Abstract
The biodiesel industry is a promising field globally, and is expanding significantly and quickly. To create a biodiesel business that is both sustainable and commercially feasible, a number of studies have been conducted on the use of non-edible oils to produce biodiesel. Thus, this study highlights biodiesel synthesis from non-edible plant oils such as pongamia and jatropha using a glycerol separation technique with an AC high voltage method through the transesterification reaction. In this context, non-edible plant oil has emerged as an alternative with a high potential for making the biodiesel process sustainable. Moreover, the study introduces how the created biodiesel fuel behaves when burned in a diesel engine. The results showed that the optimum conditions for creating biodiesel were a temperature of 60 °C, a potassium hydroxide catalyst percentage by weight of oils of 1%, and a stirring time of 60 min at a 5:1 (v/v) ratio of methanol to oil. A high-voltage procedure was used to separate glycerol and biodiesel using two electrodes of copper with different distances between them and different high voltages. The results showed that, for a batch of 15 L, the minimum separating time was 10 min when the distance between the copper electrodes was 2.5 cm, and the high voltage was 15 kV. The density, kinematic viscosity, and flash point of jatropha oil were reduced from 0.920 to 0.881 g/cm3 at 15 °C, from 37.1 to 4.38 cSt at 40 °C, and from 211 to 162 °C, respectively, for the production of biodiesel. Additionally, the density, kinematic viscosity, and flash point of pongamia oil were reduced from 0.924 to 0.888 g/cm3 at 15 °C, from 27.8 to 5.23 cSt at 40 °C, and from 222 to 158 °C, respectively, for the production of biodiesel. The calorific value of jatropha oil was increased from 38.08 to 39.65 MJ/kg for the production of biodiesel, while that of pongamia oil was increased from 36.61 to 36.94 MJ/kg. The cetane number increased from 21 for oil to 50 for biodiesel and from 32 for oil to 52 for jatropha and pongamia biodiesel, respectively. In order to run an air-cooled, single-cylinder, four-stroke diesel engine at full load, the produced biodiesel fuel was blended with diesel fuel at different percentages—10, 20, and 30%—for jatropha and pongamia methyl esters. The produced engine power values were 3.91, 3.69, and 3.29 kW for B10, B20, and B30, respectively, compared with the engine power value of jatropha methyl ester, which was 4.12 kW for diesel fuel (B00); meanwhile, the values were 3.70, 3.36, and 3.07 kW for B10, B20 and B30, respectively, for pongamia methyl ester. The findings suggest that the biodiesel derived from non-edible oils, such as pongamia and jatropha, could be a good alternative to diesel fuel.
1. Introduction
The primary energy source in the world today is fossil fuels. They are extensively utilized as raw quantifiable materials in the manufacturing of petrochemical goods and to serve a variety of industries, including transportation, agriculture, and household products [1]. The world may soon face an energy crisis based on the current energy situation. The rising global population and fast pace of economic development mean that energy consumption continues to rise, making the energy problem worse every day [2]. Frying oils, vegetable oils, animal fats, and oil created by microorganisms can all be used to make biodiesel [3,4]. However, there are a number of benefits to using second-generation biodiesel made from essential oil plants, such as its ability to facilitate high biofuel production and to lower greenhouse gas emissions [5]. The first stage in the manufacturing of biodiesel, however, is the selection of the feedstock, which requires the consideration of a number of attributes, including the composition, purity of the fuel, yield, and cost. The primary criteria for classifying biodiesel into edible, non-edible, and unused-based origins are obtainability and the kind of feedstock source used [6].
Oilseed plants called jatropha are grown in marginal semi-arid regions. Scrub can be harvested twice a year, is often disliked by cattle, and can continue to be productive for thirty to fifty years. Seeds can be obtained from the plant a year after planting, and productivity peaks after five years [7].
Pongamia (Pinnata (Karanja)) is a member of the Leguminaceae family. It is able to withstand severe weather and is widely distributed over marginal fields, riverbanks, and coastal regions. Flowering begins after 4–5 years. It is a semi-deciduous leguminous tree that fixes nitrogen and is resistant to drought [8]. The Karanja tree grows quickly, its fruits ripen 4–7 years after planting [9], and 9 to 90 kg of seeds can be produced over a period of 4 to 6 years [8].
The oil content of jatropha seeds ranges from 27 to 59% [10,11]. The major fatty acids found in the seed oil are linoleic, oleic, stearic, and palmitic acids, comprising 22.5% saturated fatty acids and 77.5% unsaturated fatty acids [12]. Amounts of 46% of oleic acid, 27% of linoleic acid, 6% of linolenic acid, and 0.1% of low-molecular-weight fatty acids, including lauric and capric acids, are found in pongamia seeds [13]. Because it can grow in a variety of soil types, pongamia possesses non-edible oil and tremendous potential for producing biodiesel [14]. The primary barriers to using vegetable oils directly as fuel are their higher molecular weights, higher viscosities, poor cold flow characteristics, low volatilities, and tendencies to form deposits due to poor combustion [15].
Since alkali catalysts require little reaction time, even at room temperature, they are frequently utilized for the transesterification of oils [16]. Bronsted acids, such as sulfonic acid and sulfuric acid, are another class of catalysts employed in transesterification. As explained in [17], transesterification is carried out using an acid catalyst if the amount of free fatty acids (FFAs) is greater than 1%. This reaction requires a lot of alcohol and the use of regulated conditions for a long time, including a high temperature of 100 °C and a pressure of 5 bar [18], lowering the triglycerides’ acid values as a result, and subsequently permitting transesterification catalyzed by alkali. Moreover, the yield is frequently greater than 95% when biodiesel is created by transesterifying triglycerides, the primary component of vegetable oils, with alcohol and an alkali catalyst at low temperatures and pressures [19]. After an ideal reaction period of 0.5–1.0 h, the separation of biodiesel from the glycerol by-product indicates a successful transesterification reaction [20,21,22]. Following transesterification processes, the biodiesel layer must be removed from the glycerol, and additional purification is required [23]. Since glycerol (1050 kg/m3) and biodiesel (approximately 880 kg/m3) have sufficiently different densities and are not mutually soluble, simple and straightforward separation techniques like centrifugation and gravitational settling can be applied [24,25]. Typically, there are traces of catalyst, glycerol, oil and its contaminants, and alcohol in the biodiesel layer [26]. Palm oil, a common raw material in the food sector, has been used to create biodiesel from fatty acids. With a molar ratio of 1:6, methyl alcohol is a solvent in which 1 weight percent of potassium hydroxide (KOH) can be used as a catalyst. A reactor chamber was used to prepare the 100 cc of substrate needed for the biodiesel synthesis reaction. The coaxial cylindrical electrode in a specially constructed chamber was made up of an outer tube electrode and an inner rod electrode. In order to compare the products with a control sample devoid of an electric field, exposure times of 5, 10, 15, and 20 min were chosen, and high voltage levels of 1.0, 2.5, and 5.0 kV were used. It was discovered that an electric field can significantly accelerate the reaction for the synthesis of biodiesel [27].
Low-quality fuel that has not been adequately purified causes serious engine issues, such as filter blockage, coking on injectors, high carbon deposits, extreme engine wear, engine banging, the thickening of lubricating oil, and the formation of lubricating oil deposits [6]. Numerous factors, including the quality of the raw materials, the composition of the fatty acids, the production process, the refining procedure, and the final production parameters, influence the fuel qualities of biodiesel. Biodiesel’s fuel qualities can be categorized using a variety of factors. The low-temperature properties (cloud point, pour point, cold filter plugging point, etc.); transport and storage properties (microbial contamination, induction period, oxidation and hydrolytic stability, flash point, temperature limit infiltration, etc.); and wear of engine parts (cleaning effect, lubrication, viscosity, compatibility with materials used in fuel system production, etc.) are the factors that have the greatest effects on engine events [28].
The three main facets of sustainability that are addressed by global biodiesel issues are social, environmental, and economic [29]. Profit maximization and reduced manufacturing costs are the main goals of economic sustainability. This calls for the development of new, ideally inedible raw materials and the application of waste- and energy-saving technology [29]. The reduced environmental burden is the main component of environmental sustainability. Employment and the utilization of local resources for the benefit of the community are the two main components of social sustainability. The studies that have been examined indicate that microalgae, fat, oils, and grease; various solid wastes; and other non-edible raw materials are viable and promising biodiesel substitutes [29]. Therefore, this study highlights biodiesel synthesis from non-edible plant oils such as pongamia and jatropha using a glycerol separation technique with an AC high voltage method through the transesterification reaction. Moreover, the objective of the experimental work presented in this paper was to evaluate and determine the ideal operating parameters for the transesterification of two non-food oils, pongamia and jatropha, as well as how these parameters affected the characteristics of the biodiesel that was produced. In addition, the study introduces how the created biodiesel fuel behaves when burned in a diesel engine.
2. Materials and Methods
2.1. A Pilot Plant for Producing Biodiesel and Experimental Location
The biodiesel production experiments were performed at the biodiesel laboratory situated at the Tractors and Farm Machinery Testing and Research Station, Alexandria Governorate, Egypt. To produce biodiesel from various vegetable oils, a prototype biodiesel plant was built locally. As shown in Figure 1, it was made up of a dry washing unit, a high voltage unit, a chemical mixing tank with an agitating pump, and a processor with a heater and pump.

Figure 1.
A pilot plant for producing biodiesel.
2.2. Raw Materials and Procedures
From the seeds of pongamia and jatropha crops, two non-edible oils were isolated (Figure 2). Depending on their free fatty acid contents, these oils can be easily turned into biodiesel utilizing a one-step or two-step procedure. Two complementary processes, mechanical and chemical, were used together to extract the oils. Pongamia pinata and jatropha curcas seeds were ground, roasted, and squeezed to obtain the oil. Solid contaminants were removed from the oil by filtering it. The ASTM D-1983 [30] test technique was used to assess the fatty acid compositions of jatropha and pongamia oils. Gas chromatography (Hewlett Packard/6890 GC) was used, along with nitrogen as a carrier gas and a flame ionization detector (FID) (Figure 3). A two-step method called base transesterification followed by acid esterification was used to create biodiesel. Methanol alcohol (CH3OH), 99%+ pure, was used as a reagent; when using H2SO4 and KOH as catalysts, they were required to be dry for acid and base reactions. The jatropha and pongamia oils were heated at 105 °C for 30 min to eliminate moisture, and then the temperature was reduced.

Figure 2.
Pongamia and jatropha seeds.

Figure 3.
GC-TSQ mass spectrometer (Thermo Scientific, Austin, TX, USA).
2.2.1. Esterification
Before directly transesterifying oils, esterification is crucial to determine the oils’ FFA contents. If the FFA percentage is more than 2%, the production of biodiesel will be reduced. Therefore, it is usually advised to perform the esterification procedure, which works as a catalyst to neutralize the oil and aid in minimizing the FFA content. In the current work, the FFA concentration was lowered such that methanol could react with jatropha and pongamia oils in the presence of the H2SO4 acid catalyst. The ratio of methanol to oil was 5:1.
