Exposure and Early Effect Biomarkers for Risk Assessment of Occupational Exposure to Formaldehyde: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion/Exclusion Criteria
2.3. Study Quality and Evaluation
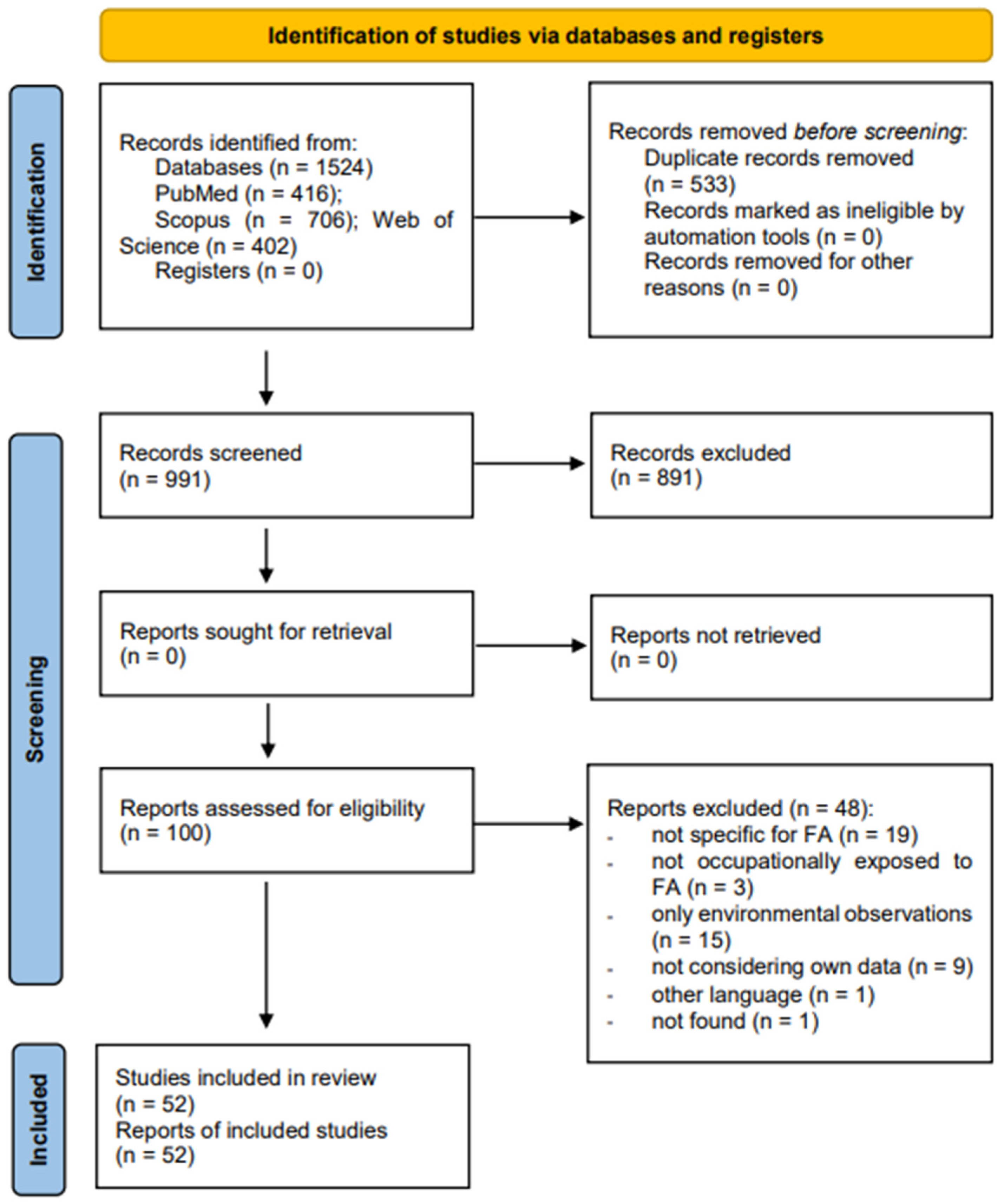
3. Results
3.1. Article Selection
3.2. Main Characteristics of the Included Studies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hulin, M.; Simoni, M.; Viegi, G.; Annesi-Maesano, I. Respiratory health and indoor air pollutants based on quantitative exposure assessments. Eur. Respir. J. 2012, 40, 1033–1045. [Google Scholar] [CrossRef] [PubMed]
- Arts, J.H.; Rennen, M.A.; de Heer, C. Inhaled formaldehyde: Evaluation of sensory irritation in relation to carcinogenicity. Regul. Toxicol. Pharmacol. 2006, 44, 144–160. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer (IARC). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Formaldehyde, 2-Butoxyethanol and 1-Tert-Butoxypropan-2-ol. 88; International Agency for Research on Cancer: Lyon, France, 2006. [Google Scholar]
- Protano, C.; Buomprisco, G.; Cammalleri, V.; Pocino, R.N.; Marotta, D.; Simonazzi, S.; Cardoni, F.; Petyx, M.; Iavicoli, S.; Vitali, M. The Carcinogenic Effects of Formaldehyde Occupational Exposure: A Systematic Review. Cancers 2021, 14, 165. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, E.; Dos Santos, A.L.A.; Peteffi, G.P.; Schneider, A.; Müller, D.; Rovaris, D.; Bau, C.H.D.; Linden, R.; Antunes, M.V.; Charão, M.F. Increase of global DNA methylation patterns in beauty salon workers exposed to low levels of formaldehyde. Environ. Sci. Pollut. Res. Int. 2019, 26, 1304–1314. [Google Scholar] [CrossRef] [PubMed]
- Cammalleri, V.; Pocino, R.N.; Marotta, D.; Protano, C.; Sinibaldi, F.; Simonazzi, S.; Petyx, M.; Iavicoli, S.; Vitali, M. Occupational scenarios and exposure assessment to formaldehyde: A systematic review. Indoor Air 2022, 32, e12949. [Google Scholar] [CrossRef] [PubMed]
- Castellani, F.; Vitali, M.; Antonucci, A.; Cofone, L.; D’Ancona, G.; Pindinello, I.; Buomprisco, G.; Petyx, M.; Ursini, C.L.; Protano, C. Effective mitigation strategies for reducing workers’ exposure to formaldehyde: A systematic review. Air Qual. Atmos. Health 2023. [Google Scholar] [CrossRef]
- Leng, J.; Liu, C.W.; Hartwell, H.J.; Yu, R.; Lai, Y.; Bodnar, W.M.; Lu, K.; Swenberg, J.A. Evaluation of inhaled low-dose formaldehyde-induced DNA adducts and DNA-protein cross-links by liquid chromatography-tandem mass spectrometry. Arch. Toxicol. 2019, 93, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Reingruber, H.; Pontel, L.B. Formaldehyde metabolism and its impact on human health. Curr. Opin. Toxicol. 2018, 9, 28–34. [Google Scholar] [CrossRef]
- Dorokhov, Y.L.; Sheshukova, E.V.; Bialik, T.E.; Komarova, T.V. Human Endogenous Formaldehyde as an Anticancer Metabolite: Its Oxidation Downregulation May Be a Means of Improving Therapy. Bioessays 2018, 40, e1800136. [Google Scholar] [CrossRef] [PubMed]
- Peteffi, G.P.; Antunes, M.V.; Carrer, C.; Valandro, E.T.; Santos, S.; Glaeser, J.; Mattos, L.; da Silva, L.B.; Linden, R. Environmental and biological monitoring of occupational formaldehyde exposure resulting from the use of products for hair straightening. Environ. Sci. Pollut. Res. Int. 2016, 23, 908–917. [Google Scholar] [CrossRef] [PubMed]
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Formaldehyde; Agency for Toxic Substances and Disease: Atlanta, GA, USA, 1999.
