Wool Agro-Waste Biomass and Spruce Sawdust: Pellets as an Organic Soil Amendment
Abstract
:1. Introduction
2. Materials and Method
2.1. Raw Materials, Grinding, and Preparation of the Pelleting Tests
2.1.1. Wool
2.1.2. Spruce Sawdust
2.1.3. Material Humidity, Density and Weight
2.1.4. Wool Grinding
2.2. Pelletizing Process
2.3. Physical Characterization of the Pellets
2.4. Microbiological and Chemical Analysis
2.5. Data Analysis
3. Results
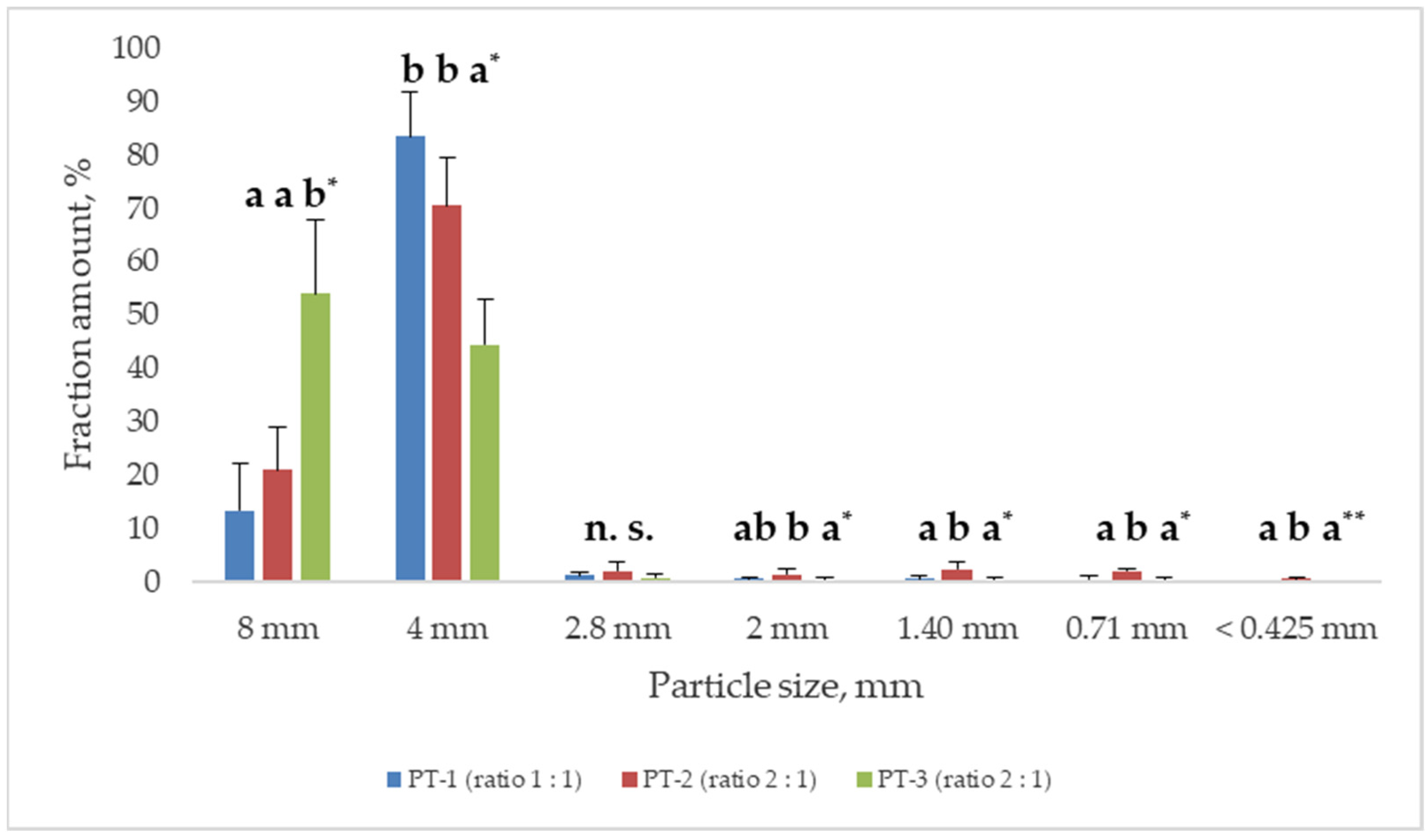
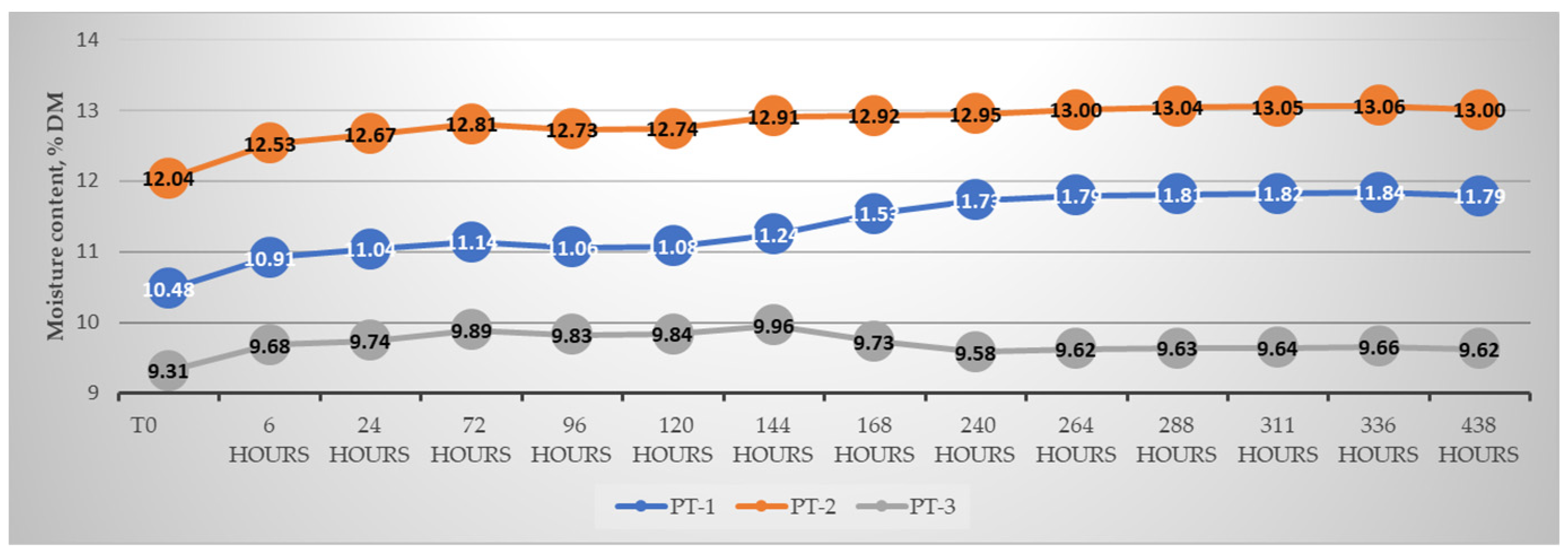
3.1. Raw Materials, Grinding, and Pellet Characterization
3.2. Microbiological and Chemical Analysis
4. Discussion
Pellet Characterization
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anas, M.; Liao, F.; Verma, K.K.; Sarwar, M.A.; Mahomood, A.; Chen, Z.; Zeng, X.; Liu, Y.; Li, Y. Fate of nitrogen in agriculture and environment: Agronomic, eco-physiological and molecular approaches to improve nitrogen use efficiency. Biol. Res. 2020, 53, 47. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zou, T.; Lassaletta, L.; Mueller, D.A.; Tubiello, F.N.; Lisk, M.D.; Lu, C.; Conant, R.T.; Dorich, C.D.; Gerber, J.; et al. Quantification of global and national nitrogen budgets for crop production. Nat. Food 2021, 2, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Cao, X.; Zhang, M.; Wei, X.; Zhang, J.; Wan, X. Plant nitrogen availability and crosstalk with phytohormones signallings and their biotechnology breeding application in crops. Plant Biotechnol. J. 2023, 21, 1320–1342. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Tian, H. Global nitrogen and phosphorus fertilizer use for agriculture production in the past half century: Shifted hot spots and nutrient imbalance. Earth Syst. Sci. 2017, 9, 181–192. [Google Scholar] [CrossRef]
- IEA. Nitrogen Demand by End Use and Scenario, 2020–2050; IEA: Paris, France, 2021; Available online: https://www.iea.org/data-and-statistics/charts/nitrogen-demand-by-end-use-and-scenario-2020-2050 (accessed on 28 September 2023).
