Variations in δ13CDIC and Influencing Factors in a Shallow Macrophytic Lake on the Qinghai–Tibetan Plateau: Implications for the Regional Carbon Cycle and Sustainable Development
Abstract
1. Introduction
2. Study Area
3. Materials and Methods
4. Results
4.1. Physical and Chemical Parameters of Lake Water
4.2. Spatial Variations in DIC Isotopic Composition in the GGH Basin Waterbodies
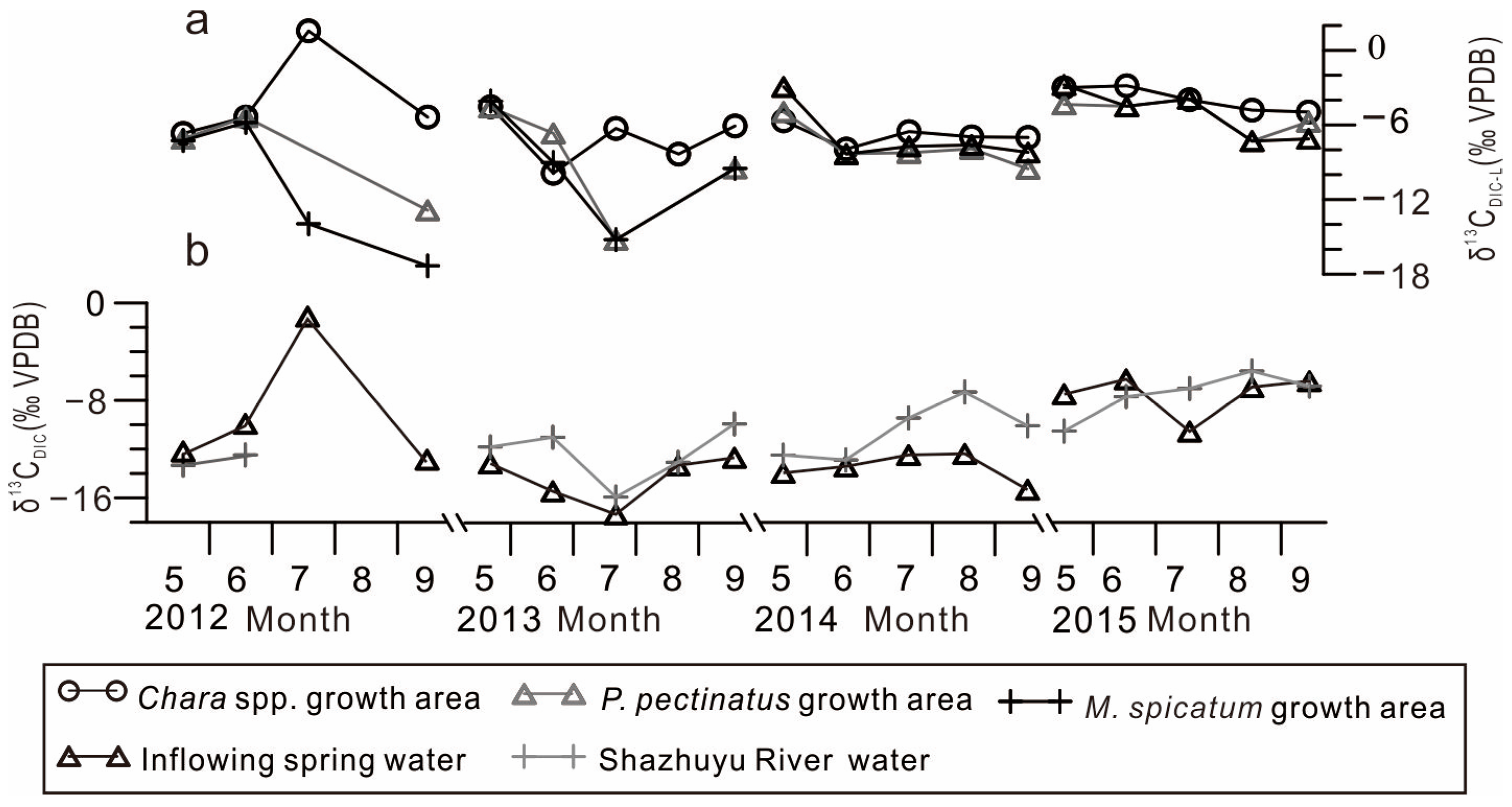
4.3. Temporal Variations in DIC Isotopic Compositions in the GGH Basin Waterbodies
5. Discussion
5.1. Factors Affecting δ13CDIC in the GGH Basin Waterbodies
- (1)
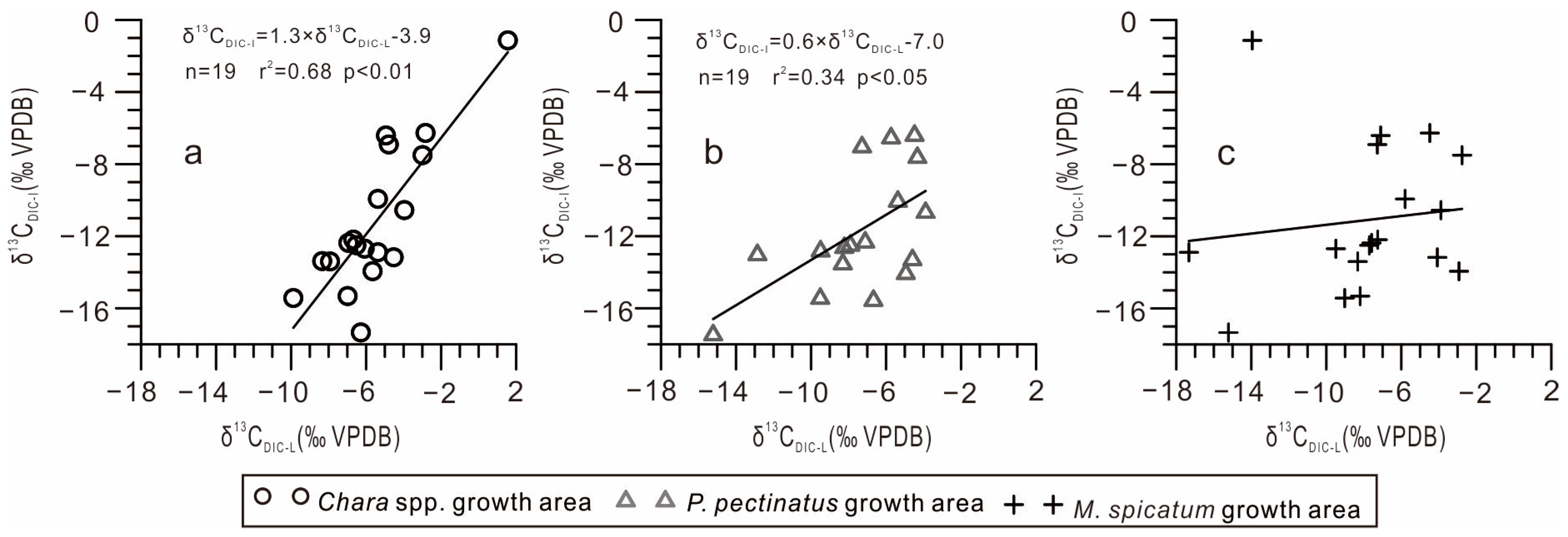
- Lake inflow carbon isotope composition. The isotopic composition of the lake inflow directly affects the isotopic composition of the lake. This is especially significant in exorheic lakes or lakes with a short water retention time. In the Chara spp. community and P. pectinatus growth area, the δ13CDIC-L and δ13CDIC-I values were positively correlated, whereas in the M. spicatum community growth area, these values showed no correlation (Figure 5). This is because the M. spicatum community was located far from the small spring-water streams, where weak exchange with spring water took place (Figure 1b). This reveals that the δ13CDIC-I values were key factors influencing the δ13CDIC-L values.
- (2)
- Exchange with atmospheric CO2. The DIC pool in the lake tends to be in isotopic equilibrium with the atmosphere via CO2 exchange. During this process, 12C-rich CO2 is preferentially released from the lake surface into the atmosphere, resulting in a DIC pool that is enriched in 13C. This exchange process is slow; therefore, its effect on the δ13CDIC-L values is more notable in endorheic lakes with a long retention time. However, it is not easily observable in lakes with short retention times or rapid circulation [23]. When the exchange between lake water and atmospheric CO2 reaches an equilibrium, the δ13CDIC-L values range from 1 to 3‰ [24,25].