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Bellisario, V.; Mengozzi, G.; Grignani, E.; Bugiani, M.; Sapino, A.; Bussolati, G.; Bono, R. Towards a formalin-free hospital. Levels of 15-F2t-isoprostane and malondialdehyde to monitor exposure to formaldehyde in nurses from operating theatres. Toxicol. Res. 2016, 5, 1122–1129. [Google Scholar] [CrossRef] [PubMed]
- Bono, R.; Romanazzi, V.; Munnia, A.; Piro, S.; Allione, A.; Ricceri, F.; Guarrera, S.; Pignata, C.; Matullo, G.; Wang, P.; et al. Malondialdehyde−deoxyguanosine adduct formation in workers of pathology wards: The role of air formaldehyde exposure. Chem. Res. Toxicol. 2010, 23, 1342–1348. [Google Scholar] [CrossRef] [PubMed]
- Bouraoui, S.; Mougou, S.; Brahem, A.; Tabka, F.; Ben Khelifa, H.; Harrabi, I.; Mrizek, N.; Elghezal, H.; Saad, A. A combination of micronucleus assay and fluorescence in situ hybridization analysis to evaluate the genotoxicity of formaldehyde. Arch. Environ. Contam. Toxicol. 2013, 64, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.; Coelho, P.; Costa, C.; Silva, S.; Mayan, O.; Santos, L.S.; Gaspar, J.; Teixeira, J.P. Genotoxic damage in pathology anatomy laboratory workers exposed to formaldehyde. Toxicology 2008, 252, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.; García-Lestón, J.; Coelho, M.; Coelho, P.; Costa, C.; Silva, S.; Porto, B.; Laffon, B.; Teixeira, J.P. Cytogenetic and immunological effects associated with occupational formaldehyde exposure. J. Toxicol. Environ. Health A 2013, 76, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.; Carvalho, S.; Costa, C.; Coelho, P.; Silva, S.; Santos, L.S.; Gaspar, J.F.; Porto, B.; Laffon, B.; Teixeira, J.P. Increased levels of chromosomal aberrations and DNA damage in a group of workers exposed to formaldehyde. Mutagenesis 2015, 30, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.; Costa, C.; Madureira, J.; Valdiglesias, V.; Teixeira-Gomes, A.; Guedes de Pinho, P.; Laffon, B.; Teixeira, J.P. Occupational exposure to formaldehyde and early biomarkers of cancer risk, immunotoxicity and susceptibility. Environ. Res. 2019, 179 Pt A, 108740. [Google Scholar] [CrossRef]
- Ghelli, F.; Cocchi, E.; Buglisi, M.; Squillacioti, G.; Bellisario, V.; Bono, R.; Santovito, A. The role of phase I, phase II, and DNA-repair gene polymorphisms in the damage induced by formaldehyde in pathologists. Sci. Rep. 2021, 11, 10507. [Google Scholar] [CrossRef] [PubMed]
- Ghelli, F.; Cocchi, E.; Bellisario, V.; Buglisi, M.; Squillacioti, G.; Santovito, A.; Bono, R. The formation of SCEs as an effect of occupational exposure to formaldehyde. Arch. Toxicol. 2022, 96, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Jakab, M.G.; Klupp, T.; Besenyei, K.; Biró, A.; Major, J.; Tompa, A. Formaldehyde-induced chromosomal aberrations and apoptosis in peripheral blood lymphocytes of personnel working in pathology departments. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2010, 698, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Ladeira, C.; Viegas, S.; Carolino, E.; Prista, J.; Gomes, M.C.; Brito, M. Genotoxicity biomarkers in occupational exposure to formaldehyde—The case of histopathology laboratories. Mutat. Res. 2011, 721, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Ladeira, C.; Viegas, S.; Carolino, E.; Gomes, M.C.; Brito, M. The influence of genetic polymorphisms in XRCC3 and ADH5 genes on the frequency of genotoxicity biomarkers in workers exposed to formaldehyde. Environ. Mol. Mutagen. 2013, 54, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Motta, O.; Charlier, B.; De Caro, F.; Coglianese, A.; De Rosa, F.; Moccia, G.; Pironti, C.; Capunzo, M.; Borrelli, A.; Filippelli, A.; et al. Environmental and biological monitoring of formaldehyde inside a hospital setting: A combined approach to manage chemical risk in workplaces. J. Public Health Res. 2021, 10, 2012. [Google Scholar] [CrossRef] [PubMed]
- Musak, L.; Smerhovsky, Z.; Halasova, E.; Osina, O.; Letkova, L.; Vodickova, L.; Polakova, V.; Buchancova, J.; Hemminki, K.; Vodicka, P. Chromosomal damage among medical staff occupationally exposed to volatile anesthetics, antineoplastic drugs, and formaldehyde. Scand. J. Work Environ. Health 2013, 39, 618–630. [Google Scholar] [CrossRef] [PubMed]
- Pala, M.; Ugolini, D.; Ceppi, M.; Rizzo, F.; Maiorana, L.; Bolognesi, C.; Schilirò, T.; Gilli, G.; Bigatti, P.; Bono, R.; et al. Occupational exposure to formaldehyde and biological monitoring of Research Institute workers. Cancer Detect. Prev. 2008, 32, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Santovito, A.; Schilirò, T.; Castellano, S.; Cervella, P.; Bigatti, M.P.; Gilli, G.; Bono, R.; DelPero, M. Combined analysis of chromosomal aberrations and glutathione S-transferase M1 and T1 polymorphisms in pathologists occupationally exposed to formaldehyde. Arch. Toxicol. 2011, 85, 1295–1302. [Google Scholar] [CrossRef] [PubMed]
- Shaham, J.; Bomstein, Y.; Meltzer, A.; Kaufman, Z.; Palma, E.; Ribak, J. DNA-protein crosslinks, a biomarker of exposure to formaldehyde—In vitro and in vivo studies. Carcinogenesis 1996, 17, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Shaham, J.; Bomstein, Y.; Melzer, A.; Ribak, J. DNA-Protein Crosslinks and Sister Chromatid Exchanges as Biomarkers of Exposure to Formaldehyde. Int. J. Occup. Environ. Health 1997, 3, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Shaham, J.; Bomstein, Y.; Gurvich, R.; Rashkovsky, M.; Kaufman, Z. DNA-protein crosslinks and p53 protein expression in relation to occupational exposure to formaldehyde. Occup. Environ. Med. 2003, 60, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Suruda, A.; Schulte, P.; Boeniger, M.; Hayes, R.B.; Livingston, G.K.; Steenland, K.; Stewart, P.; Herrick, R.; Douthit, D.; Fingerhut, M.A. Cytogenetic effects of formaldehyde exposure in students of mortuary science. Cancer Epidemiol. Biomark. Prev. 1993, 2, 453–460. [Google Scholar]