- Montanarella, L.; Panagos, P. The relevance of sustainable soil management within the European Green Deal. Land Use Policy 2021, 100, 104950. [Google Scholar] [CrossRef]
- Reichel, R.; Wei, J.; Islam, M.S.; Schmid, C.; Wissel, H.; Schröder, P.; Schloter, M.; Brüggemann, N. Potential of Wheat Straw, Spruce Sawdust, and Lignin as High Organic Carbon Soil Amendments to Improve Agricultural Nitrogen Retention Capacity: An Incubation Study. Front. Plant Sci. 2018, 9, 900. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, S.; Bardoux, G.; Abbadie, L.; Mariotti, A. Carbon input to soil may decrease soil carbon content. Ecol. Lett. 2004, 7, 314–320. [Google Scholar] [CrossRef]
- European Parliament Report on the Current Situation and Future Prospects for the Sheep and Goat Sectors in the EU. Available online: https://www.europarl.europa.eu/doceo/document/A-8-2018-0064_EN.html (accessed on 28 September 2023).
- Eurostat. Sheep Population—Annual Data. Available online: https://ec.europa.eu/eurostat/databrowser/view/APRO_MT_LSSHEEP (accessed on 28 September 2023).
- Commission Regulation (EU) No 142/2011 of 25 February 2011 Implementing Regulation (EC) No 1069/2009 of the European Parliament and of the Council Laying down Health Rules as Regards Animal By-Products and Derived Products Not Intended for Human Consumption and Implementing Council Directive 97/78/EC as Regards Certain Samples and Items Exempt from Veterinary Checks at the Border under That Directive Text with EEA Relevance. Available online: https://eur-lex.europa.eu/eli/reg/2011/142/oj (accessed on 28 September 2023).
- Camilli, F.; Crisci, A.; Mauro, A.; Bacci, L.; Di Lonardo, S.; Vagnoni, E.; Duce, P. A Preliminary Characterization of Wools from Italian Native Sheep Breeds: Opportunities for New Productions and the Development of Rural Areas. J. Nat. Fibers 2015, 12, 265–275. [Google Scholar] [CrossRef]
- Parlato, M.C.M.; Porto, S.M.C. Organized Framework of Main Possible Applications of Sheep Wool Fibers in Building Components. Sustainability 2020, 12, 761. [Google Scholar] [CrossRef]
- Decision (EU) 2022/591 of the European Parliament and of the Council of 6 April 2022 on a General Union Environment Action Programme to 2030. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32022D0591&qid=1695984091649 (accessed on 28 September 2023).