- (3)
- Organic matter decomposition in lake sediments. Sedimentary organic matter in lakes includes native aquatic plants and terrestrial organic debris transported into the lake from the surrounding watershed. Once degraded, this organic matter increases the 12C-enriched DIC composition of lake water [9]. Organic matter decomposition in Qingmuke Lake (a freshwater lake located on the Qiangtang Plateau) resulted in a DIC isotope value equal to, or even lower than, that of river water [10]. In contrast, the δ13CDIC-L values in the GGH Lake were significantly more positive than that of the Shazhuyu River, indicating that organic matter decomposition may have had a relatively small effect on the DIC lake composition. Additionally, methane produced by organic matter decomposition resulted in a more negative δ13CDIC value. Organic matter decomposition can cause a decrease in the δ13CDIC values to −50‰ [28]. This value is significantly lower than the mean δ13CDIC-L value of the GGH Lake, which indicates that the CO2 or methane produced via decomposition did not have a significant effect on seasonal or interannual changes in the δ13CDIC-L values.
- (4)
- Lake photosynthetic activity. In highly productive lakes, photosynthesis is a key factor that affects the δ13CDIC lake water values [29] (pp. 197–207), [30] (pp. 99–118). During photosynthesis, plants preferentially uptake 12C, which yields more negative δ13C values for plants and the δ13CDIC of the water body becomes more positive [31]. Charaphytes are an important submerged aquatic macrophyte. Compared with vascular plants, charaphytes have a higher photosynthetic rate and lower respiration rate. The preferential uptake of 12CO2 for photosynthetic purposes could have led to the 13C-enrichment of DIC in the lake water [32]. During intense photosynthesis, dissolved CO2 in lake water is limited [33]. When this occurs, charaphytes use HCO3 for photosynthetic activity. Compared with vascular plants, charaphytes can use HCO3− for photosynthetic activity more effectively [21]. According to Equations (1)–(3), the δ13C values of HCO3− were more positive than those of H2CO3 and CO32− in the lake water. In contrast, the photosynthetic activity of charaphytes results in carbonate precipitation in the surrounding waters, forming thick CaCO3 encrustations [33]. This also leads to 13C-enriched water in the charaphyte growth area.
- (5)
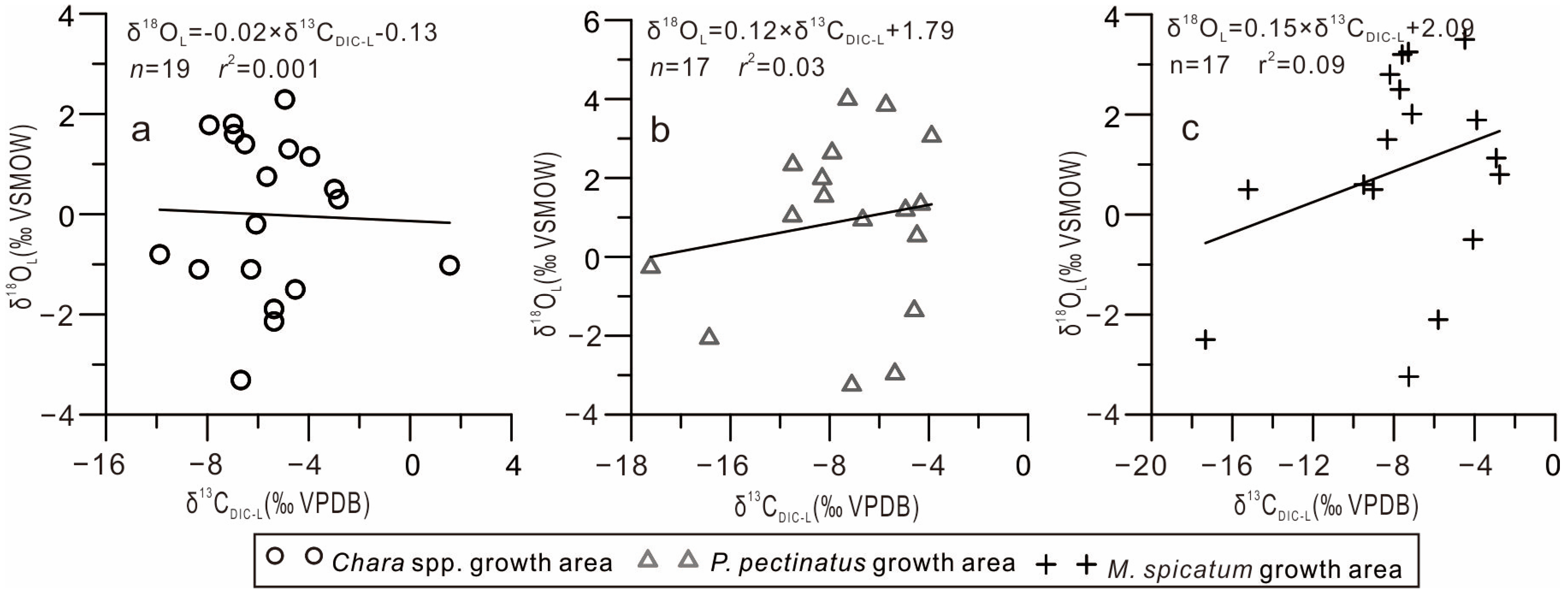