2.2.2. Transesterification
Pongamia and jatropha oils can be transformed into biodiesel using the transesterification process. This involves swapping the organic group R″ of an alcohol for the organic group R″ of an ester. The process comprises numerous sequential reversible reactions. In these reactions, triglycerides are gradually transformed into diglycerides, monoglycerides, and glycerol, which float on top and sink to the bottom. The principle of the transesterification process is shown in Zhang et al. [31].
2.2.3. Biodiesel Production
Biodiesel was produced according to the following steps:
- Alcohol (CH3OH) was mixed with KOH in the premix tank for about 10 min to form a homogenous solution of potassium methoxide (K+ −OCH3) and water (H2O);
- Jatropha or pongamia oil (30 L) was poured into the processor;
- An electric heater was used to heat the oil and prepare it for the reaction;
- In the processor tank, potassium methoxide was combined with heated vegetable oil, and the mixture was stirred for 60 min while the oil was circulated;
- The solution was left to settle through gravity for 10 h;
- After complete separation, glycerol was decanted from the bottom, and the biodiesel was washed to eliminate any impurities, such as methanol alcohol, soap, water, and glycerol.
To determine the optimum conditions for making biodiesel, some factors were varied, such as the reaction temperature (25, 30, 35, 40, 45, 50, 55, and 60 °C), stirring time (20, 30, 40, 50, and 60 min), and amount of catalyst (4.0, 4.5, 5.0, and 5.5 g), for the two seeds.
2.2.4. Time of Glycerol Separation
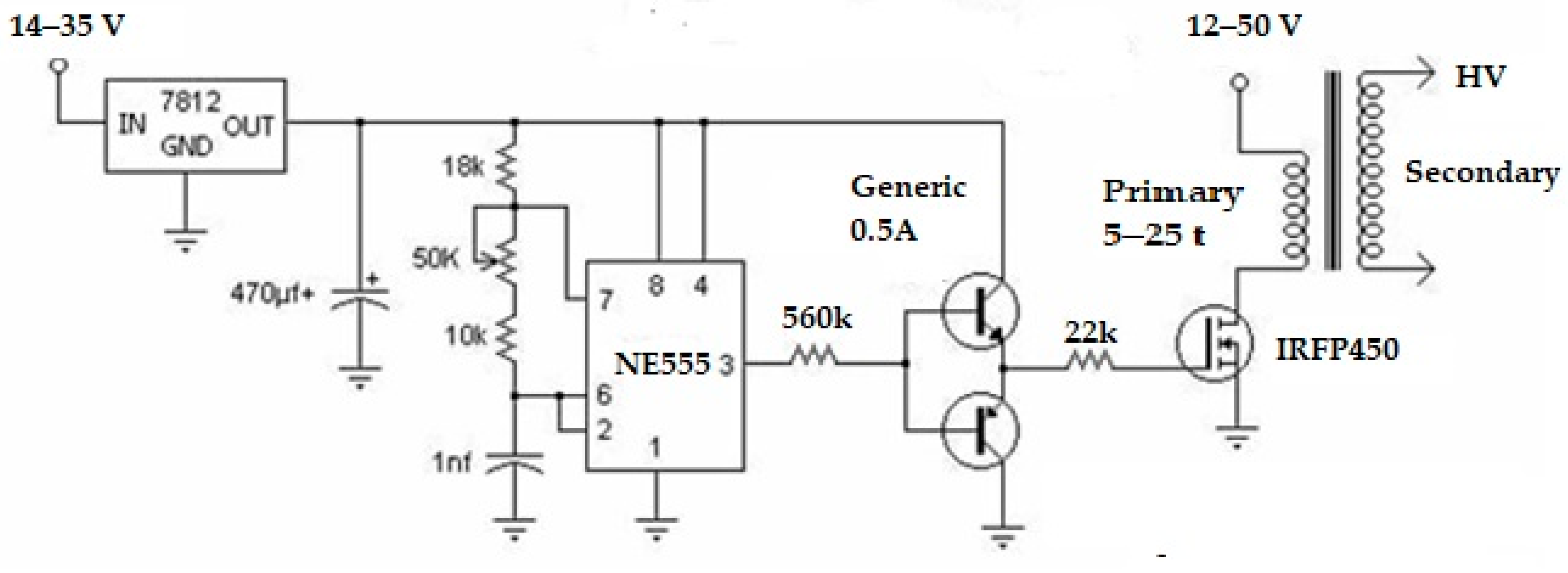
A high-voltage device was used to separate glycerol from oil, as this was faster than the gravity method. The core components of a high-voltage device are the power source and a high voltage (Figure 4). A power supply of 12 to 35 volts DC, with a 3A maximum current, was employed to feed the required electric power to the high-voltage portion. A flyback driver circuit, shown in Figure 4, was used to manufacture the high-voltage component. The circuit had a flyback transformer, ne555 transistor + heat sink, 2S547 PNP, 2S577 NPN transistor, 560-ohm 5-watt resistor, and 22-ohm 5-watt resistor. In an ideal transformer with a 1:600 turn ratio, a flyback driver circuit (Figure 5), sometimes referred to as a line, comprises principal turns with 10 laps and secondary turns with 6000 laps. Then, with a spacing of 2.5 cm between the copper electrodes, one might anticipate receiving a low amperage AC current (32 to 8 mA) and, possibly, a range of 6 kV to 21 kV with a 12 to 35V input voltage, respectively. Due to the risk of an arc forming, which could result in an explosion, the two electrodes must be completely submerged in the solution, especially when there is little space between them. Methanol vapor should also be avoided.

Figure 4.
High-voltage device.
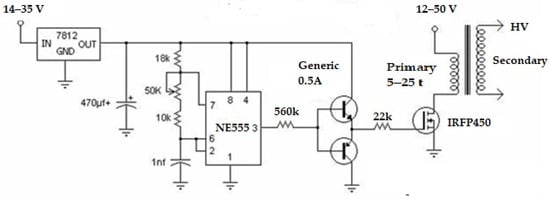
Figure 5.
Flyback driver circuit.
2.2.5. Dry Washing
The produced biodiesel was passed through two filters to eliminate any impurities. The filters were filled until they reached up to 2/3 of the total volume with sawdust, with a little press carried out by hand (Figure 6).

Figure 6.
Sawdust.
2.3. Measurements
In the laboratory of the Misr Petroleum Company in Egypt, the physical properties of diesel fuel (B00); B100 biodiesel; and B10, B20, and B30 blended biodiesels were measured using different attributes. The attributes are based on the ASTM (the American Standard for Biodiesel Testing Method, ASTM D6751) [32], which are D1298 for density kg/m3 at 15 °C; D445 for viscosity (cSt) at 40 °C mm2/s; D2500 for cloud point (°C); D93 for flash point (°C); D613 for cetane no.; and D240 for calorific value (MJ/L). These attributes were assessed using a UV-visible detector coupled with a high-pressure liquid chromatograph (HPLC, Shimadzu) to measure the quantity of free glycerol existing in the biodiesel trial (Figure 7).

Figure 7.
A high-pressure liquid chromatograph (HPLC, Shimadzu).
2.4. Performance of a Diesel Engine with the Created Biodiesel
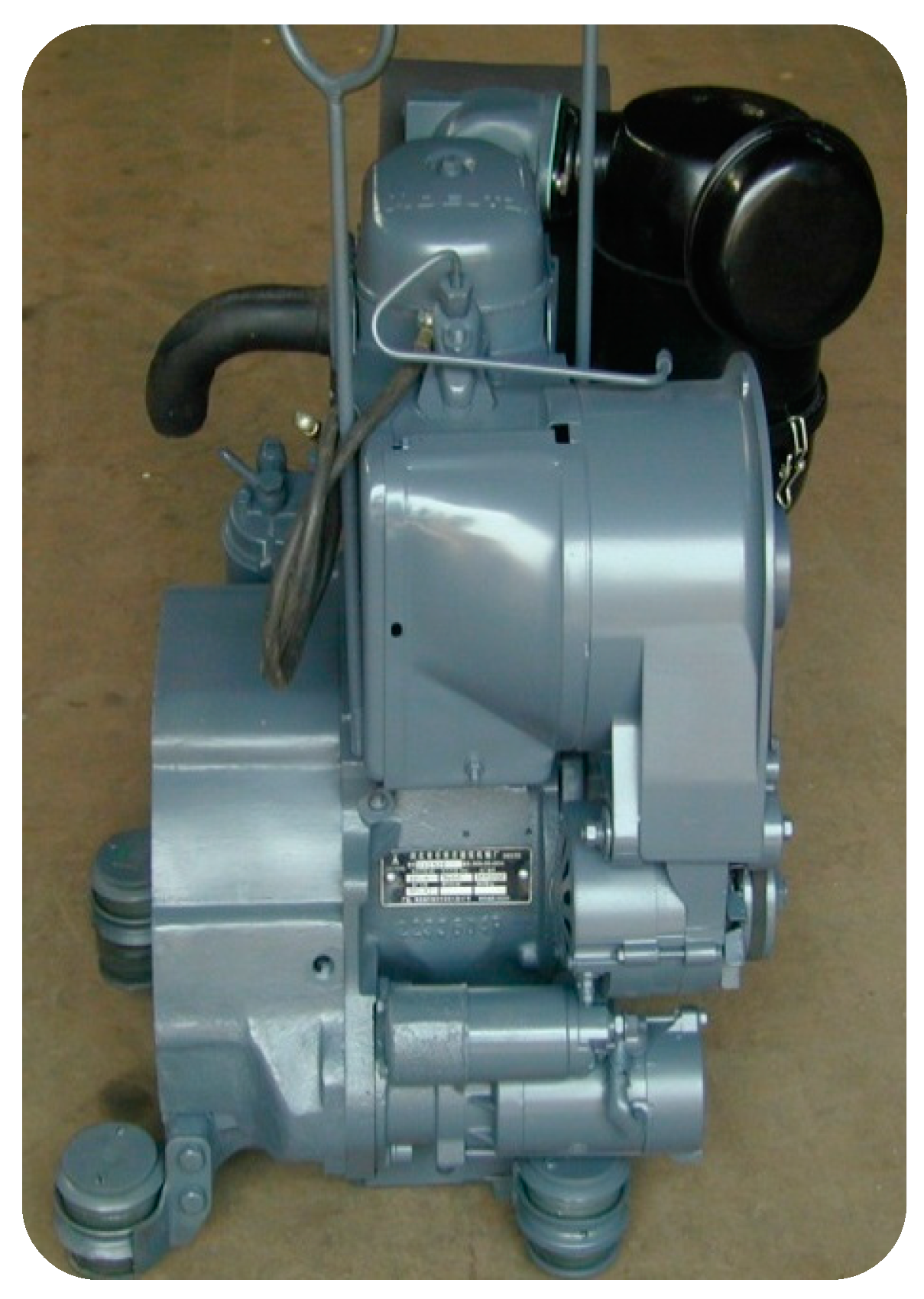
We examined the biodiesel produced from the various oils by running an engine at maximum load. For the performance tests, we used the DEUTZ engine, model F1L511, which is a single-cylinder, four-stroke, air-cooled, direct-injection engine (Figure 8). The engine performance test was conducted at the National Research Center in the Giza Governorate, Egypt. The details of the schematic diagram of the tested engine and its specifications were shown by Khalaf et al. [33]; however, the type of injection was direct injection. Figure 9 shows the engine test rig.
Figure 8.
The used diesel engine.

Figure 9.
