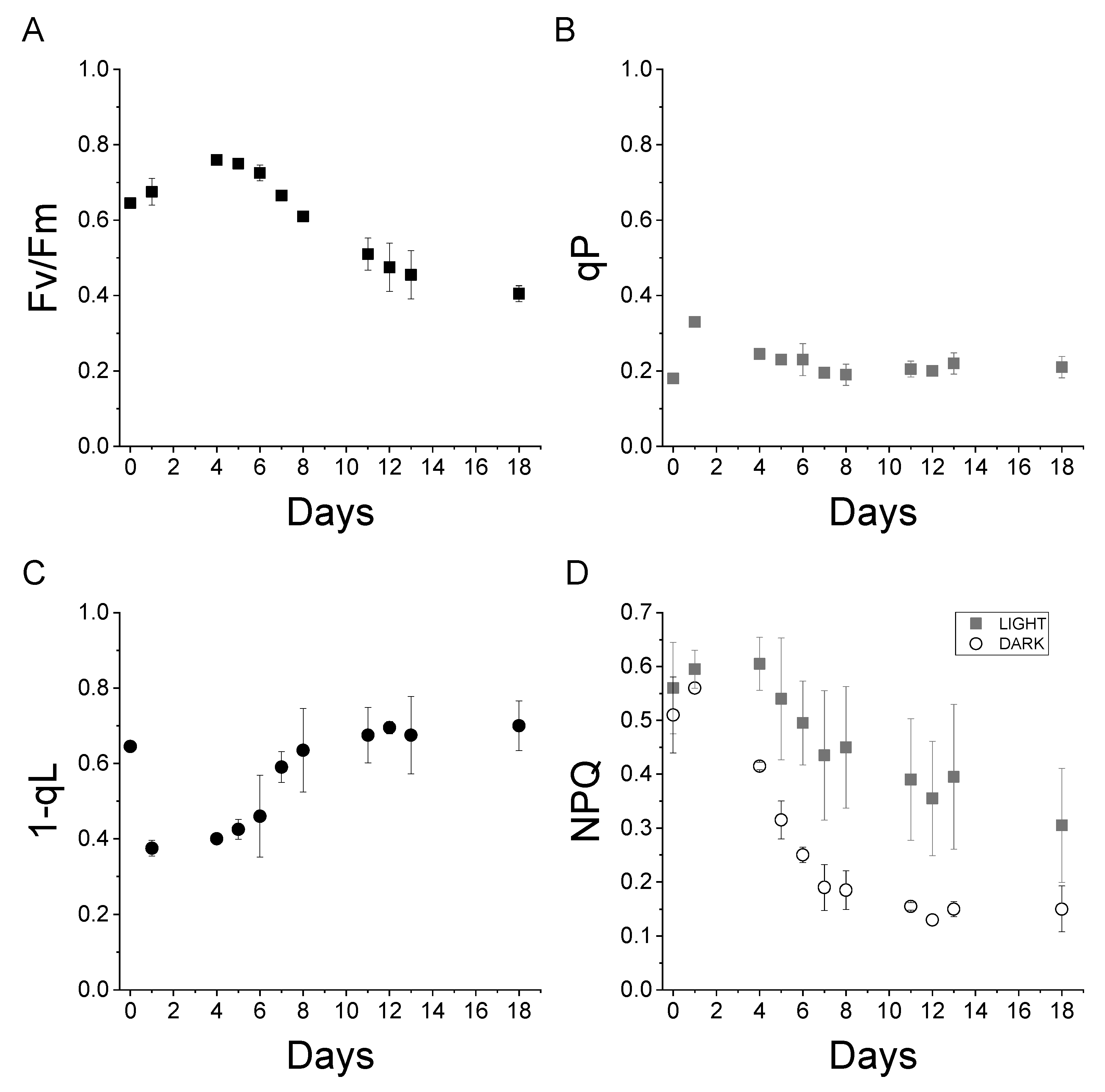Abstract
Climate change is a global critical issue. High carbon dioxide emissions and concentrations are important factors. In the construction field, concrete contributes significantly to greenhouse gas emissions. Therefore, a pioneering team of researchers has developed a new “living concrete” construction finish material capable of scrubbing carbon dioxide from the atmosphere. The material consists of ASTM (ASTM is the acronym for American Society for Testing Materials)-certified concrete block(s) with Chlorella vulgaris cultivated on the surface. Chlorella vulgaris is a common micro-algae with photosynthetic activity; these species require water, nutrients, light, and carbon dioxide to live while releasing oxygen in return. The “living concrete” block was developed in dedicated laboratories; its photosynthetic activity was quantified. Proposed as an external application assembly to a new or an existing building envelope—up to 3 m high, i.e., anthropogenic street-level emissions, or installed on roof(s) in horizontal mode—this concrete/biological composite material reverses carbon dioxide emissions and may present itself as a valid solution for climate change issues in urban moderate climates.
1. Introduction
Climate change is a critical global issue. While several greenhouse gases (GHGs) such as methane (CH4), nitrous oxide (N2O), hydrofluorocarbons (HFCs), chlorofluorocarbons (CFCs), and perfluorocarbons (PFCs) contribute noxiously to global warming, the primary agent and the largest contributor is carbon dioxide (CO2) [1]. In Europe, 36% of CO2 emanates from the building sector, which consumes 40% of the energy [2]. CO2 being by far the most significant greenhouse gas (GHG) emitted [3], the World Green Building Council (WGBC) moreover estimates that 39% of these carbon emissions are released during the life cycle of buildings [4]. Factually, concrete is the most used construction material worldwide [5]. Cement manufacture, the basic component of concrete, releases five to eight percent of the global greenhouse gas [6]. In Europe, the production of 1 kg of Portland Cement Type I emits, on estimate, 800 g of CO2, 2.4 g of nitrogen oxides (NOx), 0.5 g of sulfur dioxide (SO2), and 0.2 g to 0.3 g of dust [7]. Globally, CO2 emissions from cement production are estimated to be three billion tons per year [8]. According to the National Aeronautics and Space Administration (NASA), CO2, once added to the atmosphere, remains between 300 years to 1000 years [9].
