Sustainable Water Use in a Fruit Processing Plant: Evaluation of Microbiological and Physicochemical Properties of Wash Water after Application of a Modular Water Recovery System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Description of the Collection Site and Water Samples
2.2. Microbiological Analysis
2.3. Physicochemical Analysis
- -
- pH level was measured potentiometrically (Metrohm OMNIS titrator, Herisau, Switzerland) under the PN-EN ISO 10523:2012 standard [20],
- -
- the hardness of the water (calculated as CaCO3) was determined using the PN-ISO 6059:1999 titration method (Methrom OMNIS titrator) [21],
- -
- conductivity of water was determined using the conductivity method (Metrohm OMNIS titrator, Herisau, Switzerland) in accordance with the PN-EN 27888:1999 standard [22],
- -
- bromides were determined by the photometric method (Lovibond PM620 photometer, Lovibond, Dortmund, Germany) in accordance with the PN-ISO 10304-1:2009 standard [23],
- -
- ammonium ion concentration was determined by a photometric method (Lovibond PM620 photometer, Lovibond, Dortmund, Germany) according to the PN-ISO 7150-1:2002 standard [24],
- -
- nitrates were determined by the photometric method (Lovibond PM620 photometer, Lovibond, Dortmund, Germany) according to the PN-EN ISO 13395:2001 standard [25],
- -
- turbidity was determined by the nephelometric method (Methrom OMNIS titrator, Metrohm OMNIS titrator, Herisau, Switzerland) according to the PN-EN ISO 7027-1:2016 standard [26].
2.4. Water Recovery System
3. Results
3.1. Results of Microbiological Tests
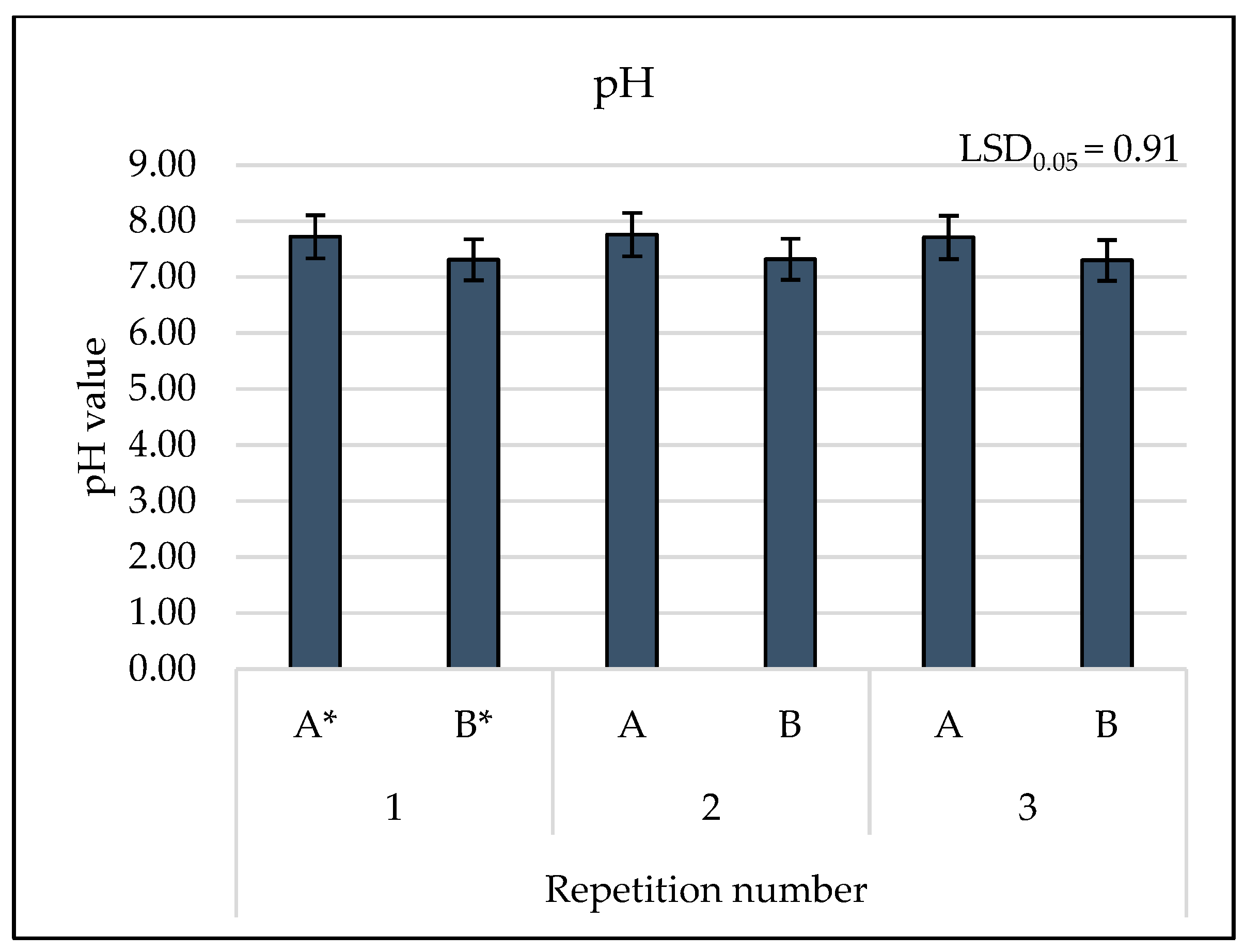