The engine test rig.
The tested engine was linked to a generator to gauge the engine’s power output and rotary speed. Both the engine rotary speed and fuel consumption could be measured using the engine. Air was supplied to the engine by using an air box with a measurement aperture to deliver air. The pressure differential between the two sides of the orifice was measured using a pressure differential meter. A glass burette and stopwatch were used to calculate the fuel consumption rate. A digital tachometer was utilized to measure the engine speed. A number of electric lights used the generator’s output power. All data were obtained after the engine had warmed up. The trials were started, and measurements were taken after the engine achieved a steady condition. First, diesel fuel was used to run the engine. Second, for jatropha and pongamia at varying loads, the engine was run at a 20% biodiesel percentage. The engine speed was measured and altered for each running situation, from idling to full load adjustment.
2.4.1. Fuel Consumption Apparatus
Fuel consumption (kg/h) was determined directly using a graduated glass pipe. The time needed for the engine to measure an exact volume of oil was noted. The fuel consumption (FC) for each biodiesel percentage was determined as follows:
where FC is the rate of consumed fuel (kg/h), V is the spent fuel in the glass bulb (50 cm3), and t is the time needed to deliver the quantity of fuel (min).
FC = (V × 60)/(t)
2.4.2. Engine Test Procedure
The trials were performed according to the following steps, in order:
- Each measuring device was examined to ensure that there were no reading modifications;
- The engine was run;
- Using diesel fuel, the engine was warmed up for fifteen minutes without any load;
- The engine was allowed to stabilize for a certain amount of time before starting;
- The engine was adjusted to a full load at variable engine speeds ranging from idling speeds of 800 to 1500 rpm;
- The fuel was changed to a tested fuel, like a blend of diesel and biodiesel;
- All the instrument readings were recorded, and by the end of this step, one complete measurement point was obtained at the engine variable load;
- The air flow and fuel flow rates were measured under these conditions.
3. Results and Discussion
3.1. Oil Extraction
The yields of oil extracted from jatropha and pongamia seeds were 24.8 and 26.89%, respectively. The moisture percentages of the pongamia and jatropha seeds were 16.9% and 7.3%, respectively. These low moisture contents are advantageous for extending the seeds’ shelf lives. For jatropha and pongamia oils, the FFA values were discovered to be 2.8% and 5.4%, respectively. Low yields of biodiesel are caused by soap production and difficulties in the transesterification of glycerides, which are often caused by a high FFA content (>1–2% w/w) [34]. To unlock the complexity and improve system performances, it is crucial to consider the complexity of the biodiesel production processes. This includes process design, quantitative evaluation, and optimization of the biodiesel from the perspectives of entire systems [35]. The primary obstacle to the broad commercialization of biodiesel is its restricted feedstock and the several processes involved in producing it, including pre-treatment of the feedstock, transesterification reactions, and biodiesel purification [36]. Therefore, to make biodiesel and its blend a sustainable commercial fuel, it is important to have detailed knowledge about such processes.
3.2. Fatty Acid Composition of Jatropha and Pongamia Oils
Saturated and unsaturated fatty acids are distinguished by the quantities of double bonds they contain. Triglycerides can contain any of the three main types of fatty acids, saturated (Cn:0), monounsaturated (Cn:1), and polyunsaturated fatty acids (Cn:2,3), which have two or three double bonds. Every biodiesel feedstock has a unique fatty acid structure, and nearly every important fuel attribute of a biodiesel fuel is highly dependent on the feedstock’s fatty acid composition [37]. According to Table 1, the results of the Gas Chromatography (GC) analysis by weight showed that the most abundant fatty acids in jatropha seed oil were oleic (44.7%) and linoleic (32.8%) acids, followed by palmitic (14.2%) and stearic (6.88%) acids, which, together, constituted 98.58% of the total fatty acid content. In contrast, the most abundant fatty acids in pongamia seed oil were oleic (51.59%) and linoleic (21.14%) acids, followed by palmitic (11.65%) and stearic (7.5%) acids, which, together, constituted 91.88% of the total fatty acid content.

Table 1.
Fatty acid conformation of both pongamia and jatropha oils.
Saturated fats solidify at low temperatures and create solid crystals that clog gasoline lines, whereas compounds with more unsaturated double bonds break down more readily when they react with airborne oxygen [38,39]. The amount of energy in a fatty acid is determined by its saturation level. Compared to saturated fat, which only comprises single bonds, unsaturated fat has double bonds, which lowers the molecular energy. About 3.5 eV is the bond energy of a single bond, and 6.4 eV is the bond energy of a double bond. As a result, breaking down two single bonds releases more energy (7eV against 6.4 eV) than breaking up one double bond. This indicates that saturated fats have more energy than unsaturated fats [38,40].
3.3. Factors Affecting Ester Conversion
Experiments were conducted to determine how the reaction temperature, amount of catalyst, and stirring time affected the percentage of oil ester conversion in pongamia and jatropha oils. The quantity of biodiesel produced divided by the entire mass of vegetable oil involved in the process is the ester conversion percentage.
3.3.1. Effect of Temperature on Reaction
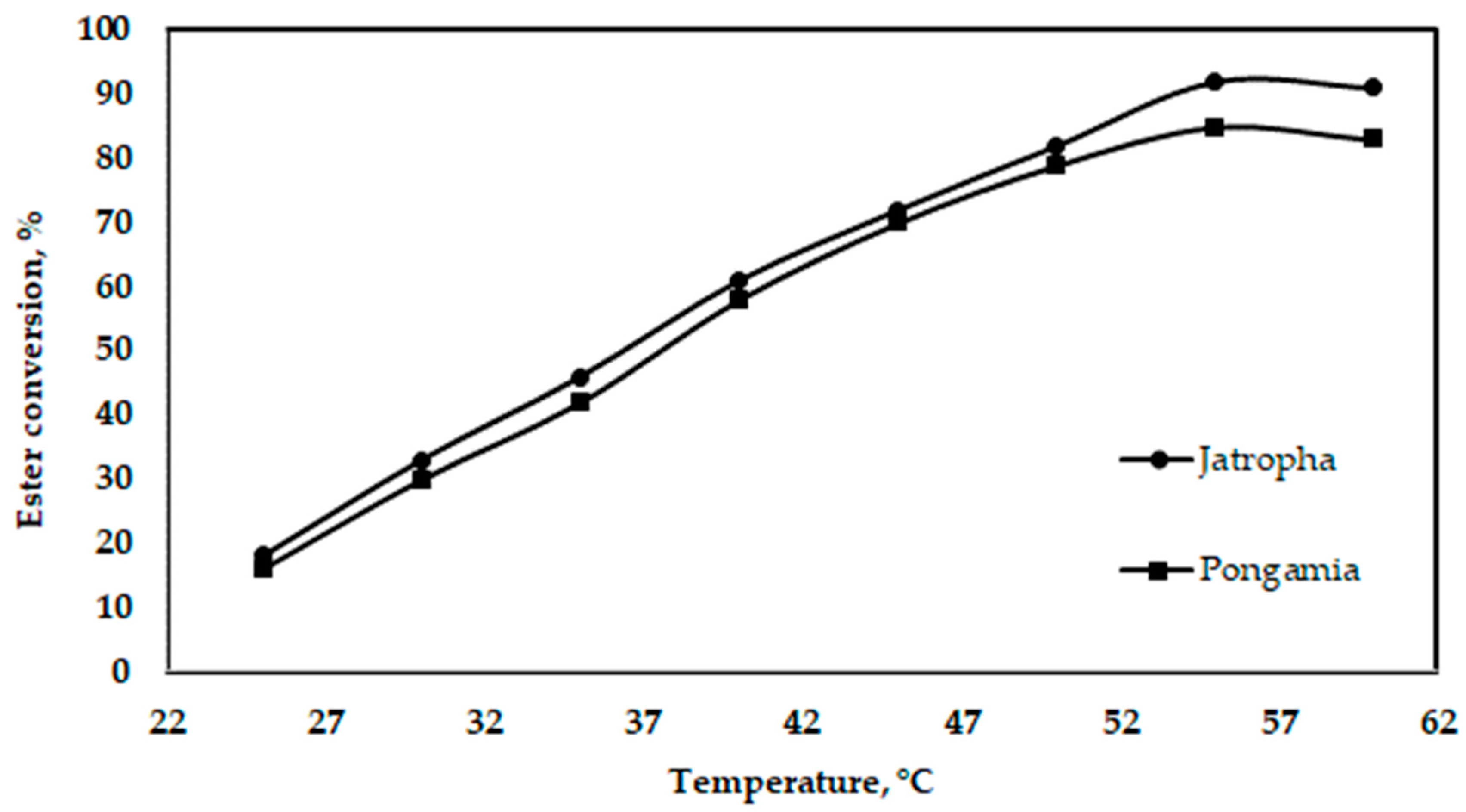
The increase in reaction temperature and the increase in the amount of yielded biodiesel are shown in Figure 10. The variation seems to be linear, except at the end, where the trend changes to a downward one. The maximum temperature needed to obtain the maximum yield was 55 °C. The boiling temperature of methyl alcohol is 64.7 °C, which is when methanol begins to evaporate. For this reason, the biodiesel content begins to decrease. Thus, one may conclude that the optimum temperature for the ester process is 55 °C. The alcohol’s boiling point (60–70 °C) is the only temperature limit during the reaction. If this limit is exceeded, the alcohol’s evaporation or saponification reaction is accelerated, reducing the amount of final product that is obtained [41].
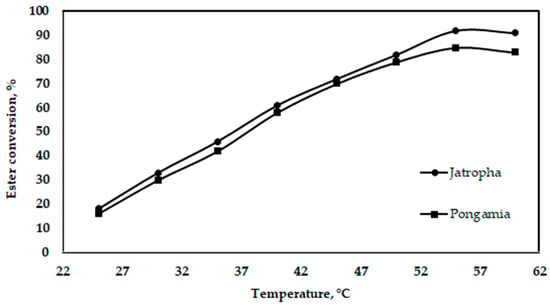
Figure 10.
Effect of oil temperature on ester conversion for jatropha and pongamia oils at an oil-to-methanol ratio of 5:1 (v/v) (stirring time was 60 min).