- Rawson, T. A hierarchical Bayesian quantitative microbiological risk assessment model for Salmonella in the sheep meat food chain. Food Microbiol. 2022, 104, 103975. [Google Scholar] [CrossRef]
- Górecki, R.S.; Górecki, M.T. Utilization of waste wool as substrate amendment in pot cultivation of tomato, sweet pepper, and eggplant. Pol. J. Environ. Stud. 2010, 19, 1083–1087. [Google Scholar]
- Böhme, M.; Pinker, I.; Grüneberg, H.; Herfort, S. Sheep wool as fertilizer for vegetables and flowers in organic farming. Acta Hortic. 2012, 933, 195–202. [Google Scholar] [CrossRef]
- Abdallah, A.M.; Ugolini, F.; Baronti, S.; Maienza, A.; Ungaro, F.; Camilli, F. Assessment of Two Sheep Wool Residues from Textile Industry as Organic Fertilizer in Sunflower and Maize Cultivation. J. Soil Sci. Plant Nutr. 2019, 19, 793–807. [Google Scholar] [CrossRef]
- Abdallah, A.M.; Ugolini, F.; Baronti, S.; Maienza, A.; Camilli, F.; Bonora, L.; Martelli, F.; Primicerio, J.; Ungaro, F. The potential of recycling wool residues as an amendment for enhancing the physical and hydraulic properties of a sandy loam soil. Int. J. Recycl. Org. Waste Agric. 2019, 8, 131–143. [Google Scholar] [CrossRef]
- Broda, J.; Gawlowski, A.; Rom, M.; Kobiela-Mendrek, K. Utilisation of waste wool from mountain sheep as fertiliser in winter wheat cultivation. J. Nat. Fibers 2023, 20, 2. [Google Scholar] [CrossRef]
- Gousterova, A.; Nustorova, M.; Goshev, I.; Christov, P.; Braikova, D.; Tishinov, K.; Haertlé, T.; Nedkov, P. Alkaline hydrolysate of waste sheep wool aimed as fertilizer. Biotechnol. Biotechnol. Equip. 2003, 17, 140–145. [Google Scholar] [CrossRef]
- Gogos, A.; Schäffer, A.; Schulin, R. Hydrolysed wool: A novel soil amendment for zinc and iron biofortification of wheat. N. Z. J. Agric. Res. 2013, 56, 130–141. [Google Scholar] [CrossRef]
- Bradshaw, T.; Hagen, K. Wool Pellets Are a Viable Alternative to Commercial Fertilizer for Organic Vegetable Production. Agronomy 2022, 12, 1210. [Google Scholar] [CrossRef]
- Kostov, O.; Rankov, V.; Atanacova, G.; Lynch, J.M. Decomposition of sawdust and bark treated with cellulose-decomposing microorganisms. Biol. Fertil. Soils. 1991, 11, 105–110. [Google Scholar] [CrossRef]
- Tocci, R.; Pippi, E.; Campostrini, M.; Martini, A.; Bozzi, R.; Benvenuti, D.; Bonelli, A.; Pezzati, A.; Sargentini, C. Characterization of meat from Pecora dell’Amiata and Pomarancina light lamb slaughtered at 20 kg of live weight. Large Anim. Rev. 2017, 23, 131–140. [Google Scholar]
- PN-EN ISO 17828:2016-02; Solid Biofuels—Determination of Bulk Density. ISO: Geneva, Switzerland, 2016.
- PN-R-64798:2009; Feed—Determination of Fragmentation. Polish Committee for Standardization: Warsaw, Poland, 2009.
- Obidziński, S.; Joka Yildiz, M.; Dąbrowski, S.; Jasiński, J.; Czekała, W. Application of Post-Flotation Dairy Sludge in the Production of Wood Pellets: Pelletization and Combustion Analysis. Energies 2022, 15, 9427. [Google Scholar] [CrossRef]
- ISO/IEC 17025:2017; General Requirements for the Competence of Testing and Calibration Laboratories. Available online: https://www.iso.org/standard/66912.html (accessed on 28 September 2023).
- ISO 15213-2:2023; Microbiology of the Food Chain Horizontal Method for the Detection and Enumeration of Clostridium spp. Available online: https://www.iso.org/standard/36588.html (accessed on 28 September 2023).
- UNI ISO 16649-2:2010; Microbiologia di Alimenti e Mangimi per Animali—Metodo Orizzontale per la Conta di Escherichia coli beta Glucuronidasi-Positiva—Parte 2: Tecnica Della Conta Delle Colonie a 44 °C che Utilizza 5-bromo-4-cloro-3-Indolil Beta-D-Glucuronide. Available online: https://store.uni.com/uni-iso-16649-2-2010 (accessed on 28 September 2023).
- BS EN ISO 11290-1:2017; Microbiology of the Food Chain. Horizontal Method for the Detection and Enumeration of Listeria monocytogenes and of Listeria spp. Detection Method. Available online: https://www.en-standard.eu/bs-en-iso-11290-1-2017-microbiology-of-the-food-chain-horizontal-method-for-the-detection-and-enumeration-of-i-listeria-i-i-monocytogenes-i-and-of-i-listeria-i-spp-detection-method/?msclkid=c3d15f18fd611c8d55183af0aa15111b (accessed on 28 September 2023).