- Tompa, A.; Jakab, M.; Biró, A.; Magyar, B.; Fodor, Z.; Klupp, T.; Major, J. Chemical safety and health conditions among Hungarian hospital nurses. Ann. N. Y. Acad. Sci. 2006, 1076, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Attia, D.; Mansour, N.; Taha, F.; Seif El Dein, A. Assessment of lipid peroxidation and p53 as a biomarker of carcinogenesis among workers exposed to formaldehyde in the cosmetic industry. Toxicol. Ind. Health 2016, 32, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Bono, R.; Vincenti, M.; Schiliro’, T.; Scursatone, E.; Pignata, C.; Gilli, G. N-Methylenvaline in a group of subjects occupationally exposed to formaldehyde. Toxicol. Lett. 2006, 161, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Bono, R.; Romanazzi, V.; Pirro, V.; Degan, R.; Pignata, C.; Suppo, E.; Pazzi, M.; Vincenti, M. Formaldehyde and tobacco smoke as alkylating agents: The formation of N-methylenvaline in pathologists and in plastic laminate workers. Sci. Total Environ. 2012, 414, 701–707. [Google Scholar] [CrossRef] [PubMed]
- Bono, R.; Munnia, A.; Romanazzi, V.; Bellisario, V.; Cellai, F.; Peluso, M.E.M. Formaldehyde-induced toxicity in the nasal epithelia of workers of a plastic laminate plant. Toxicol. Res. 2016, 5, 752–760. [Google Scholar] [CrossRef] [PubMed]
- Burgaz, S.; Erdem, O.; Cakmak, G.; Erdem, N.; Karakaya, A.; Karakaya, A.E. Cytogenetic analysis of buccal cells from shoe-workers and pathology and anatomy laboratory workers exposed to n-hexane, toluene, methyl ethyl ketone and formaldehyde. Biomarkers 2002, 7, 151–161. [Google Scholar] [CrossRef] [PubMed]
- El Far, M.; El Naggar, M.; Elkhawaga, O.A.; Yahya, R.; Allam, A.; Khalifa, A. Carcinoembryonic antigen, alpha-fetoprotein, and prostate-specific antigen in the sera of industrial workers exposed to phenol, formaldehyde, urea, and mixed vapors. Inhal. Toxicol. 2006, 18, 1041–1046. [Google Scholar] [CrossRef] [PubMed]
- Ghelli, F.; Bellisario, V.; Squillacioti, G.; Grignani, E.; Garzaro, G.; Buglisi, M.; Bergamaschi, E.; Bono, R. Oxidative stress induction in woodworkers occupationally exposed to wood dust and formaldehyde. J. Occup. Med. Toxicol. 2021, 16, 4. [Google Scholar] [CrossRef] [PubMed]
- Hosgood, H.D., 3rd; Zhang, L.; Tang, X.; Vermeulen, R.; Hao, Z.; Shen, M.; Qiu, C.; Ge, Y.; Hua, M.; Ji, Z.; et al. Occupational exposure to formaldehyde and alterations in lymphocyte subsets. Am. J. Ind. Med. 2013, 56, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Lan, Q.; Smith, M.T.; Tang, X.; Guo, W.; Vermeulen, R.; Ji, Z.; Hu, W.; Hubbard, A.E.; Shen, M.; McHale, C.M.; et al. Chromosome-wide aneuploidy study of cultured circulating myeloid progenitor cells from workers occupationally exposed to formaldehyde. Carcinogenesis 2015, 36, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Lyapina, M.; Zhelezova, G.; Petrova, E.; Boev, M. Flow cytometric determination of neutrophil respiratory burst activity in workers exposed to formaldehyde. Int. Arch. Occup. Environ. Health 2004, 77, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Maniscalco, M.; Paris, D.; Melck, D.; Chiariello, N.; Di Napoli, F.; Manno, M.; Iavicoli, I.; Motta, A. Biomonitoring of workers using nuclear magnetic resonance-based metabolomics of exhaled breath condensate: A pilot study. Toxicol. Lett. 2018, 298, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Orsière, T.; Sari-Minodier, I.; Iarmarcovai, G.; Botta, A. Genotoxic risk assessment of pathology and anatomy laboratory workers exposed to formaldehyde by use of personal air sampling and analysis of DNA damage in peripheral lymphocytes. Mutat. Res. 2006, 605, 30–41. [Google Scholar] [CrossRef]
- Oztan, O.; Tutkun, L.; Turksoy, V.A.; Deniz, S.; Dip, A.; Iritas, S.B.; Eravci, D.B.; Alaguney, M.E. The relationship between impaired lung functions and cytokine levels in formaldehyde exposure. Arch. Environ. Occup. Health 2020, 76, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Peteffi, G.P.; da Silva, L.B.; Antunes, M.V.; Wilhelm, C.; Valandro, E.T.; Glaeser, J.; Kaefer, D.; Linden, R. Evaluation of genotoxicity in workers exposed to low levels of formaldehyde in a furniture manufacturing facility. Toxicol. Ind. Health 2016, 32, 1763–1773. [Google Scholar] [CrossRef] [PubMed]
- Phillips, R.V.; Wei, L.; Cardenas, A.; Hubbard, A.E.; McHale, C.M.; Vermeulen, R.; Wei, H.; Smith, M.T.; Zhang, L.; Lan, Q.; et al. Epigenome-wide association studies of occupational exposure to benzene and formaldehyde. Epigenetics 2022, 17, 2259–2277. [Google Scholar] [CrossRef] [PubMed]
- Regazzoni, L.G.; Grigoryan, H.; Ji, Z.; Chen, X.; Daniels, S.I.; Huang, D.; Sanchez, S.; Tang, N.; Sillé, F.C.; Iavarone, A.T.; et al. Using lysine adducts of human serum albumin to investigate the disposition of exogenous formaldehyde in human blood. Toxicol. Lett. 2017, 268, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Romanazzi, V.; Pirro, V.; Bellisario, V.; Mengozzi, G.; Peluso, M.; Pazzi, M.; Bugiani, M.; Verlato, G.; Bono, R. 15-F₂t isoprostane as biomarker of oxidative stress induced by tobacco smoke and occupational exposure to formaldehyde in workers of plastic laminates. Sci. Total Environ. 2013, 442, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Seow, W.J.; Zhang, L.; Vermeulen, R.; Tang, X.; Hu, W.; Bassig, B.A.; Ji, Z.; Shiels, M.S.; Kemp, T.J.; Shen, M.; et al. Circulating immune/inflammation markers in Chinese workers occupationally exposed to formaldehyde. Carcinogenesis 2015, 36, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Van der Laan, L.; Cardenas, A.; Vermeulen, R.; Fadadu, R.P.; Hubbard, A.E.; Phillips, R.V.; Zhang, L.; Breeze, C.; Hu, W.; Wen, C.; et al. Epigenetic aging biomarkers and occupational exposure to benzene, trichloroethylene and formaldehyde. Environ. Int. 2022, 158, 106871. [Google Scholar] [CrossRef] [PubMed]
- Zendehdel, R.; Fazli, Z.; Mazinani, M. Neurotoxicity effect of formaldehyde on occupational exposure and influence of individual susceptibility to some metabolism parameters. Environ. Monit. Assess. 2016, 188, 648. [Google Scholar] [CrossRef] [PubMed]
- Zendehdel, R.; Vahabi, M.; Sedghi, R. Estimation of formaldehyde occupational exposure limit based on genetic damage in some Iranian exposed workers using benchmark dose method. Environ. Sci. Pollut. Res. Int. 2018, 25, 31183–31189. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Tang, X.; Rothman, N.; Vermeulen, R.; Ji, Z.; Shen, M.; Qiu, C.; Guo, W.; Liu, S.; Reiss, B.; et al. Occupational exposure to formaldehyde, hematotoxicity, and leukemia-specific chromosome changes in cultured myeloid progenitor cells. Cancer Epidemiol. Biomark. Prev. 2010, 19, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Zhitkovich, A.; Lukanova, A.; Popov, T.; Taioli, E.; Cohen, H.; Costa, M.; Toniolo, P. DNA-protein crosslinks in peripheral lymphocytes of individuals exposed to hexavalent chromium compounds. Biomarkers 1996, 1, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Aglan, M.A.; Mansour, G.N. Hair straightening products and the risk of occupational formaldehyde exposure in hairstylists. Drug Chem. Toxicol. 2020, 43, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Norbäck, D.; Wålinder, R.; Wieslander, G.; Smedje, G.; Erwall, C.; Venge, P. Indoor air pollutants in schools: Nasal patency and biomarkers in nasal lavage. Allergy 2000, 55, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Squillacioti, G.; Bellisario, V.; Grosso, A.; Ghelli, F.; Piccioni, P.; Grignani, E.; Corsico, A.; Bono, R. Formaldehyde, Oxidative Stress, and FeNO in Traffic Police Officers Working in Two Cities of Northern Italy. Int. J. Environ. Res. Public Health 2020, 17, 1655. [Google Scholar] [CrossRef] [PubMed]
