The Effects of the Interaction of Pesticides with Humin Fraction as Influencing the Sustainable Development of Agroecosystems
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Sampling and Characteristics
2.2. Isolation of HUM and Saturation with Pesticides
2.3. Chemical Composition
2.4. Electron Paramagnetic Resonance (EPR) Method
2.5. Fluorescence Method
2.6. Delayed Luminescence Method
2.7. Statistical Analysis
3. Results
3.1. Chemical Composition
3.2. Electron Paramagnetic Resonance (EPR)
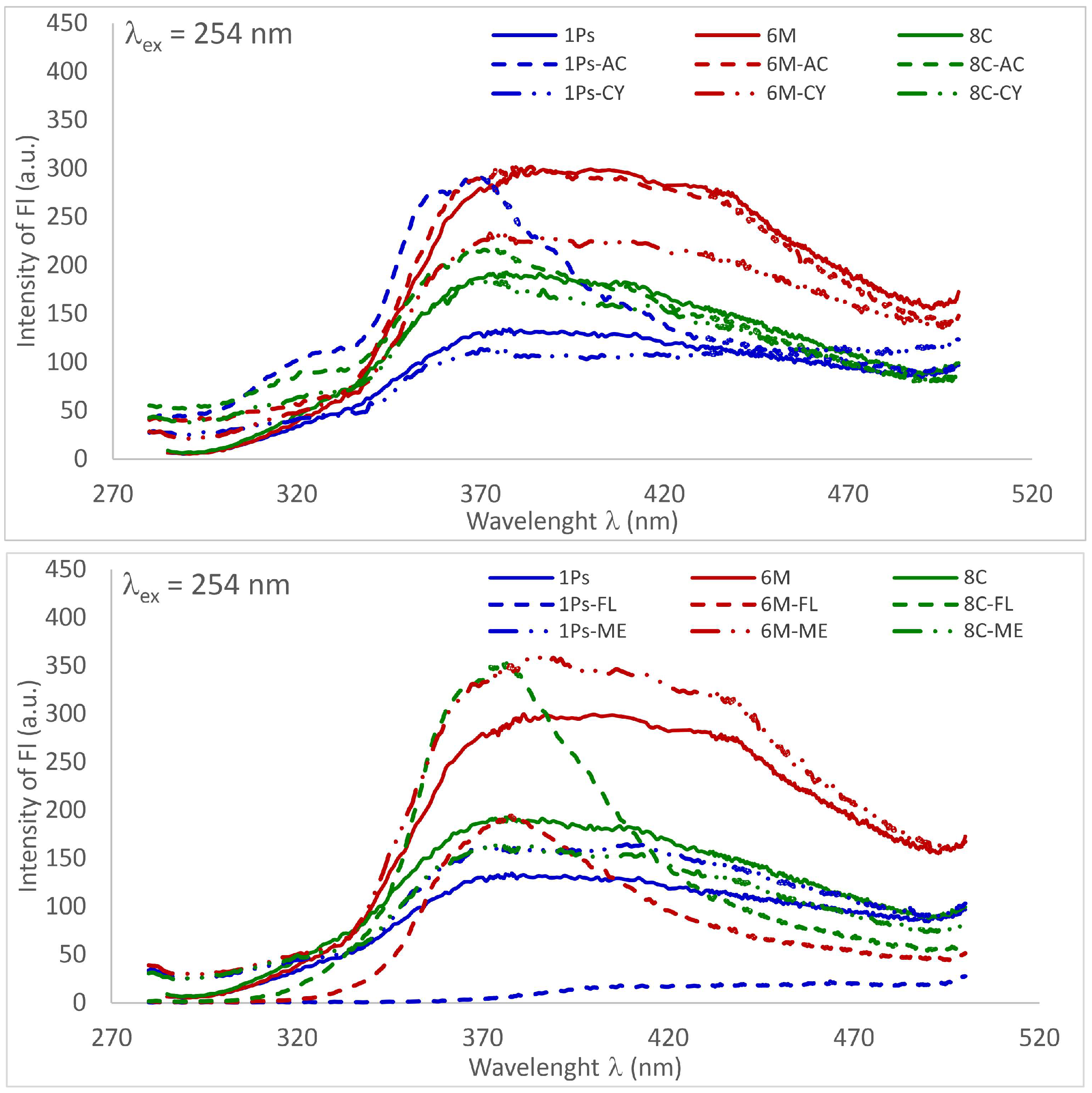
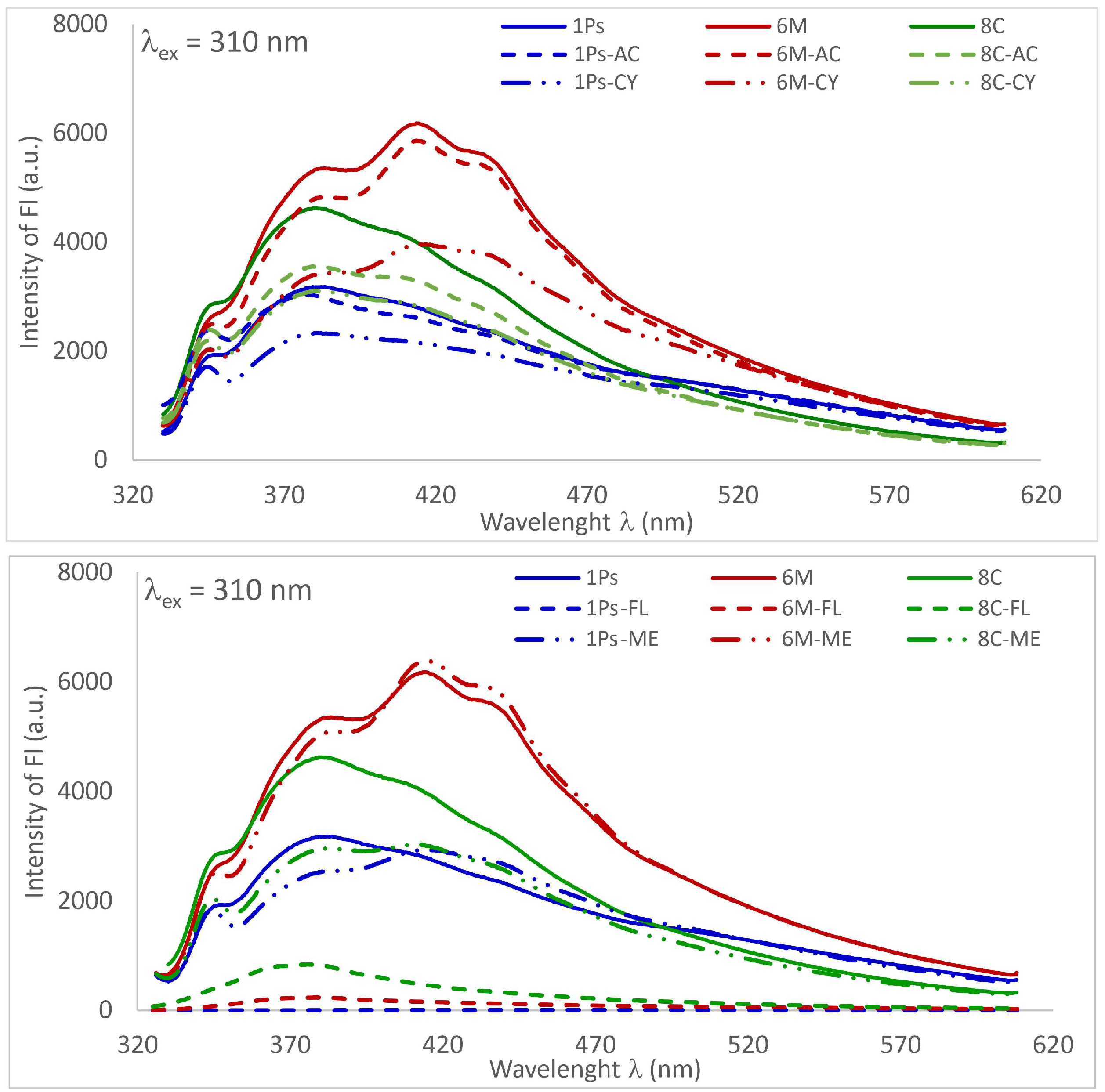
3.3. Fluorescence
3.4. Delayed Luminescence
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- European Commission, Directorate-General for Research and Innovation; Veerman, C.; Correia, T.P.; Bastioli, C.; Biro, B.; Bouma, J.; Cienciala, E.; Emmett, B.; Frison, E.A.; Grand, A.; et al. Caring for Soil Is Caring for Life: Ensure 75% of Soils Are Healthy by 2030 for Healthy food, People, Nature and Climate: Interim Report of the Mission Board for Soil Health and Food, Publications Office; European Commission: Brussels, Belgium, 2020. [Google Scholar] [CrossRef]
- Rasool, S.; Raool, T.; Gani, K.M. A review of interactions of pesticides within various interfaces of intrinsic and organic residue amended soil environment. Chem. Eng. J. Adv. 2022, 11, 100301. [Google Scholar] [CrossRef]
- Silva, V.; Mol, H.; Zomer, P.; Tienstra, M.; Ritsema, C.; Geissen, W. Pesticide residues in European agricultural soils—A hidden reality unfolded. Sci. Total Environ. 2019, 653, 1532–1545. [Google Scholar] [CrossRef] [PubMed]
- COM(2020) 380 Final (2020) EU Biodiversity Strategy for 2030 Bringing Nature Back into Our Lives; European Commission: Brussels, Belgium, 2020.
- COM(2006) 231 Final (2006) Soil Thematic Strategy Impact Assessment. COM(2006)231—SEC(2006) 1165; European Commission: Brussels, Belgium, 2006.
- COM(2023) 416 Final (2023) Directive of the European Parliament and of the Council on Soil Monitoring and Resilience (Soil Monitoring Law); European Commission: Brussels, Belgium, 2023.