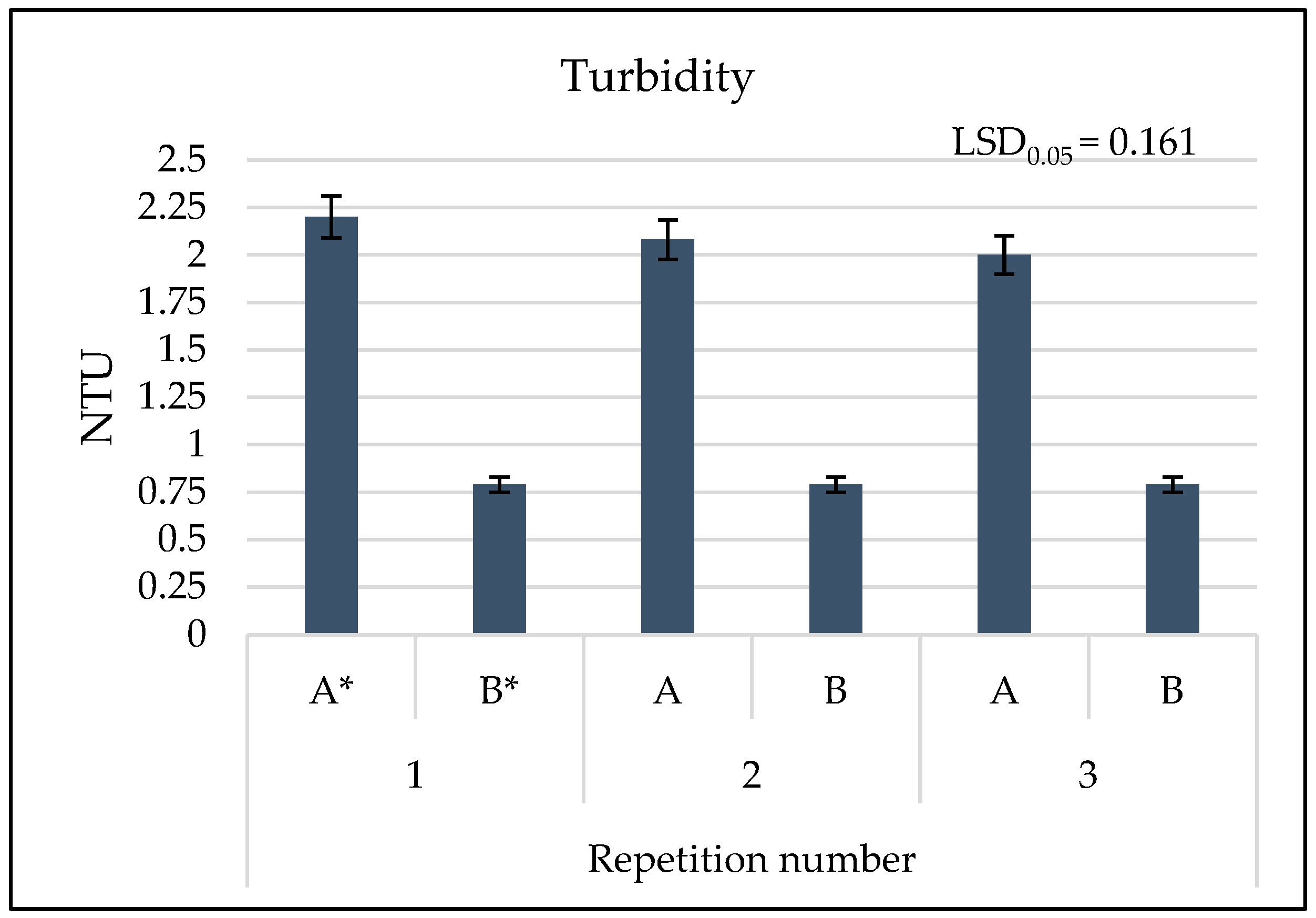
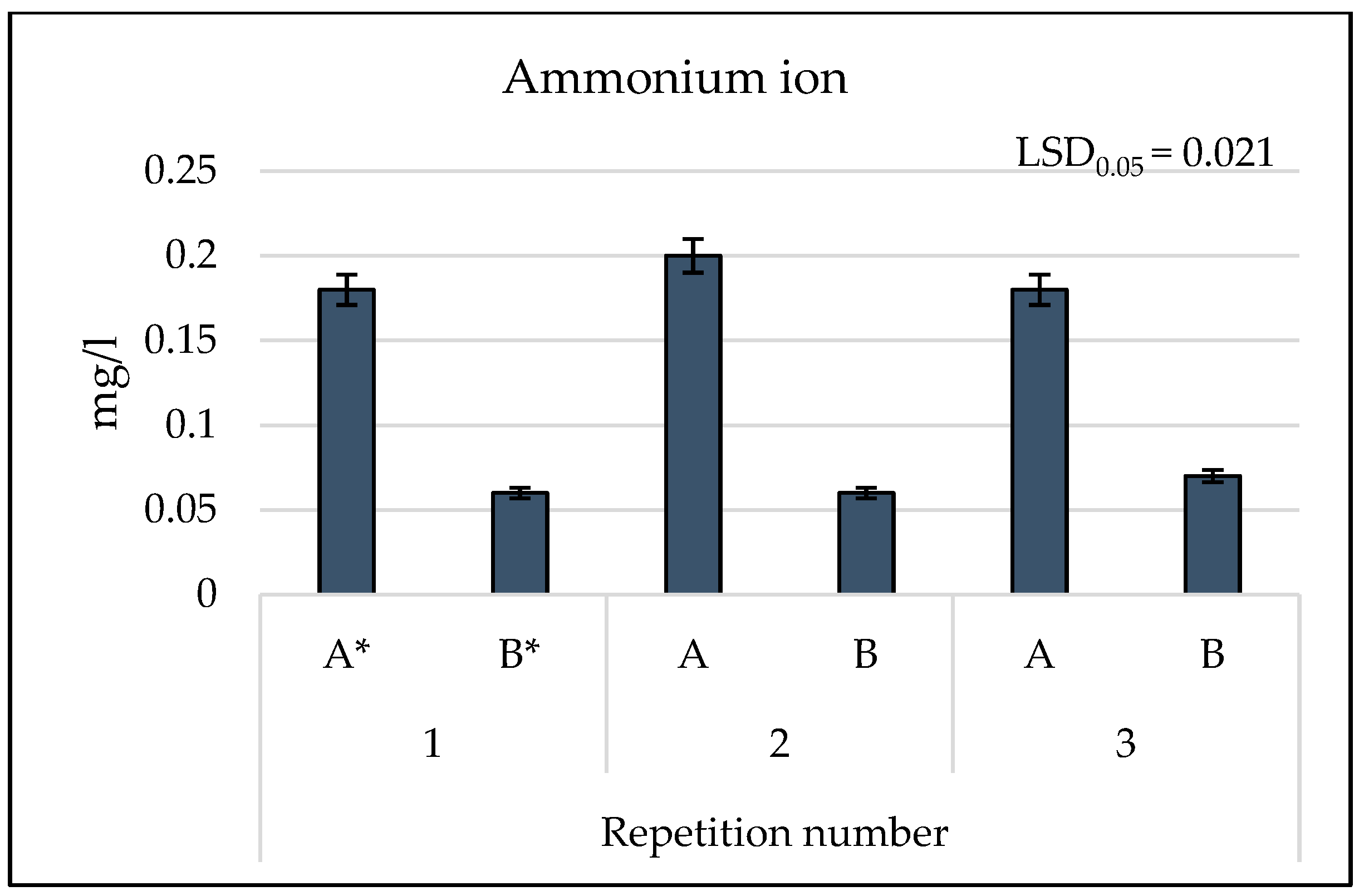
3.2. Results of Physicochemical Tests
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- UN Water. Sustainable Development Goal 6: Synthesis Report 2018 on Water and Sanitation; United Nations: New York, NY, USA, 2018; ISBN 978-92-1-101370-2. [Google Scholar]
- Vanham, D.; Alfieri, L.; Flörke, M.; Grimaldi, S.; Lorini, V.; de Roo, A.; Feyen, L. The Number of People Exposed to Water Stress in Relation to How Much Water is Reserved for the Environment: A global modelling study. Lancet Planet. Health 2021, 5, e766–e774. [Google Scholar] [CrossRef] [PubMed]
- Aivazidou, E.; Tsolakis, N.; Vlachos, D.P.; Iakovou, E. Water Footprint Mitigation Strategies for Agrifood Products: The Application of System Dynamics in Green Marketing. In Proceedings of the Strategic Innovative Marketing; Kavoura, A., Sakas, D.P., Tomaras, P., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 275–281. [Google Scholar]
- Gil, M.I.; Selma, M.V.; López-Gálvez, F.; Allende, A. Fresh-Cut Product Sanitation and Wash Water Disinfection: Problems and Solutions. Int. J. Food Microbiol. 2009, 134, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Jagtap, S.; Skouteris, G.; Choudhari, V.; Rahimifard, S. Improving Water Efficiency in the Beverage Industry With the Internet of Things. Available online: https://www.igi-global.com/chapter/improving-water-efficiency-in-the-beverage-industry-with-the-internet-of-things/www.igi-global.com/chapter/improving-water-efficiency-in-the-beverage-industry-with-the-internet-of-things/287162 (accessed on 27 June 2022).