3.3.2. Effect of Catalyst Quantity
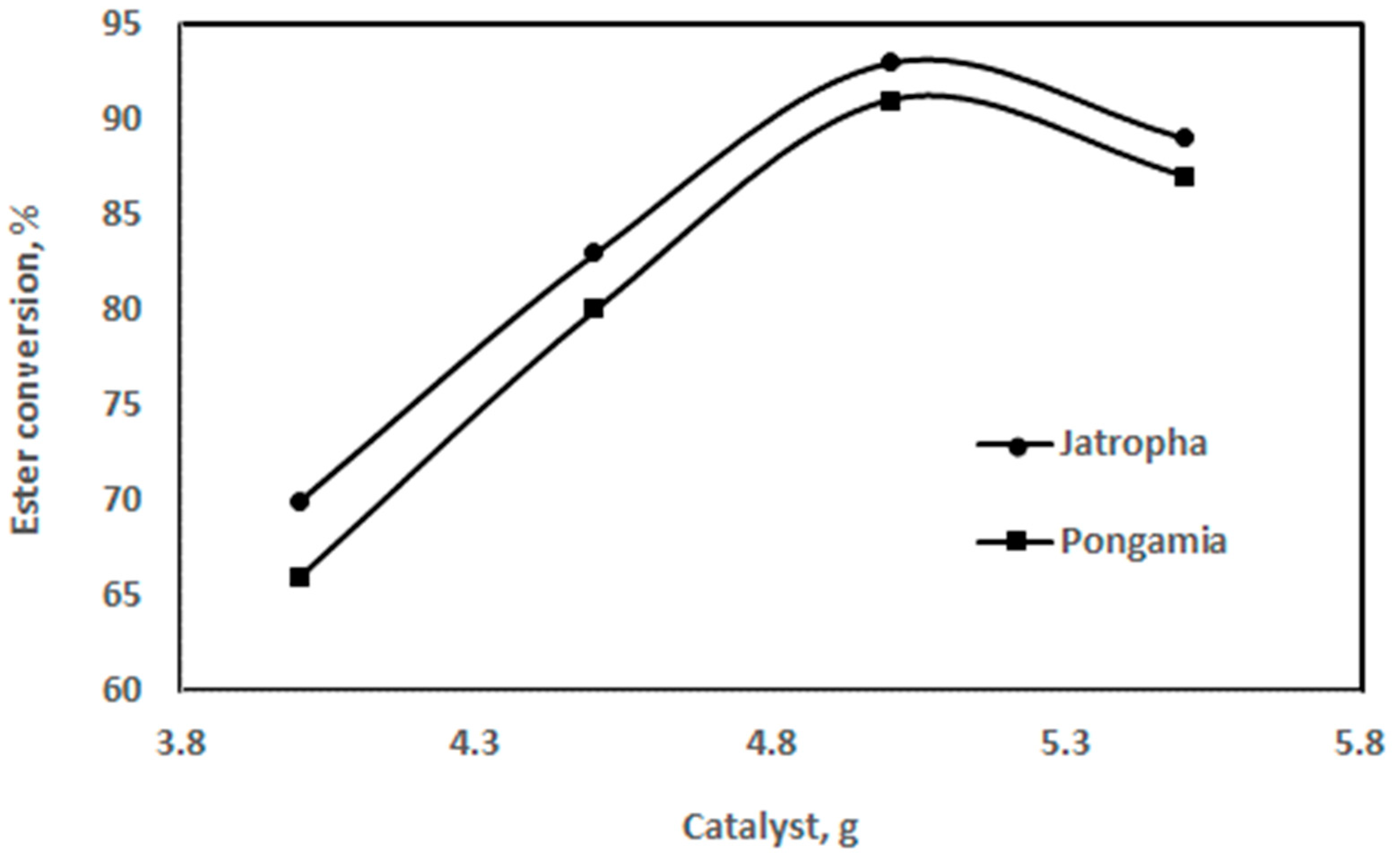
The impact of varying the catalyst quantity on the biodiesel yield is shown in Figure 11. The bulk of the catalyst boosts ester formation. With an increasing catalyst mass, the ester formation rate first increased and subsequently decreased. The oil-to-methanol ratio (v/v) was 5:1 during the reaction, which was conducted at 55 °C. The study’s findings show that, when a 1.0–1.4% NaOH concentration is applied, 90–98% of jatropha oil is converted into methyl ester [42].
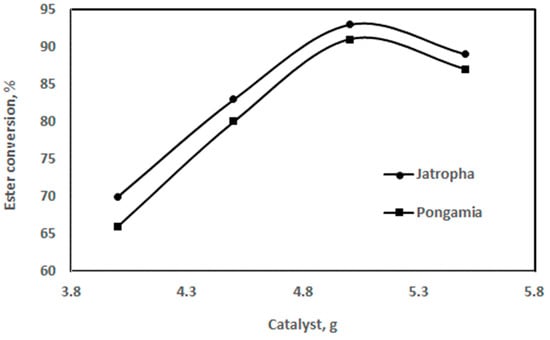
Figure 11.
Effect of catalyst amount on ester conversion for jatropha and pongamia oils at a temperature of 55 °C and ratio of 5:1 (v/v) (stirring time was 60 min).
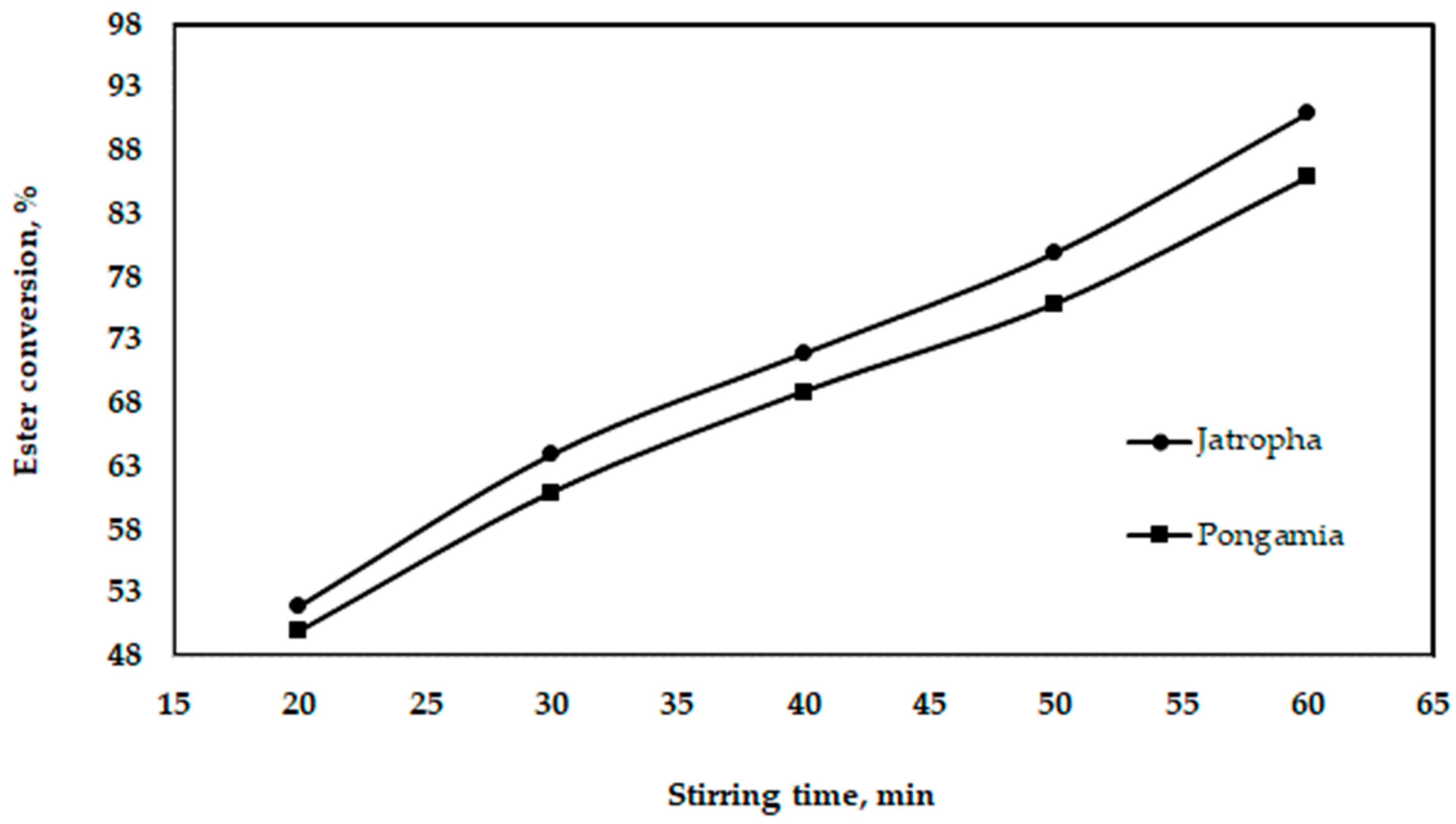
3.3.3. Effect of Stirring Time
Figure 12 shows the influence of the stirring time, showing that, as the stirring time increases, the output of biodiesel also increases. Only until the alcohol phases have been combined into a single, homogenous phase of oil can a high degree of conversion be achieved.
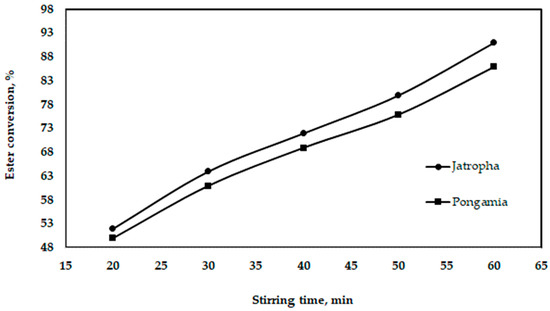
Figure 12.
Effect of stirring time on ester conversion for jatropha and pongamia oils at a temperature of 55 °C and ratio of 5:1 (v/v).
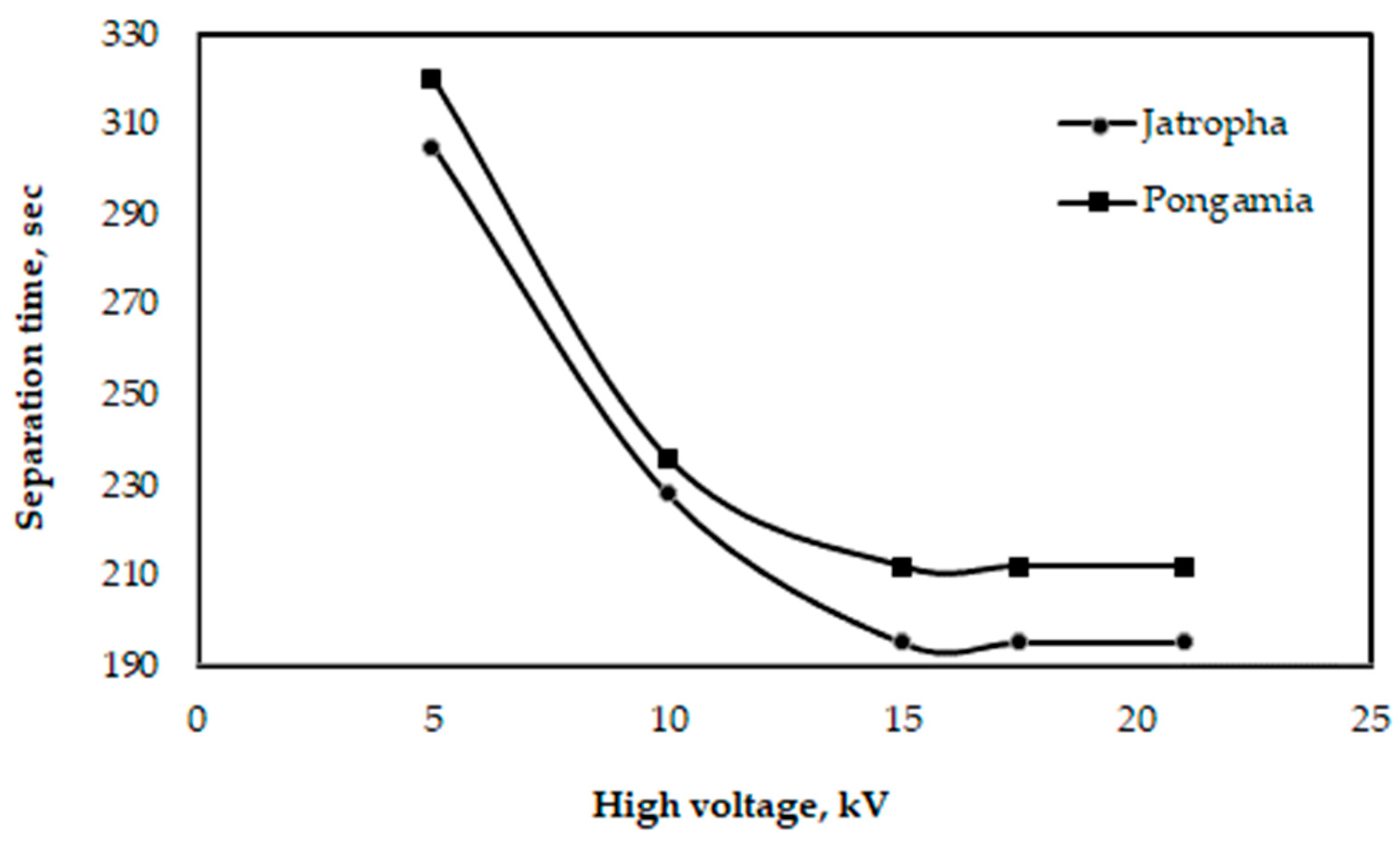
3.4. Separation Time
Glycerol may be separated from oil more quickly with a high-voltage device than by using the gravity approach. Because it can speed up the process of separating biodiesel from glycerol and other undesirable chemicals, the separation process, assisted by a voltage electric current, can yield positive results [43,44]. Figure 13 illustrates the impact of a high voltage on the duration of glycerol separation for pongamia and jatropha oils at a distance of 2.5 cm between electrodes. According to the data, the lowest separation times for pongamia and jatropha were 212 and 195 s, respectively, at a distance of 2.5 cm between electrodes and with a high voltage of 21 kV. According to the study by Isamil et al. [44], while utilizing frying cooking oil to make biodiesel, the minimum time needed to separate glycerol was 210 s when the distance between the electrodes was 2 cm, and the high voltage was 7.2 kV for a combination of 10 L.
