Comparison of the Effects of Different Organic Amendments on the Immobilization and Phytoavailability of Lead
Abstract
1. Introduction
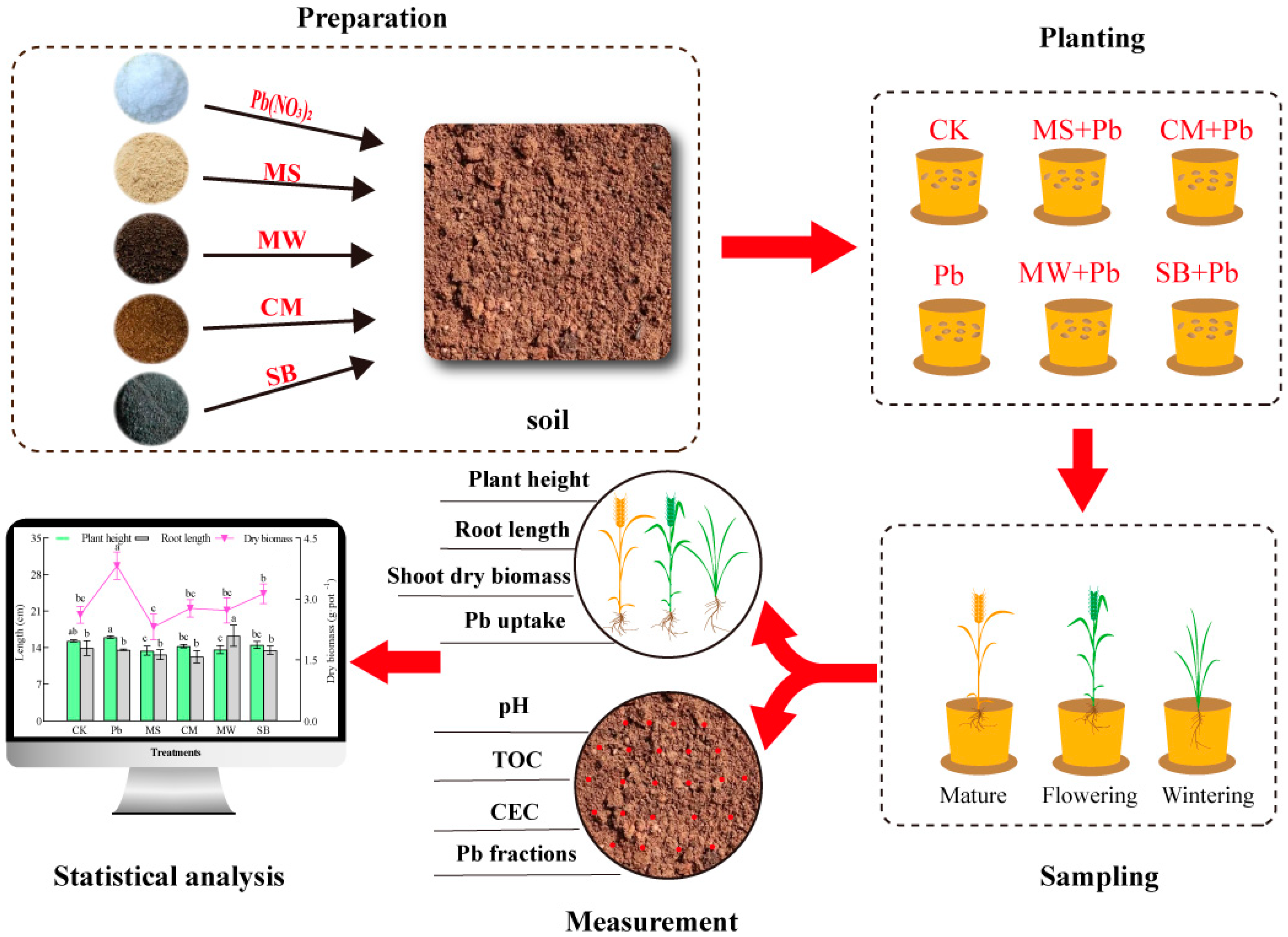
2. Materials and Methods
2.1. Preparation of Soils and Amendments
2.2. Pot Experimental Design
2.3. Pb Analysis
2.4. Characterization and Analysis
- (1)
- Organic amendment characterization
- (2)
- Physico-chemical property analysis
- (3)
- Growth parameter analysis
2.5. Statistical Analysis
3. Results
3.1. Organic Amendment Characterization
3.1.1. Basic Properties of the Organic Amendments
3.1.2. SEM Results of Organic Amendments
3.1.3. FTIR Spectrometry Results of Organic Amendments
3.2. Soil Physico-Chemical Property Analysis
3.2.1. Basic Soil Characteristics
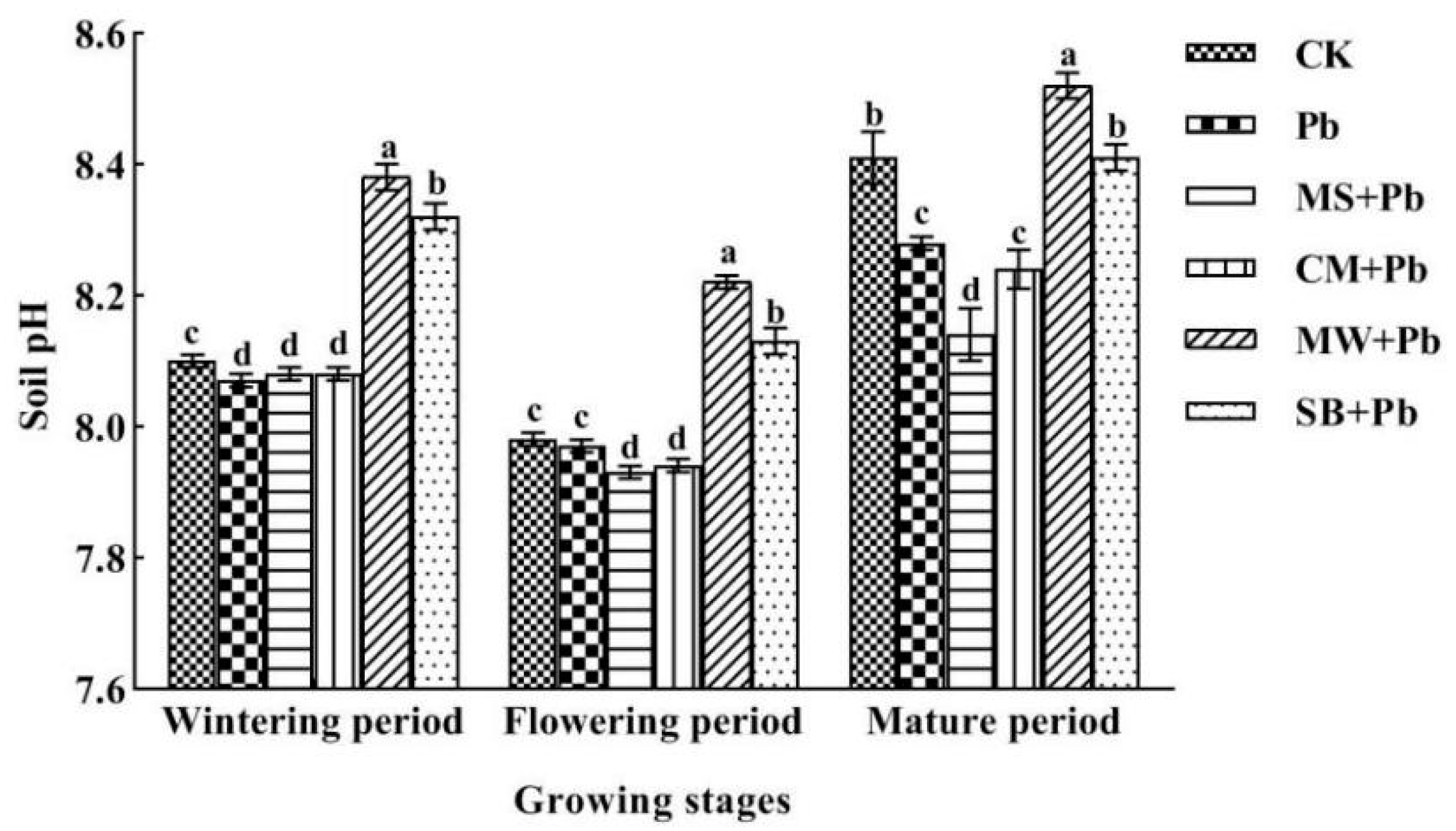
3.2.2. Impact of Organic Amendments on soil Chemical Properties
- (1)
- Soil pH
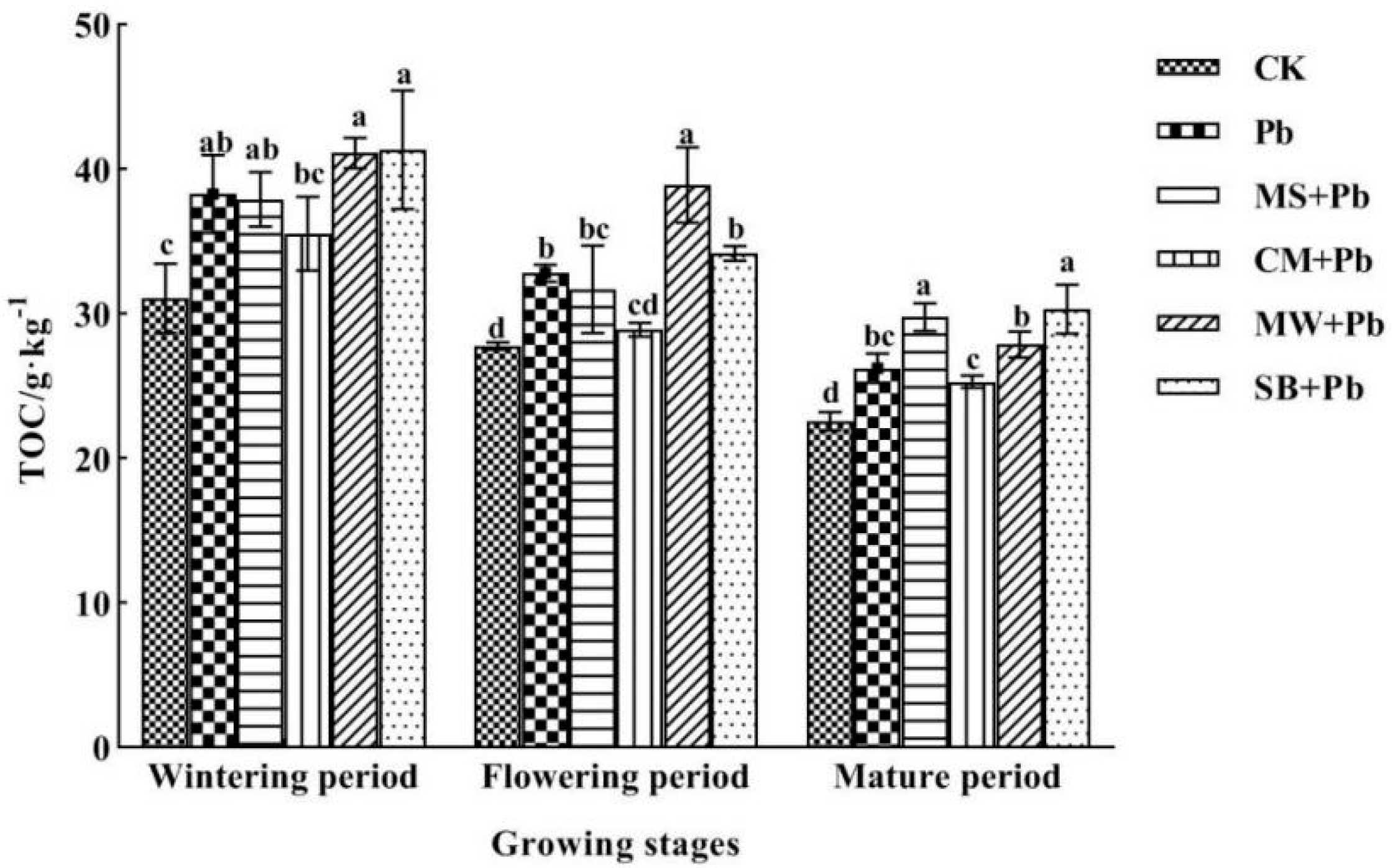
- (2)
- Content of TOC
- (3)
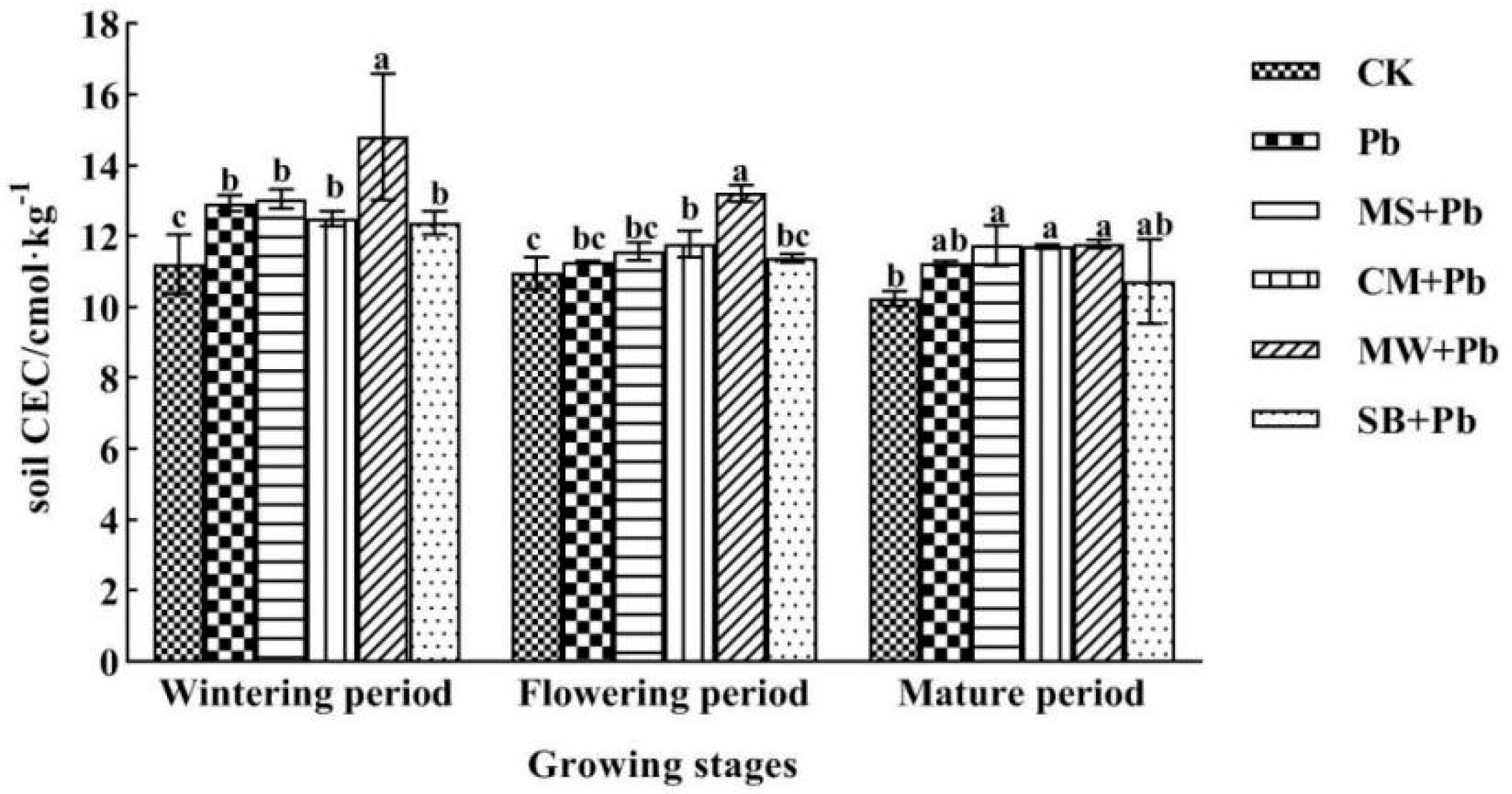
- Capacity of CEC
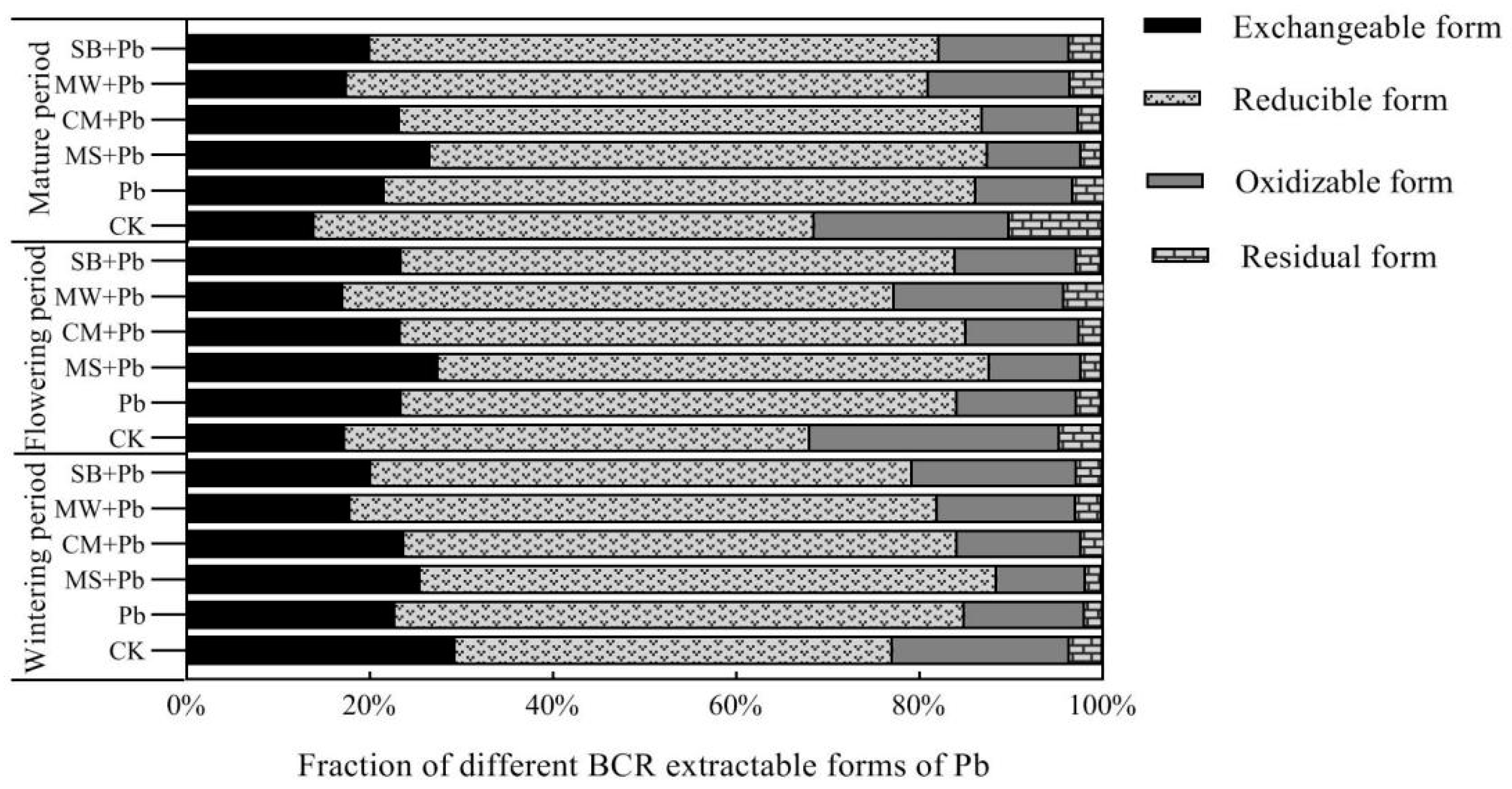
3.3. Impact of Organic Amendments on Soil Pb Fractions
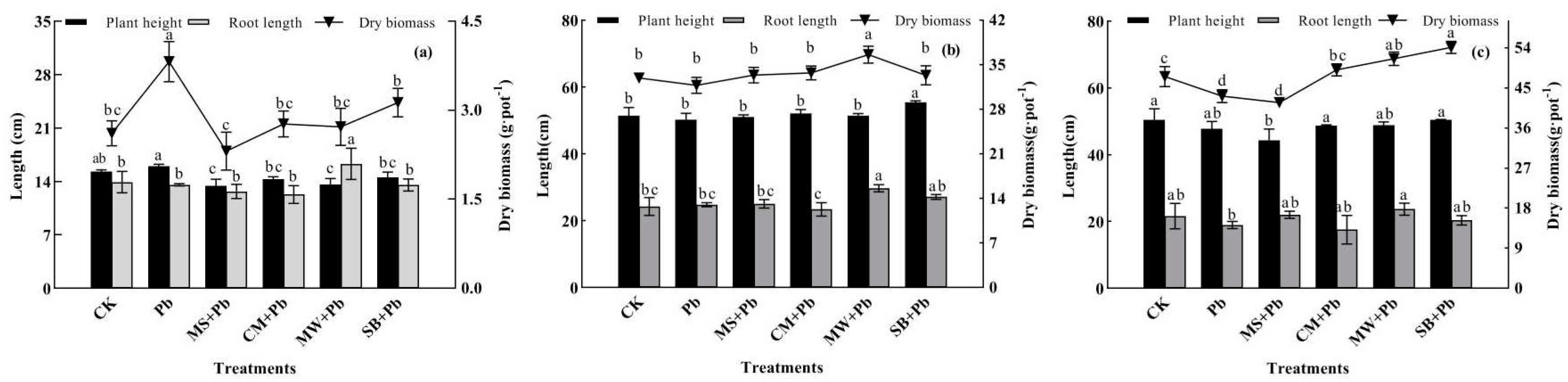
3.4. Impact of Organic Amendments on Plant Growth
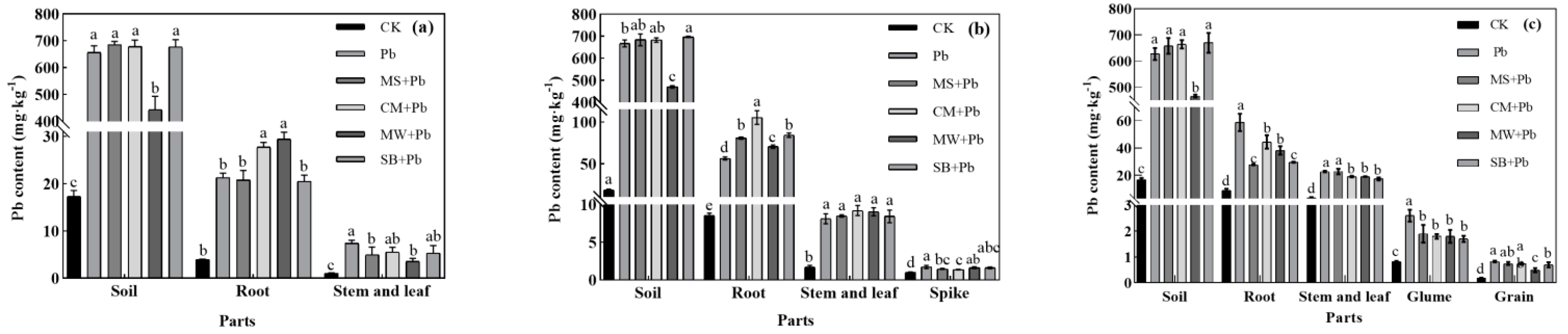
3.5. Impact of Organic Amendments on Pb Uptake
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mu, M.Q.; Wang, Z.C.; Chen, Z.R.; Wu, Y.C.; Nie, W.T.; Zhao, S.W.; Yin, X.J.; Teng, X.H. Physiological characteristics, rhizosphere soil properties, and root-related microbial communities of Trifolium repens L. in response to Pb toxicity. Sci. Total Environ. 2023, 907, 167871. [Google Scholar] [CrossRef] [PubMed]
- Giti, T.F.; David, A.R.; Ursula, K.; Saideh, G. Environmental and human health risks of potentially harmful elements in mining-impacted soils: A case study of the Angouran Zn–Pb Mine, Iran. J. Environ. Manag. 2023, 334, 117470. [Google Scholar]
- Huang, C.Y.; Guo, Z.H.; Li, T.S.; Xu, R.; Peng, C.; Gao, Z.L.; Zhong, L.J. Source identification and migration fate of metal(loid)s in soil and groundwater from an abandoned Pb/Zn mine. Sci. Total Environ. 2023, 895, 165037. [Google Scholar] [CrossRef]