- Lehto, M.; Sipilä, I.; Alakukku, L.; Kymäläinen, H.-R. Water Consumption and Wastewaters in Fresh-Cut Vegetable Production. Agric. Food Sci. 2014, 23, 246–256. [Google Scholar] [CrossRef]
- Mundi, G.S.; Zytner, R.G.; Warriner, K.; Gharabaghi, B. Predicting Fruit and Vegetable Processing Wash-Water Quality. Water Sci. Technol. 2018, 2017, 256–269. [Google Scholar] [CrossRef] [PubMed]
- Hoekstra, A.Y.; Mekonnen, M.M.; Chapagain, A.K.; Mathews, R.E.; Richter, B.D. Global Monthly Water Scarcity: Blue Water Footprints versus Blue Water Availability. PLoS ONE 2012, 7, e32688. [Google Scholar] [CrossRef] [PubMed]
- Ansorge, L.; Stejskalová, L. Water Footprint as a Tool for Selection of Alternatives (Comments on “Food Recommendations for Reducing Water Footprint”). Sustainability 2022, 14, 6317. [Google Scholar] [CrossRef]
- Campos, D.A.; Gómez-García, R.; Vilas-Boas, A.A.; Madureira, A.R.; Pintado, M.M. Management of Fruit Industrial By-Products—A Case Study on Circular Economy Approach. Molecules 2020, 25, 320. [Google Scholar] [CrossRef]
- Geisendorf, S.; Pietrulla, F. The Circular Economy and Circular Economic Concepts—A Literature Analysis and Redefinition. Thunderbird Int. Bus. Rev. 2018, 60, 771–782. [Google Scholar] [CrossRef]
- Nallapaneni, M.K.; Haque, A.; Patwary, S. It Is Time to Synergize the Circularity of Circular Bioeconomy with Sustainability and Resiliency Principles. Sustainability 2023, 15, 12239. [Google Scholar] [CrossRef]
- Allende, A.; Selma, M.V.; López-Gálvez, F.; Villaescusa, R. Impact of Wash Water Quality on Sensory and Microbial Quality, Including Escherichia coli Cross-Contamination, of Fresh-Cut Escarole. J. Food Prot. 2008, 71, 2514–2518. [Google Scholar] [CrossRef]
- Lapidot, A.; Romling, U.; Yaron, S. Biofilm Formation and the Survival of Salmonella Typhimurium on Parsley. Int. J. Food Microbiol. 2006, 109, 229–233. [Google Scholar] [CrossRef]
- Mritunjay, S.K.; Kumar, V. A Study on Prevalence of Microbial Contamination on the Surface of Raw Salad Vegetables. 3 Biotech 2017, 7, 13. [Google Scholar] [CrossRef]
- Selma, M.V.; Allende, A.; Lopez-Galvez, F.; Conesa, M.A.; Gil, M.I. Disinfection Potential of Ozone, Ultraviolet-C and Their Combination in Wash Water for the Fresh-Cut Vegetable Industry. Food Microbiol. 2008, 25, 809–814. [Google Scholar] [CrossRef]
- Studziński, W.; Poćwiardowski, W.; Osińska, W. Application of the Swimming Pool Backwash Water Recovery System with the Use of Filter Tubes. Molecules 2021, 26, 6620. [Google Scholar] [CrossRef]
- European Commission. Directive 98/79/EC of the European Parliament and of the Council of 27 October 1998 on in Vitro Diagnostic Medical Devices. Off. J. Eur. Commun. 1998, 331, 1–37. [Google Scholar]
- ISO PN-ISO 5667-5:2017-10; Jakość Wody—Pobieranie Próbek—Część 5: Wytyczne Dotyczące Pobierania Próbek Wody do Picia ze Stacji Uzdatniania i z Systemów Dystrybucji. Polish Committee for Standardisation: Warsaw, Poland, 2017.
- ISO PN-EN ISO 10523; Jakość Wody—Oznaczanie pH. Polish Committee for Standardisation: Warsaw, Poland, 2012.
- ISO PN-ISO 6059:1999; Jakość Wody—Oznaczanie Sumarycznej Zawartości Wapnia i Magnezu—Metoda Miareczkowa z EDTA. Polish Committee for Standardisation: Warsaw, Poland, 1999.
- ISO PN-EN 27888:1999; Jakość Wody—Oznaczanie Przewodności Elektrycznej Właściwej. Polish Committee for Standardisation: Warsaw, Poland, 1999.
- ISO PN-EN ISO 10304-1:2009; Jakość Wody—Oznaczanie Rozpuszczonych Anionów za Pomocą Chromatografii Jonowej—Część 1: Oznaczanie Bromków, Chlorków, Fluorków, Azotanów, Azotynów, Fosforanów i Siarczanów. Polish Committee for Standardisation: Warsaw, Poland, 2009.
- ISO PN-ISO 7150-1:2002; Jakość Wody—Oznaczanie Azotu Amonowego—Część 1: Manualna Metoda Spektrometryczna. Polish Committee for Standardisation: Warsaw, Poland, 2002.