In this context, finding efficient negative (by removal, absorption, and/or scrubbing) CO2 emissions techniques is mandatory. Therefore, we elaborated a “negative thinking (methodological research for CO2 scrubbing solutions)” approach by researching efficient and affordable technical solutions for CO2 removal from the atmosphere. To reverse the noxious emissions phenomenon, we developed an applicable finding: the “living concrete” block material, i.e., an advanced symbiotic green concrete construction material with photosynthetic activity that absorbs carbon dioxide while releasing oxygen in return. Concrete is an inert construction material; however, our innovative ASTM [10] certified concrete block “lives” by the cultivation of micro-algae on its surface. Micro-algae are unicellular photosynthetic organisms that use light energy to fix CO2 into organic matter.
Micro-algae are often cultivated on an industrial scale in artificial systems to generate desired biomolecules, including pigments, food additives, bio-stimulants, and biochemicals. Among the various algal species and strains, Chlorella vulgaris, a green alga, is particularly favored for industrial applications due to its rapid growth and high resistance to both biotic and abiotic stresses.
Researching possible interactions between the biological realm and the concrete building field is a new scientific professional trend. Bacteria-based self-healing concrete (self-healing concrete or self-repairing concrete possesses the ability to repair its cracks autonomously) [11], i.e., with living organisms, has been developed by several European companies to repair concrete. Lately, living concrete has been under research in many universities with cyanobacteria [12]. Investigations are being conducted by international universities to develop concrete suitable for algae growth [13]. This researched concrete material is defined as bio-receptive, i.e., possessing the aptitude to be colonized by one or several groups of living organisms without necessarily undergoing any biodeterioration [13]. These few studies focus more on concrete composition than on necessary conditions for micro-algae sustainability.
Focusing on green concrete [14,15] to develop greener buildings, this article exposes the conceptualizing of a novel methodology for symbiosing the concrete with micro-biology to reverse noxious GHG emissions trends. Compared to current scientific studies, this paper proposes a methodological experiment disclosing life settings of the innovative symbiotic concrete material as well as growth symbiotic conditions of the C. vulgaris in laboratories. Moreover, measured photosynthetic activities are reported. Additionally, the discussion sheds light on possible future research paths to obtain an environmentally adaptable practical finish concrete material that can efficiently scrub CO2 and emit oxygen in return, under various non-controlled conditions. The “living concrete” block is a promising green concrete finish solution to the building envelope. Applied judiciously in moderate climatic zones, it may reverse climate change in urban cities.
2. Materials and Method
With the aim of reversing detrimental CO2 emission trends from the construction sector, our experiment focused on developing a symbiotic finish concrete material that “lives” by cultivating common micro-algae C. vulgaris on its surface. Selecting standard certified concrete finish block samples, the methodology adopted consisted, first, of symbiosing the inert material with C. vulgaris micro-organisms under controlled favorable living conditions; second, it implied testing and measuring the growth of C. vulgaris micro-living organisms through photosynthetic technical measurements; finally, it inferred conclusions and proposed future research paths for building applications. The following paragraphs describe the specifications of the selected concrete blocks and the micro-algae; they also present the steps of the experiment and the measurement techniques implemented.
2.1. Selected Concrete Blocks Specifications
As a first step and to conduct the experiment, four ASTM-certified concrete block samples were chosen from the PPB Structures concrete manufacturing firm, referenced PAVER 10 × 10; 14 0006 00 07 [16]; size: 10 × 10 × 6 cm; color: grey—please refer to Appendix A—Figure A1. The block samples were labeled A, B, C, and D.
Several assessment criteria were thoroughly evaluated before the final selection of the concrete finish block(s): (1) adequacy for external application; (2) porosity; (3) humidity retainment; (4) modularity; (5) portability; (6) size and dimensions; (7) durability; and (8) carbonation property. Effectively, the concrete blocks possessed the required physical properties for external application under moderate weathering conditions. They could be fixed on stainless steel mounting assemblies as an external layer to the building envelope. Their ability to retain humidity would support micro-algae life conditions. Their practical dimensions and their portability would facilitate maintenance and replacement through a modular design accommodating various external building shapes. Concrete is a durable building material. Positioned as a finish block, it would undergo carbonation, i.e., a process that involves sequestering CO2 from the surrounding air.
The ASTM-certified concrete blocks perform at a compressive strength of 250 kg/cm2 in 28 days; the concrete mix is composed of 370 kg/m3 cement content; the free water—cement ratio by mass is 0.28; the ratio of fine (0–3 mm) to total fine aggregates is 0.123; the maximum nominal size of the aggregate is 5 mm; the fine aggregates to cement ratio by weight is 4.96. For further specifications of the concrete mix of these blocks, please refer to Appendix A—Table A1. The blocks may be laid, in horizontal mode, over non-walkable surface areas such as roofs. They may be mounted, in vertical mode, as an external building finish layer. In the latter case, the blocks should be mechanically fixed on a stainless-steel modular frame mounting system. The assembly would be applied as an external layer to the new or the existing building envelope. In all cases, necessary structural measures, waterproofing, and protection of the building envelope must be considered.
2.2. Micro-Algae Specifications and Experiment Brief
The term “algae” covers many different organisms capable of producing oxygen through photosynthesis, which is the process of harvesting light energy from the sun to generate carbohydrates [17]. Genera, such as Chlorella, Scenedesmus, and Chlorococcum, proved effective in capturing CO2 from effluents and emissions from industrial activities [18]. The selection of C. vulgaris was based on the availability and ability to thrive in mild growth conditions [19]. The most favorable conditions for C. vulgaris algae growth are daylight with a temperature of 25 °C, in BG-11 culture medium. This solution is a cultivation media for green algae; it is composed of essential nutrients including nitrate, phosphate, and micro-elements required for algae cultivation [20,21].
The C. vulgaris strain herein used is the 211/11p strain [22] obtained from the Culture Collection of Algae at Göttingen University; then, this Chlorella vulgaris strain was cultivated at the laboratories of the Biotechnology Department at the University of Verona. For the experiment, approximately 5 × 109 C. vulgaris cells were collected from a fresh culture in BG-11 (Blue Green 11) growth medium [23]. Please refer to Appendix B—Table A2—for BG-11 composition.
The experiment consisted of spreading concentrated C. vulgaris, allowing adherence, and studying conditions for keeping it alive on the concrete samples.
2.3. Cultivation on Block Samples
The step-by-step procedure of the experiment is described here below:
- Sanitization of Samples A and B concrete blocks: Samples A and B were sanitized with 70% alcohol. They were, then, desiccated for an hour at 60 °C temperature. Samples C and D were left aside for future comparative structural tests (Samples C and D were not utilized during the experiment; they will serve as reference samples for future performance structural comparative tests with Samples A and B).
