Changes in Land Use through Eucalyptus Plantations Impact Soil Fauna Communities in Brazilian Savannas
Abstract
1. Introduction
2. Materials and Methods
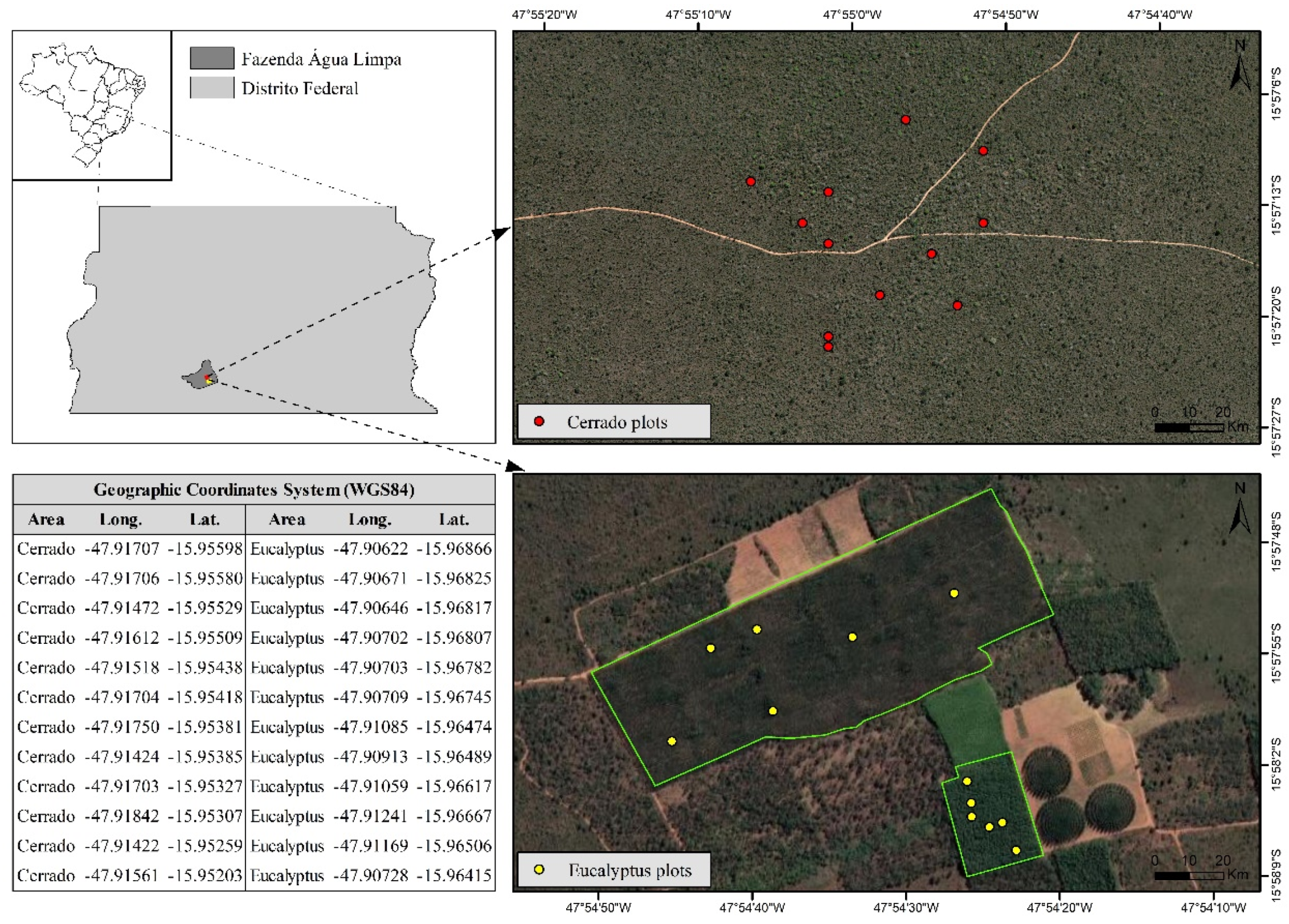
2.1. Study Sites Description
2.2. Soil Mesofauna Abundance and Diversity
2.3. Soil Chemical Analyses
2.4. Statistics
3. Results
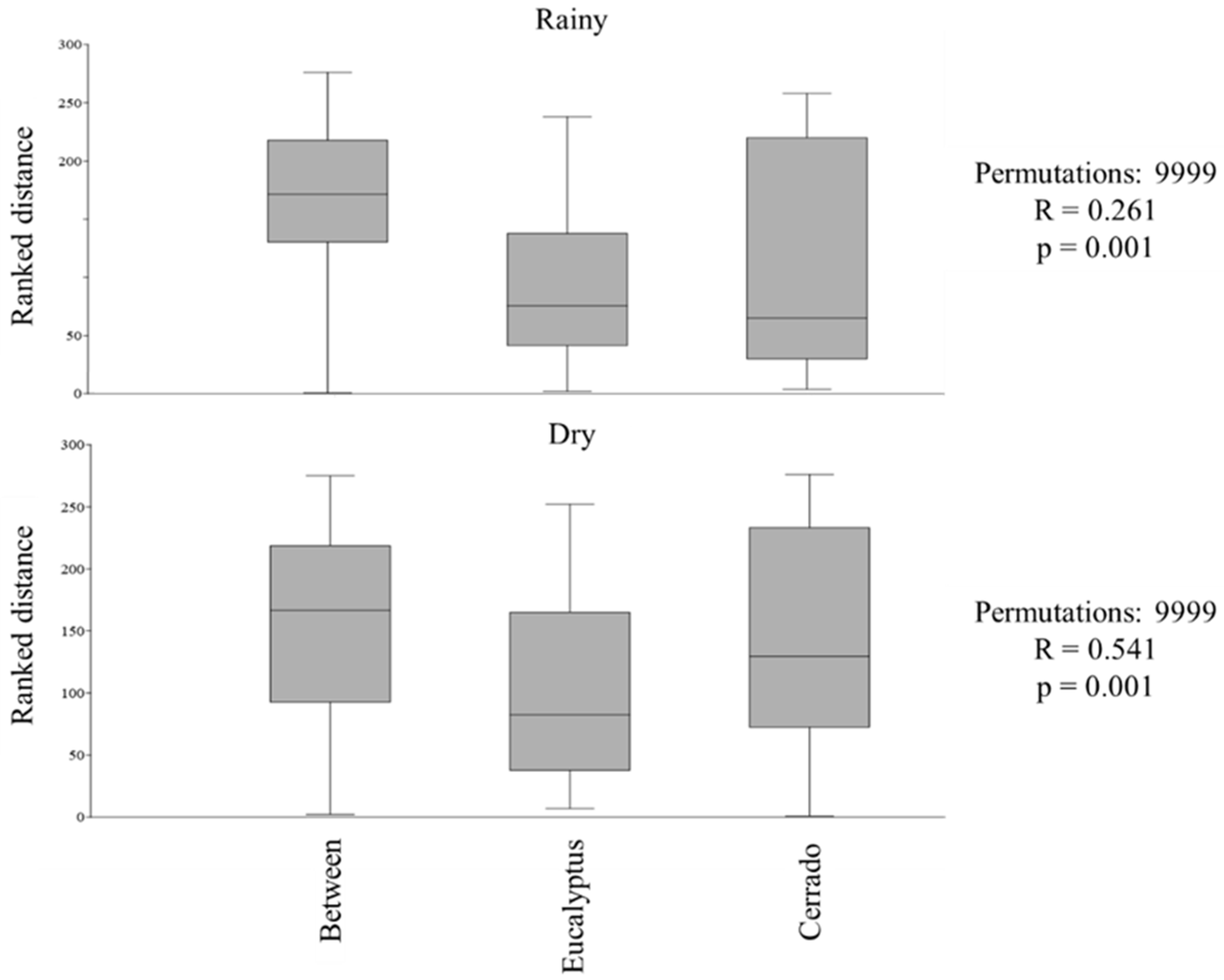
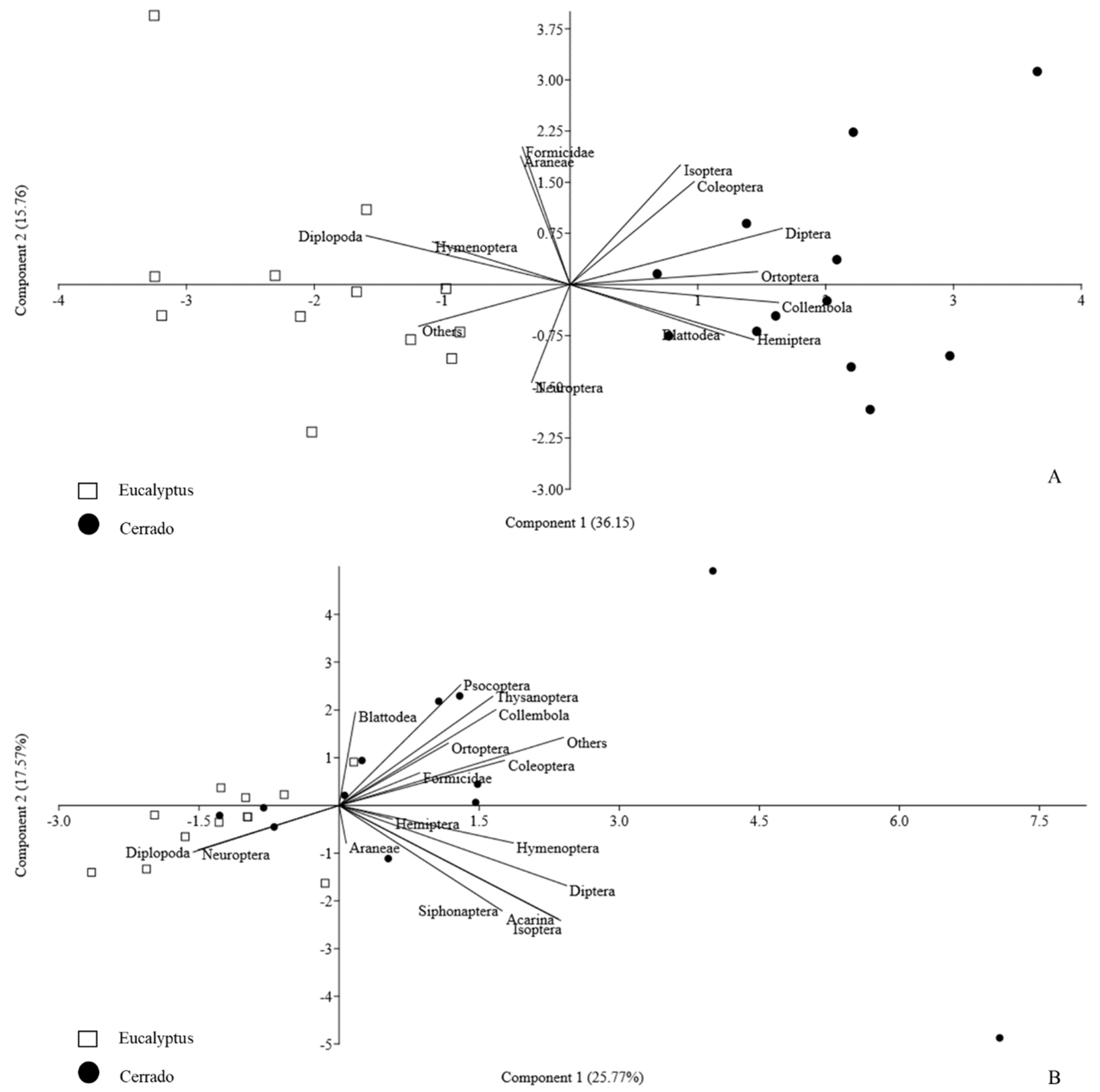
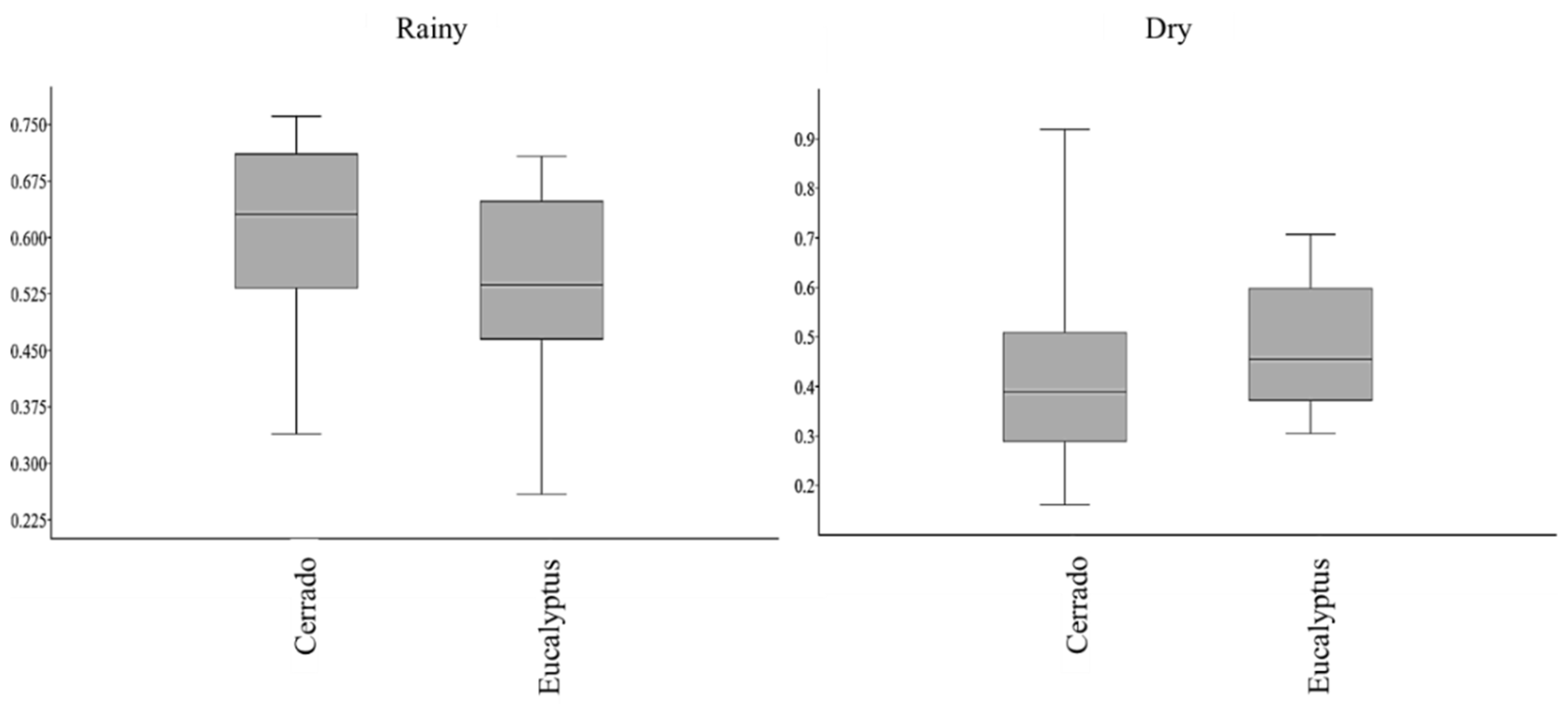
3.1. Diversity in Rainy and Dry Seasons
3.2. Correlations between Soil Chemistry Parameters and Soil Mesofauna
4. Discussion
4.1. Soil Mesofauna Presented Differences between eucalyptus and Cerrado Areas in Both Seasons
4.2. Organic Matter, Potassium, Calcium, Phosphorous, and pH Are Correlated to Soil Mesofauna
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Rainy Collections | |||||
|---|---|---|---|---|---|
| Overrall Average Dissimilarity—58.3 | |||||
| Taxon | Average Dissimilarity | Contribution % | Cumulative % | Mean Eucalyptus | Mean Cerrado |