- Triebig, G.; Schaller, K.H.; Beyer, B.; Müller, J.; Valentin, H. Formaldehyde exposure at various workplaces. Sci. Total Environ. 1989, 79, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Vargová, M.; Janota, S.; Karelová, J.; Baranèokova, M.; Sulcová, M. The evaluation of possible health risk associated with occupational exposure to formaldehyde. SPIE 1993, 1716, 447–454. [Google Scholar] [CrossRef]
- Viegas, S.; Ladeira, C.; Nunes, C.; Malta-Vacas, J.; Gomes, M.; Brito, M.; Mendonca, P.; Prista, J. Genotoxic effects in occupational exposure to formaldehyde: A study in anatomy and pathology laboratories and formaldehyde-resins production. J. Occup. Med. Toxicol. 2010, 5, 25. [Google Scholar] [CrossRef] [PubMed]
- Schmid, K.; Schaller, K.H.; Angerer, J.; Lehnert, G. The Importance of formic acid Excretion in the urine for environmental and occupational medicine questions. Zentralblatt Hyg. Umweltmed. 1994, 196, 139–152. [Google Scholar]
- Boeniger, M.F. Formate in urine as a biological indicator of formaldehyde exposure: A review. Am. Ind. Hyg. Assoc. J. 1987, 48, 900–908. [Google Scholar] [CrossRef] [PubMed]
- Santoro, P.M.; Borrelli, I.; Gualano, M.R.; Amantea, C.; Tumminello, A.; Daniele, A.; Rossi, M.F.; Moscato, U. Occupational hazards and gender differences: A narrative review. Ital. J. Gend.-Specif. Med. 2022, 8, 154–162. [Google Scholar]
- Anderson, D. Factors contributing to biomarker responses in exposed workers. Mutat. Res.-Fund. Mech. Mutagen. 1999, 428, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Nagaraju, R.; Kalahasthi, R.; Balachandar, R.; Bagepally, B.S. Cadmium exposure and DNA damage (genotoxicity): A systematic review and meta-analysis. Crit. Rev. Toxicol. 2022, 52, 786–798. [Google Scholar] [CrossRef] [PubMed]
- Villarini, M.; Gianfredi, V.; Levorato, S.; Vannini, S.; Salvatori, T.; Moretti, M. Occupational exposure to cytostatic/antineoplastic drugs and cytogenetic damage measured using the lymphocyte cytokinesis-block micronucleus assay: A systematic review of the literature and meta-analysis. Mutat. Res. Rev. Mutat. Res. 2016, 770 Pt A, 35–45. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, K.; Wang, B.; Pu, Y.; Zhang, J. Occupational benzene exposure and the risk of genetic damage: A systematic review and meta-analysis. BMC Public Health 2020, 20, 1113. [Google Scholar] [CrossRef] [PubMed]
- da Silva Augusto, L.G.; Lieber, S.R.; Ruiz, M.A.; de Souza, C.A. Micronucleus monitoring to assess human occupational exposure to organochlorides. Environ. Mol. Mutagen. 1997, 29, 46–52. [Google Scholar] [CrossRef]
- Deeks, J.J.; Higgins, J.P.T.; Altman, D.G. Chapter 10: Analysing Data and Undertaking Meta-Analyses. In Cochrane Handbook for Systematic Reviews of Interventions; Version 6.2 (updated February 2021); Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; Cochrane: London, UK, 2021. [Google Scholar]
| Reference Country Working Context | Sample Size Age (Mean Value ± SD and/or Range) Gender (%) | Biomarkers of Internal Dose and/or Early Effect | Confounding and Interfering Factors | Main Results | Quality Evaluation According to NOS Scale |
|---|---|---|---|---|---|
| Bellisario et al., 2016 [14] Italy Hospital operating theatre | 94; 30 exposed and 64 controls; 45 ± 8 years; 0 males (0%) and 94 females (100%) | Biomarkers of effect: levels of 15-F2t-isoprostane and malondialdehyde in urine samples | Smoking habits, log-FA, UVS, urinary cotinine, age, body mass index | Statistically significant increases in urinary levels of 15-F2t-isoprostane and malondialdehyde in nurses using FA, in particular in those workers using liquid FA | Fair |
| Bono et al., 2010 [15] Italy Pathology wards | 76; 44 exposed and 32 controls; 32% <30 years, 31% 30–39 years, 37% >39 years; 22 males (29%) and 53 females (71%) | Biomarkers of effect: levels of leukocyte malondialdehyde-deoxyguanosine adducts | Gender, age, smoking habits, exposure status, air-FA measurements | Statistically significant increases in malondialdehyde-deoxyguanosine adducts in exposed with respect to controls. The effect becomes stronger when the evaluation of air-FA exposure was based on personal samplers | Fair |
| Bouraoui et al., 2012 [16] Tunisia Hospital pathology anatomy laboratory | 62; 31 exposed and 31 controls; 43 ± 9 years; 21 males (40%) and 41 females (60%) | Biomarkers of effect: micronucleus frequencies in peripheral lymphocytes | Gender, age, smoking habits, use of individual protection, professional class, presence of respiratory and ocular effects | Statistically significant increases in micronucleus frequency in the exposed group compared to controls | Fair |
| Costa et al., 2008 [17] Portugal Hospital pathology anatomy laboratory | 60; 30 exposed and 30 controls; 38 ± 9 years; 20 males (33%) and 40 females (67%) | Biomarkers of effect: micronucleus frequencies, sister chromatid exchanges, comet tail length in peripheral lymphocytes | Age, gender, smoking habits, years of employment | Statistically significant increases in micronucleus frequency, sister chromatid exchanges, and comet tail length in the exposed group compared to controls | Fair |
| Costa et al., 2013 [18] Portugal Hospital pathology anatomy laboratory | 70; 35 exposed and 35 controls; 40 ± 10 years; 11 males (16%) and 59 females (84%) | Biomarkers of effect: micronucleus frequencies and sister chromatid exchanges in peripheral lymphocytes, T-cell receptor mutations in mononuclear leukocytes | Age, gender, smoking habits, years of employment | Statistically significant increases in micronucleus frequency and sister chromatid exchanges in the exposed group compared to controls. No significant differences were found for T-cells receptor mutations | Fair |