- Shi, Z.Y.; Lu, J.P.; Liu, T.X.; Zhao, X.Q.; Liu, Y.H.; Mi, J.H.; Zhao, X.Z. Risk assessment and source apportionment of available atmospheric heavy metal in a typical sandy area reservoir in Inner Mongolia, China. Sci. Total Environ. 2023, 912, 168960. [Google Scholar] [CrossRef]
- Yang, D.J.; Yang, Y.; Hua, Y.P. Source anaysis base on the positive matrix factorization models and risk assessment of heavy metals in agricultural soil. Sustainability 2023, 15, 13225. [Google Scholar] [CrossRef]
- Beiyuan, J.; Awad, Y.M.; Beckers, F.; Tsang, D.C.W.; Ok, Y.S.; Rinklebe, J. Mobility and phytoavailability of As and Pb in a contaminated soil using pine sawdust biochar under systematic change of redox conditions. Chemosphere 2017, 178, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Zeng, G.; Gong, J.; Liang, J.; Xu, P.; Liu, Z.; Zhang, Y.; Zhang, C.; Cheng, M.; Liu, Y.; et al. Evaluation methods for assessing effectiveness of in situ remediation of soil and sediment contaminated with organic pollutants and heavy metals. Environ. Int. 2017, 105, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Hannan, F.; Huang, Q.; Farooq, M.A.; Ayyaz, A.; Ma, J.; Zhang, N.; Ali, B.; Deyett, E.; Zhou, W.; Islam, F. Organic and inorganic amendments for the remediation of nickel contaminated soil and its improvement on Brassica napus growth and oxidative defense. J. Hazard. Mater. 2021, 416, 125921. [Google Scholar] [CrossRef] [PubMed]
- Palansooriya, K.N.; Shaheen, S.M.; Chen, S.S.; Tsang, D.C.; Hashimoto, Y.; Hou, D.; Bolan, N.S.; Rinklebe, J.; Ok, Y.S. Soil amendments for immobilization of potentially toxic elements in contaminated soils: A critical review. Environ. Int. 2020, 134, 105046. [Google Scholar] [CrossRef]
- Berhane, M.; Xu, M.; Liang, Z.Y.; Shi, J.L.; Wei, G.H.; Tian, X.H. Effects of long term straw return on soil organic carbon storage and sequestration rate in North China upland crops: A meta-analysis. Glob. Chang. Biol. 2020, 4, 26. [Google Scholar] [CrossRef]