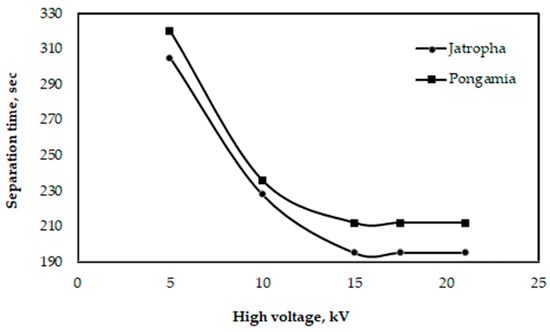
Figure 13.
Effect of high voltage on the time needed for glycerol separation when the distance between electrodes is 2.5 cm for pongamia and jatropha.
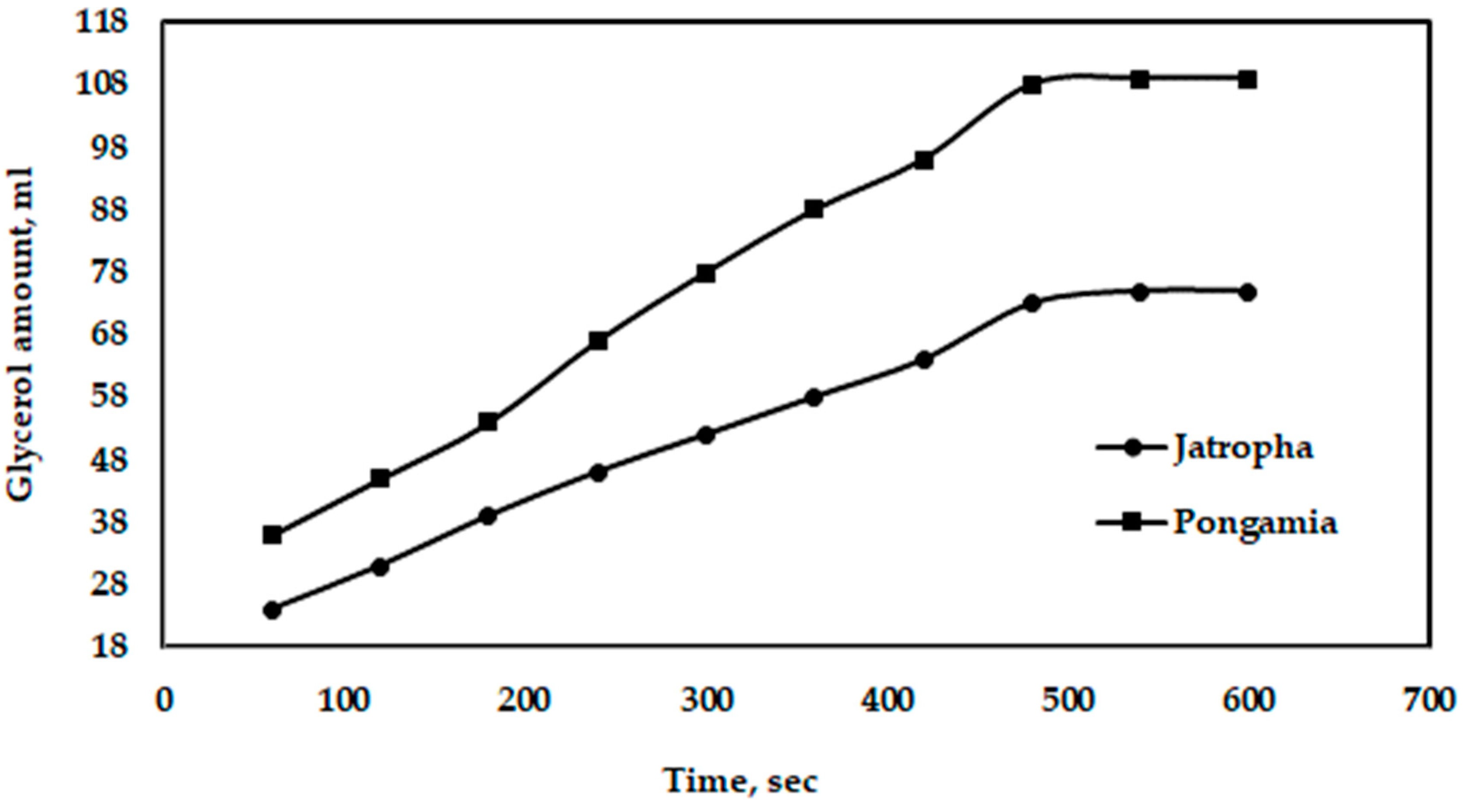
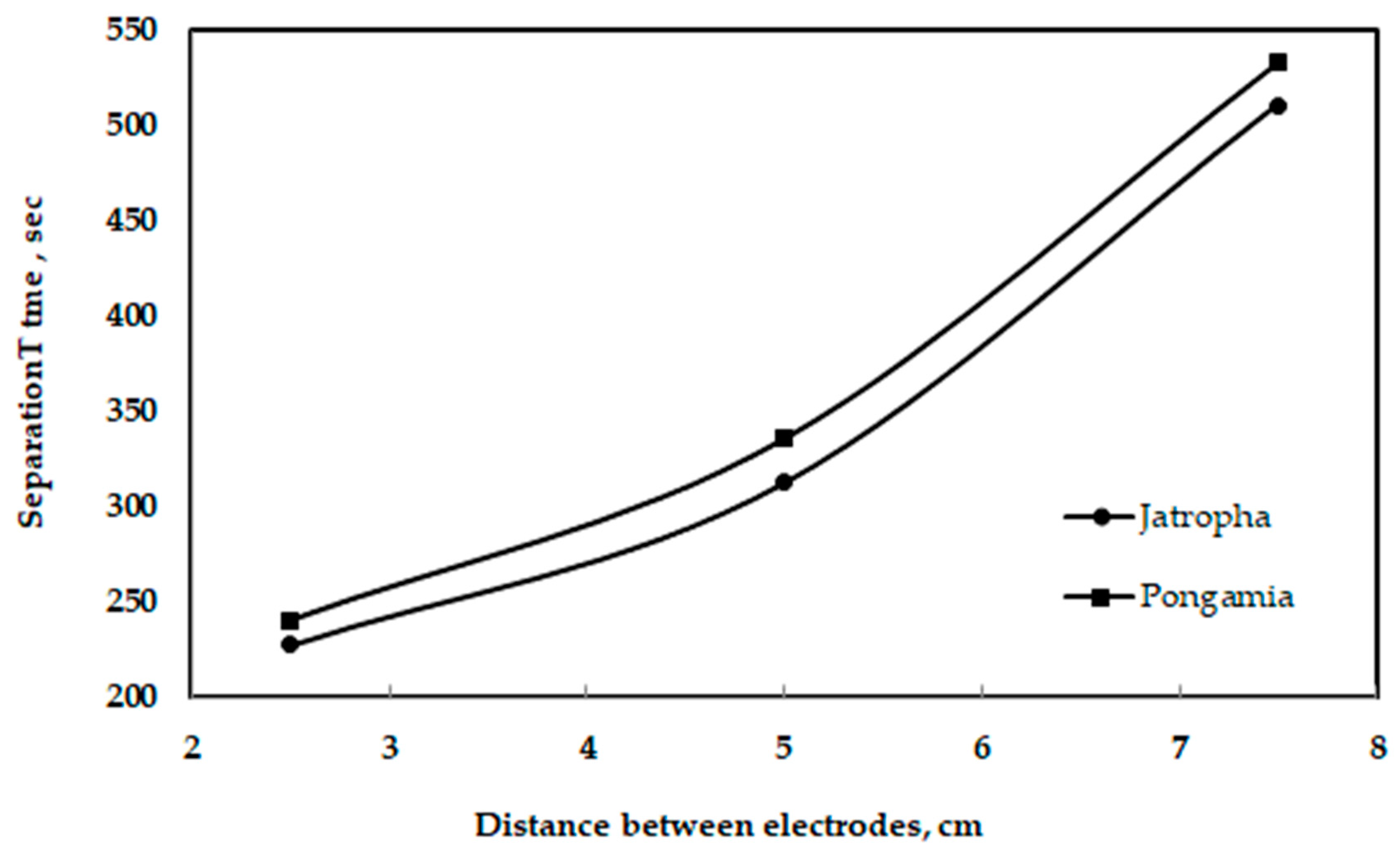
Figure 14 shows the effect of gravity on the separation of glycerol from jatropha and pongamia oils. It is clear that the maximum amounts of glycerol separated by gravity were 109 and 75 mL for pongamia oil and jatropha oil, respectively. Additionally, Figure 15 shows the relationship between the distances of electrodes when using a high voltage and a longer separation time. However, compared to a traditional gravitational settling separation, the electrically driven separation technology with a high voltage alternating current source technique had a better efficiency when removing glycerol and other pollutants [45]. Moreover, the high-voltage technique has been demonstrated to be capable of quickly inducing glycerol fallout, according to Isamil et al. [44].