- Water retention time. In arid regions, with extended lake water residence times, strong evaporation leads to the preferential loss of the light 12CO2 and 16O2 isotopes, yielding more positive δ13CDIC-L and oxygen (δ18O) isotopic lake water compositions; furthermore, a significant positive correlation was observed between δ13CDIC-L and δ18OL [35]. Monitoring results revealed that the δ18OL values of the GGH Lake significantly deviated from the global meteoric water line but were consistent with the local evaporation line, indicating that evaporation affected the δ18OL lake water composition [19,36]. However, this study found that the δ13CDIC-L and δ18OL values of the GGH Lake were not correlated (Figure 6), indicating that evaporation may have had only a minimal effect on the δ13CDIC-L value of the lake.
5.2. Isotopic Composition of DIC in the GGH Basin Groundwater and Shazhuyu River
5.3. Implications of Lake Water DIC Isotopic Composition on the Carbon Cycle
6. Conclusions
- (1)
- For the overall DIC isotopic composition in the GGH Basin, we found that δ13CDIC-I was the most negative, followed by δ13CDIC-R; δ13CDIC-L was the most positive. This was caused by isotope fractionation resulting from the photosynthesis of aquatic plants after spring water inflow into the lake.
- (2)
- Owing to variations in the photosynthetic activity intensity of different aquatic plants, there were also significant variations in the δ13CDIC-L values in areas with different aquatic plants. This likely occurred because Chara spp. plants have a higher photosynthetic rate and are more capable of using CO2 for photosynthetic activity, converting them into plant organisms.
- (3)
- Variations in the δ13CDIC-L were primarily affected by the δ13CDIC-I and aquatic plant photosynthesis. The change in δ13CDIC-I to a more positive value resulted from carbon isotope equilibration between 13C from carbonate weathering in the watershed and 12CO2 from soil respiration.
- (4)
- The changes in the δ13CDIC-L composition of the GGH Lake indicated that the DIC from lake inflow and the photosynthesis of aquatic plants were the key components in the carbon cycle of the lake. This provides more supportive evidence to estimate the regional carbon budget and sustainable development.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Holgerson, M.A.; Raymond, P.A. Large contribution to inland water CO2 and CH4 emissions from very small ponds. Nat. Geosci. 2016, 9, 222. [Google Scholar] [CrossRef]
- Cole, J.J.; Caraco, N.F.; Kling, G.W.; Kratz, T.K. Carbon dioxide supersaturation in the surface waters of lakes. Science 1994, 265, 1568–1570. [Google Scholar] [CrossRef] [PubMed]
- Ran, L.; Lu, X.X.; Liu, S. Dynamics of riverine CO2 in the Yangtze River fluvial network and their implications for carbon evasion. Biogeosciences 2017, 14, 2183–2198. [Google Scholar] [CrossRef]
- Yan, F.; Sillanpää, M.; Kang, S.; Aho, K.S.; Qu, B.; Wei, D.; Raymond, P.A. Lakes on the Tibetan Plateau as conduits of greenhouse gases to the atmosphere. J. Geophys. Res.-Biogeosci. 2018, 123, 2091–2103. [Google Scholar] [CrossRef]