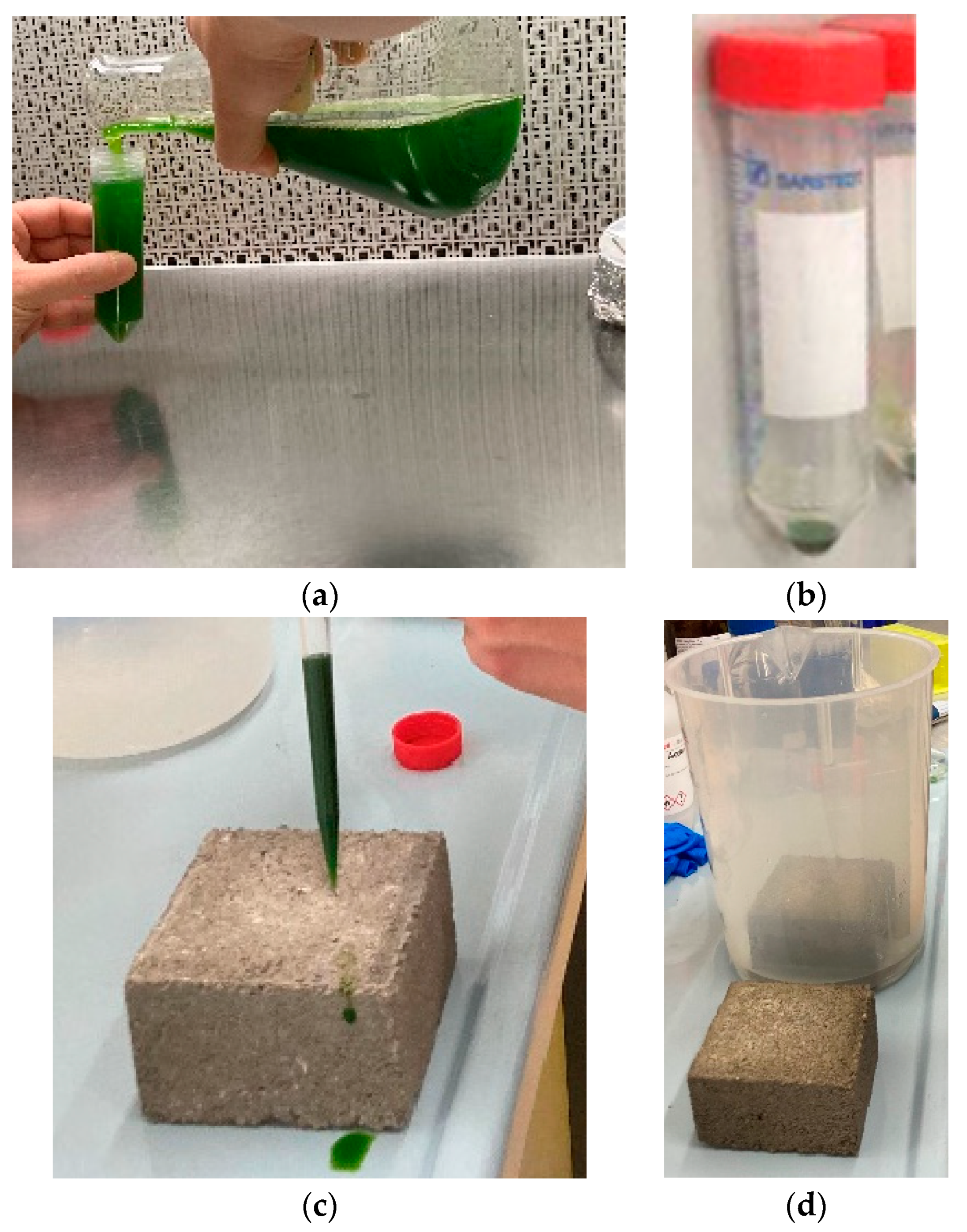
- Collection of the micro-algae and centrifuging: C. vulgaris cells were collected from a saturated culture (1 × 108 cells/mL) grown at 100 µmol photons m−2 s−1 in continuous light in BG-11 growth medium and poured into 50 mL centrifuge tubes—Figure 1a below. Afterwards, the tubes were centrifuged for 5 min at 3000× g to pellet the cells—Figure 1b below. This procedure substituted the exhausted medium with the fresh medium and increased cell concentration.
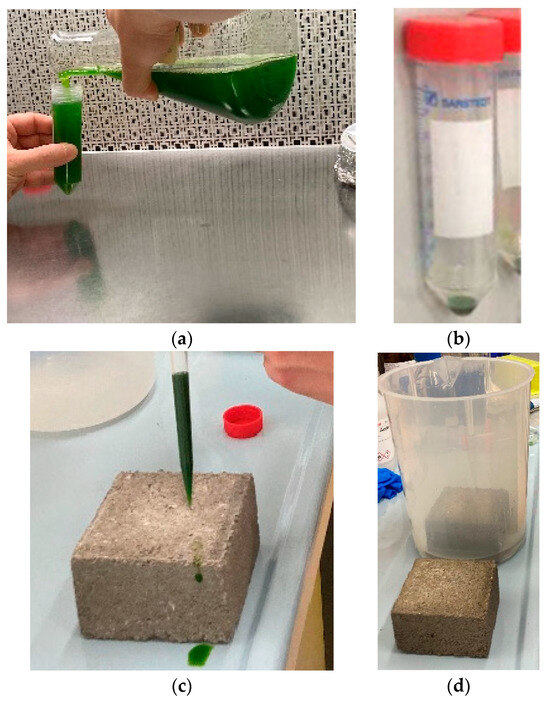 Figure 1. The living concrete experiment: (a) collecting Chlorella vulgaris liquid cultures; (b) concentrated solutions of Chlorella vulgaris; (c) spreading the concentrated solution on the concrete sample; (d) partially immersing one of the samples.
Figure 1. The living concrete experiment: (a) collecting Chlorella vulgaris liquid cultures; (b) concentrated solutions of Chlorella vulgaris; (c) spreading the concentrated solution on the concrete sample; (d) partially immersing one of the samples. - Re-suspension of the micro-algae solution: Pelleted cells were, then, resuspended in 5 mL of fresh BG-11 medium. The resulting cell concentration obtained was 1 × 109 cell/mL.