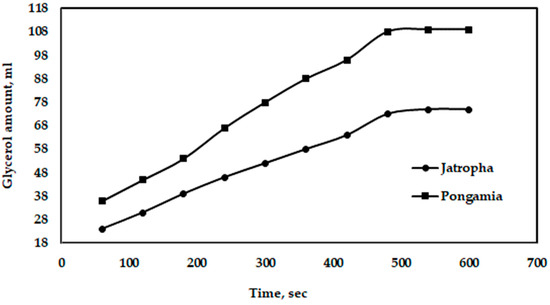
Figure 14.
Effect of gravity on the amount of glycerol separated from jatropha and pongamia oils.
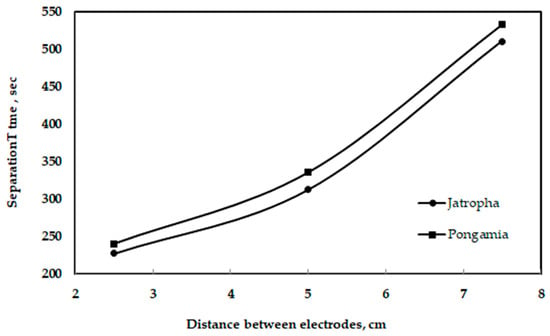
Figure 15.
Relationship between the distance between the electrodes and separation time.
3.5. Dry Washing
From Table 2, it is clear that dry washing using sawdust was an effective method for reducing the water content to the allowable limits. Moreover, the rest of the impurities were within the allowable levels according to the ASTM standards.

Table 2.
Effect of sawdust on the purity of biodiesel in the case of jatropha and pongamia oils.
3.6. Oil and Its Biodiesel Properties
The density, kinematic viscosity, and flash point of jatropha oil were reduced from 0.920 to 0.881 g/cm3 at 15 °C, from 37.1 to 4.38 cSt 40 °C, and from 211 to 162 °C, respectively, for the production of biodiesel, as shown in Table 3. Also, the density, kinematic viscosity, and flash point of pongamia oil were reduced from 0.924 to 0.888 g/cm3 at 15 °C, from 27.8 to 5.23 cSt at 40 °C, and from 222 to 158 °C, respectively, for the production of biodiesel, as shown in Table 3. In general, biodiesel derived from different feedstock generations has a density ranging from 832 to 982 kg/m3. Regular diesel has a flash point of 55–65 °C, but biodiesel fuels have a flash point of above 150 °C. However, 47 is the lowest figure for the cetane number. Additionally, the calorific value of biodiesel fuels is often lower than that of petroleum diesel [49]. Moreover, the kinematic viscosity of pure biodiesels is higher than that of petrol diesels [50], and according to the ASTM guidelines, the kinematic viscosity should be between 1.9 and 6.0 cST [51]. The obtained kinematic viscosity from jatropha and pongamia oils was within the recommended guidelines. Kinematic viscosity, however, is a crucial and significant aspect of an engine fuel’s flow [51]. The calorific value of jatropha oil was increased from 38.08 to 39.65 MJ/kg for the production of biodiesel, while that of pongamia oil was increased from 36.61 to 36.94 MJ/kg. However, Khalaf et al. [33] reported that the heating value of jatropha oil was 42.1 MJ/kg. Additionally, the cetane number increased from 21 for oil to 50 for the production of biodiesel and from 32 for oil to 52 for jatropha and pongamia biodiesels, respectively, as shown in Table 3. Furthermore, Table 4 and Table 5 show the fuel properties of jatropha and pongamia biodiesel and their blends, respectively. By inspection of Table 4 and Table 5, it can be seen that the kinematic viscosity and density of jatropha and pongamia biodiesels are higher than those of diesel fuel; however, this fact was reported by Zheng and Cho [52], who stated that these characteristics have an effect on the fuel’s flow and atomization. In addition, the calorific value of jatropha and pongamia biodiesels is generally lower than that of diesel fuel; however, this fact was reported by Zheng and Cho [52], who stated that the calorific value makes the fuel consumption of biodiesel blends higher than that of diesel fuel. The flash point values of jatropha and pongamia biodiesels are higher than those of diesel fuel; however, biodiesel has a major benefit in terms of storage and transportation due to its high flash point [52]. The cloud point values of jatropha and pongamia biodiesels are higher than those of diesel fuel, as shown in Table 4 and Table 5; however, in the study of Sarin et al. [53], the jatropha and pongamia biodiesels had cloud points of 4.0 and −1.0 °C, respectively. General, blends of jatropha and pongamia biodiesels and diesel share many physical and chemical characteristics, which makes them a great substitute fuel.

Table 3.
Oil and its biodiesel properties for jatropha and pongamia, compared with the diesel ASTM standards and EN 14214.

Table 4.
Fuel properties of jatropha biodiesel and its blends.

Table 5.
Fuel properties of pongamia biodiesel and its blends.
3.7. Engine Performance
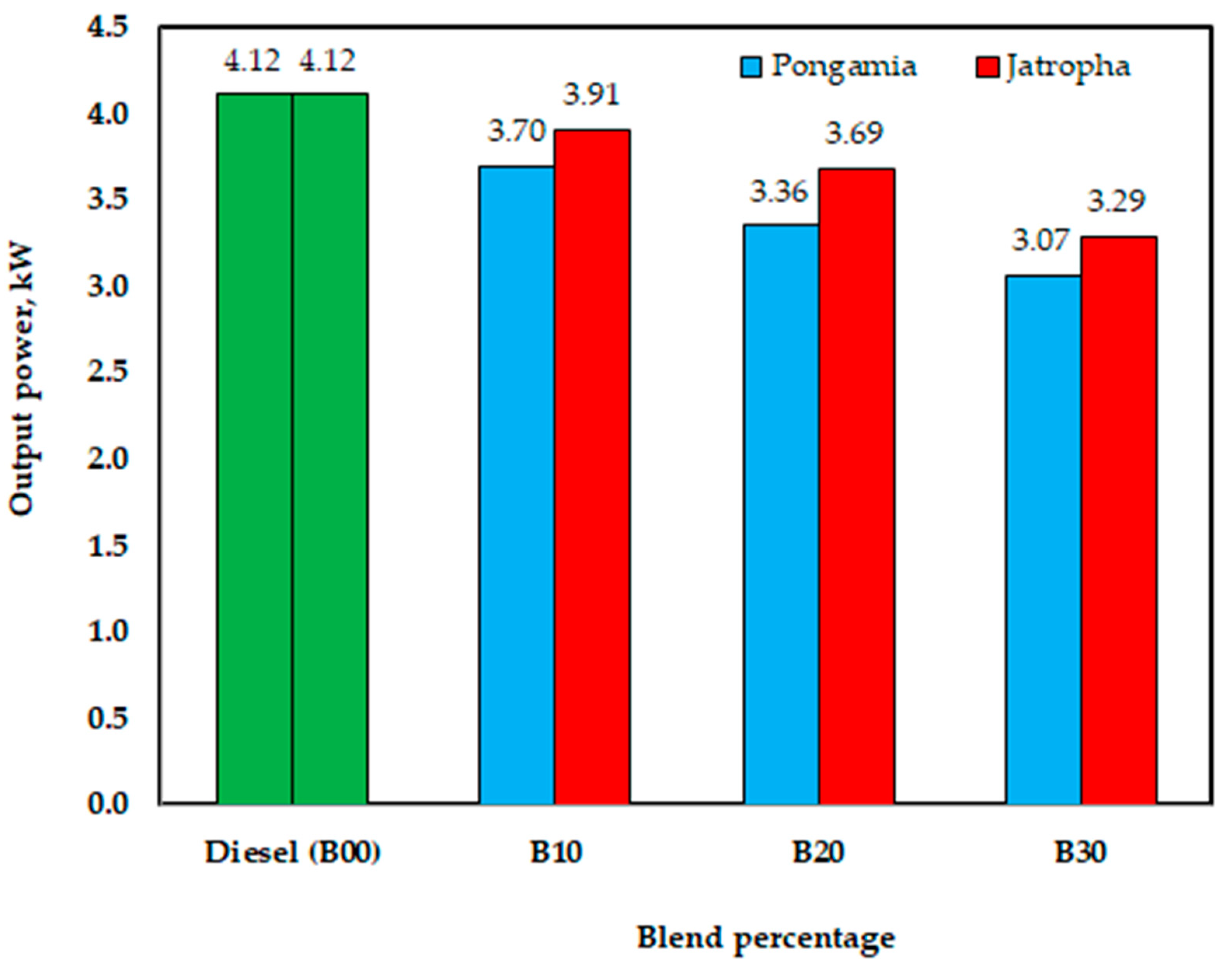
The investigated engine performance with the produced biodiesel, to support the sustainable biodiesel industry and the sustainability of biodiesel production, can run a diesel engine. However, according to the engine’s load performances, the engine’s power values when using jatropha methyl ester were 3.91, 3.69, and 3.29 kW for B10, B20, and B30, respectively, while its power with diesel fuel (B00) was 4.12 kW. However, with pongamia methyl ester, the values were 3.70, 3.36, and 3.07 kW for B10, B20, and B30, respectively. As seen in Figure 16, this drop might point to a reduced calorific value for methyl ester. According to the experimental results, under the testing settings for jatropha biodiesel, the maximum loss in output power was 20% lower than that of diesel fuel for B30; under the testing conditions for pongamia biodiesel, the maximum decrease in output power was 25% lower than that of diesel fuel for B30. El-Emam et al.’s results [54] showed that utilizing biodiesel made from jatropha seeds could reduce the output power by up to 16% at a 750-engine rpm when compared to diesel fuel for B80.
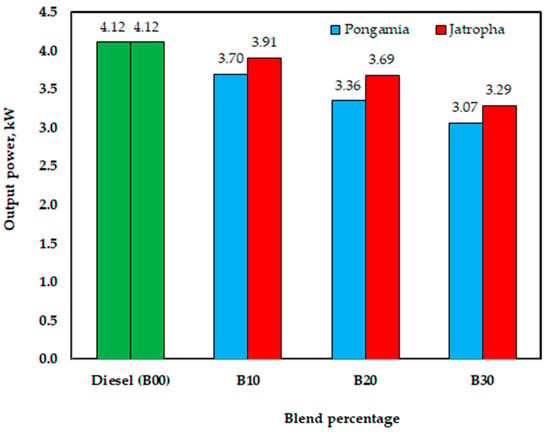
Figure 16.
Effect of blend percentage on engine output power with conventional diesel fuel (B00) and jatropha and pongamia biodiesels.