| Costa et al., 2015 [19] Portugal Hospital pathology anatomy laboratory | 171; 84 exposed and 87 controls; 39 ± 10 years; 39 males (23%) and 132 females (77%) | Biomarkers of effect: chromosomal aberrations in peripheral lymphocytes and percentage of DNA in the comet tail in peripheral mononuclear cells | Health conditions, general medical history, medication, diagnostic tests (X-rays, etc), age, gender, smoking habits, alcohol consumption, dietary habits | Statistically significant increases in chromosomal aberrations and percentage of DNA in the comet tail in the exposed group compared to controls | Fair |
| Costa et al., 2019 [20] Portugal Hospital pathology anatomy laboratory | 172; 85 exposed and 87 controls; 40 ± 10 years; 138 males (80%) and 34 females (20%) | Biomarkers of internal dose: urinary formic acid concentrations Biomarkers of effect: micronucleus frequencies in peripheral lymphocytes and in exfoliated buccal cells, sister chromatid exchanges in peripheral lymphocytes, T-cell receptor mutations in mononuclear leukocytes, percentages of different lymphocyte subpopulations | Health conditions, general medical history, medication, diagnostic tests (X-rays, etc), age, gender, smoking habits, alcohol consumption, dietary habits | Statistically significant increases in urinary formic acid, micronucleus frequency and sister chromatid exchanges in the exposed group compared to controls. Statistically significant alteration of percentages of different lymphocyte subpopulations in the exposed group compared to controls. No significant differences were found for T-cells receptor mutations | Fair |
| Ghelli et al., 2021 [21] Italy Hospital pathology laboratory | 105; 57 exposed and 48 controls; 41.4 ± 9.3 years; 54 males (52%) and 51 females (48%) | Biomarkers of effect: formation of chromosomal aberrations on peripheral blood lymphocytes | Sex, age, personal habits (smoking) during the last year, work characteristics (length in years of service working and type of work) | Statistically significant increases in chromosomal aberrations in the exposed group compared to controls. Significant positive correlations were found between chromosomal aberrations frequency and air-FA concentration | Fair |
| Ghelli et al., 2022 [22] Italy Hospital pathology laboratory | 105; 57 exposed and 48 controls; 41.4 ± 9.3 years; 54 males (52%) and 51 females (48%) | Biomarkers of effect: formation of sister-chromatid exchanges in peripheral blood lymphocytes | Sex, age, smoking habits referring to the last year, working characteristics (working years and task and personal protective equipment use). | Statistically significant increases of sister-chromatid exchanges in the exposed group compared to controls | Good |
| Jakab et al., 2010 [23] Hungary Hospital pathology laboratory | 74; 37 exposed and 37 controls; 43.2 ± 2.0 years; 0 males (0%) and 74 females (100%) | Biomarkers of effect: formation of chromosomal aberrations, sister-chromatid exchange, HPRT mutations, UV-induced unscheduled DNA-repair synthesis, premature centromere division and of cells with a high frequency of SCE in peripheral blood lymphocytes | Age, medication, lifestyle (smoking and drinking habits), medical and work histories in relation to known or suspected chemical mutagens and/or to exposure to ionizing radiation, use of protective devices during work | Statistically significant increases in the apoptotic activity and chromosomal aberrations levels in the exposed group compared to controls | Fair |
| Ladeira et al., 2011 [24] Portugal Hospital-associated histopathology laboratories | 141; 56 exposed and 85 controls; 35.9 ± 9.8 years; 50 males (35%) and 91 females (65%) | Biomarkers of effect: micronuclei in peripheral blood lymphocytes and exfoliated cells from the buccal mucosa, nucleoplasmic bridges, common poor repair and/or telomere fusion and nuclear buds in peripheral blood lymphocytes | Age, gender, smoking habits and alcohol consumption, use of protective devices during work | Statistically significant increases in the investigated biomarkers in the exposed group compared to controls | Good |
| Ladeira et al., 2013 [25] Portugal Hospital-associated histopathology laboratories | 136; 54 exposed and 82 controls; 36.3 ± 9.8 years; 48 males (35%) and 88 females (65%) | Biomarkers of effect: micronuclei in peripheral blood lymphocytes and exfoliated cells from the buccal mucosa, nucleoplasmic bridges and nuclear buds in peripheral blood lymphocytes | Age, gender, smoking habits, alcohol consumption | Statistically significant increases in the investigated biomarkers in the exposed group compared to controls | Good |
| Motta et al., 2021 [26] Italy Anatomic pathology unit | 16 exposed | Biomarkers of internal dose: urinary formaldehyde concentrations | - | Workers’ urinary formaldehyde levels were minimal, but the statistical analysis highlighted a slight weekly accumulation | Poor |
| Musak et al., 2013 [27] Czech Republic Hospital laboratories | 355; 105 exposed and 250 controls; 39 ± 10 years; 50 males (14%) and 305 females (86%) | Biomarkers of effect: structural chromosomal aberrations in peripheral blood lymphocytes | Age, gender, job category, smoking habits | Statistically significant increases in the chromosomal aberrations levels in the exposed group compared to controls | Good |
| Pala et al., 2008 [28] Italy Cancer research institute laboratories | 36; 27 low exposed and 9 high exposed; 40.14 (range 27–52) years; 12 males (33%) and 24 females (67%) | Biomarkers of internal dose: formaldehyde human serum albumin conjugate Biomarkers of effect: chromosome aberrations, micronuclei, sister chromatid exchanges in peripheral blood lymphocytes | Age, gender, smoking habits, exposure to other chemicals | Statistically significant increase in the biomarker of exposure in subjects with high exposure, but not of the biomarkers of effect | Poor |
| Santovito et al., 2011 [29] Italy Pathology wards | 36; 20 exposed and 16 controls; 43.9 ± 2.34 years; 13 males (36%) and 23 females (64%) | Biomarkers of effect: frequency of chromosomal aberrations in peripheral blood lymphocytes | Age, years of employment | Statistically significant increase in the frequency of chromosomal aberrations per cell and in the percentage of cells with aberrations in peripheral lymphocytes in the exposed group compared to controls | Poor |