- Ju, J.; Cai, Y.X.; Zuo, W.G.; Hai, T.Z.; Yang, H.J.; Mao, W.; Yu, H.S.; Ke, F. Effects of nitrogen management on soil nitrogen content and rice grain yield in double cropping rice production area with continuous full amount of straw returning. Commun. Soil Sci. Plant Anal. 2019, 50, 2655–2668. [Google Scholar] [CrossRef]
- Shan, A.; Pan, J.; Kang, K.J.; Pan, M.; Wang, G.; Wang, M.; He, Z.; Yang, X. Effects of straw return with N fertilizer reduction on crop yield, plant diseases and pests and potential heavy metal risk in a Chinese rice paddy: A field study of 2 consecutive wheat-rice cycles. Environ. Pollut. 2021, 288, 117741. [Google Scholar] [CrossRef] [PubMed]
- Aritonang, S.P.; Panjaitan, E.; Tondang, F. Cucumber plants (Cucumis sativus L.) growth and crop yield of chicken manure fertilized with plant spacing. IOP Conf. Ser. Earth Environ. Sci. 2018, 130, 012045. [Google Scholar] [CrossRef]
- Wan, Y.; Huang, Q.; Wang, Q.; Yu, Y.; Su, D.; Qiao, Y.; Li, H. Accumulation and bioavailability of heavy metals in an acid soil and their uptake by paddy rice under continuous application of chicken and swine manure. J. Hazard. Mater. 2020, 384, 121293. [Google Scholar] [CrossRef] [PubMed]
- Han, L.Z.; Chen, Y.H.; Chen, M.L.; Wu, Y.F.; Su, R.K.; Du, L.; Liu, Z.M. Mushroom residue modification enhances phytoremediation potential of Paulownia fortunei to lead-zinc slag. Chemosphere 2020, 253, 126774. [Google Scholar] [CrossRef] [PubMed]
- Castanho, N.R.C.M.; Oliveira, R.A.; Batista, B.L.; Freire, B.M.; Lange, C.; Lopes, A.M.; Jozala, A.F.; Grotto, D. Comparative Study on Lead and Copper Biosorption Using Three Bioproducts from Edible Mushrooms Residues. J. Fungi 2021, 7, 441. [Google Scholar] [CrossRef] [PubMed]
- Azeem, M.; Hayat, R.; Hussain, Q.; Ahmed, M.; Pan, G.X.; Tahir, M.I.; Imran, M.; Irfan, M.; Hassan, M.U. Biochar improves soil quality and N-2-fixation and reduces net ecosystem CO2 exchange in a dryland legume-cereal cropping system. Soil Tillage Res. 2019, 186, 172–182. [Google Scholar] [CrossRef]