- Orton, T.; Saby, N.; Arrouays, D.; Jolivet, C.; Villanneau, E.; Marchant, B.; Caria, G.; Barriuso, E.; Bispo, A.; Briand, O. Spatial distribution of Lindane concentration in topsoil across France. Sci. Total Environ. 2013, 443, 338–350. [Google Scholar] [CrossRef] [PubMed]
- Masia, A.; Vasquez, K.; Campo, J.; Pico, Y. Assessment of two extraction methods to determine pesticides in soils, sediments and sludges. Application to the Turia River Basin. J. Chromatogr. A 2015, 1378, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Pose-Juan, E.; Sanchez-Martin, M.J.; Andrades, M.S.; Rodriguez-Cruz, M.S.; Herrero-Hernandez, E. Pesticide residues in vineyard soils from Spain: Spatial and temporal distributions. Sci. Total Environ. 2015, 514, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Qu, C.; Albanese, S.; Chen, W.; Lima, A.; Doherty, A.L.; Piccolo, A.; Arienzo, M.; Qi, S.; De Vivo, B. The status of organochlorine pesticide contamination in the soils of the Campanian Plain, southern Italy, and correlations with soil properties and cancer risk. Environ. Pollut. 2016, 216, 500–511. [Google Scholar] [CrossRef] [PubMed]
- Hvězdová, M.; Kosubová, P.; Košíková, M.; Scherr, K.E.; Šimek, Z.; Brodský, L.; Šudoma, M.; Škulcová, L.; Sáňka, M.; Svobodová, M.; et al. Currently and recently used pesticides in Central European arable soils. Sci. Total Environ. 2018, 613, 361–370. [Google Scholar] [CrossRef]
- Abd El-Ghany, N.M.; Abd El-Aziz, S.E.; Marei, S.S. A review: Application of remote sensing as a promising strategy for insect pests and diseases management. Environ. Sci. Pollut. Res. 2020, 27, 33503–33515. [Google Scholar] [CrossRef]
- Barchańska, H.; Czaplicka, M.; Kyzioł-Komosińska, J. Interaction of selected pesticides with mineral and organic soil components. Arch. Environ. Prot. 2020, 46, 80–91. [Google Scholar]
- Levine, M. Fluorescence-Based Sensing of Pesticides Using Supramolecular Chemistry. Front. Chem. 2021, 9, 616815. [Google Scholar] [CrossRef]
- Wen, B.; Zhang, J.; Zhang, S.; Shan, X.; Khan, S.U.; Xing, B. Phenanthrene sorption to soil humic acid and different humin fractions. Environ. Sci. Technol. 2007, 41, 3165–3171. [Google Scholar] [CrossRef] [PubMed]