| Collembola | 18.1 | 31.05 | 31.05 | 15.5 | 82.9 |
| Isoptera | 13.3 | 22.82 | 53.87 | 1.17 | 168 |
| Formicidae | 11.69 | 20.05 | 73.91 | 78.8 | 68.3 |
| Diptera | 6.824 | 11.7 | 85.62 | 12.5 | 40.3 |
| Hemiptera | 3.78 | 6.484 | 92.1 | 3.58 | 16.3 |
| Coleoptera | 1.492 | 2.559 | 94.66 | 1.42 | 9.42 |
| Araneae | 1.122 | 1.925 | 96.59 | 6.5 | 5.5 |
| Ortoptera | 0.8821 | 1.513 | 98.1 | 0.5 | 3.92 |
| Hymenoptera | 0.2816 | 0.4831 | 98.58 | 1.08 | 0.25 |
| Others | 0.2481 | 0.4256 | 99.01 | 0.917 | 0 |
| Diplopoda | 0.241 | 0.4134 | 99.42 | 0.917 | 0 |
| Blattodea | 0.236 | 0.4048 | 99.83 | 0.0833 | 0.833 |
| Neuroptera | 0.1014 | 0.1739 | 100 | 0.333 | 0.0833 |
| Dry season | |||||
| Overrall average dissimilarity—62.79 | |||||
| Taxon | Average dissimilarity | Contribution % | Cumulative % | Mean Eucalyptus | Mean Cerrado |
| Formicidae | 28.75 | 45.8 | 45.8 | 91.8 | 198 |
| Isoptera | 13.98 | 22.27 | 68.06 | 2 | 159 |