- UNI EN ISO 4833-1:2022; Microbiologia Della Catena Alimentare—Metodo Orizzontale per la Conta dei Microrganismi—Parte 1: Conta Delle Colonie a 30 °C con la Tecnica Dell’inseminazione in Profondità. Available online: https://store.uni.com/p/UNI1610703/uni-en-iso-4833-1-2022/UNI1610703. (accessed on 28 September 2023).
- ISO 6579-1:2017/Amd 1:2020; Microbiology of the Food Chain Horizontal Method for the Detection, Enumeration and Serotyping of Salmonella. Available online: https://www.iso.org/standard/76671.html (accessed on 28 September 2023).
- ISO 6888-2:2021; Microbiology of the Food Chain Horizontal Method for the Enumeration of Coagulase-Positive Staphylococci (Staphylococcus aureus and Other Species). Available online: https://www.iso.org/standard/76673.html (accessed on 28 September 2023).
- Castrica, M.; Menchetti, L.; Balzaretti, C.M.; Branciari, R.; Ranucci, D.; Cotozzolo, E.; Vigo, D.; Curone, G.; Brecchia, G.; Miraglia, D. Impact of Dietary Supplementation with Goji Berries (Lycium barbarum) on Microbiological Quality, Physico-Chemical, and Sensory Characteristics of Rabbit Meat. Foods 2020, 9, 1480. [Google Scholar] [CrossRef] [PubMed]
- Di Gioia, F.; De Bellis, P.; Mininni, C.; Santamaria, P.; Serio, F. Physicochemical, agronomical and microbiological evaluation of alternative growing media for the production of rapini (Brassica rapa L.) microgreens. J. Sci. Food Agric. 2016, 97, 1212–1219. [Google Scholar] [CrossRef] [PubMed]
- Scatassa, M.L.; Cardamone, C.; Miraglia, V.; Lazzara, F.; Fiorenza, G.; Macaluso, G.; Arcuri, L.; Settanni, L.; Mancuso, I. Characterisation of the Microflora Contaminating the Wooden Vats Used for Traditional Sicilian Cheese Production. Ital. J. Food Saf. 2015, 4, 4509. [Google Scholar] [CrossRef] [PubMed]
- Rea, S.; Marino, L.; Stocchi, R.; Branciari, R.; Loschi, A.R.; Miraglia, D.; Ranucci, D. Differences in Chemical, Physical and Microbiological Characteristics of Italian Burrata Cheeses Made in Artisanal and Industrial Plants of Apulia Region. Ital. J. Food Saf. 2016, 5, 5879. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 36, 27–31. [Google Scholar] [CrossRef]
- Duca, D.; Maceratesi, V.; Fabrizi, S.; Toscano, G. Valorising Agricultural Residues through Pelletisation. Processes 2022, 10, 232. [Google Scholar] [CrossRef]
- Samuelsson, R.; Larsson, S.H.; Thyrel, M.; Lestander, T.A. Moisture content and storage time influence the binding mechanisms in biofuel wood pellets. Appl. Energy 2012, 99, 109–115. [Google Scholar] [CrossRef]
- Gilvari, H.; van Battum, C.H.H.; van Dijk, S.A.; de Jong, W.; Schott, D.L. Large-scale transportation and storage of wood pellets: Investigation of the change in physical properties. Particuology 2021, 57, 146–156. [Google Scholar] [CrossRef]
- Liu, Z.; Mi, B.; Jiang, Z.; Fei, B.; Cai, Z.; Liu, X. Improved bulk density of bamboo pellets as biomass for energy production. Renew. Energy 2016, 86, 1–7. [Google Scholar] [CrossRef]
- Yub Harun, N.; Parvez, A.M.; Afzal, M.T. Process and Energy Analysis of Pelleting Agricultural and Woody Biomass Blends. Sustainability 2018, 10, 1770. [Google Scholar] [CrossRef]
- Tumuluru, J.S. Effect of pellet die diameter on density and durability of pellets made from high moisture woody and herbaceous biomass. Carbon. Resour. Convers. 2018, 1, 44–54. [Google Scholar] [CrossRef]
- Leo Gallico. La lana, 2000. Edito da Eventi & Progetti Editore. Available online: https://www.hoepli.it/libro/la-lana-/9786001587849.html (accessed on 14 February 2024).