- Riva, J.; Juarez, A.; Yudi, L. Interactions between herbicides and humic acids present in soils. In Herbicides: Properties, Crop Protection, and Environmental Hazards; Nova Science Publ.: New York, NY, USA, 2011; Chapter 6; pp. 1–20. [Google Scholar]
- Senesi, N. Binding mechanisms of pesticides to soil humic substances. Sci. Total Environ. 1992, 123–124, 63–76. [Google Scholar] [CrossRef]
- Ukalska-Jaruga, A.; Bejger, R.; Smreczak, B.; Weber, J.; Mielnik, L.; Jerzykiewicz, M.; Ćwieląg-Piasecka, I.; Jamroz, E.; Dębicka, M.; Kocowicz, A.; et al. The Interaction of Pesticides with Humin Fractions and Their Potential Impact on Non-Extractable Residue Formation. Molecules 2023, 28, 7146. [Google Scholar] [CrossRef] [PubMed]
- Bilala, M.; Iqbal, H.M.N.; Barceló, D. Persistence of pesticides-based contaminants in the environment and their effective degradation using laccase-assisted biocatalytic systems. Sci. Total Environ. 2019, 695, 133896. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.; Mylotte, R.; Swift, R. Humin: Its Composition and Importance in Soil Organic Matter. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Burlington, MA, USA, 2017; Volume 143, pp. 47–138. ISBN 978-0-12-812421-5. [Google Scholar]
- Hayes, M.B.H.; Malcolm, L.R. Considerations of Compositions and of Aspects of the Structures of Humic Substances. In Humic Substances and Chemical Contaminants; Soil Science Society of America: Madison, WI, USA, 2001; Chapter 1. [Google Scholar]
- Steinberg, C.E.W. Ecology of Humic Substances in Freshwaters—Determinants from Geochemistry to Ecological Niches; Springer: Berlin/Heidelberg, Germany, 2003; p. 440. [Google Scholar]
- Senesi, N.; Miano, T.M.; Provenzano, M.R.; Brunetti, G. Characterization, differentiation, and classification of humic substances by fluorescence spectroscopy. Soil Sci. 1991, 152, 259–271. [Google Scholar] [CrossRef]
- Coble, P.G. Characterization of marine and terrestrial DOM in seawater using excitation–emission matrix spectroscopy. Mar. Chem. 1996, 51, 325–346. [Google Scholar] [CrossRef]