| Diplopoda | 6.779 | 10.8 | 78.86 | 28 | 0.167 |
| Diptera | 3.723 | 5.93 | 84.79 | 9.67 | 30.3 |
| Coleoptera | 2.262 | 3.602 | 88.39 | 4.5 | 11.3 |
| Araneae | 2.033 | 3.239 | 91.63 | 12.8 | 3.75 |
| Hymenoptera | 1.025 | 1.633 | 93.26 | 2.25 | 6.17 |
| Collembola | 0.919 | 1.464 | 94.73 | 1.17 | 3.92 |
| Others | 0.7098 | 1.13 | 95.86 | 0.167 | 2.83 |
| Hemiptera | 0.5647 | 0.8994 | 96.76 | 2.17 | 3 |
| Thysanoptera | 0.5268 | 0.8391 | 97.59 | 0 | 1.92 |
| Ortoptera | 0.4608 | 0.734 | 98.33 | 1.25 | 1.92 |
| Neuroptera | 0.4186 | 0.6668 | 99 | 1.67 | 0 |
| Acarina | 0.2436 | 0.3879 | 99.38 | 0.25 | 3.67 |
| Psocoptera | 0.159 | 0.2532 | 99.64 | 0 | 0.75 |
| Blattodea | 0.1367 | 0.2177 | 99.85 | 0.417 | 0.333 |
| Siphonaptera | 0.09155 | 0.1458 | 100 | 0.25 | 0.417 |
References
- Hurtt, G.C.; Chini, L.; Sahajpal, R.; Frolking, S.; Bodirsky, B.L.; Calvin, K.; Doelman, J.C.; Fisk, J.; Fujimori, S.; Goldewijk, K.K.; et al. Harmonization