- Xiao, L.; Yuan, G.D.; Feng, L.R.; Bi, D.X.; Wei, J. Soil properties and the growth of wheat (Triticum aestivum L.) and maize (Zea mays L.) in response to reed (Phragmites communis) biochar use in a salt-affected soil in the Yellow River Delta. Agric. Ecosyst. Environ. 2020, 303, 107124. [Google Scholar] [CrossRef]
- Cooper, J.; Greenberg, I.; Ludwig, B.; Hippich, L.; Fischer, D.; Glaser, B.; Kaiser, M. Effect of biochar and compost on soil properties and organic matter in aggregate size fractions under field conditions. Agric. Ecosyst. Environ. 2020, 295, 106882. [Google Scholar] [CrossRef]
- Mehmood, I.; Qiao, L.; Chen, H.; Tang, Q.; Woolf, D.; Fan, M. Biochar addition leads to more soil organic carbon sequestration under a maize-rice cropping system than continuous flooded rice. Agric. Ecosyst. Environ. 2020, 298, 106965. [Google Scholar] [CrossRef]
- Fungo, B.; Lehmann, J.; Kalbitz, K.; Thiongo, M.; Okeyo, I.; Tenywa, M.; Neufeldt, H. Aggregate size distribution in a biochar-amended tropical Ultisol under conventional hand-hoe tillage. Soil Tillage Res. 2017, 165, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Huang, Y.; Lin, J.; Chen, X.; Li, C.; Zhang, J. Biochar applied to consolidated land increased the quality of an acid surface soil and tobacco crop in Southern China. J. Soils Sediments 2020, 20, 3091–3102. [Google Scholar] [CrossRef]
- Sun, C.; Wang, D.; Shen, X.; Li, C.; Liu, J.; Lan, T.; Wang, W.; Xie, H.; Zhang, Y. Effects of biochar, compost and straw input on root exudation of maize (Zea mays L.): From function to morphology. Agric. Ecosyst. Environ. 2020, 297, 106952. [Google Scholar] [CrossRef]
- Raj, A.; Yadav, A.; Arya, S.; Sirohi, R.; Kumar, S.; Rawat, A.P.; Thakur, R.S.; Patel, D.K.; Bahadur, L.; Pandey, A. Preparation, characterization and agri applications of biochar produced by pyrolysis of sewage sludge at different temperatures. Sci. Total Environ. 2021, 795, 148722. [Google Scholar] [CrossRef] [PubMed]