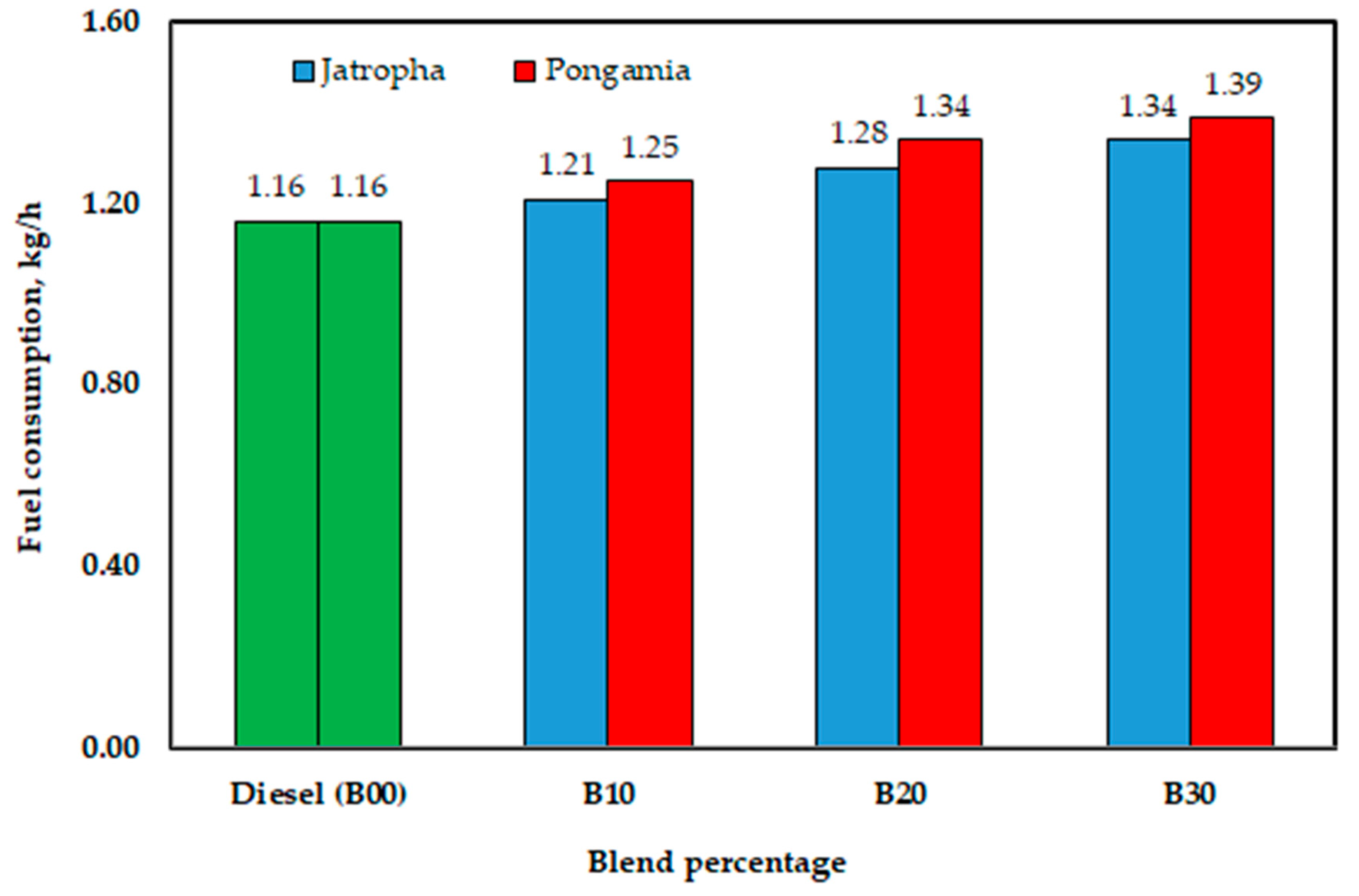
As shown, the engine used 1.21, 1.28, and 1.34 kg/h of jatropha methyl ester for B10, B20, and B30, respectively, whereas the diesel fuel (B00) consumed 1.16 kg/h. As seen in Figure 17, the values were 1.25, 1.34, and 1.39 kg/h for B10, B20, and B30, respectively, when using pongamia methyl ester. The decreased calorific value of biodiesel could be the main cause of this increased fuel consumption. However, this is most likely not the sole factor contributing to the increase in the grams of biodiesel consumed per unit of energy produced. It makes sense to associate the lower specific fuel consumption with the increased oxygen content of the blends under consideration. Fuel-based oxygen has an innate ability to speed up processes within incredibly fuel-rich spray patterns, resulting in more thorough burning.
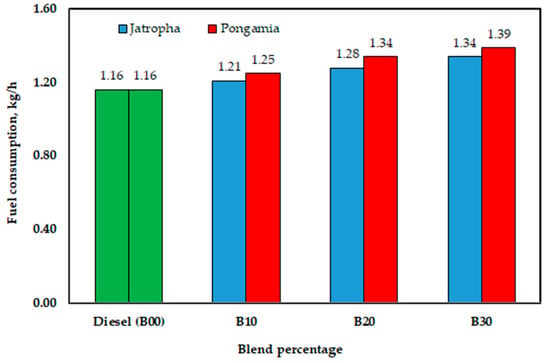
Figure 17.
Effect of blend percentage on fuel consumption with conventional diesel fuel (B00) and jatropha and pongamia biodiesels.
4. Conclusions
This study transformed two non-edible oils extracted from the seeds of jatropha and pongamia crops into biodiesel fuel; these oils are considered environmentally responsible value-added products. The effects of the reaction temperature, quantity of catalyst, and stirring time on the percentages of oil ester conversion for jatropha and pongamia oils were studied. The conclusions are summarized as follows:
- The yields of oils extracted from jatropha and pongamia seeds by using the mentioned procedures were 24.8% and 26.89%, respectively;
- The amount of free fatty acids (FFA) values were found to be 2.8% and 5.4% for jatropha oil and pongamia oil, respectively;
- The most abundant fatty acids in jatropha seed oil were oleic (44.7%) and linoleic (32.8%) acids, followed by palmitic (14.2%) and stearic (6.88%) acids, which, together, comprised 98.58% of the total fatty acid content, while the most abundant fatty acids of pongamia seed oil were oleic (51.59%) and linoleic (21.14%) acids, followed by palmitic (11.65%) and stearic (7.5%) acids, which, together, comprised 91.88% of the total fatty acid content;
- The density, kinematic viscosity, and flash point of jatropha oil were reduced from 0.920 to 0.881 g/cm3 at 15 °C, from 37.1 to 4.38 cSt at 40 °C, and from 211 to 162 °C, respectively, for the production of biodiesel. Also, the density, viscosity, and flash point of pongamia oil were reduced from 0.924 to 0.888 g/cm3 at 15 °C, from 27.8 to 5.23 cSt at 40 °C, and from 222 to 158 °C, respectively, for the production of biodiesel;
- The fuel consumption of each of the involved oils was evaluated and quantified using an air-cooled, single-cylinder, four-stroke diesel engine at full load, resulting in values of 1.21, 1.28, and 1.34 kg/h for B10, B20, and B30, respectively, with jatropha methyl ester compared to 1.16 kg/h for diesel fuel (B00), while the fuel consumption values were 1.25, 1.34, and 1.39 kg/h for B10, B20, and B30, respectively, with pongamia methyl ester.
We conclude that biodiesel can be produced directly from non-edible oils, specifically jatropha and pongamia oils, via mechanical and chemical methods. Furthermore, the produced biodiesel can be used in tractor engines for agricultural production applications.
Author Contributions
Conceptualization, A.I.M., S.S.A., M.M.D. and M.F.Z.; methodology A.I.M., M.M.D. and M.F.Z.; software, A.M.A., S.S.A., S.M.A.-S. and M.M.D.; formal analysis, A.M.A., M.M.D. and M.F.Z.; validation, A.I.M., S.S.A., M.M.D. and M.F.Z.; visualization, A.I.M., S.S.A., M.M.D., M.F.Z. and A.M.A.; investigation, S.M.A.-S., A.I.M., S.S.A., M.M.D., M.F.Z. and A.M.A.; resources, S.M.A.-S., A.I.M., S.S.A., M.M.D., M.F.Z. and A.M.A.; data curation, A.I.M., S.S.A., M.M.D. and M.F.Z.; writing—original draft preparation, A.I.M., S.S.A., S.M.A.-S., M.M.D. and M.F.Z.; supervision, A.I.M., S.S.A., M.M.D. and M.F.Z.; funding acquisition, S.M.A.-S., A.I.M., S.S.A., M.M.D. and M.F.Z.; writing—review and editing, all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Taghizadeh-Hesary, F.; Yoshino, N.; Abdoli, G.; Farzinvash, A. An estimation of the impact of oil shocks on crude oil exporting economies and their trade partners. Front. Econ. China 2013, 8, 571–591. [Google Scholar] [CrossRef]
- Hajjari, M.; Tabatabaei, M.; Aghbashlo, M.; Ghanavati, H. A review on the prospects of sustainable biodiesel production: A global scenario with an emphasis on waste-oil biodiesel utilization. Renew. Sustain. Energy Rev. 2017, 72, 445–464. [Google Scholar] [CrossRef]
- Mahdavi, M.; Abedini, E.; Darabi, A.H. Biodiesel synthesis from oleic acid by nanocatalyst (ZrO2/Al2O3) under high voltage conditions. RSC Adv. 2015, 5, 55027–55032. [Google Scholar] [CrossRef]
- Sales, M.B.; Borges, P.T.; Ribeiro Filho, M.N.; Miranda da Silva, L.R.; Castro, A.P.; Sanders Lopes, A.A.; Chaves de Lima, R.K.; de Sousa Rios, M.A.; Santos, J.C.S.d. Sustainable Feedstocks and Challenges in Biodiesel Production: An Advanced Bibliometric Analysis. Bioengineering 2022, 9, 539. [Google Scholar] [CrossRef] [PubMed]
- Biberci, M.A. Bibliometric Analysis of the Use of Biodiesel Production from Essential Oils as Biofuels. Processes 2023, 11, 974. [Google Scholar] [CrossRef]
- Demirbas, A. Political, economic and environmental impacts of biofuels: A review. Appl. Energy 2009, 86 (Suppl. S1), S108–S117. [Google Scholar] [CrossRef]
- Ong, H.C.; Silitonga, A.; Masjuki, H.; Mahlia, T.; Chong, W.; Boosroh, M. Production and comparative fuel properties of biodiesel from non-edible oils: Jatropha curcas, Sterculia foetida and Ceiba pentandra. Energy Convers. Manag. 2013, 73, 245–255. [Google Scholar] [CrossRef]
- Shabban, M.A.; Aly, H.M. Production and performance evaluation of biodiesel from pongamia tree oil. J. Soil Sci. Agric. Eng. Mansoura Univ. 2016, 7, 201–211. [Google Scholar] [CrossRef]
- Harreh, D.; Saleh, A.A.; Reddy, A.N.R.; Hamdan, S. An Experimental Investigation of Karanja Biodiesel Production in Sarawak, Malaysia. J. Eng. 2018, 2018, 4174205. [Google Scholar] [CrossRef]
- Rodrigues, J.; Miranda, I.; Gominho, J.; Vasconcelos, M.; Barradas, G.; Pereira, H.; Bianchi-de-Aguiar, F.; Ferreira-Dias, S. Modeling and optimization of laboratory-scale conditioning of Jatropha curcas L. seeds for oil expression. Ind. Crops Prod. 2016, 83, 614–619. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, Z.; Liang, J.; Tang, L.; Chen, F. Detoxification of Jatropha curcas seed cake in solid-state fermentation of newly isolated endophytic strain and nutrition assessment for its potential utilizations. Int. Biodeterior. Biodegrad. 2016, 109, 202–210. [Google Scholar] [CrossRef]
- Kumar, P.; Srivastava, V.C.; Jha, M.K. Jatropha curcas phytotomy and applications: Development as a potential biofuel plant through biotechnological advancements. Renew. Sustain. Energy Rev. 2016, 59, 818–838. [Google Scholar] [CrossRef]
- Malaikozhundan, B.; Vaseeharan, B.; Vijayakumar, S.; Pandiselvi, K.; Kalanjiam, R.; Murugan, K.; Benelli, G. Biological therapeutics of Pongamia pinnata coated zinc oxide nanoparticles against clinically important pathogenic bacteria, fungi and MCF-7 breast cancer cells. Microb. Pathog. 2017, 104, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, G.; Sharma, M.P. Effect of metal on stability and cold flow property of pongamia biodiesel. Mater. Today Proc. 2015, 2, 1421–1426. [Google Scholar] [CrossRef]
- Jaichandar, S.; Annamalai, K. The status of biodiesel as an alternative fuel for diesel engine—An overview. J. Sustain. Energy Environ. 2011, 1, 71–75. [Google Scholar]
- Encinar, J.M.; Gonzalez, J.F.; Rodriguez, J.J.; Tajedor, A. Biodiesel fuel from vegetable oils: Transesterification of Cynara cardunculuc L. oil with ethanol. Energy Fuel 2002, 16, 443–450. [Google Scholar] [CrossRef]
- Goff, M.J.; Bauer, N.S.; Lopes, S.; Sutterlin, W.R.; Suppes, G.J. Acid catalyzed alcoholysis of soybean oil. J. Am. Oil Chem. Soc. 2004, 81, 415–420. [Google Scholar] [CrossRef]