- Hanson, P.C.; Pollard, A.I.; Bade, D.L.; Predick, K.; Carpenter, S.R.; Foley, J.A. A model of carbon evasion and sedimentation in temperate lakes. Glob. Chang. Biol. 2004, 10, 1285–1298. [Google Scholar] [CrossRef]
- Balmer, M.B.; Downing, J.A. Carbon dioxide concentrations in eutrophic lakes: Undersaturation implies atmospheric uptake. Inland Waters 2011, 1, 125–132. [Google Scholar] [CrossRef]
- Wang, T.; Piao, S.L. Estimate of terrestrial carbon balance over the Tibetan Plateau: Progresses, challenges and perspectives. Quat. Sci. 2023, 43, 313–323, (In Chinese with English abstract). [Google Scholar]
- Zhang, J.; Quay, P.D.; Wilbur, D.O. Carbon isotope fractionation during gas-water exchange and dissolution of CO2. Geochim. Cosmochim. Acta 1995, 59, 107–114. [Google Scholar] [CrossRef]
- Myrbo, A.; Shapley, M.D. Seasonal water-column dynamics of dissolved inorganic carbon stable isotopic compositions (δ13CDIC) in small hardwater lakes in Minnesota and Montana. Geochim. Cosmochim. Acta 2006, 70, 2699–2714. [Google Scholar] [CrossRef]
- Lei, Y.; Yao, T.; Sheng, Y.; Zhang, E.; Wang, W.; Li, J. Characteristics of δ13CDIC in lakes on the Tibetan Plateau and its implications for the carbon cycle. Hydrol. Process. 2012, 26, 535–543. [Google Scholar] [CrossRef]
- Mu, C.; Zhang, T.; Wu, Q.; Peng, X.; Zhang, P.; Yang, Y.; Cheng, G. Dissolved organic carbon, CO2, and CH4 concentrations and their stable isotope ratios in thermokarst lakes on the Qinghai-Tibetan Plateau. J. Limnol. 2016, 75, 313–319. [Google Scholar] [CrossRef]
- Fang, Y.; Cheng, W.; Zhang, Y.; Wang, N.; Zhao, S.; Zhou, C.; Bao, A. Changes in inland lakes on the Tibetan Plateau over the past 40 years. J. Geogr. Sci. 2016, 26, 415–438. [Google Scholar] [CrossRef]
- Zhang, G.; Yao, T.; Chen, W.; Zheng, G.; Shum, C.K.; Yang, K.; Piao, S.; Sheng, Y.; Yi, S.; Li, J. Regional differences of lake evolution across China during 1960s-2015 and its natural and anthropogenic causes. Remote Sens. Environ. 2019, 221, 386–404. [Google Scholar] [CrossRef]
- Dong, G.R.; Gao, S.Y.; Jin, J. Desertification and Controls in the Gonghe Basin, Qinghai Province; Science Press: Beijing, China, 1993; pp. 1–166. [Google Scholar]
- Winkler, M.G.; Wang, P.K. The late-Quaternary vegetation and climate of China. In Global Climates Since the Last Glacial Maximum; Wright, H.E., Jr., Kutzbach, J.E., Webb, T., III, Ruddiman, W.F., Street-Perrott, F.A., Bartlein, P.J., Eds.; University of Minnesota Press: Minneapolis, MN, USA, 1993; pp. 221–264. [Google Scholar]
- Qiang, M.; Song, L.; Chen, F.; Li, M.; Liu, X.; Wang, Q. A 16-ka lake-level record inferred from macrofossils in a sediment core from Genggahai Lake, northeastern Qinghai–Tibetan Plateau (China). J. Paleolimnol. 2013, 49, 575–590. [Google Scholar] [CrossRef]