- Soaking, spreading the solution, and partial immersing: Both Samples A and B were soaked in BG-11 medium to ensure adequate moisturization with nutrients. Afterwards, the re-suspended cells were spread on the surface of concrete block Samples A and B to assess C. vulgaris growth—Figure 1c. However, to observe two different growth conditions, Sample A was left in a beaker, partially immersed in BG-11 solution up to 2 cm as measured from the bottom; this Sample A was designated as the “Wet Block”. The 2 cm were refilled five days a week to keep the immersion level constant. Sample B was not subject to immersion; it was designated as the “Dry Block”—Figure 1d.
- Growth observation conditions: For daily observation, both Samples A and B were positioned on a counter in a sealed chamber at a temperature of 23 °C at a constant light of 100 µmol photons m−2 s−1. The chamber was a sanitized room of 3 m × 1.7 m located at the laboratory premises of the Biotechnology Department—Verona University.
2.4. Photosynthetic Measurements
Photosynthetic measurements were performed in a dedicated chamber, five days a week. At each instance, Samples A and B were retrieved and then re-positioned back into their observation chamber room. Photosynthetic parameters Fv/Fm, qP, 1-qL, and NPQ (these are the common photosynthetic parameters; they are used for the characterization of photosynthetic organisms (refer to the Results section)) were characterized by measuring chlorophyll fluorescence of intact cells at room temperature. This was conducted with a closed FluorCam FC 800-C instrument [24,25]. Fv/Fm was measured on dark-adapted cells; qP, NPQ, and 1-qL were measured upon 10 min of illumination [26] with an actinic light of 1200 µmol m−2 s−1. This illumination was followed by 10 min of dark recovery. In all cases, a saturating light of 4000 μmol photons m−2 s−1 was utilized.
3. Results
In summary, two 10 cm × 10 cm × 6 cm concrete finish block samples—Sample A and Sample B—were soaked in a fresh BG-11 medium. Sample A or the “Wet Block” was, then, partially immersed in a beaker. The immersion level was 2 cm as measured from the bottom. The solution was continuously refilled with fresh BG-11 growth medium to keep the level constant. Sample B or the “Dry Block” was not immersed—Figure 1d. The porosity of the concrete blocks retained moisture of the nutrient solution, which is a favorable condition for micro-algae growth.
Due to capillary action, soaking Sample A (Wet Block) maintained moisture saturation with BG-11 growth medium and sustained the life of C. vulgaris cell culture initially spread on its surface. However, in the case of the “Dry Block” (Sample B), the cells ran down from the surface into the inner pores of the block and were almost completely absorbed by the concrete matrix.
Soaking entirely both concrete blocks—Sample A and Sample B—in BG-11 medium prior to pouring C. vulgaris micro-algae solution on the surface limited the run-down of the solution into concrete block pores. Observation revealed that as the humidity/saturation of the soaked concrete block increases, the runoff of the concentrated C. vulgaris cell culture becomes more restricted. As mentioned earlier, Sample A, or the “Wet Block”, was partially immersed in growth medium—up to 2 cm from the bottom—with fresh medium renewed once a day, five days a week, in a sealed chamber at 23 °C and under constant light for further observation. Sample B, or the “Dry Block”, was provided daily with fresh BG-11 medium under the same conditions but without immersing.
The initial results of both living concrete blocks are shown in Figure 2 below.

Figure 2.
(a) The partially immersed “Wet Block”; (b) the “Dry Block”.
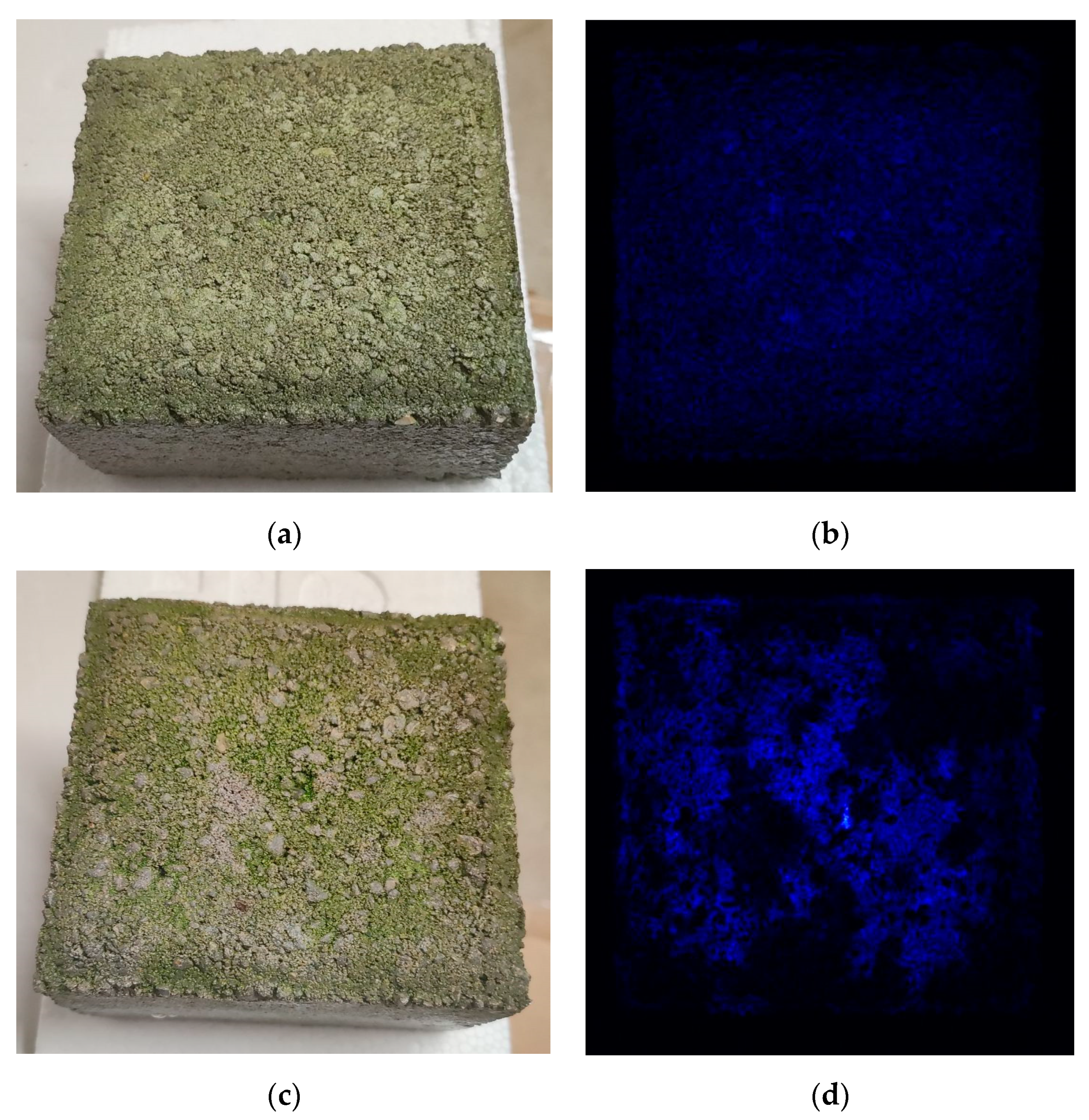
Sample B, or the “Dry Block”, exhibited a loss of green color during the growth period. After five days, death of the micro-algae was observed. Therefore, the experiment continued over Sample A, or the “wet” concrete block, to explore the potential of sustaining C. vulgaris growth as long as possible. During this experiment, the fluorescence emitted by the chlorophylls present in C. vulgaris was recorded to investigate their photosynthetic properties.