- ISO PN-EN ISO 13395:2001; Jakość Wody—Oznaczanie Azotu Azotynowego i Azotanowego Oraz ich Sumy Metodą Analizy Przepływowej (CFA i FIA) z Detekcją Spektrometryczną. Polish Committee for Standardisation: Warsaw, Poland, 2001.
- ISO PN-EN ISO 7027-1; Jakość Wody—Oznaczanie Mętności—Część 1: Metody Ilościowe. Polish Committee for Standardisation: Warsaw, Poland, 2016.
- Poćwiardowski, W. The Potential of Swimming Pool Rinsing Water for Irrigation of Green Areas: A Case Study. Environ. Sci. Pollut. Res. 2023, 30, 57174–57177. [Google Scholar] [CrossRef]
- López-Gálvez, F.; Tudela, J.A.; Allende, A.; Gil, M.I. Microbial and Chemical Characterization of Commercial Washing Lines of Fresh Produce Highlights the Need for Process Water Control. Innov. Food Sci. Emerg. Technol. 2019, 51, 211–219. [Google Scholar] [CrossRef]
- Nüesch-Inderbinen, M.; Zurfluh, K.; Peterhans, S.; Hächler, H.; Stephan, R. Assessment of the Prevalence of Extended-Spectrum β-Lactamase-Producing Enterobacteriaceae in Ready-to-Eat Salads, Fresh-Cut Fruit, and Sprouts from the Swiss Market. J. Food Prot. 2015, 78, 1178–1181. [Google Scholar] [CrossRef] [PubMed]
- Banach, J.; Van Der Fels-Klerx, H. Microbiological Reduction Strategies of Irrigation Water for Fresh Produce. J. Food Prot. 2020, 83, 1072–1087. [Google Scholar] [CrossRef] [PubMed]
- Byappanahalli, M.N.; Nevers, M.B.; Korajkic, A.; Staley, Z.R.; Harwood, V.J. Enterococci in the Environment. Microbiol. Mol. Biol. Rev. 2012, 76, 685–706. [Google Scholar] [CrossRef] [PubMed]
- Toc, D.A.; Pandrea, S.L.; Botan, A.; Mihaila, R.M.; Costache, C.A.; Colosi, I.A.; Junie, L.M. Enterococcus raffinosus, Enterococcus durans and Enterococcus avium Isolated from a Tertiary Care Hospital in Romania—Retrospective Study and Brief Review. Biology 2022, 11, 598. [Google Scholar] [CrossRef]
- Endicott-Yazdani, T.R.; Dhiman, N.; Benavides, R.; Spak, C.W. Myroides Odoratimimus Bacteremia in a Diabetic Patient. Bayl. Univ. Med. Cent. Proc. 2015, 28, 342–343. [Google Scholar] [CrossRef] [PubMed]
- Kurt, A.F.; Mete, B.; Houssein, F.M.; Tok, Y.; Kuskucu, M.A.; Yucebag, E.; Urkmez, S.; Tabak, F.; Aygun, G. A Pan-Resistant Myroides Odoratimimus Catheter-Related Bacteremia in a COVID-19 Patient and Review of the Literature. Acta Microbiol. Immunol. Hung. 2022, 69, 164–170. [Google Scholar] [CrossRef]
- Espinoza, V.; Valdez, M.; Burcovschii, S.; Fong, I.; Petersen, G.; Heidari, A. The First Case Report of Endocarditis Caused by Serratia fonticola. J. Investig. Med. High Impact Case Rep. 2021, 9, 23247096211044916. [Google Scholar] [CrossRef]
- Hai, P.D.; Hoa, L.T.V.; Tot, N.H.; Phuong, L.L.; Quang, V.; Thuyet, B.T.; Son, P.N. First Report of Biliary Tract Infection Caused by Multidrug-Resistant Serratia fonticola. New Microbes New Infect. 2020, 36, 100692. [Google Scholar] [CrossRef]
- Katib, A.A.; Shaikhomar, O.; Dajam, M.; Alqurashi, L. Serratia Fonticola Microbe Presented as a Community-Acquired Urinary Tract Infection (UTI): A Case Report. J. Ideas Health 2020, 3, 226–227. [Google Scholar] [CrossRef]