- Li, Y.; Qiang, M.; Jin, Y.; Liu, L.; Zhou, A.; Zhang, J. Influence of aquatic plant photosynthesis on the reservoir effect of Genggahai Lake, northeastern Qinghai-Tibetan Plateau. Radiocarbon 2018, 60, 561–569. [Google Scholar] [CrossRef]
- Wachniew, P.; Różański, K. Carbon budget of a mid-latitude, groundwater-controlled lake: Isotopic evidence for the importance of dissolved inorganic carbon recycling. Geochim. Cosmo-Chim. Acta 1997, 61, 2453–2465. [Google Scholar] [CrossRef]
- Song, L.; Qiang, M.; Lang, L.; Liu, X.; Wang, Q.; Li, M. Changes in palaeoproductivity of Genggahai Lake over the past 16 ka in the Gonghe Basin, northeastern Qinghai-Tibetan Plateau. Chin. Sci. Bull. 2012, 57, 2595–2605. [Google Scholar] [CrossRef]
- Jin, Y.; Qiang, M.; Liu, Y.; Li, Y.; Li, H.; Li, F. Variations in carbon and oxygen isotopes of carbonate and water environments: A case study from Genggahai Lake, northeastern Qinghai-Tibetan Plateau. Chin. Sci. Bull. 2015, 60, 847–856. (In Chinese) [Google Scholar]
- Van den Berg, M.S.; Coops, H.; Simons, J.; Pilon, J. A comparative study of the use of inor-ganic carbon resources by Chara aspera and Potamogeton pectinatus. Aquat. Bot. 2002, 72, 219–233. [Google Scholar] [CrossRef]
- Bade, D.L.; Carpenter, S.R.; Cole, J.J.; Hanson, P.C.; Hesslein, R.H. Controls of δ13C-DIC in lakes: Geochemistry, lake metabolism, and morphometry. Limnol. Oceanogr. 2004, 49, 1160–1172. [Google Scholar] [CrossRef]
- Shen, J.; Yuan, H.; Liu, E.; Wang, J.; Wang, Y. Spatial distribution and stratigraphic character-istics of surface sediments in Taihu Lake. China Sci. Bull. 2011, 56, 179–187. [Google Scholar] [CrossRef]
- Deuser, W.G.; Degens, E.T. Carbon isotope fractionation in the system CO2(gas)—CO2(aqueous)—HCO3−(aqueous). Nature 1967, 215, 1033. [Google Scholar] [CrossRef]
- Leng, M.J.; Marshall, J.D. Palaeoclimate interpretation of stable isotope data from lake sediment archives. Quat. Sci. Rev. 2004, 23, 811–831. [Google Scholar] [CrossRef]
- Das, A.; Krishnaswami, S.; Bhattacharya, S.K. Carbon isotope ratio of dissolved inorganic carbon (DIC) in rivers draining the Deccan Traps, India: Sources of DIC and their magnitudes. Earth Planet. Sci. Lett. 2005, 236, 419–429. [Google Scholar] [CrossRef]
- Stumm, W.; Morgan, J.J. Aquatic Chemistry: An Introduction Emphasizing Chemical Equilibria in Natural Waters, 3rd ed.; Wiley: New York, NY, USA, 1970; p. 583. [Google Scholar]
- Sun, Q.; Xie, M.; Shi, L.; Zhang, Z.; Lin, Y.; Shang, W.; Chu, G. Alkanes, compound-specific carbon isotope measures and climate variation during the last millennium from varved sediments of Lake Xiaolongwan, northeast China. J. Paleolimnol. 2013, 50, 331–344. [Google Scholar] [CrossRef]