Figure 3 depicts the fluorescence emitted by the chlorophylls. This fluorescence emission increased noticeably from day 4 onwards, indicating increased chlorophyll concentration and, consequently, cell growth.
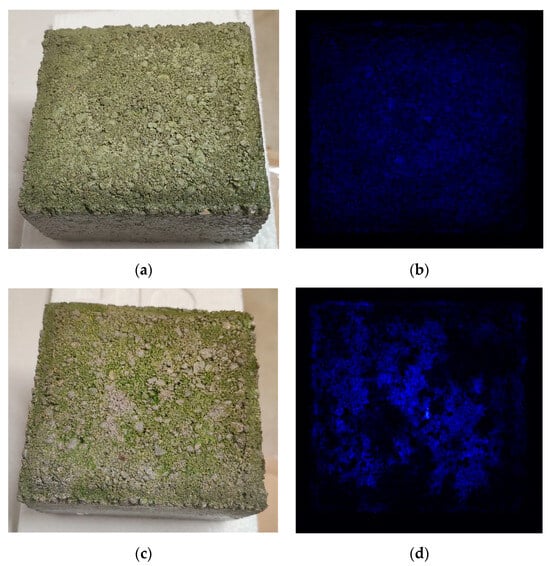
Figure 3.
Increase in chlorophyll fluorescence on Sample A; (a) the “wet” partially immersed living concrete block on day 0; (b) fluorescence imaging showing chlorophyll fluorescence emission on day 0; (c) the “wet” partially immersed living concrete block on day 4; (d) fluorescence imaging showing chlorophyll fluorescence emission on day 4.
Maintaining a constant BG-11 partial immersion level, Sample A sustained the life of C. vulgaris micro-algae for more than two weeks. The photosynthetic activities are reported in Figure 4. These activities are measured from the chlorophyll fluorescence of dark-adapted cells illuminated with an actinic light of 1200 µmol m−2 s−1. The fluorescence measured corresponds to the fluorescence emitted by one of the photosystems present in the chloroplast of Chlorella cells, Photosystem II. Photosystems, Photosystem II and Photosystem I, are protein complexes where photochemical reactions occur during the light phase of photosynthesis. The fluorescence quantum yield of the two photosystems is different: the fluorescence quantum yield of Photosystem I is much lower when compared to Photosystem II at room temperature. The measurement of Photosystem II fluorescence fluctuations, upon exposure to different light intensities, allows the retrieval of the common photosynthetic parameters used for the characterization of photosynthetic organisms such as Fv/Fm, qP, 1-qL, and NPQ [26,27].
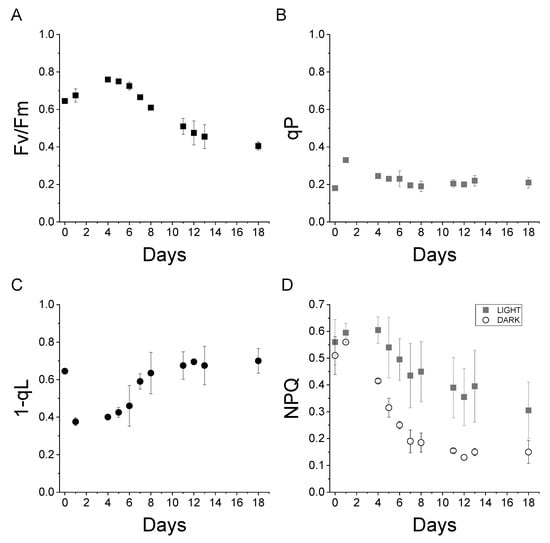
Figure 4.
Photosynthetic parameters measured on Chlorella vulgaris cells on the “Wet Block”.
The Fv/Fm parameter represents the maximum Photosystem II quantum yield. This parameter indicates the maximum efficiency of photochemical activity occurring at the level of Photosystem II in dark-adapted cells [26]. As depicted in Figure 4A, Fv/Fm increased from day 0 to day 4, and it remained above 0.4 until the end of the experiment. Fv/Fm values surpassing 0.4 indicate that the cells are alive and photosynthetically active.
qP is a photosynthetic parameter indicating the photosynthetic activity in light-adapted cells [26]. Non-zero 0 qP values were measured during the experiment; this demonstrated photosynthetically active cells—Figure 4B.
1-qL indicates the redox state of plastoquinones, the primary electron acceptor from Photosystem II. A 1-qL value of 0 signifies a fully reduced plastoquinone pool and, consequently, a saturated electron transport chain, while a value of 1 indicates complete oxidation [28]. As reported in Figure 4C, 1-qL initially decreased from 0.6 to 0.4 on day 1 but, subsequently, increased to a stable value of 0.6. This result indicates that not only are the cells photosynthetically active, but also the photosynthetic electron transport is not saturated.
Finally, NPQ (non-photochemical quenching) is a fluorescence-derived parameter that indicates the thermal dissipation of the light absorbed by the cells [29]. NPQ is, usually, triggered upon exposure to high-intensity light as a photoprotective mechanism, reducing the risk of saturation of the photosynthetic electron transport chain. While NPQ is induced upon light exposure, a long-living NPQ component may also be present in the dark. As illustrated in Figure 4D, NPQ values ranging between 0.3 and 0.6 were measured during the experiments in light-exposed cells. Upon dark recovery, the NPQ was similar to the light-adapted cells at day 0 and day 1. However, it significantly decreased, indicating the onset of an NPQ component specifically triggered by exposure to light, commonly called qE, which relaxes within a few minutes upon dark recovery.