- Goodman, D.; Murphy, D.; Dorairaj, J. Case Study: Soft Tissue Infection with Raoultella ornithinolytica. JPRAS Open 2022, 33, 17–20. [Google Scholar] [CrossRef]
- Yuan, Y.; Gao, M. Genomic Analysis of a Ginger Pathogen Bacillus pumilus Providing the Understanding to the Pathogenesis and the Novel Control Strategy. Sci. Rep. 2015, 5, 10259. [Google Scholar] [CrossRef]
- Luo, A.; Wang, F.; Sun, D.; Liu, X.; Xin, B. Formation, Development, and Cross-Species Interactions in Biofilms. Front. Microbiol. 2022, 12, 757327. [Google Scholar] [CrossRef]
- Gao, J.; Jang, H.; Huang, L.; Matthews, K.R. Influence of Product Volume on Water Antimicrobial Efficacy and Cross-Contamination during Retail Batch Washing of Lettuce. Int. J. Food Microbiol. 2020, 323, 108593. [Google Scholar] [CrossRef]
- Wang, R.Y.; Shen, X.; Su, Y.; Critzer, F.; Zhu, M.-J. Chlorine and Peroxyacetic Acid Inactivation of Listeria Monocytogenes in Simulated Apple Dump Tank Water. Food Control. 2022, 144, 109314. [Google Scholar] [CrossRef]
- İzgördü, ÖK.; Darcan, C.; Kariptaş, E. Overview of VBNC, a Survival Strategy for Microorganisms. 3 Biotech 2022, 12, 307. [Google Scholar] [CrossRef] [PubMed]
- Sha’Arani, S.; Azizan, S.N.F.; Akhir, F.N.M.; Yuzir, M.A.M.; Othman, N.; Zakaria, Z.; Noor, M.J.M.M.; Hara, H. Removal Efficiency of Gram-Positive and Gram-Negative Bacteria Using a Natural Coagulant during Coagulation, Flocculation, and sedimentation Processes. Water Sci. Technol. 2019, 80, 1787–1795. [Google Scholar] [CrossRef] [PubMed]
- Xiangli, Q.; Zhenjia, Z.; Nongcun, W.; Wee, V.; Low, M.; Loh, C.; Hing, N.T. Coagulation Pretreatment for a Large-Scale Ultrafiltration Process Treating Water from the Taihu River. Desalination 2008, 230, 305–313. [Google Scholar] [CrossRef]
- Pfannes, K.R.; Langenbach, K.M.W.; Pilloni, G.; Stührmann, T.; Euringer, K.; Lueders, T.; Neu, T.R.; Müller, J.A.; Kästner, M.; Meckenstock, R.U. Selective elimination of Bacterial Faecal Indicators in the Schmutzdecke of Slow sand Filtration Columns. Appl. Microbiol. Biotechnol. 2015, 99, 10323–10332. [Google Scholar] [CrossRef]
- Hembach, N.; Alexander, J.; Hiller, C.; Wieland, A.; Schwartz, T. Dissemination Prevention of Antibiotic Resistant and Facultative Pathogenic Bacteria by Ultrafiltration and Ozone Treatment at an Urban Wastewater Treatment Plant. Sci. Rep. 2019, 9, 12843. [Google Scholar] [CrossRef]
- Mota, V.C.; Brenne, H.; Kojen, M.; Marhaug, K.R.; Jakobsen, M.E. Evaluation of an ultrafiltration membrane for the removal of fish viruses and bacteria in aquaculture water. Front. Mar. Sci. 2022, 9, 1037017. [Google Scholar] [CrossRef]
- Ersoy, Z.G.; Barisci, S.; Dinc, O. Mechanisms of the Escherichia coli and Enterococcus Faecalis Inactivation by Ozone. LWT 2019, 100, 306–313. [Google Scholar] [CrossRef]
- Farrell, C.; Hassard, F.; Jefferson, B.; Leziart, T.; Nocker, A.; Jarvis, P. Turbidity Composition and the Relationship with Microbial Attachment and UV Inactivation Efficacy. Sci. Total. Environ. 2018, 624, 638–647. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Nan, J. Study on the Impact of Particle Size Distribution on Turbidity in Water. Desalination Water Treat. 2012, 41, 26–34. [Google Scholar] [CrossRef]
