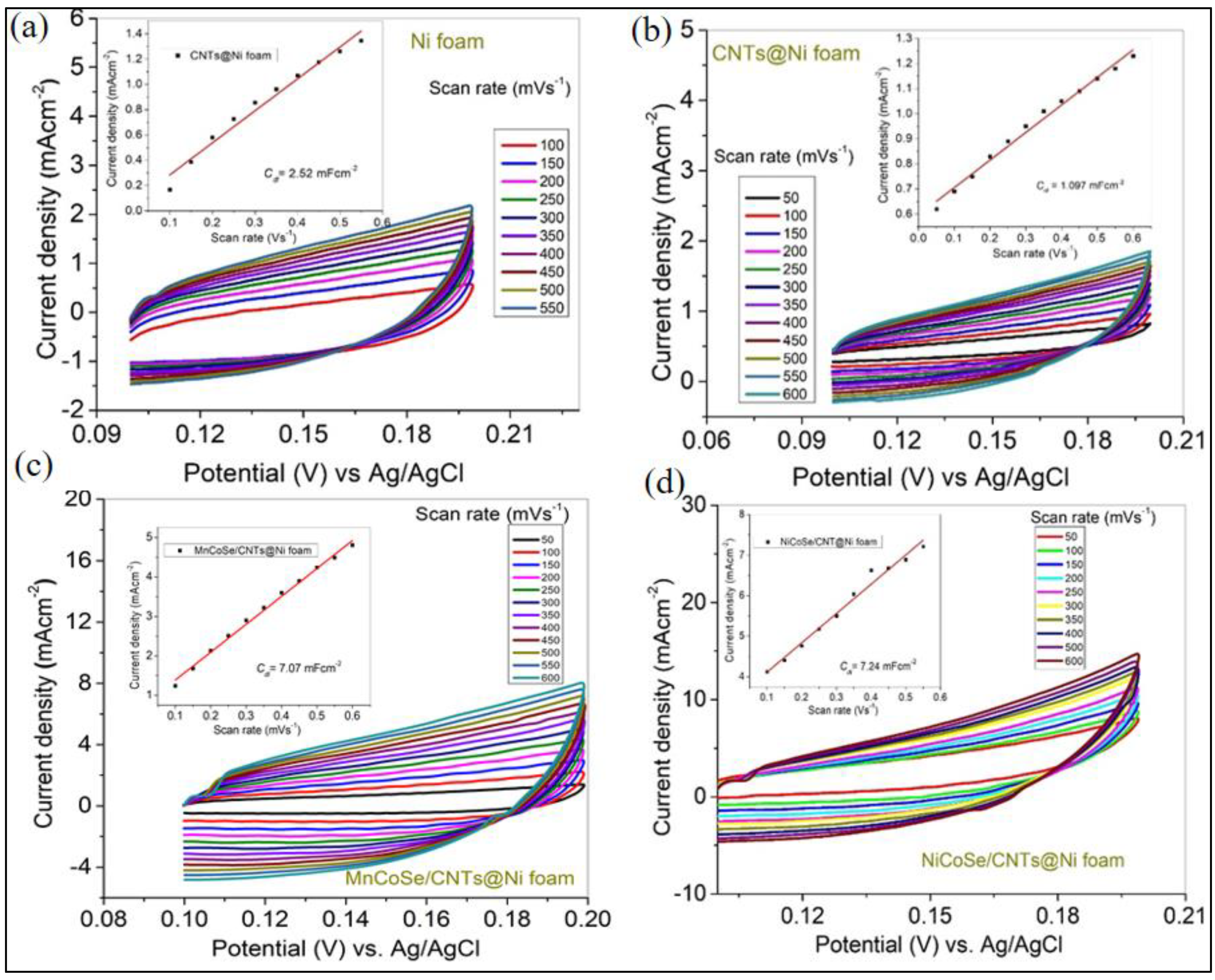
| Electrocatalyst | η100 (V) | Tafel Slop (mV/dec) | Cdl (mFcm−2) | Rct (Ω) |
|---|---|---|---|---|
| Ni foam | 0.619 | 312 | 2.52 | 2.66 |
| CNTs@ Ni foam | 0.698 | 282 | 1.09 | 1.50 |
| MnCoSe/CNTs@Ni foam | 0.612 | 177 | 7.07 | 1.03 |
| NiCoSe/CNTs@Ni foam | 0.560 | 163 | 7.24 | 0.90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riaz, S.; Anjum, M.S.; Ali, A.; Mehmood, Y.; Ahmad, M.; Alwadai, N.; Iqbal, M.; Akyürekli, S.; Hassan, N.; Shoukat, R. Carbon Nanotube Composites with Bimetallic Transition Metal Selenides as Efficient Electrocatalysts for Oxygen Evolution Reaction. Sustainability 2024, 16, 1953. https://doi.org/10.3390/su16051953
Riaz S, Anjum MS, Ali A, Mehmood Y, Ahmad M, Alwadai N, Iqbal M, Akyürekli S, Hassan N, Shoukat R. Carbon Nanotube Composites with Bimetallic Transition Metal Selenides as Efficient Electrocatalysts for Oxygen Evolution Reaction. Sustainability. 2024; 16(5):1953. https://doi.org/10.3390/su16051953
Chicago/Turabian StyleRiaz, Shamas, Muhammad Shafiq Anjum, Abid Ali, Yasir Mehmood, Muhammad Ahmad, Norah Alwadai, Munawar Iqbal, Salih Akyürekli, Noor Hassan, and Rizwan Shoukat. 2024. "Carbon Nanotube Composites with Bimetallic Transition Metal Selenides as Efficient Electrocatalysts for Oxygen Evolution Reaction" Sustainability 16, no. 5: 1953. https://doi.org/10.3390/su16051953
APA StyleRiaz, S., Anjum, M. S., Ali, A., Mehmood, Y., Ahmad, M., Alwadai, N., Iqbal, M., Akyürekli, S., Hassan, N., & Shoukat, R. (2024). Carbon Nanotube Composites with Bimetallic Transition Metal Selenides as Efficient Electrocatalysts for Oxygen Evolution Reaction. Sustainability, 16(5), 1953. https://doi.org/10.3390/su16051953