The photosynthetic parameters measured demonstrate that C. vulgaris cells were photosynthetically active when cultivated on the “wet” concrete block. This photosynthetic activity persisted throughout the eighteen-day experiment—until the latter was deliberately concluded.
4. Discussion
The conducted experiment demonstrated the successful cultivation of Chlorella vulgaris on concrete blocks measuring 10 cm × 10 cm × 6 cm; this success depended on maintaining an optimal concentration of micro-algae solution. Compared to the “Dry Block” (non-immersed), the “Wet Block” or the partially immersed block—up to 2 cm from the bottom—exhibited better moisture retainment, creating more favorable life conditions on the surface. Pouring BG-11 five days a week onto the surface sustained the life of the Chlorella vulgaris micro-algae, i.e., the living concrete block. It is noteworthy that the living concrete block “lived” for eighteen days; in other words, C. vulgaris cells remained photosynthetically active until the deliberate ending of the experiment.
The fluorescence emission by the chlorophylls of C. vulgaris cells over the “wet” concrete block—Sample A—shows a noticeable increase from day 4 onwards, indicating increased chlorophyll concentration and, consequently, cell growth. This increased activity supports the conclusion that the cells are growing on the block (more cells, more chlorophylls, more fluorescence). Based on fluorescence imaging results, the photosynthetic parameters related to the photosynthetic activity were retrieved.
Micro-algae living condition constraints may be impeded due to the following factors: stress may be exhibited in cases of water or nutrient shortage when the temperature falls outside the range of 20 °C to 30 °C, or when the light intensity is either insufficient or exceeds the tolerable threshold. Should one or more of these impeding conditions be present, C. vulgaris species would die; death can be identified by a discoloration process, transitioning from deep green to brown and eventually to white.
Limitations of this study stem from laboratory-controlled conditions. Temperature and light were maintained at 23 °C and 100 µmol m−2 s−1, while humidity control was limited to the range of 40–60%. However, exposing the living concrete block to uncontrolled atmospheric conditions necessitates comprehensive independent testing and measurements under various specific conditions before considering market promotion.
Additional limitations are associated with the size and the specifications of the selected concrete blocks. For instance, custom-made concrete blocks of 10 × 10 × 3 cm may yield better results. This would be attributed to the proximity of the biological living surface to the partially immersing medium, i.e., the micro-algae life-sustaining source or its nutrients. However, further empirical evidence is required to support the suggested size variation with comparative analytical tests. Moreover, alternate concrete mix compositions might yield unexpected results. According to concrete specialists, moisture may not impact the durability of the concrete finish. However, BG-11 composition may affect the strength performance of the concrete; this possibility necessitates further tests and measurements.
Further experimental research will start, first, by testing the symbiotic living concrete under normal atmospheric conditions in mild climates. Other studies could be conducted to compare the structural properties of Blocks A and B to the reference Blocks C and D, after prolonged sustained C. vulgaris life conditions. Furthermore, chemical tests such as carbonation should be conducted for similar comparative assessments.
Cultivating micro-algae on the surface of the finish concrete block has no impact on the structural performance of the waterproofed building envelope, particularly if a 3 cm to 5 cm gap is maintained between the latter and the stainless-steel mounting system. In addition, maintenance and replacement of the block are practical due to its compact size and mechanical fixation, eliminating adhesion requirements.
A separate analysis could focus on developing living concrete with cultivated cyanobacteria or other micro-algae species that may exhibit higher resistance to challenging growth conditions, particularly for applying concrete finish blocks in environments with high temperatures and/or intense light exposure. Thermophilic cyanobacteria may present more sturdy capacities to withstand extreme light and temperature conditions: the fast-growing strain Synechococcus PCC 11901, which was proven to have an extraordinary growth rate at temperatures up to 45 °C [30]. Alternatively, Chlorella ohadii was reported as an extremely fast-growing green algal strain thriving under conditions of high light intensity and high temperatures [31]. Moreover, by using fast-growing strains, it will be possible to increase the carbon sequestration efficiency of the concrete block. The suitability of these fast-growing strains for cultivation on concrete block surfaces requires specific research efforts.
The living concrete blocks construction system is proposed as an outer layer applied assembly to new building envelopes or existing ones—up to 3 m high, i.e., anthropogenic street-level emissions. The system can also be laid on non-walkable roof areas. Compared to green walls and green roofs, the “living” concrete solutions are cheaper and more practical. They require little maintenance and provide the convenience of replacing individual blocks with ease. Factually, living concrete reverses carbon dioxide emissions pollution and may present itself as a valid solution for climate change issues in urban moderate climates.
5. Conclusions
The “living” concrete experiment has proven the possibility of sustaining the life of Chlorella vulgaris micro-algae on ASTM-certified concrete blocks partially immersed in BG-11 nutrient solution up to 2 cm from the bottom, pouring nutrients five days a week, in a controlled laboratory environment, a temperature of 23 °C, and constant light. For this “wet” partially immersed concrete block, chlorophyll fluorescence emissions of Chlorella vulgaris cells were recorded, for eighteen days, using chlorophyll fluorescence imaging.
The living concrete wall or roof is a practical construction system requiring less maintenance than green walls and green roofs. In urban moderate climates, the photosynthetic activities of the “living” concrete—walls and/or roofs—may efficiently reverse carbon dioxide emissions and may present a valid solution for climate change issues.
6. Patents
E-Soleau—92 Institut National de la Propriété Industrielle (INPI)—DSO2023012898.
Author Contributions
Conceptualization, J.N.T. and R.A.H.; research, J.N.T. and R.A.H.; methodology, R.A.H.; issues solving, R.A.H.; concrete provision, R.A.H.; experiment, R.A.H. and F.B.; writing, R.A.H. and F.B.; writing review, J.N.T. and M.B.; laboratories, M.B. and F.B.; experiment follow-up, F.B. and R.A.H.; supervision, J.N.T.; laboratories supervision, M.B.; technical study and impact analysis, R.A.H.; paper publication funding acquisition, J.N.T. All authors have read and agreed to the published version of the manuscript.