- Řezáčová, V.; Gryndler, M. Fluorescence spectroscopy: A tool to characterize humic substances in soil colonized by microorganisms? Folia Microbiol. 2006, 51, 215–221. [Google Scholar] [CrossRef]
- Bu, X.; Wang, L.; Ma, W.; Yu, X.; McDowell, W.H.; Ruan, H. Spectroscopic characterization of hot-water extractable organic matter from soils under four different vegetation types along an elevation gradient in the Wuyi Mountains. Geoderma 2010, 159, 139–146. [Google Scholar] [CrossRef]
- D’Orazio, V.; Miano, T. Fluorescence properties of humic acid interaction products with s-triazine and bipyridilium herbicides and their Cu complexes: A multivariate approach. J. Soils Sediments 2018, 18, 1347–1354. [Google Scholar] [CrossRef]
- Liu, D.; Yu, H.; Yang, F.; Liu, L.; Gao, H.; Cui, B. Characterizing humic substances from native halophyte soils by fluorescence spectroscopy combined with Parallel L. Sustainability 2020, 12, 9787. [Google Scholar] [CrossRef]
- Gao, J.; Liu, L.; Shi, Z.; Lv, J. Characteristics of Fluorescent Dissolved Organic Matter in Paddy Soil Amended With Crop Residues After Column (0–40 cm) Leaching. Front. Environ. Sci. 2022, 10, 766795. [Google Scholar] [CrossRef]
- Wei, Z.; Wang, X.; Zhao, X.; Xi, B.; Wei, Y.; Zhang, X.; Zhao, Y. Fluorescence characteristics of molecular weight fractions of dissolved organic matter derived from composts. Int. Biodeterior. Biodegrad. 2016, 113, 187–194. [Google Scholar] [CrossRef]
- Mielnik, L.; Asensio, C. Using delayed luminescence to characterize humic acids from lake sediments. J. Soil Sediments 2018, 18, 2844–2850. [Google Scholar] [CrossRef]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World map of the Köppen-Geiger climate classification updated. Meteorol. 2006, 15, 259–263. [Google Scholar] [CrossRef]
- Weber, J.; Jamroz, E.; Kocowicz, A.; Dębicka, M.; Bekier, J.; Ćwieląg-Piasecka, I.; Ukalska-Jaruga, A.; Mielnik, L.; Bejger, R.; Jerzykiewicz, M. Optimized isolation method of humin fraction from mineral soil material. Environ. Geochem. Health 2022, 44, 1289–1298. [Google Scholar] [CrossRef]