of global land use change and management for the period 850–2100 (LUH2) for CMIP6. Geosci. Model Dev. 2020, 13, 5425–5464. [Google Scholar] [CrossRef]
- Pompeo, P.N.; Oliveira Filho, L.C.I.D.; Klauberg Filho, O.; Mafra, Á.L.; Baretta, D. Coleoptera diversity and soil properties in land use systems. Floresta Ambiente 2020, 27, 1–10. [Google Scholar] [CrossRef]
- Rosa, M.G.D.; Klauberg Filho, O.; Bartz, M.L.C.; Mafra, Á.L.; Sousa, J.P.F.A.D.; Baretta, D. Macrofauna edáfica e atributos físicos e químicos em sistemas de uso do solo no planalto catarinense. Rev. Bras. Ciênc. Solo 2015, 39, 1544–1553. [Google Scholar] [CrossRef]
- Beaumelle, L.; Thouvenot, L.; Hines, J.; Jochum, M.; Eisenhauer, N.; Phillips, H.R. Soil fauna diversity and chemical stressors: A review of knowledge gaps and roadmap for future research. Ecography 2021, 44, 845–859. [Google Scholar] [CrossRef]
- Morandi, P.S.; Marimon, B.S.; Marimon-Junior, B.H.; Ratter, J.A.; Feldpausch, T.R.; Colli, G.R.; Munhoz, C.B.R.; da Silva Júnior, M.C.; de Souza Lima, E.; Haidar, R.F.; et al. Tree diversity and above-ground biomass in the South America Cerrado biome and their conservation implications. Biodivers. Conserv. 2020, 29, 1519–1536. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; Da Fonseca, G.A.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Assis, L.F.; Ferreira, K.R.; Vinhas, L.; Maurano, L.; Almeida, C.; Carvalho, A.; Rodrigues, J.; Maciel, A.; Camargo, C. TerraBrasilis: A spatial data analytics infrastructure for large-scale thematic mapping. ISPRS Int. J. Geo-Inf. 2019, 8, 513. [Google Scholar] [CrossRef]
- Zuin, V.G. What can be learnt from the Brazilian Cerrado? In Biomass Burning in Sub-Saharan Africa; Springer: Dordrecht, The Netherlands, 2020; pp. 143–160. [Google Scholar] [CrossRef]
- MAPBIOMAS. Coleção MapBiomas. 2020. Available online: https://brasil.mapbiomas.org/produtos (accessed on 4 December 2023).