Funding
The paper publication is funded by HARMONIA (harmonia-project.eu). The HARMONIA project has received funding from the EU Horizon 2020 research and innovation programme under agreement No. 101003517.
Data Availability Statement
Data is contained within the article.
Acknowledgments
This experimental research is part of the Ph.D. thesis “Designing the Green Urban Building Envelope in the Mediterranean Climate: An Architectural Approach” conducted by Rola Hasbini under the supervision of Julia Nerantzia Tzortzi at the Department of Architecture, Built Environment, and Construction Engineering at Politecnico Di Milano, Italy. The actual experiment was conducted at the laboratories of the University of Verona—Department of Biotechnology. The authors contributed equally to this work. The authors are grateful to Carlo Andrea Castiglioni for his continuous supervision and valuable technical support of this advanced concrete material research and experiment. They are also thankful to the academic society of the Department of Architecture, Built Environment, and Construction Engineering at Politecnico Di Milano for providing a conducive rich academic environment. The authors are also grateful to Alex Costa from the University of Milan—Plant Physiology Department—for channeling the experiment as well as the research society of the University of Verona Department of Biotechnology for their advanced support and offering of laboratories. They are also grateful for the technical review and advice received from Natia Anastasi, Neapolis University, Pafos. The authors express sincere gratitude to Derviche Haddad PPB Structures S.A.L.—Tony Hneiné—for the concrete products provided and for the certified product declaration.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A

Figure A1.
The concrete block samples.
Figure A1.
The concrete block samples.


Table A1.
Technical specifications of the concrete blocks as per the manufacturer PPB Structures.
Table A1.
Technical specifications of the concrete blocks as per the manufacturer PPB Structures.
| PPB Structures Derviche Haddad | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Concrete Pavers Mix Design | ||||||||||||
| Concrete Pavers—Standard Mix | ||||||||||||
| Concrete Mix Design Criteria | ||||||||||||
| f’cu @ 28 days (kg/cm2) | 250 | Cement Content kg/m3 | 370 | |||||||||
| Free Water Cement Ratio by mass | 0.28 | Ratio of fine (0–3 mm) to Total Fine Aggregates | 0.123 | |||||||||
| Maximum nom. size of aggregate (MM) | 5 | Fine Aggregates to Cement Ratio By Weight | 4.96 | |||||||||
| Initial Slump mm. | SD | Slump Retention/Minutes | NS | |||||||||
| Concrete Pavers, Mix Proportions | ||||||||||||
| Mix Ingredients Description | Material’s Properties | Weights kg/m3 | ||||||||||
| Dry Bulk Density | Dry Specific Gravity | SSD Specific Gravity | Absorption % | Moisture Content % | Batch Volume (L) | Weights Dry (kg) | Weights SSD (kg) | Weights Corrections (kg) | Mix Ingredients 1/m3 | Batch Volume (m3) | ||
| Portland Cement | Pa-l 42.5 | 3.157 | 117.2 | 370 | 370 | 370 | 185 | |||||
| Total Water | Clean Water | 0.999 | 103.60 | 133 | 104 | 133 | 66 | |||||
| Crushed Sand | 0–3 mm | 1.479 | 2.57 | 2.61 | 1.58% | 0.0% | 94.61 | 243 | 247 | 0 | 243 | 122 |
| Crushed Sand | 0–5 mm | 1.520 | 2.59 | 2.63 | 1.60% | 0.0% | 674.59 | 1594 | 1619 | 0 | 1594 | 797 |
| Medium Agg. | 3–10 mm | 1.690 | 2.69 | 2.71 | 0.63% | 0.0% | 0.0 | 0 | 0 | 0 | 0 | 0 |
| Coarse Agg. | 10–19 mm | 1.595 | 2.65 | 2.67 | 0.60% | 0.0% | 0.0 | 0 | 0 | 0 | 0 | 0 |
| Epsilon I21 | 0.00% | 0.00 | 0.00 | 0.00 | 0 | 0 | ||||||
| Air Content % | 1.00 | 10.00 | 10.00 | 10.00 | 10 | 5 | ||||||
| Totals | 1000 | 2340 | 2340 | 2340 | 1170 | |||||||
Appendix B

Table A2.
BG-11 Chemical composition.
Table A2.
BG-11 Chemical composition.
| Component | Molar Concentration in Final Medium |
|---|---|
| Fe-Citrate | 3.12 × 10−5 M |
| NaNO3 | 1.76 × 10−2 M |
| K3HPO4 | 2.24 × 10−4 M |
| MgSO4·7H2O | 3.04 × 10−4 M |
| CaCl2·2H2O | 1.84 × 10−4 M |
| NOHCO3 | 1.89 × 10−4 M |
| H3BO3 | 46.253 |
| MnCl2·4H2O | 9.146 |
| ZnSO4·7H2O | 0.765 |
| NaMoO4·2H2O | 1.781 |
| CuSO4·5H2O | 0.316 |
| CaCl2·6H2O | 0.161 |
References
- Agents of Global Warming, Ask NASA, NASA Global Climate Change, Last Modified 8 February 2010. Available online: https://climate.nasa.gov/explore/ask-nasa-climate/260/agents-of-global-warming/ (accessed on 14 August 2023).
- In Focus: Energy Efficiency in Buildings, News Article, European Commission, Last Modified 17 February 2020. Available online: https://commission.europa.eu/news/focus-energy-efficiency-buildings-2020-02-17_en (accessed on 14 August 2023).
- Carbon Dioxide Levels Are at a Record High. Here’s What You Need to Know, Environment|Explainer, National Geographic, Last Modified 13 May 2019. Available online: https://www.nationalgeographic.com/environment/article/greenhouse-gases#:~:text=Carbon%20dioxide%20(CO2)%3A,atmosphere%20for%20thousands%20of%20years (accessed on 8 August 2023).
- Bringing Embodied Carbon Upfront, Advancing Net Zero, World Green Building Council, 2019 Report. Available online: https://worldgbc.org/advancing-net-zero/embodied-carbon/ (accessed on 6 August 2023).