- Zaccone, C.; Plaza, C.; Ciavatta, C.; Miano, T.M.; Shotyk, W. Advances in the determination of humification degree in peat since Achard (1786): Applications in geochemical and paleoenvironmental studies. Earth-Sci. Rev. 2018, 185, 163–178. [Google Scholar] [CrossRef]
- Stevenson, F.J. Humus Chemistry. Genesis, Composition, Reactions, 2nd ed.; John Wiley and Sons, Inc.: New York, NY, USA, 1994; p. 512. ISBN 978-0-471-59474-1. [Google Scholar]
- Milori, D.M.B.P.; Bayer, C.; Bagnato, V.S.; Mielniczuk, J.; Martin-Neto, L. Humification degree of soil humic acids determined by fluorescence spectroscopy. Soil Sci. 2002, 167, 739–749. [Google Scholar] [CrossRef]
- Mielnik, L.; Weber, J.; Podlasinski, M.; Kocowicz, A. Fluorescence properties of humic substances transformed in ectohumus horizons of Podzols affected by alkaline fly-ash. Land Degrad. Dev. 2021, 32, 3487–3497. [Google Scholar] [CrossRef]
- Frimmel, F.H. Photochemical aspects related to humic substances. Environ. Int. 1994, 20, 373–385. [Google Scholar] [CrossRef]
- Aguer, J.P.; Richard, C.; Trubetskaya, O.; Trubetskoj, O.; Leveque, J.F.; Andreux, F. Photoinductive efficiency of soil extracted humic and fulvic acids. Chemosphere 2002, 49, 259–262. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro Dick, D.; Burb, P.; Herzog, H. Influence of Extractant and Soil Type on Molecular Characteristics of Humic Substances From Two Brazilian Soils. J. Braz. Chem. Soc. 1999, 10, 140–145. [Google Scholar]
- Sanchez-Monedero, M.A.; Cegarra, J.; Garcia, D.; Roig, A. Chemical and structural evolution of humic acids during organic waste composting. Biodegradation 2002, 13, 361–371. [Google Scholar] [CrossRef]
- Senesi, N.; Loffredo, E.; D’Orazio, V.; Brunetti, G.; Miano, T.M.; La Cava, P. Adsorption of pesticides by humic acids from organic amendments and soils. In Humic Substances and Chemical Contaminants; Clapp, C.E., Hayes, M.H.B., Senesi, N., Bloom, P.R., Jardine, P.M., Eds.; SSSA: Madison, WI, USA, 2001; pp. 129–154. [Google Scholar]
- Kawski, A. Photoluminescence of Solutions; Scientific Publishing House PWN: Warsaw, Poland, 1992; p. 369. ISBN 83-01-10173-3. (In Polish) [Google Scholar]
- Ayawei, N.; Ebelegi, A.N.; Wankasi, D. Modelling and Interpretation of Adsorption Isotherms. J. Chem. 2017, 2017, 3039817. [Google Scholar] [CrossRef]