- Bogusz, A.; Oleszczuk, P. Effect of biochar addition to sewage sludge on cadmium, copper and lead speciation in sewage sludge-amended soil. Chemosphere 2020, 239, 124719. [Google Scholar] [CrossRef] [PubMed]
- Cang, L.; Xing, J.F.; Liu, C.; Wang, Y.J.; Zhou, D.M. Effects of different water management strategies on the stability of cadmium and copper immobilization by biochar in rice-wheat rotation system. Ecotoxicol. Environ. Saf. 2020, 202, 110887. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Shaheen, S.M.; Guo, D.; Li, Y.; Xiao, R.; Wahid, F.; Azeem, M.; Sohail, K.; Zhang, T.; Rinklebe, J.; et al. Apricot shell-and apple tree-derived biochar affect the fractionation and bioavailability of Zn and Cd as well as the microbial activity in smelter contaminated soil. Environ. Pollut. 2020, 264, 114773. [Google Scholar] [CrossRef] [PubMed]
- Nemati, K.; Bakar, N.K.A.; Abas, M.R.; Sobhanzadeh, E. Speciation of heavy metals by modified BCR sequential extraction procedure in different depths of sediments from Sungai Buloh, Selangor, Malaysia. J. Hazard. Mater. 2011, 192, 402–410. [Google Scholar] [CrossRef]
- Bremner, J.M. Determination of nitrogen in soil by the Kjeldahl method. J. Agric. Sci. 1960, 55, 11–33. [Google Scholar] [CrossRef]
- Li, W.K.; Niu, S.K.; Liu, X.D.; Wang, J.M. Short-term response of the soil bacterial community to differing wildfire severity in Pinus tabulaeformis stands. Sci. Rep. 2019, 9, 1148. [Google Scholar] [CrossRef] [PubMed]
- Pehlivan, E.; Altun, T.; Cetin, S.; Bhanger, M.I. Lead sorption by waste biomass of hazelnut and almond shell. J. Hazard. Mater. 2009, 167, 1203–1208. [Google Scholar] [CrossRef] [PubMed]
- Singha, B.; Das, S.K. Removal of Pb (II) ions from aqueous solution and industrial effluent using natural biosorbents. Environ. Sci. Pollut. Res. 2012, 19, 2212–2226. [Google Scholar] [CrossRef] [PubMed]