- McKenzie, J.A. Carbon-13 Cycle in Lake Greifen: A Model for Restricted Ocean Basins. In Nature and Origin of Cretaceous Carbon-Rich Facies; Schlanger, S.O., Cita, M.B., Eds.; Academic Press: London, UK, 1982; pp. 197–207. [Google Scholar]
- McKenzie, J.A. Carbon Isotopes and Productivity in the Lacustrine and Marine Environment. In Chemical Processes in Lakes; Stumm, W., Ed.; Wiley: New York, NY, USA, 1985; pp. 99–118. [Google Scholar]
- Andrews, J.E.; Coletta, P.; Pentecost, A.; Riding, B.; Dennis, S.; Dennis, P.F.; Spiro, B. Equilibrium and disequilibrium stable isotope effects in modern charophyte calcites: Implications for palaeoenvironmental studies. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2004, 204, 101–114. [Google Scholar] [CrossRef]
- Pełechaty, M.; Apolinarska, K.; Pukacz, A.; Krupska, J.; Siepak, M.; Boszke, P.; Sinkowski, M. Stable isotope composition of Chara rudis incrustation in Lake Jasne, Poland. Hydrobiologia 2010, 656, 29–42. [Google Scholar] [CrossRef]
- Herczeg, A.L.; Fairbanks, R.G. Anomalous carbon isotope fractionation between atmospheric CO2 and dissolved inorganic carbon induced by intense photosynthesis. Geochim. Cosmochim. Acta 1987, 51, 895–899. [Google Scholar] [CrossRef]
- Pentecost, A.; Andrews, J.E.; Dennis, P.F.; Marca-Bel, A.; Dennis, S. Charophyte growth in small temperate water bodies: Extreme isotopic disequilibrium and implications for the palaeoecology of shallow marl lakes. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2006, 240, 389–404. [Google Scholar] [CrossRef]
- Li, H.C.; Ku, T.L. δ13C–δ18C covariance as a paleohydrological indicator for closed-basin lakes. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1997, 133, 69–80. [Google Scholar] [CrossRef]
- Qiang, M.; Song, L.; Jin, Y.; Li, Y.; Liu, L.; Zhang, J.; Chen, F. A 16-ka oxygen-isotope record from Genggahai Lake on the northeastern Qinghai-Tibetan Plateau: Hydroclimatic evolution and changes in atmospheric circulation. Quat. Sci. Rev. 2017, 162, 72–87. [Google Scholar] [CrossRef]
- Jin, Y.; Jin, X.; Yang, D.; Mao, X. Controlling Factors of Surface Water Ionic Composition Characteristics in the Lake Genggahai Catchment, NE Qinghai–Tibetan Plateau, China. Water 2019, 11, 1329. [Google Scholar] [CrossRef]
- Brunet, F.; Gaiero, D.; Probst, J.L.; Depetris, P.J.; Gauthier Lafaye, F.; Stille, P. δ13C tracing of dissolved inorganic carbon sources in Patagonian rivers (Argentina). Hydrol. Process. 2005, 19, 3321–3344. [Google Scholar] [CrossRef]
- Weynell, M.; Wiechert, U.; Zhang, C. Chemical and isotopic (O, H, C) composition of surface waters in the catchment of Lake Donggi Cona (NW China) and implications for paleoenvironmental reconstructions. Chem. Geol. 2016, 435, 92–107. [Google Scholar] [CrossRef]