| Shaham et al., 1996 [30] Israel Anatomy and pathology laboratories | 20; 12 exposed and 8 controls; 42.5 ± 10.1 years | Biomarker of effect: amount of DNA–protein crosslinks in white blood cells | Age, smoking habits, medical history, hygiene habits | Statistically significant increase in the levels of DNA–protein crosslinks in peripheral white blood cells in the exposed group comapred to controls. Linear positive relationship between years of exposure and the amount of DNA–protein crosslinks | Poor |
| Shaham et al., 1997 [31] Israel Anatomy and pathology department | 33; 13 exposed and 20 controls; 40.5 ± 12 years | Biomarker of effect: sister chromatid exchanges in peripheral blood lymphocytes | Age, gender, smoking habits, years of FA exposure, occupational and medical histories, hygiene habits | Statistically significant increase in the mean numbers of sister chromatid exchanges in the exposed group compared to controls | Poor |
| Shaham et al., 2003 [32] Israel Pathology wards | 399; 186 exposed and 213 controls; 43.9 ± 10.3 years; 186 males (47%) and 213 females (53%) | Biomarkers of effect: DNA–protein crosslinks and p53 “wild type” and mutant (pantropic p53) in peripheral lymphocytes | Age, gender, smoking habits, years of education, origin | Statistically significant higher level of pantropic p53 in the exposed group compared to controls | Fair |
| Suruda et al., 1993 [33] USA Anatomy laboratory | 29; 23.6 years; 22 males (76%) and 7 females (24%) | Biomarkers of effect: micronuclei in buccal cells, nasal cells, and peripheral blood lymphocyte; lymphocyte sister chromatid exchange | Age, gender, smoking status, performed embalming in 90 days prior to study | Statistically significant increase in micronucleus frequency during the study period compared to pre-exposure levels in epithelial cells from the buccal area, nasal cells, and blood cells in the low exposed group, and a decrease in lymphocyte sister chromatid exchange | Poor |
| Tompa et al., 2006 [34] Hungary Hospital operating theater | 180; 86 exposed and 94 controls; 42.4 ± 1.7 years; 14 males (7.8%) and 166 females (92.2%) | Biomarkers of effect: chromosome aberrations, sister chromatid exchange, ratio of lymphocyte subpopulations, lymphocyte activation markers and leukocyte oxidative burst | Age, smoking, drinking, exposure to known or suspected mutagens, occupational history, use of protective devices during work | Statistically significant increase in the mean sister chromatid exchange frequency was observed in the exposed group compared to controls | Fair |
| Reference Country Working Context | Sample Size Age (Mean Value ± SD and/or Range) Gender (%) | Biomarkers of Internal Dose and/or Early Effect | Confounding and Interfering Factors | Main Results | Quality Evaluation According to NOS Scale |
|---|---|---|---|---|---|
| Attia et al., 2016 [35] Egypt Cosmetic industry | 60; 40 exposed and 20 controls; 29 ± 11 years; 12 males (20%) and 48 females (80%) | Biomarkers of internal dose: urinary formic acid concentration Biomarkers of effect: levels of p53 mutations and malondialdehyde in serum samples | Gender, age, smoking habits, alcohol consumption | Statistically significant increase in serum malondialdehyde in exposed workers compared to controls | Fair |
| Bono et al., 2006 [36] Italy Plywood and laminate factory | 51; 21 exposed and 30 controls; 35 ± 8 years; 37 males (73%) and 14 females (27%) | Biomarkers of effect: alkylation of hemoglobin to form a terminal N-methylenvaline residue | Age, gender, residence, smoking habits, professional activity | Statistically significant higher prevalence of N-methylenvaline in the exposed group compared to controls | Fair |
| Bono et al., 2012 [37] Italy Laboratory and plastic laminate plant | 173; 95 exposed and 78 controls; 40 years; 89 males (51%) and 84 females (49%) | Biomarkers of effect: alkylation of hemoglobin to form a terminal N-methylenvaline residue | Age, gender, smoking habits, place of residence, hobbies, therapies, smoking habits, profession, environmental and personal protective equipment | Statistically significant higher concentration of N-methylenvaline in the exposed group compared to controls | Fair |
| Bono et al., 2016 [38] Italy Plastic laminate plant | 95; 50 exposed and 45 controls; 44 ± 10 years; 95 males (100%) and 0 females (0%) | Biomarkers of effect: frequency of 3 (2-deoxy-β-D-erythro-pentafuranosyl) pyrimido [1,2-α] purin-10 (3H)-one deoxyguanosine adducts | Age and smoking habits, jobs, personal formaldehyde exposure | Statistically significant increase in frequency of deoxyguanosine adduct in the exposed group compared to controls. | Good |
| Burgaz et al., 2002 [39] Turkey Shoes factory and anatomy and pathology laboratory | 68; 50 exposed and 18 controls; 30 ± 8 years; 55 males (81%) and 13 females (19%) | Biomarkers of effect: micronucleus frequencies in epithelial buccal cells | Gender, age and smoking habits, duration of exposure | Statistically significant increases in micronucleus frequency in the exposed group compared to controls | Fair |
| El Far et al., 2006 [40] Egypt Chemical industries | 80; 65 exposed and 15 controls; 26–60 years; 80 males (100%) and 0 females (0%) | Biomarkers of effect: levels of carcinoembryonic antigen, alpha-fetoproteins, prostate-specific antigen | - | Statistically significant higher serum concentration of carcinoembryonic antigen, alpha-fetoproteins, and prostate-specific antigen in the exposed group compared to controls | Fair |
| Ghelli et al., 2021 [41] Italy Wood industry plants | 238; 127 exposed and 111 controls; 42 ± 16 years; 161 males (68%) and 77 females (32%) | Biomarkers of effect: levels of oxidative stress markers as 15-F2t-IsoP and 8-oxo-dGuo in urine samples | Age, gender, body mass index, smoking habits, residence, working years, wheezing, asthma-like symptoms, allergies, eczema, personal protective equipment use | Statistically significant higher concentrations of 15-F2t-IsoP and 8-oxo-dGuo in the exposed group compared to controls | Good |
| Hosgood et al., 2012 [42] China Melamine resins and plastic utensils factories | 94; 43 exposed and 51 controls; 30.5 ± 6.5 years; 81 males (86%) and 13 females (14%) | Biomarkers of effect: major lymphocyte subsets | Age, gender, smoking habits, alcohol consumption, recent infections (flu or respiratory infections in the previous month), body mass index | Statistically significant decrease in counts of NK cells, regulatory T cells, and CD8+ effector memory T cells in the exposed group compared to controls | Fair |