- Gashaw, A.; Lakachew, A. Production of biodiesel from non edible oil and its properties. Int. J. Sci. Environ. 2014, 3, 1544–1562. [Google Scholar]
- Aboumosalam, M.; Gad, A.; Barakat, Y.; Ismail, H. Accelerating the separation of emulsified oil products using high electrostatic fields. Egypt. J. Pet. 2020, 29, 219–225. [Google Scholar] [CrossRef]
- Ali, E.N.; Tay, C.I. Characterization of biodiesel produced from palm oil via base catalyzed transesterification. Proc. Eng. 2013, 53, 7–12. [Google Scholar] [CrossRef]
- Banerjee, N.; Ramakrishnan, R.; Jash, T. Biodiesel production from used vegetable oil collected from shops selling fritters in Kolkata. Energy Proc. 2014, 54, 161–165. [Google Scholar] [CrossRef]
- Peiter, A.S.; Lins, P.V.; Meili, L.; Soletti, J.I.; Carvalho, S.H.; Pimentel, W.R.; Meneghetti, S.M.P. Stirring and mixing in ethylic biodiesel production. J. King Saud Univ.-Sci. 2020, 32, 54–59. [Google Scholar] [CrossRef]
- Bateni, H.; Karimi, K. Biodiesel production from castor plant integrating ethanol production via a biorefinery approach. Chem. Eng. Res. Des. 2016, 107, 4–12. [Google Scholar] [CrossRef]
- Nigatu, S.G.; Marchetti, J.M. Review Biodiesel production technologies: Review. AIMS Energy 2017, 5, 425–457. [Google Scholar] [CrossRef]
- Atadashi, I.M.; Aroua, M.K.; Abdul Aziz, A.R.; Sulaiman, N.M.N. Production of biodiesel using high free fatty acid feedstocks. Renew. Sustain. Energy Rev. 2012, 16, 3275–3285. [Google Scholar] [CrossRef]
- Bateni, H.; Karimi, K.; Zamani, A.; Benakashani, F. Castor plant for biodiesel, biogas, and ethanol production with a bio-refinery processing perspective. Appl. Energy 2014, 136, 14–22. [Google Scholar] [CrossRef]
- Ratanabuntha, T.; Tonmitr, K.; Suksri, A. Acceleration in biodiesel production from palm oil process by high voltage electric field. Int. J. Smart Grid Clean Energy 2018, 7, 225–230. [Google Scholar] [CrossRef]
- Barabás, I.; Todorut, I.-A. Biodiesel Quality, Standards and Properties, Biodiesel Quality, Emissions and By-Products; Montero, G., Ed.; InTech: Vienna, Austria, 2011; ISBN 978-953-307-784-0. Available online: http://www.intechopen.com/books/biodiesel-quality-emissions-and-by-products/biodiesel-qualitystandards-and-properties (accessed on 20 January 2024).
- Mizik, T.; Gyarmati, G. Economic and Sustainability of Biodiesel Production—A Systematic Literature Review. Clean Technol. 2021, 3, 19–36. [Google Scholar] [CrossRef]
- ASTM. D1983: Standard test methods for fatty acid composition by gas-liquid chromatography of methyl esters. In Annual Book of ASTM Standards; ASTM: Philadelphia, PA, USA, 1990; Volume 06.03, pp. 433–436. [Google Scholar]
- Zhang, W.; Wang, C.; Luo, B.; He, P.; Zhang, L.; Wu, G. Efficient and economic transesterification of waste cooking soybean oil to biodiesel catalyzed by outer surface of ZSM-22 supported different Mo catalyst. Biomass Bioenergy 2022, 167, 106646. [Google Scholar] [CrossRef]
- ASTM D6751-15c; Standard Specification for Biodiesel Fuel Blend Stock (B100) for Middle Distillate Fuels. ASTM International: West Conshohocken, PA, USA, 2010; pp. 1–10.
- Khalaf, M.; Abdel-Fadeel, W.; Abd Elhady, S.; Esmail, M.F.C. Performance and emissions of a diesel engine fueled with a biofuel extracted from jatropha seeds. Int. J. Appl. Energy Syst. 2022, 4, 40–50. [Google Scholar] [CrossRef]
- Akbar, E.; Yaakob, Z.; Kamarudin, S.K.; Ismail, M.; Salimon, J. Characteristic and composition of Jatropha curcas oil seed from Malaysia and its potential as biodiesel feedstock. Eur. J. Sci. Res. 2009, 29, 396–403. [Google Scholar]
- Pasha, M.K.; Dai, L.; Liu, D.; Guo, M.; Du, W. An overview to process design, simulation and sustainability evaluation of biodiesel production. Biotechnol. Biofuels 2021, 14, 129. [Google Scholar] [CrossRef] [PubMed]
- Khanna, A.; Lamba, B.Y.; Jain, S.; Bolshev, V.; Budnikov, D.; Panchenko, V.; Smirnov, A. Biodiesel Production from Jatropha: A Computational Approach by Means of Artificial Intelligence and Genetic Algorithm. Sustainability 2023, 15, 9785. [Google Scholar] [CrossRef]
- Lang, X.; Dalai, A.K.; Bakhshi, N.N.; Reaney, M.J.; Hertz, P.B. Preparation and characterization of bio-diesels from various bio-oils. Bioresour. Technol. 2001, 80, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Zumdahl, S. Chemical Principles; Houghton Mifflin Harcourt Trade & Reference Publishers: Boston, MA, USA, 1995; ISBN 10: 0669393215/13: 9780669393217. [Google Scholar]
- Goering, C.E.; Schwab, A.W.; Daugherty, M.J.; Pryde, E.H.; Heakin, A.J. Fuel properties of eleven vegetable oils. Tran. ASAE 1982, 25, 1472–1477. [Google Scholar] [CrossRef]
- Corinna, W.U. Vegetable oils are moving from the kitchen table to the car engine. Sci. News 1998, 154, 364. [Google Scholar]
- Abdel Fatah, M.; Farag, H.A.; Ossman, M.E. Production of biodiesel from non-edible oil and effect of blending with diesel on fuel properties. Int. J. Eng. Sci. Technol. 2012, 2, 583–591. [Google Scholar]
- Agarwal, A.; Gupta, P. Rajdeep: Biodiesel production for CI engine from various non-edible oils: A review. Int. J. Emerg. Eng. Res. Technol. 2015, 3, 8–16. [Google Scholar]
- Trisnaliani, L.; Zikri, A. Separation of Glycerol from biodiesel oil products using high voltage electrolysis method. Indones. J. Fundam. Appl. Chem. 2018, 3, 7–11. [Google Scholar] [CrossRef]
- Ismail, Z.E.; Moussa, A.I.; Deef, M.M. Utilization of high voltage to separate glycerol during producing biodiesel. J. Soil Sci. Agric. Eng. Mansoura Univ. 2018, 9, 329–332. [Google Scholar] [CrossRef]
- Ampairojanawong, R.; Boripun, A.; Ruankon, S.; Suwanasri, T.; Cheenkachorn, K.; Kangsadan, T. Separation Process of Biodiesel-Product Mixture from Crude Glycerol and Other Contaminants Using Electrically Driven Separation Technique with AC High Voltage. Electrochem 2023, 4, 123–144. [Google Scholar] [CrossRef]
- ASTM D6584-21; Standard Test Method for Determination of Total Monoglycerides, Total Diglycerides, Total Triglycerides, and Free and Total Glycerin in B-100 Biodiesel Methyl Esters by Gas Chromatography. ASTM: West Conshohocken, PA, USA, 2021.
- European Committee for Standardization. EN 14110 Fat and Oil Derivatives—Fatty Acid Methyl Esters (FAME)—Determination of Methanol Content; European Committee for Standardization: Brussels, Belgium, 2003. [Google Scholar]
- EN 14214:2013 V2+A2:2019; Liquid Petroleum Products—Fatty Acid Methyl Esters (FAME) for Use in Diesel Engines and Heating App lications—Requirements and Test Methods. European Committee for Standardization: Brussels, Belgium, 2019.
- Neupane, D.; Bhattarai, D.; Ahmed, Z.; Das, B.; Pandey, S.; Solomon, J.K.Q.; Qin, R.; Adhikari, P. Growing Jatropha (Jatropha curcas L.) as a Potential Second-Generation Biodiesel Feedstock. Inventions 2021, 6, 60. [Google Scholar] [CrossRef]
- Reddy, A.N.R.; Saleh, A.A.; Islam, M.S.; Hamdan, S.; Rezaur Rahman, M.; Masjuki, H.H. Experimental evaluation of fatty acid composition influence on Jatropha biodiesel physicochemical properties. J. Renew. Sustain. Energy 2018, 10, 013103. [Google Scholar] [CrossRef]
- Tanner, A.; Baranek, M.; Eastlack, T.; Butts, B.; Beazley, M.; Hampton, M. Biodiesel production directly from rapeseeds. Water 2023, 15, 2595. [Google Scholar] [CrossRef]
- Zheng, F.; Cho, H.M. The Effect of Different Mixing Proportions and Different Operating Conditions of Biodiesel Blended Fuel on Emissions and Performance of Compression Ignition Engines. Energies 2024, 17, 344. [Google Scholar] [CrossRef]
- Sarin, A.; Arora, R.; Singh, N.P.; Sarin, R.; Malhotra, R.K.; Kundu, K. Effect of blends of Palm-Jatropha-Pongamia biodiesels on cloud point and pour point. Energy 2009, 34, 2016–2021. [Google Scholar] [CrossRef]
- El-Emam, S.H.; Okasha, F.M.O. Abdel-Salam, H.A.; Shaban, A. Performance of diesel engine using jatropha biodiesel fuel blends. Mansoura Eng. J. (MEJ) 2010, 35, M39–M50. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).