- Regione Emilia Romagna. Deliberazione della Giunta Regionale 16/06/99 n. 960. Available online: https://www.cittametropolitana.bo.it/imprese/Engine/RAServeFile.php/f/Suap/normativa/del_rer_960-1999.pdf (accessed on 1 March 2024).
- Ranford, S.; Swan, P.; van Koten, C. Chemical residue trends for Australian and New Zealand wool. Sci. Rep. 2022, 12, 768. [Google Scholar] [CrossRef]
- Bhavsar, P.; Zoccola, M.; Dalla Fontana, G.; Pallavicini, M.; Roda, G.; Bolchi, C. Sustainable Routes for Wool Grease Removal Using Green Solvent Cyclopentyl Methyl Ether in Solvent Extraction and Biosurfactant Wool Protein Hydrolyzate in Scouring. Processes 2023, 11, 1309. [Google Scholar] [CrossRef]
- Directive 2003/99/EC of the European Parliament and of the Council of 17 November 2003 on the Monitoring of Zoonoses and Zoonotic Agents, Amending Council Decision 90/424/EEC and Repealing Council Directive 92/117/EEC. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32003L0099 (accessed on 1 March 2024).
- Ronga, D.; Mantovi, P.; Pacchioli, M.T.; Pulvirenti, A.; Bigi, F.; Allesina, G.; Pedrazzi, S.; Tava, A.; Dal Prà, A. Combined Effects of Dewatering, Composting and Pelleting to Valorize and Delocalize Livestock Manure, Improving Agricultural Sustainability. Agronomy 2020, 10, 661. [Google Scholar] [CrossRef]



| Agent | Method |
|---|---|
| Clostridium perfringens (bacterial count) | ISO 15213-2:2023 [30] |
| Escherichia coli (Β-glucuronidase producing strains) | UNI ISO 16649-2: 2010 [31] |
| Listeria monocytogenes | UNI EN ISO 11290-1: 2017 [32] |
| Total bacterial count (30 °C) | UNI EN ISO 4833-1:2022 [33] |
| Salmonella spp. | ISO 6579-1:2017/Amd 1:2020 [34] |
| Coagulase positive Staphylococcus | ISO 6888-2:2021 [35] |
| Items | PT-1 (Ratio 1:1) | PT-2 (Ratio 2:1) | PT-3 (Ratio 2:1) | Mean | |
|---|---|---|---|---|---|
| Mean (SE) | Mean (SE) | Mean (SE) | p-Value | ||
| DM, % | 90.80 | 89.61 | 88.12 | 89.51 | - |
| Bulk density (a.p.p.), (Kg·m−3) | 475.67 (12.89) | 458.12 (13.22) | 397.42 (16.90) | 444.07 | n.s. |
| 1.000-pellet weight, g | 464.14 (1.87) b | 496.40 (1.08) a | 501.05 (1.07) a | 487.20 | 0.01 |
| Proximal diameter, mm | 3.64 (0.08) | 3.70 (0.09) | 3.82 (0.09) | 3.72 | n.s. |
| Distal diameter, mm | 3.71 (0.10) | 4.21 (0.09) | 3.91 (0.09) | 3.94 | n.s. |
| Diameter, ratio | 1.02 (0.41) | 1.13 (0.13) | 1.02 (0.01) | 1.06 | n.s. |
| Length, mm | 17.06 a (0.71) | 16.95 a (0.99) | 20.87 b (0.99) | 18.28 | 0.04 |
| Items | Wool | Mixture 1:1 | Mixture 2:1 | PT-1 | PT-2 | PT-3 |
|---|---|---|---|---|---|---|
| Clostridium perfringens, CFU | <1.00 × 101 | <1.50 × 102 | <1.00 × 101 | <1.00 × 101 | <1.00 × 101 | <1.00 × 101 |