| Lan et al., 2015 [43] China Melamine resins plant | 52; 29 exposed and 23 controls; 31 ± 5 years; 47 males (90%) and 5 females (10%) | Biomarkers of effect: chromosomal aneuploidy and structural chromosome aberrations in myeloid progenitor cells | Age, gender, smoking habits, alcohol consumption, recent infections (flu or respiratory infections in the previous week), use of medication, body mass index | Statistically significant increase in the frequencies of monosomy, trisomy, tetrasomy, and structural chromosome aberrations of multiple chromosomes in exposed group compared to controls | Good |
| Lyapina et. al. 2004 [44] Bulgaria Carbamide FA glue employees | 50; 29 exposed and 21 controls; 38.5 ± 12.5 years; Exposed: 13 males (26%) and 16 females (32%) | Biomarkers of effect: neutrophil respiratory burst activity; haematologic alterations | Age, gender, smoking habits | Statistically significant negative correlation between the duration of exposure to formaldeyde and erythrocyte count and haematocrit level, and lower neutrophil respiratory burst activity in the exposed group with upper respiratory tract findings and frequent and long-lasting infectious inflammatory relapses | Poor |
| Maniscalco et al., 2018 [45] Italy Friction system manufacturing plant | 30; 20 exposed and 10 controls; 36.5 ± 6.5 years; 30 males (100%) and 0 females (0%) | Biomarkers of effect: changes in metabolic profiles in exhaled breath condensate | Smoking habits | Statistically significant increase in the concentration of propionate, isopropanol, lactate, acetoin, methanol, 1,2-propanediol, ethylene glycol, 3-hydroxyisobutyrate, and phenylalanine in the exposed group compared to controls | Good |
| Orsiere et al., 2006 [46] France Anatomy laboratory | 96; 59 exposed and 37 controls; 44.3 ± 8.3 years; 20 males (20.9%) and 76 females (79.1%) | Biomarkers of effect: DNA damage by chemiluminescence microplate assay and cytokinesis-blocked micronucleus assay in peripheral lymphocytes | Age, gender, smoking habits, alcohol consumption, recent X-ray diagnostic or radiotherapy history, use of mutagenic or reprotoxic drugs | Statistically significant higher frequency of micronuclei in the exposed group compared to controls | Fair |
| Oztan et al., 2020 [47] Turkey Fiber manufacturing company | 198; 116 exposed and 82 controls; 35.3 ± 6.68 years; 198 males (100%) and 0 females (0%) | Biomarkers of internal dose: urinary formic acid concentration Biomarkers of effect: proinflammatory cytokines, pulmonary function tests, serum AST, ALT, GGT, and creatinine | Chronic disease, medications, smoking status | Statistically significant increase in mean level of FA, TNF-α, and IL-6; significant decrease in FEV1 and FVC in the exposed group compared to controls | Fair |
| Peteffi et al., 2016 [48] Brazil Furniture manufacturing facility | 91; 46 exposed and 45 controls; 35 ± 11.4 years; 41 males (45%) and 50 females (55%) | Biomarkers of internal dose: urinary formic acid concentration Biomarkers of effect: micronucleus test in exfoliated buccal cells and comet assay in peripheral lymphocytes | Age, gender, smoking habits, alcohol consumption | Statistically significant increase in damage frequency and damage index in the comet assay, frequency of micronuclei, and formic acid concentration in urine in the exposed group compared to controls | Poor |
| Phillips et al., 2022 [49] USA Factories | 71; 31 exposed and 40 controls; 30.6 ± 6.7 years; 59 males (83%) and 12 females (17%) | Biomarkers of effect: DNA methylation in peripherical blood cells | Age, gender, occupational and medical history, environmental exposures, smoking habits, alcohol consumption | Statistically significant decrease in methylation variability in the DUSP22 gene promoter and hypomethylation of the HOXA5 promoter region in the exposed group compared to controls | Fair |
| Regazzoni et al., 2017 [50] Italy FA production factory | 30; 15 exposed and 15 controls; 9 males (30%) and 21 females (70%) | Biomarkers of internal dose: formaldeyde human serum albumin conjugate | Smoking habits | No increase of formyl adducts in exposed subjects compared to controls | Fair |
| Romanazzi et al., 2013 [51] Italy Decorative laminates industry | 105; 51 exposed and 54 controls; 40 ± 10 years; 105 males (100%) and 0 females (0%) | Biomarkers of effect: levels of 15-F2t-isoprostane in urine samples | Age, place of residence, hobbies, therapies, smoking habits, professional use of environmental and personal protective devices | Statistically significant increase in 15-F2t-isoprostane in exposed group compared to controls | Poor |
| Seow et al., 2015 [52] China Resins and plastic factories | 94; 43 exposed and 51 controls; 30.5 ± 6.5 years; 43 males (46%) and 51 females (54%) | Biomarkers of effect: circulating immune/inflammation markers | Age, gender, smoking habits, alcohol consumption, recent infections or medication use, body mass index | Statistically significant decrease in immunomodulating markers in the exposed workers compared to controls | Good |
| Van der Laan et al., 2022 [53] China Resins and plastic factories | 70; 31 exposed and 39 controls; 30.7 ± 6.7 years; 12 males (17%) and 58 females (83%) | Biomarkers of effect: DNA methylation in peripheral blood cells | body mass index, smoking habits, alcohol consumption, self-reported recent infection | No statistically significant differences in methylation in peripheral blood cells were observed in the exposed group compared to controls | Fair |
| Zendehdel et al., 2016 [54] Iran Melamine dish preparation plant | 67; 35 exposed and 32 controls; 17–59 years | Biomarkers of effect: erythrocyte acetylcholinesterase activity | Age, gender, smoking habits, socioeconomic status, genotype of acetylcholinesterase | Statistically significant increase in the acetylcholinesterase activity in the exposed group compared to controls | Fair |
| Zendehdel et al., 2018 [55] Iran Melamine tableware plant | 87; 53 exposed and 34 controls; 28.8 ± 7.9 years; 80 males (92%) and 7 females (8%) | Biomarkers of effect: DNA damage by comet assay | Age, gender, smoking habits, alcohol consumption, work experience | Statistically significant increase in DNA tail lengths at comet assay in the exposed group | Good |