- Pignatello, J. Dynamic interactions of natural organic matter and organic compounds. J. Soils Sediments 2012, 12, 1241–1256. [Google Scholar] [CrossRef]
- Novotny, E.H.; Turetta, A.P.D.; Resende, M.F.; Rebello, C.M. The quality of soil organic matter, accessed by 13C solid state nuclear magnetic resonance, is just as important as its content concerning pesticide sorption. Environ. Pollut. 2020, 266, 115298. [Google Scholar] [CrossRef] [PubMed]
- Sadegh-Zadeh, F.; Abd Wahid, S.; Jalili, B. Sorption, degradation and leaching of pesticides in soils amended with organic matter: A review. Adv. Environ. Technol. 2017, 3, 119–132. [Google Scholar] [CrossRef]
- Ehlers, G.; Loibner, A. Linking organic pollutant (bio) availability with geosorbent properties and biomimetic methodology: A review of geosorbent characterization and (bio)availability prediction. Environ. Pollut. 2006, 141, 494–512. [Google Scholar] [CrossRef]


| Soil | pH (KCl) | CaCO3 | TOC | TN | TOC/TN | CEC 1 | HA 2 | FA 3 | HUM 4 | USDA Textural Class |
|---|---|---|---|---|---|---|---|---|---|---|
| g kg−1 | % of C | |||||||||
| 1Ps | 7.71 | 14.6 | 13.3 | 1.06 | 12.5 | 28.3 | 45.20 | 17.72 | 37.09 | sandy loam |
| 6M | 7.52 | 15.3 | 21.2 | 1.60 | 13.2 | 33.4 | 35.64 | 18.79 | 45.57 | loam |
| 8C | 7.39 | 10.3 | 26.1 | 2.03 | 12.8 | 21.6 | 27.32 | 29.74 | 42.94 | silt loam |
| Sample | C | N | H | O | H/C | O/H | C/N | RC·10−16 [Spins g−1] | g-Paramater |
|---|---|---|---|---|---|---|---|---|---|
| Atomic % of Ash Free Mass | |||||||||
| 1Ps | 41.26 | 1.87 | 41.82 | 18.17 | 0.93 | 0.44 | 22.04 | 8.32 | 2.0030 |
| 1Ps-AC *1 | 33.71 | 1.61 | 31.17 | 33.39 | 0.92 | 1.07 | 20.98 | 4.03 | 2.0028 |
| 1Ps-CY *2 | 33.01 | 1.54 | 30.89 | 34.25 | 0.94 | 1.04 | 21.50 | 6.57 | 2.0030 |
| 1Ps-FL *3 | 28.98 | 1.50 | 36.57 | 32.15 | 1.26 | 0.88 | 19.36 | 3.82 | 2.0030 |
| 1Ps-ME *4 | 32.64 | 1.72 | 33.16 | 31.01 | 1.02 | 0.94 | 18.94 | 4.54 | 2.0028 |
| 6M | 38.98 | 1.99 | 41.83 | 20.36 | 0.97 | 0.52 | 19.62 | 12.40 | 2.0030 |
| 6M-AC *1 | 36.06 | 1.75 | 36.97 | 24.25 | 1.03 | 0.66 | 20.58 | 5.06 | 2.0030 |