- Han, R.P.; Zhang, L.J.; Chen, S.; Zhang, M.M.; Zhu, H.M.; Zhan, L.J. Characterization of modified wheat straw, kinetic and equilibrium study about copper ion and methylene blue adsorption in batch mode. Carbohydr. Polym. 2010, 79, 1140–1149. [Google Scholar] [CrossRef]
- El-Naggar, A.; Lee, M.H.; Hur, J.; Lee, Y.H.; Igalavithana, A.D.; Shaheen, S.M.; Ryu, C.; Rinklebe, J.; Tsang, D.C.W.; Ok, Y.S. Biochar-induced metal immobilization and soil biogeochemical process: An integrated mechanistic approach. Sci. Total Environ. 2020, 698, 134112. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.P.; Shen, R.; Ji, S.B.; Xie, L.K.; Zhang, H.B. Effects of biochar derived from sewage sludge and sewage sludge/cotton stalks on the immobilization and phytoavailability of Pb, Cu, and Zn in sandy loam soil. J. Hazard. Mater. 2021, 419, 126468. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.H.; Hu, L.L.; Zhang, C.A.; Wang, S.S.; Wang, X.Z.; Huo, Z.Y. Preparation of biochar -interpenetrated iron-alginate hydrogel as a pH-independent sorbent for removal of Cr(VI) and Pb(II). Environ. Pollut. 2021, 287, 117303. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.T.; Xiao, Q.; Malik, Z.; Su, Y.; Wang, Y.F.; Lu, S.G. Crop straw-derived biochar alleviated cadmium and copper phytotoxicity by reducing bioavailability and accumulation in a field experiment of rice-rape-corn rotation system. Chemosphere 2021, 280, 130830. [Google Scholar] [CrossRef]
- Yue, Y.; Shen, C.; Ge, Y. Biochar accelerates the removal of tetracyclines and their intermediates by altering soil properties. J. Hazard. Mater. 2019, 380, 120821. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.K.; Zheng, G.D.; Chen, T.B.; Shi, X.X.; Wang, Y.W.; Nie, E.Q.; Liu, J.W. Effect of phosphate amendments on improving the fertilizer efficiency and reducing the mobility of heavy metals during sewage sludge composting. J. Environ. Manag. 2019, 235, 124–132. [Google Scholar] [CrossRef]