| Zhang et al., 2010 [56] China Melamine resins plant | 94; 43 exposed and 51 controls; 30.5 ± 6.5 years; 81 males (86%) and 13 females (14%) | Biomarkers of effect: hematopoietic function disruption and leukemia-related chromosome changes by fluorescence in situ hybridization (FISH) | Smoking habits, alcohol consumption, age, gender, flu or respiratory infections in the previousmonth, body mass index | Statistically significant decrease in total white blood cell counts in the exposed group | Fair |
| Zhitkovich et al., 1996 [57] Bulgaria Chrome-platers factory | 16; 10 exposed and 6 controls; 37.8 ± 6.8 years | Biomarkers of effect: DNA-protein crosslinks in peripheral blood lymphocytes | Smoking habits, age, gender, weight, alcohol consumption, occupational exposure to chromium | No statistically significant differences of levels of DNA-protein crosslinks in peripheral lymphocytes in the exposed group | Poor |
| Reference Country Working Context | Sample Size Age (Mean Value ± SD and/or Range) Gender (%) | Biomarkers of Internal Dose and/or Early Effect | Confounding and Interfering Factors | Main Results | Quality Evaluation According to NOS Scale |
|---|---|---|---|---|---|
| Aglan and Mansour 2020 [58] Egypt Hairdressing salon | 120; 60 exposed and 60 controls; 20–36 years; 0 males (0%) and 120 females (100%) | Biomarkers of effect: micronucleus frequencies in epithelial buccal cells and peripheral blood lymphocytes | Age, residency, nutritional habits, socio-economic standard | Statistically significant increase in micronucleus frequency in hairstylists involved in hair straightening procedure for >5 years with respect to the controls | Fair |
| Barbosa et al., 2019 [5] Brazil Hairdressing salon | 49; 8 males (16%) and 41 females (84%) | Biomarkers of effect: global DNA methylation in whole blood | Age, gender, alcohol consumption, smoking habits | Statistically significant increase in global DNA methylation in higher-exposed group. | Fair |
| Norbak et al., 2000 [59] Sweden School buildings | 234 | Biomarkers of effect: acoustic rhinometry and eosinophil cationic protein, myeloperoxidase, lysozyme, albumin in nasal lavage | Age, gender, smoking habits, atopy and mean classroom temperature in the school | Statistically significant increase in eosinophil cationic protein and of lysozyme in the exposed group. A lower degree of nasal patency was found at higher concentrations of respirable dust, nitrogen dioxide, and formaldehyde | Fair |
| Peteffi et al., 2016 [11] Brazil Hairdressing salon | 50 | Biomarkers of internal dose: urinary formic acid concentration Biomarkers of effect: micronucleus test in exfoliated buccal cells and comet assay in peripheral lymphocytes | Age, gender, weight, smoking, smoking-related habits, allergic symptoms, whether they wear personal protection equipment | Statistically significant variation in damage frequency and damage index in the comet assay, frequency of micronuclei, and formic acid concentration in urine before and after the exposure, respectively | Fair |
| Squillacioti et al., 2020 [60] Italy Traffic police officers | 154; 85 outdoor workers and 69 indoor workers; 45.8 ± 7.7 years; 88 males (57%) and 66 females (43%) | Biomarkers of effect: urinary F2t-isoprostane; FeNO as a marker of airway eosinophils inflammation | Age, gender, smoking habits, body mass index, sampling location, job duties, cities | Statistically significant positive correlation between the air concentration of formaldehyde and 15-F2t-isoprostane | Fair |
| Triebig et al., 1989 [61] Netherlands Anatomic theatres, pathological laboratories, chipboard manufacturers | 153 | Biomarkers of internal dose: urinary formic acid concentration | Workplaces | No significant relationship between FA exposure and formic acid excretion in urine | Poor |
| Vargova et al., 1993 [62] Slovakia Woodsplinter materials plant | 39; 20 exposed and 19 controls; 42.3 years | Biomarkers of effect: structural chromosome aberrations in peripheral blood lymphocytes | Age, lifestyle factors, social status, health conditions | Statistically significant higher percent of aberrant cells and breaks per cell in the exposed group compared to control | Poor |
| Viegas et al., 2010 [63] Portugal Resin production plant, pathology and anatomy laboratories | 165; 80 exposed and 85 controls; 34.8 ± 8.9 years; 79 males (48%) and 86 females (52%) | Biomarkers of effect: micronucleus test in exfoliated epithelial cells from buccal mucosa and peripheral blood lymphocytes | Age, gender, smoking habits, health conditions, medical history, medication, lifestyle factors | Statistically significant higher frequency of micronuclei in the exposed group, both in peripheral blood lymphocytes and in epithelial buccal cells in the exposed group compared to control. Moderate positive correlation between years of exposure and frequency of micronuclei in peripheral blood lymphocytes and in epithelial cells | Good |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Protano, C.; Antonucci, A.; De Giorgi, A.; Zanni, S.; Mazzeo, E.; Cammalleri, V.; Fabiani, L.; Mastrantonio, R.; Muselli, M.; Mastrangeli, G.; et al. Exposure and Early Effect Biomarkers for Risk Assessment of Occupational Exposure to Formaldehyde: A Systematic Review. Sustainability 2024, 16, 3631. https://doi.org/10.3390/su16093631
Protano C, Antonucci A, De Giorgi A, Zanni S, Mazzeo E, Cammalleri V, Fabiani L, Mastrantonio R, Muselli M, Mastrangeli G, et al. Exposure and Early Effect Biomarkers for Risk Assessment of Occupational Exposure to Formaldehyde: A Systematic Review. Sustainability. 2024; 16(9):3631. https://doi.org/10.3390/su16093631
Chicago/Turabian StyleProtano, Carmela, Arianna Antonucci, Andrea De Giorgi, Stefano Zanni, Elisa Mazzeo, Vincenzo Cammalleri, Leila Fabiani, Riccardo Mastrantonio, Mario Muselli, Giada Mastrangeli, and et al. 2024. "Exposure and Early Effect Biomarkers for Risk Assessment of Occupational Exposure to Formaldehyde: A Systematic Review" Sustainability 16, no. 9: 3631. https://doi.org/10.3390/su16093631
APA StyleProtano, C., Antonucci, A., De Giorgi, A., Zanni, S., Mazzeo, E., Cammalleri, V., Fabiani, L., Mastrantonio, R., Muselli, M., Mastrangeli, G., Ursini, C. L., Cavallo, D., Poli, D., Gennaro, G. D., De Palma, G., & Vitali, M. (2024). Exposure and Early Effect Biomarkers for Risk Assessment of Occupational Exposure to Formaldehyde: A Systematic Review. Sustainability, 16(9), 3631. https://doi.org/10.3390/su16093631