- Boeno, D.; Silva, R.F.; Almeida, H.S.; Rodrigues, A.C.; Vanzan, M.; Andreazza, R. Influence of eucalyptus development under soil fauna. Braz. J. Biol. 2019, 80, 345–353. [Google Scholar] [CrossRef]
- Yang, X.; Shao, M.; Li, T.; Gan, M.; Chen, M. Community characteristics and distribution patterns of soil fauna after vegetation restoration in the northern Loess Plateau. Ecol. Indic. 2021, 122, 107236. [Google Scholar] [CrossRef]
- Baretta, D.; Santos, J.C.P.; Mafra, Á.L.; do Prado Wildner, L.; Miquelluti, D.J. Fauna edáfica avaliada por armadilhas e catação manual afetada pelo manejo do solo na região oeste catarinense. Rev. Ciênc. Agrovet. 2003, 2, 97–106. [Google Scholar]
- Rieff, G.G.; Natal-da-Luz, T.; Sousa, J.P.; Wallau, M.O.; Hahn, L.; de Sá, E.L.S. Collembolans and Mites communities as a tool for assessing soil quality: Effect of eucalyptus plantations on soil mesofauna biodiversity. Curr. Sci. 2016, 110, 713–719. [Google Scholar] [CrossRef]
- da Silva, P.H.M.; Poggiani, F.; Laclau, J.P. Applying sewage sludge to Eucalyptus grandis plantations: Effects on biomass production and nutrient cycling through litterfall. Appl. Environ. Soil Sci. 2011, 2011, 710614. [Google Scholar] [CrossRef]
- Gonçalves, F.; Carlos, C.; Crespo, L.; Zina, V.; Oliveira, A.; Salvação, J.; Pereira, J.A.; Torres, L. Soil Arthropods in the Douro Demarcated Region Vineyards: General Characteristics and Ecosystem Services Provided. Sustainability 2021, 13, 7837. [Google Scholar] [CrossRef]
- Souza, S.T.D.; Cassol, P.C.; Baretta, D.; Bartz, M.L.C.; Klauberg Filho, O.; Mafra, Á.L.; Rosa, M.G.D. Abundance and diversity of soil macrofauna in native forest, eucalyptus plantations, perennial pasture, integrated crop-livestock, and no-tillage cropping. Rev. Bras. Ciênc. Solo 2016, 40, 1–14. [Google Scholar] [CrossRef]
- Bardgett, R.; Van der Putten, W. Belowground biodiversity and ecosystem functioning. Nature 2014, 515, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Guerra, C.A.; Heintz-Buschart, A.; Sikorski, J.; Chatzinotas, A.; Guerrero-Ramírez, N.; Cesarz, S.; Beaumelle, L.; Rillig, M.C.; Maestre, F.T.; Delgado-Baquerizo, M.; et al. Blind spots in global soil biodiversity and ecosystem function research. Nat. Commun. 2020, 11, 3870. [Google Scholar] [CrossRef]
- Portilho, I.I.R.; Crepaldi, R.A.; Borges, C.D.; Silva, R.F.D.; Salton, J.C.; Mercante, F.M. Fauna invertebrada e atributos físicos e químicos do solo em sistemas de integração lavoura-pecuária. Pesqui. Agropecu. Bras. 2011, 46, 1310–1320. [Google Scholar] [CrossRef]
- Silveira, E.R. Diversidade e papel funcional da macrofauna do solo na integração lavoura-pecuária. Rev. Téc.-Cient. 2016, 4, 1–16. [Google Scholar]
- Vendrame, P.R.S.; Marchão, R.L.; Brito, O.R.; Guimarães, M.D.F.; Becquer, T. Relationship between macrofauna, mineralogy and exchangeable calcium and magnesium in Cerrado Oxisols under pasture. Pesqui. Agropecu. Bras. 2009, 44, 996–1001. [Google Scholar] [CrossRef]
- de Faria, A.C.G.S.; da Silva, M.F.R.; Oliveira, D.M.S.; Vanin, L.G.S. Macrofauna edáfica em área de Cerrado regenerado. Rev. Biol. Ciênc. Terra 2021, 21, 39–45. [Google Scholar]
- Baretta, D.; Bartz, M.L.C.; Fachini, I.; Anselmi, R.; Zortéa, T.; Baretta, C.R.D.M. Soil fauna and its relation with environmental variables in soil management systems. Rev. Ciênc. Agron. 2014, 45, 871–879. [Google Scholar] [CrossRef]
- Haridasan, M. Nutritional adaptations of native plants of the Cerrado biome in acid soils. Braz. J. Plant Physiol. 2008, 20, 183–195. [Google Scholar] [CrossRef]
- Nimer, E. Climatologia do Brasil; Departamento de Recursos Naturais e Estudos Ambientais: Rio de Janeiro, Brazil, 1989; 421p. [Google Scholar]
- IUSS Working Group WRB. World Reference Base for Soil Resources. In World Soil Resources Report 103; FAO: Rome, Italy, 2006. [Google Scholar]
- Santos, H.G.; Jacomine, P.K.T.; Anjos, L.H.C.; Oliveira, V.A.; Lumbreras, J.F.L.; Coelho, M.R.; Almeida, J.A.; Araújo Filho, J.C.; Oliveira, J.B.; Cunha, T. Brazilian System of Soil Classification, 5th ed.; Embrapa: Brasília, Brazil, 2018; Available online: https://www.redeilpf.org.br/arquivos/SiBCS-2018-ISBN-9788570358219-english.pdf (accessed on 2 December 2023).