- Jin, J.W.; Wang, M.Y.; Cao, Y.C.; Wu, S.C.; Liang, P.; Li, Y.N.; Zhang, J.Y.; Zhang, J.; Wong, M.H.; Shan, S.D.; et al. Cumulative effects of bamboo sawdust addition on pyrolysis of sewage sludge: Biochar properties and environmental risk from metals. Bioresour. Technol. 2017, 228, 218–226. [Google Scholar] [CrossRef]
- Liu, X.S.; Zhang, X.; Li, R.; Wang, G.L.; Jin, Y.; Xu, W.Y.; Wang, H.M.; Qu, J.J. Organic amendment improves rhizosphere environment and shapes soil bacterial community in black and red soil under lead stress. J. Hazard. Mater. 2021, 416, 125805. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.F.; Liu, D.X.; Ma, J.H.; Jin, B.Y.; Peng, J.B.; He, X.L. Assessing the influence of immobilization remediation of heavy metal contaminated farmland on the physical properties of soil. Sci. Total. Environ. 2021, 781, 146773. [Google Scholar] [CrossRef] [PubMed]
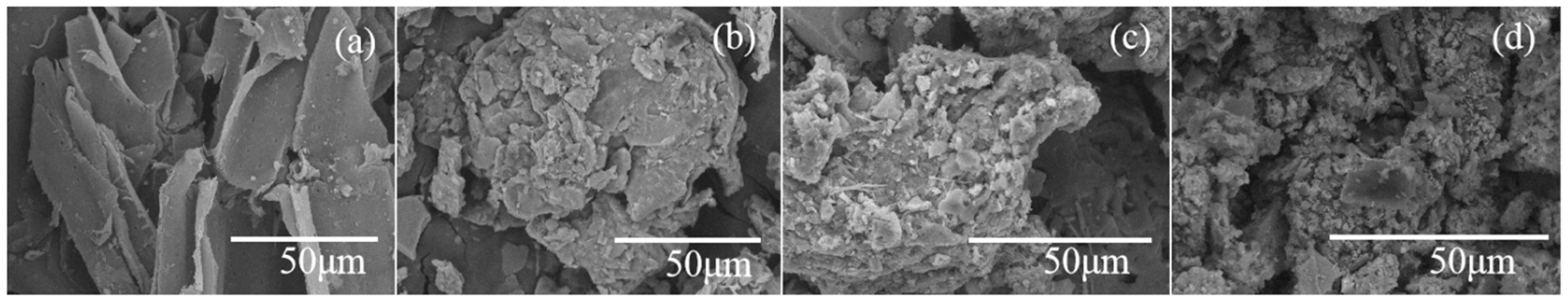

| Fraction | Phase(s) | Extraction Reagent(s) | Procedures |
|---|---|---|---|
| Exchangeable form | Soil solution, carbonates, exchangeable metals | 0.11 mol·L−1 HOAc | 40 mL of acetic acid is added to 1 g of the sample in a centrifuge tube and shaken for 16 h at 25 °C. The extract is separated from solid residues via centrifugation and then filtered for measurement. |
| Reducible form | Oxides Fe/Mn | 0.5 mol·L−1 NH2OH·HCl (pH = 2) | 40 mL of freshly prepared hydroxylammonium chloride is added to the residue from step 1 and re-suspended by shaking for 16 h at 25 °C. The remaining operations are the same as in step 1. |
| Oxidizable form | Organic matter and sulfides | (1) 30% H2O2 | The residue from step 2 is treated with hydrogen peroxide and digested for 1 h at room temperature and 1 h at 85 °C several times, until the volume is reduced to 2–3 mL. Then, 50 mL of 1.0 M ammonium acetate is added to the cold mixture and the rest of the operations are the same as in step 1. |
| (2) 1 mol·L−1 NH4OAc (pH = 2) | |||
| Residual form | Remaining, non-silicate-bound metals | HNO3/HCl | The residue from step 3 is microwave digested using a mixture of HNO3 and HCl. |
| Parameter | MS | CM | MW | SB |