- Petek Gursel, A.; Masanet, E.; Horvath, A.; Stadel, A. Life-Cycle Inventory Analysis of Concrete Production: A Critical Review. Cem. Concr. Compos. 2014, 51, 38–48. [Google Scholar] [CrossRef]
- Kajaste, R.; Hurme, M. Cement Industry Greenhouse Gas Emissions—Management Options and Abatement Cost. J. Clean. Prod. 2016, 112, 4041–4052. [Google Scholar] [CrossRef]
- Marinković, S.B. Life cycle assessment (LCA) aspects of concrete. In Eco-Efficient Concrete; Pacheco-Torgal, F., Jalali, S., Labrincha, J., John, V.M., Eds.; Woodhead Publishing: Philadelphia, PA, USA, 2013; pp. 45–80. [Google Scholar]
- Naqi, A.; Jang, G.C. Recent Progress in Green Cement Technology Utilizing Low-Carbon Emission Fuels and Raw Materials: A Review. Sustainability 2019, 11, 537. [Google Scholar] [CrossRef]
- The Atmosphere: Getting a Handle on Carbon Dioxide, News, Global Climate Change, Last Modified 9 October 2019. Available online: https://climate.nasa.gov/news/2915/the-atmosphere-get-ting-a-handle-on-carbon-dioxide/#:~:text=Carbon%20dioxide%20is%20a%20different,be-tween%20300%20to%201%2C000%20years (accessed on 8 August 2023).
- ASTM International. Available online: https://www.astm.org/ (accessed on 20 August 2023).
- Bacteria-Based Self-Healing Concrete, Numero 1, In Genium, Revistas, Sedici—Repositorio Intitucional de la UNLP, Last Modified May 2021. Available online: http://sedici.unlp.edu.ar/handle/10915/119709 (accessed on 8 August 2023).
- Bricks Alive! Scientists Create Living Concrete, Science, the New York Times, Last Modified 15 January 2020. Available online: https://www.nytimes.com/2020/01/15/science/construction-concrete-bacteria-photosynthesis.html (accessed on 12 August 2023).
- Veeger, M.; Ottelé, M.; Prieto, A. Making Bioreceptive Concrete: Formulation and Testing of Bioreceptive Concrete Mixtures. J. Build. Eng. 2021, 44, 102545. [Google Scholar] [CrossRef]
- Tzortzi, J.N.; Hasbini, R. A Classification Review on Green Concrete. Adv. Mater. Res. 2021, 1166, 113–123. [Google Scholar] [CrossRef]
- Tzortzi, J.N.; Hasbini, R. A Classification Review on Green Concrete. In Green Concrete; Kryvenko, P., Kolisnychenko, S., Eds.; Trans Tech Publication: Stafa-Zurich, Switzerland, 2023; pp. 3–13. [Google Scholar]
- Derviche Haddad PPB Structures—S.A.L. Floor Covering & Concrete Products; Exterior—Interlock Paver; Derviche Haddad PPB Structures—S.A.L.: Beirut, Lebanon, 2003. [Google Scholar]
- What Are Algae, Planet Earth, Live Science, Last Modified 4 June 2016. Available online: https://www.livescience.com/54979-what-are-algae.html (accessed on 8 August 2023).
- Onyeaka, H.; Miri, T.; Obileke, K.C.; Hart, A.; Anumudu, C.; Al-Sharify, Z. Minimizing Carbon Footprint via Microalgae as a Biological Capture. Carbon Capture Sci. Technol. 2021, 1, 100007. [Google Scholar] [CrossRef]
- Safim, C.; Zebib, B.; Merah, O.; Pontalier, P.-Y.; Vaca-Garcia, C. Morphology, Composition, Production, Processing and Applications of Chlorella vulgaris: A review. Renew. Sustain. Energy Rev. 2014, 35, 265–278. [Google Scholar] [CrossRef]
- Allaguvatova, R.; Myasina, Y.; Zakharenko, V.; Gaysina, L. A Simple Method for the Cultivation of Algae Chlorella Vulgaris Bejerinck. IOP Conf. Ser. Earth Environ. Sci. 2019, 390, 012020. [Google Scholar] [CrossRef]
- Pandey, S.; Narayanan, I.; Vinayagam, R.; Selvaraj, R.; Varadavenkatesan, T.; Pugazhendhi, A. A Review on the Effect of Blue Green 11 Medium and its Constituents on Microalgal Growth and Lipid Production. J. Environ. Chem. Eng. 2023, 11, 109984. [Google Scholar] [CrossRef]
- Cecchin, M.; Marcolungo, L.; Rossato, M.; Girolomoni, L.; Cosentino, E.; Cuine, S.; Li-Beisson, Y.; Delledonne, M.; Ballottari, M. Chlorella Vulgaris Genome Assembly and Annotation Reveals the Molecular Basis for Metabolic Acclimation to High Light Conditions. Plant J. 2019, 100, 1289–1305. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.M.; Stanier, R.Y. Growth and Division of Some Unicellular Blue-Green Algae. J. Gen. Microbiol. 1968, 51, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Photon System Instruments, Czech Republic. Available online: https://fluorcams.psi.cz/ (accessed on 10 December 2023).
- Nowicka, B. Practical aspects of the measurements of non-photochemical chlorophyll fluorescence quenching in green microalgae Chlamydomonas reinhardtii using Open FluorCam. Physiol. Plant. 2019, 168, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Baker, N.R. Chlorophyll Fluorescence: A Probe of Photosynthesis in Vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef] [PubMed]
- Van Kooten, O.; Snel, J.F.H. The Use of Chlorophyll Fluorescence Nomenclature in Plant Stress Physiology. Photosynth. Res. 1990, 25, 147–150. [Google Scholar] [CrossRef]
- Kramer, D.M.; Johnson, G.; Kiirats, O.; Edwards, G.E. New Fluorescence Parameters for the Determination of QA Redox State and Excitation Energy Fluxes. Photosynth. Res. 2004, 79, 209. [Google Scholar] [CrossRef] [PubMed]
- Horton, P. Nonphotochemical quenching of chlorophyll fluorescence. In Light as an Energy Source and Information Carrier in Plant Physiology; Jennings, R.C., Ed.; Plenum Press: New York, NY, USA, 1996; pp. 99–111. [Google Scholar]
- Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7205611/ (accessed on 16 December 2023).
- Levin, G.; Kulikovsky, S.; Liveanu, V.; Eichenbaum, B.; Meir, A.; Isaacson, T.; Tadmor, Y.; Adir, N.; Schuster, G. The Desert Green Algae Chlorella ohadii Thrives at Excessively High Light Intensities by Exceptionally Enhancing the Mechanisms that Protect Photosynthesis from Photoinhibition. Plant J. 2021, 106, 1260–1277. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).