- Felfili, J.M.; Ribeiro, J.F.; Fagg, C.W.; Machado, J.W.B. Recuperação de Matas de Galeria. 2000. Available online: https://www.infoteca.cnptia.embrapa.br/infoteca/bitstream/doc/564008/1/doc21.pdf (accessed on 4 December 2023).
- Mota, F.M. Biomassa, Fluxos de Carbono e Energia em Área de Cerrado Sentido Restrito e Plantio de Eucalipto no Distrito Federal. Ph.D. Thesis, Faculdade de Tecnologia, Universidade de Brasília, Brasília, Brazil, 2017. [Google Scholar]
- Sauvadet, M.; Chauvat, M.; Brunet, N.; Bertrand, I. Can changes in litter quality drive soil fauna structure and functions? Soil Biol. Biochem. 2017, 107, 94–103. [Google Scholar] [CrossRef]
- Lavelle, P.; Spain, A.V. Soil organisms. In Soil Ecology; Springer: Berlin/Heidelberg, Germany, 2001; pp. 201–356. [Google Scholar]
- Ribeiro, J.F.; Walter, B.M.T. Fitofisionomias do bioma Cerrado: Os biomas do Cerrado. In Cerrado: Ambiente e Flora; Embrapa: Planaltina, Brazil, 1998; pp. 89–166. [Google Scholar]
- Assad, M.L.L. Fauna do solo. In Biologia dos Solos dos Cerrados, 1st ed; EMBRAPA: Planaltina, Brazil, 1977; pp. 363–443. [Google Scholar]
- Ribeiro, F.P.; Gatto, A.; Oliveira, A.D.; Pulrolnik, K.; Ferreira, E.A.B.; Carvalho, A.D.; Bussinguer, Â.P.; Muller, A.G.; Moraes-neto, S.D. Litter dynamics in Eucalyptus and native forest in the Brazilian Cerrado. J. Agric. Sci. 2018, 10, 29–43. [Google Scholar] [CrossRef]
- Mesibov, R. Cambaloid millipedes of Tasmania, Australia, with remarks on family-level classification and descriptions of two new genera and four new species (Diplopoda, Spirostreptida). ZooKeys 2019, 827, 1–17. [Google Scholar] [CrossRef]
- Silva, V.M.; Antoniolli, Z.I.; Jacques, R.J.S.; Ott, R.; da Silva Rodrigues, P.E.; Andrade, F.V.; Passos, R.R.; de Sá Mendonça, E. Influence of the tropical millipede, Glyphiulus granulatus (Gervais, 1847), on aggregation, enzymatic activity, and phosphorus fractions in the soil. Geoderma 2017, 289, 135–141. [Google Scholar] [CrossRef]
- Prastyaningsih, S.R.; Hardiwinoto, S.; Musyafa, M.; Koranto, C.A.D. Diversity of termites (Isoptera) on industrial forest plantation of Eucalyptus pellita stands of tropical ecosystem in Riau, Indonesia. Biodiversitas J. Biol. Divers. 2020, 21, 5498–5505. [Google Scholar] [CrossRef]
- Barros, E.; Pashanasi, B.; Constantino, R.; Lavelle, P. Effects of land-use system on the soil macrofauna in western Brazilian Amazonia. Biol. Fertil. Soils 2002, 35, 338–347. [Google Scholar] [CrossRef]
- Martins, L.; de Morais Pereira, J.; Tonelli, M.; Baretta, D. Composição da macrofauna do solo sob diferentes usos da terra (cana-de-açúcar, eucalipto e mata nativa) em Jacutinga (MG). Rev. Agrogeoambient. 2017, 9, 11–22. [Google Scholar] [CrossRef][Green Version]
- Tacca, D.; Klein, C.; Preuss, J.F. Artropodofauna do solo em um bosque de eucalipto e um remanescente de mata nativa no sul do Brasil. Rev. Thema 2017, 14, 249–261. [Google Scholar] [CrossRef][Green Version]
- Oliveira Filho, L.C.I.; Klauberg Filho, O.; Baretta, D.; Tanaka, C.A.S.; Sousa, J.P. Collembola community structure as a tool to assess land use effects on soil quality. Rev. Bras. Ciênc. Solo 2016, 40, 1–18. [Google Scholar] [CrossRef]
- Hopkin, S.P. Biology of the Springtails (Insecta: Collembola); Oxford University Press: Oxford, UK, 1997; 344p. [Google Scholar]
- Tavares, P.D.; da Silva, C.F.; Pereira, M.G.; da Silva, E.M.R. Composition of the soil fauna community and leaf litter stock in agro-forestry systems and secondary forestry. Biosci. J. 2020, 36, 1377–1389. [Google Scholar] [CrossRef]