| E. coli beta-glucuronidase positive test, CFU | <1.00 × 101 | <5.90 × 102 | <5.70 × 104 | <1.00 × 101 | <1.00 × 101 | <1.00 × 101 |
| Listeria monocytogenes | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Total viable count (TVC), CFU | <3.20 × 105 | <2.20 × 104 | <1.20 × 107 | <3.50 × 103 | <1.00 × 101 | <1.00 × 101 |
| Salmonella spp., CFU | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Coagulase-positive staphylococcus (CPS), CFU | <1.00 × 101 | <1.00 × 101 | <1.00 × 101 | <1.00 × 101 | <1.00 × 101 | <1.00 × 101 |
| Items | PT-1 (Ratio 1:1) | PT-2 (Ratio 2:1) | PT-3 (Ratio 2:1) | Mean | |
|---|---|---|---|---|---|
| Mean ± s.d. (CV) | p-Value | ||||
| Total N, % DM | 1.79 ± 0.05 (2.64) a | 1.26 ± 0.02 (1.85) b | 1.71 ± 0.04 (2.37) a | 1.58 | 0.002 |
| TOC, % DM | 44.43 ± 2.58 (5.82) | 44.04 ± 3.20 (7.28) | 43.89 ± 1.23 (2.81) | 44.11 | n.s. |
| Ca, mg kg−1 | 1011.5 ± 33.23 (3.28) b | 1225.5 ± 33.23 (2.71) a | 962.00 ± 28.28 (2.94) b | 1066.33 | 0.007 |
| Cu, mg kg−1 | n.d. | 2.49 ± 0.08 (3.41) a | 1.71 ± 0.42 (3.67) b | 1.40 | <0.001 |
| Fe, mg kg−1 | 144.50 ± 3.54 (2.45) c | 293.00 ± 11.31 (3.86) a | 173.50 ± 6.36 (3.67) b | 203.67 | <0.001 |
| K, mg kg−1 | 6689.00 ± 117.38 (1.75) a | 6687.00 ± 120.21 (1.76) a | 5769.50 ± 116.67 (2.02) b | 6428.50 | 0.005 |
| Mg, mg kg−1 | 231.00 ± 2.83 (1.22) b | 222.00 ± 5.66 (2.55) b | 258.00 ± 9.90 (3.84) a | 338.00 | 0.027 |
| Mn, mg kg−1 | 70.70 ± 2.40 (3.40) b | 81.40 ± 1.56 (1.91) a | 69.10 ± 3.11 (4.50) b | 73.73 | 0.027 |
| Na, mg kg−1 | 58.20 ± 3.96 (6.80) b | 72.30 ± 3.11 (4.30) a | 58.80 ± 2.26 (3.84) b | 63.10 | 0.035 |
| Zn, mg kg−1 | 15.80 ± 0.28 (1.79) b | 18.30 ± 0.71 (3.86) a | 14.50 ± 0.42 (2.93) b | 16.20 | 0.011 |
| P, mg kg−1 | 95.80 ± 1.84 (1.92) a | 33.30 ± 2.40 (7.22) c | 51.40 ± 1.31 (2.20) b | 60.17 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dal Prà, A.; Ugolini, F.; Negri, M.; Bortolu, S.; Duce, P.; Macci, C.; Lombardo, A.; Benedetti, M.; Brajon, G.; Guazzini, L.; et al. Wool Agro-Waste Biomass and Spruce Sawdust: Pellets as an Organic Soil Amendment. Sustainability 2024, 16, 2228. https://doi.org/10.3390/su16062228
Dal Prà A, Ugolini F, Negri M, Bortolu S, Duce P, Macci C, Lombardo A, Benedetti M, Brajon G, Guazzini L, et al. Wool Agro-Waste Biomass and Spruce Sawdust: Pellets as an Organic Soil Amendment. Sustainability. 2024; 16(6):2228. https://doi.org/10.3390/su16062228
Chicago/Turabian StyleDal Prà, Aldo, Francesca Ugolini, Martino Negri, Sara Bortolu, Pierpaolo Duce, Cristina Macci, Andrea Lombardo, Martina Benedetti, Giovanni Brajon, Lucia Guazzini, and et al. 2024. "Wool Agro-Waste Biomass and Spruce Sawdust: Pellets as an Organic Soil Amendment" Sustainability 16, no. 6: 2228. https://doi.org/10.3390/su16062228