| 6M-CY *2 | 37.75 | 1.83 | 35.01 | 23.80 | 0.93 | 0.63 | 20.63 | 3.20 | 2.0030 |
| 6M-FL *3 | 34.11 | 1.75 | 38.46 | 25.28 | 1.13 | 0.66 | 19.51 | 3.49 | 2.0030 |
| 6M-ME *4 | 35.62 | 1.97 | 39.25 | 19.73 | 1.10 | 0.50 | 18.04 | 3.59 | 2.0030 |
| 8C | 42.84 | 2.30 | 40.81 | 15.92 | 0.88 | 0.37 | 18.65 | 11.70 | 2.0030 |
| 8C-AC *1 | 28.57 | 1.48 | 29.43 | 40.01 | 1.03 | 1.36 | 19.36 | 5.60 | 2.0030 |
| 8C-CY *2 | 38.46 | 1.62 | 33.91 | 25.15 | 0.88 | 0.65 | 23.68 | 7.03 | 2.0030 |
| 8C-FL *3 | 38.90 | 1.77 | 34.20 | 22.69 | 0.88 | 0.58 | 21.92 | 3.69 | 2.0030 |
| 8C-ME *4 | 40.86 | 1.54 | 39.06 | 17.66 | 0.96 | 0.45 | 26.55 | 5.48 | 2.0030 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mielnik, L.; Weber, J.; Ukalska-Jaruga, A.; Bejger, R.; Jerzykiewicz, M.; Kocowicz, A.; Ćwieląg-Piasecka, I.; Jamroz, E.; Debicka, M.; Bekier, J. The Effects of the Interaction of Pesticides with Humin Fraction as Influencing the Sustainable Development of Agroecosystems. Sustainability 2024, 16, 1983. https://doi.org/10.3390/su16051983
Mielnik L, Weber J, Ukalska-Jaruga A, Bejger R, Jerzykiewicz M, Kocowicz A, Ćwieląg-Piasecka I, Jamroz E, Debicka M, Bekier J. The Effects of the Interaction of Pesticides with Humin Fraction as Influencing the Sustainable Development of Agroecosystems. Sustainability. 2024; 16(5):1983. https://doi.org/10.3390/su16051983
Chicago/Turabian StyleMielnik, Lilla, Jerzy Weber, Aleksandra Ukalska-Jaruga, Romualda Bejger, Maria Jerzykiewicz, Andrzej Kocowicz, Irmina Ćwieląg-Piasecka, Elżbieta Jamroz, Magdalena Debicka, and Jakub Bekier. 2024. "The Effects of the Interaction of Pesticides with Humin Fraction as Influencing the Sustainable Development of Agroecosystems" Sustainability 16, no. 5: 1983. https://doi.org/10.3390/su16051983
APA StyleMielnik, L., Weber, J., Ukalska-Jaruga, A., Bejger, R., Jerzykiewicz, M., Kocowicz, A., Ćwieląg-Piasecka, I., Jamroz, E., Debicka, M., & Bekier, J. (2024). The Effects of the Interaction of Pesticides with Humin Fraction as Influencing the Sustainable Development of Agroecosystems. Sustainability, 16(5), 1983. https://doi.org/10.3390/su16051983