- Viana, G.G.; Albuquerque, G.S. Polimorfismo no padrão de manchas tegumentares de larvas e adultos de Ceraeochrysa caligata (Neuroptera: Chrysopidae) e redescrição dos instares larvais. Zool. Curitiba 2009, 26, 166–174. [Google Scholar] [CrossRef]
- Tauber, C.A.; Tauber, M.J.; Albuquerque, G.S. Neuroptera (lacewings, antlions). In Encyclopedia of Insect; Elsevier: Amsterdam, The Netherlands, 2009; pp. 695–707. [Google Scholar]
- Freitas, S.; Penny, N.D. Neuroptera Linnaeus, 1758. In Insetos do Brasil: Diversidade e Taxonomia; Holos Editora: Ribeirão Preto, Brazil, 2012; pp. 537–546. [Google Scholar]
- Zhu, X.; Zhu, B. Diversity and abundance of soil fauna as influenced by long-term fertilization in cropland of purple soil, China. Soil Tillage Res. 2015, 146, 39–46. [Google Scholar] [CrossRef]
- Lavelle, P.; Chauvel, A.; Fragoso, C. Faunal Activity in Acid Soils. In Plant-Soil Interactions at Low pH: Principles and Management, Proceedings of the Third International Symposium on Plant-Soil Interactions at Low, pH, Brisbane, Australia; Springer: Amsterdam, The Netherlands, 1993; pp. 12–16. [Google Scholar]
- Carvalho, T.A.; Reis, P.R.; Bernardi, L.F.; Marafeli, P.P.; Martinez, P.A. Edaphic mites and their response to the incorporation of organic matter from various species of fabaceae into the soil beneath coffee trees. Acarina 2018, 26, 183–195. [Google Scholar] [CrossRef]
- Kime, R.D.; Golovatch, S.I. Trends in the ecological strategies and evolution of millipedes (Diplopoda). Biol. J. Linn. Soc. 2000, 69, 333–349. [Google Scholar] [CrossRef]
- Francisco da Silva, E.; Pinheiro Reis Lourente, E.; Estevão Marchetti, M.; Martins Mercante, F.; Karolina Teixeira Ferreira, A.; Carneiro Fujii, G. Frações Lábeis e Recalcitrantes Da Matéria Orgânica Solos Sob Integração Lavoura pecuária. Pesq. Agropec. Bras. 2011, 46. [Google Scholar] [CrossRef]
- Susser, J.R.; Pelini, S.L.; Weintraub, M.N. Can we reduce phosphorus runoff from agricultural fields by stimulating soil biota? J. Environ. Qual. 2020, 49, 933–944. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Yang, W.; Yue, K.; Tan, B.; Wu, F. Impacts of soil fauna on nitrogen and phosphorus release during litter decomposition were differently controlled by plant species and ecosystem type. J. For. Res. (Harbin) 2019, 30, 921–930. [Google Scholar] [CrossRef]
- Juhos, K.; Czigány, S.; Madarász, B.; Ladányi, M. Interpretation of soil quality indicators for land suitability assessment—A multivariate approach for Central European arable soils. Ecol. Indic. 2019, 99, 261–272. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inkotte, J.; Bomfim, B.; Rosa, M.G.d.; Valadão, M.B.X.; Gatto, A.; Santos, J.A.; Pereira, R.S. Changes in Land Use through Eucalyptus Plantations Impact Soil Fauna Communities in Brazilian Savannas. Sustainability 2024, 16, 2943. https://doi.org/10.3390/su16072943
Inkotte J, Bomfim B, Rosa MGd, Valadão MBX, Gatto A, Santos JA, Pereira RS. Changes in Land Use through Eucalyptus Plantations Impact Soil Fauna Communities in Brazilian Savannas. Sustainability. 2024; 16(7):2943. https://doi.org/10.3390/su16072943
Chicago/Turabian StyleInkotte, Jonas, Barbara Bomfim, Márcio Gonçalves da Rosa, Marco Bruno Xavier Valadão, Alcides Gatto, Juscelina Arcanjo Santos, and Reginaldo Sergio Pereira. 2024. "Changes in Land Use through Eucalyptus Plantations Impact Soil Fauna Communities in Brazilian Savannas" Sustainability 16, no. 7: 2943. https://doi.org/10.3390/su16072943
APA StyleInkotte, J., Bomfim, B., Rosa, M. G. d., Valadão, M. B. X., Gatto, A., Santos, J. A., & Pereira, R. S. (2024). Changes in Land Use through Eucalyptus Plantations Impact Soil Fauna Communities in Brazilian Savannas. Sustainability, 16(7), 2943. https://doi.org/10.3390/su16072943