|---|---|---|---|---|
| BET surface area (m2·g−1) | 2.4 | 4.83 | 6.9 | 174.85 |
| Total pore volume (cm−3·g−1) | 0.0077 | 0.013 | 0.02 | 0.1139 |
| Average pore diameter (nm) | 12.94 | 10.75 | 11.61 | 2.61 |
| Organic matter content (g·kg−1) | 789.94 | 265.34 | 647.13 | 443.74 |
| pH | 6.91 | 5.18 | 8.49 | 9.88 |
| Pb content (mg·kg−1) | 9.62 | 11.76 | 14.09 | 30.98 |
| Functional Groups | MS | CM | MW | SB |
|---|---|---|---|---|
| Surface O-H stretching | 3406 | 3411 | 3407 | 3435 |
| Aliphatic C-H stretching | 2921 | 2923 | 2924 | — |
| Aliphatic acid C=O stretching | 1732 | — | 1793 | 1796 |
| Unsaturated group like alkene | 1637 | 1630 | 1635 | — |
| Aromatic C-NO2 stretching | 1515 | — | — | — |
| Carboxylic C-O stretching | 1426 | — | 1426 | 1431 |
| Alcoholic and phenolic C-O stretching | 1042 | 1033 | 1035 | 1040 |
| OM (g·kg−1) | TN (g·kg−1) | AN (mg·kg−1) | pH | CEC (cmol·kg−1) | Pb Content (mg·kg−1) |
|---|---|---|---|---|---|
| 52.54 | 1.43 | 30.26 | 8.05 | 13.56 | 17.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Chen, J.; An, J.; Wang, X.; Shao, Y. Comparison of the Effects of Different Organic Amendments on the Immobilization and Phytoavailability of Lead. Sustainability 2024, 16, 2981. https://doi.org/10.3390/su16072981
Wang X, Chen J, An J, Wang X, Shao Y. Comparison of the Effects of Different Organic Amendments on the Immobilization and Phytoavailability of Lead. Sustainability. 2024; 16(7):2981. https://doi.org/10.3390/su16072981
Chicago/Turabian StyleWang, Xiaojie, Jingwen Chen, Jiahui An, Xueping Wang, and Yun Shao. 2024. "Comparison of the Effects of Different Organic Amendments on the Immobilization and Phytoavailability of Lead" Sustainability 16, no. 7: 2981. https://doi.org/10.3390/su16072981
APA StyleWang, X., Chen, J., An, J., Wang, X., & Shao, Y. (2024). Comparison of the Effects of Different Organic Amendments on the Immobilization and Phytoavailability of Lead. Sustainability, 16(7), 2981. https://doi.org/10.3390/su16072981