- Tranvik, L.J.; Downing, J.A.; Cotner, J.B.; Loiselle, S.A.; Striegl, R.G.; Ballatore, T.J.; Dillon, P.; Finlay, K.; Fortino, K.; Knoll, L.B.; et al. Lakes and reservoirs as regulators of carbon cycling and climate. Limnol. Oceanogr. 2009, 54, 2298–2314. [Google Scholar] [CrossRef]
- Herczeg, A.L.; Leaney, F.W.; Dighton, J.C.; Lamontagne, S.; Schiff, S.L.; Telfer, A.L.; English, M.C. A modern isotope record of changes in water and carbon budgets in a groundwater-fed lake: Blue Lake, South Australia. Limnol. Oceanogr. 2003, 48, 2093–2105. [Google Scholar] [CrossRef]
- Striegl, R.G.; Kortelainen, P.; Chanton, J.P.; Wickland, K.P.; Bugna, G.C.; Rantakari, M. Carbon dioxide partial pressure and 13C content of north temperate and boreal lakes at spring ice melt. Limnol. Oceanogr. 2001, 46, 941–945. [Google Scholar] [CrossRef]
- Liu, W.; Li, X.; An, Z.; Xu, L.M.; Zhang, Q.L. Total organic carbon isotopes: A novel proxy of lake level from Lake Qinghai in the Qinghai-Tibet Plateau, China. Chem. Geol. 2013, 347, 153–160. [Google Scholar] [CrossRef]
- Andrews, J.E.; Riding, R.; Dennis, P.F. The stable isotope record of environmental and climatic signals in modern terrestrial microbial carbonates from Europe. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1997, 129, 171–189. [Google Scholar] [CrossRef]
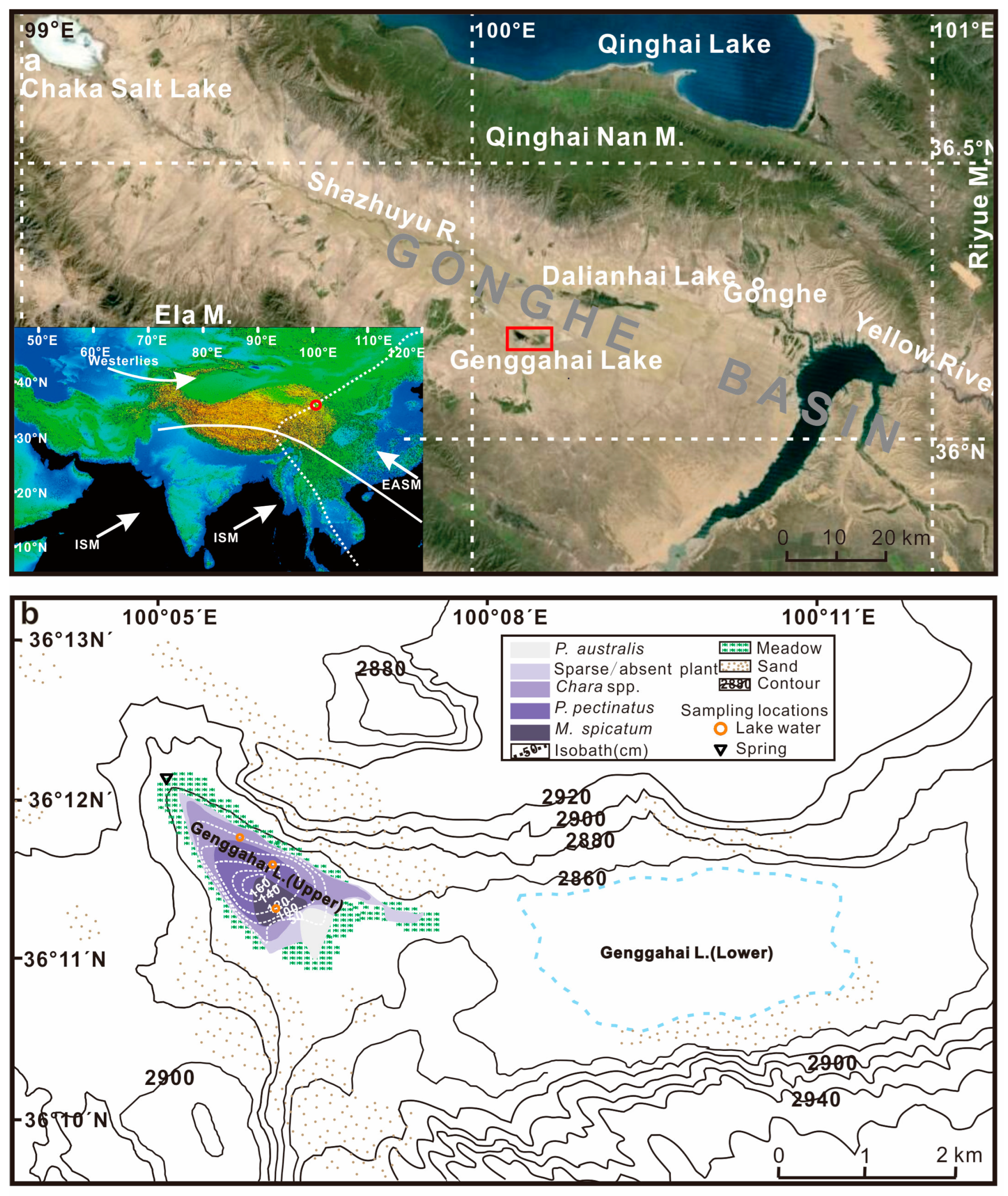


| Water Sample Types | Measured Parameters |
|---|---|
| Lake water Groundwater River water | Water temperature pH DO carbon isotopes oxygen isotopes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, Y.; Jin, X. Variations in δ13CDIC and Influencing Factors in a Shallow Macrophytic Lake on the Qinghai–Tibetan Plateau: Implications for the Regional Carbon Cycle and Sustainable Development. Sustainability 2024, 16, 3350. https://doi.org/10.3390/su16083350
Jin Y, Jin X. Variations in δ13CDIC and Influencing Factors in a Shallow Macrophytic Lake on the Qinghai–Tibetan Plateau: Implications for the Regional Carbon Cycle and Sustainable Development. Sustainability. 2024; 16(8):3350. https://doi.org/10.3390/su16083350
Chicago/Turabian StyleJin, Yanxiang, and Xin Jin. 2024. "Variations in δ13CDIC and Influencing Factors in a Shallow Macrophytic Lake on the Qinghai–Tibetan Plateau: Implications for the Regional Carbon Cycle and Sustainable Development" Sustainability 16, no. 8: 3350. https://doi.org/10.3390/su16083350
APA StyleJin, Y., & Jin, X. (2024). Variations in δ13CDIC and Influencing Factors in a Shallow Macrophytic Lake on the Qinghai–Tibetan Plateau: Implications for the Regional Carbon Cycle and Sustainable Development. Sustainability, 16(8), 3350. https://doi.org/10.3390/su16083350