- Mundi, G.S.; Zytner, R.G.; Warriner, K. Fruit and Vegetable Wash-Water Characterization, Treatment Feasibility Study and Decision Matrices. Can. J. Civ. Eng. 2017, 44, 971–983. [Google Scholar] [CrossRef]
- Gombas, D.; Luo, Y.; Brennan, J.; Shergill, G.; Petran, R.; Walsh, R.; Hau, H.; Khurana, K.; Zomorodi, B.; Rosen, J.; et al. Guidelines To Validate Control of Cross-Contamination during Washing of Fresh-Cut Leafy Vegetables. J. Food Prot. 2017, 80, 312–330. [Google Scholar] [CrossRef] [PubMed]






| Tested Microorganism | Unit | Sample ID | |||||
|---|---|---|---|---|---|---|---|
| S | C | P | |||||
| A * | B * | A | B | A | B | ||
| Escherichia coli | cfu/100 mL | 1.3 × 101 | n.d. | 1.2 × 102 | n.d. | n.d. | n.d. |
| Enterobacteriaceae family | cfu/100 mL | 1.2 × 102 | n.d. | 1.8 × 103 | n.d. | n.d. | n.d. |
| Staphylococcus spp. | 1.5 × 101 | n.d. | 2.1 × 102 | n.d. | 1.5 × 101 | n.d. | |
| Staphylococcus aureus | cfu/100 mL | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Pseudomonas aeruginosa | cfu/100 mL | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Legionella spp. | cfu/100 mL | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Enterococcus spp. | cfu/100 mL | 2.24 × 102 | n.d. | 2.2 × 102 | n.d. | 2.7 × 102 | n.d. |
| Salmonella spp. | cfu/100 mL | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Total number of microorganisms, 36 ± 2 °C after 48 h | cfu/1 mL | 1.6 × 104 | n.d. | 1.1 × 104 | n.d. | 5.1 × 104 | n.d. |
| Organism (Best Match) | Score Value | Organism (Second-Best Match) | Score Value |
|---|---|---|---|
| Myroides odoratimimus | 2.35 | Myroides odoratimimus | 2.30 |
| Enterococcus durans | 2.47 | Enterococus durans | 2.37 |
| Serratia fonticola | 2.38 | Serratia fonticola | 2.38 |
| Raoultella ornithinolytica | 1.93 | Enterobacter kobei | 1.87 |
| Bacillus pumilus | 1.79 | No organism identification possible | 1.67 |
| Staphylococcus xylosus | 2.10 | No organism identification possible | 1.59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanarek, P.; Breza-Boruta, B.; Poćwiardowski, W.; Szulc, J. Sustainable Water Use in a Fruit Processing Plant: Evaluation of Microbiological and Physicochemical Properties of Wash Water after Application of a Modular Water Recovery System. Sustainability 2024, 16, 2181. https://doi.org/10.3390/su16052181
Kanarek P, Breza-Boruta B, Poćwiardowski W, Szulc J. Sustainable Water Use in a Fruit Processing Plant: Evaluation of Microbiological and Physicochemical Properties of Wash Water after Application of a Modular Water Recovery System. Sustainability. 2024; 16(5):2181. https://doi.org/10.3390/su16052181
Chicago/Turabian StyleKanarek, Piotr, Barbara Breza-Boruta, Wojciech Poćwiardowski, and Joanna Szulc. 2024. "Sustainable Water Use in a Fruit Processing Plant: Evaluation of Microbiological and Physicochemical Properties of Wash Water after Application of a Modular Water Recovery System" Sustainability 16, no. 5: 2181. https://doi.org/10.3390/su16052181





