Yam Nematodes as Production Constraints in Ghana: A Socio-Economic Perspective
Abstract
1. Introduction
2. Methodology
2.1. Study Area and Sample Selection
2.2. Sampling Procedure
2.3. Data Collection and Analysis
2.4. Extraction of Nematodes from Soils Collected from Yam Farms and from Tubers
3. Results and Discussion
3.1. Socio-Demographic Characteristics of Yam Farmers
3.2. Yam Farmers’ Experience and Intensity of Land Use
3.3. Farmers’ Perception and Knowledge of Yam Nematodes
3.4. Yam Farmers’ Knowledge in Control and Management of Nematode Infestation
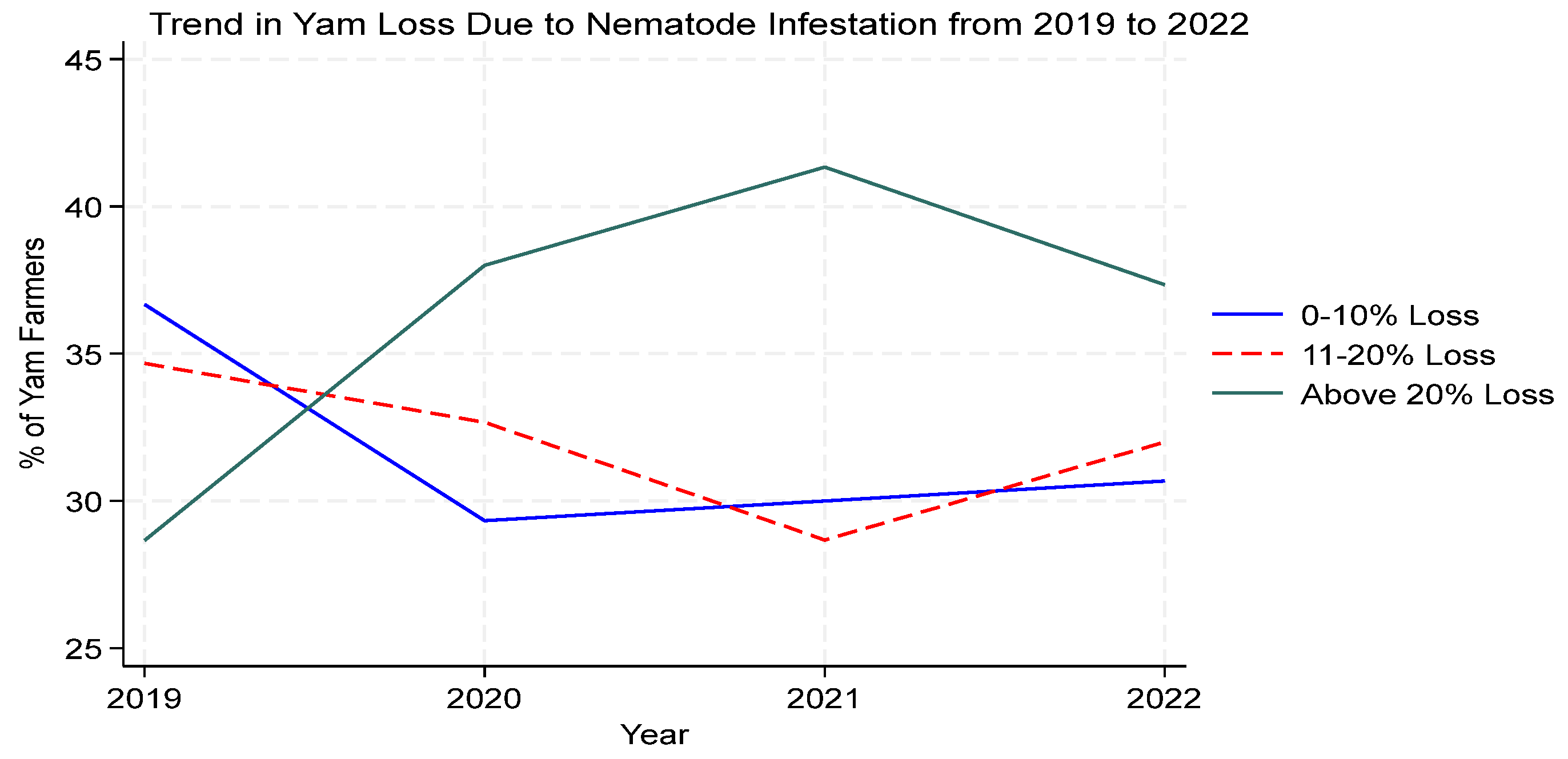
3.5. Loss of Yam Tubers in Storage Due to Nematode Infection
3.6. Plant Parasitic Nematode Genera Associated with Soils and Yam Tubers
4. Conclusions and Implications
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nweke, F.; Aidoo, R.; Okoye, B. Yam consumption patterns in West Africa. Gates Open Res. 2019, 3, 637. [Google Scholar]
- Ghana Export Promotion Authority (GEPA). Analysis of 2021 Non-Traditional Exports Statistics. 2022. Available online: https://www.gepaghana.org/export-statistic/nte-annual-report-2021-full/ (accessed on 17 October 2024).
- Wu, Z.G.; Jiang, W.; Nitin, M.; Bao, X.Q.; Chen, S.L.; Tao, Z.M. Characterizing diversity based on nutritional and bioactive compositions of yam germplasm (Dioscorea spp.) commonly cultivated in China. J. Food Drugs Anal. 2016, 24, 367–375. [Google Scholar] [CrossRef]
- Mignouna, D.B.; Abdoulaye, T.; Alene, A.; Akinola, A.A.; Maroya, N. Baseline Protocols: The Case of Yam Improvement for Income and Food Security in West Africa (YIIFSWA) Project; YIIFSWA working paper series, No. 4; SIDALC: Turrialba, Costa Rica, 2014. [Google Scholar]
- Owusu Danquah, E.; Danquah, F.O.; Frimpong, F.; Dankwa, K.O.; Weebadde, C.K.; Ennin, S.A.; Opoku, A.Y. Sustainable intensification and climate-smart yam production for improved food security in West Africa: A review. Front. Agron. 2022, 4, 858114. [Google Scholar] [CrossRef]
- Carsky, R.J.; Asiedu, R.; Cornet, D. Review of soil fertility management for yam-based systems in West Africa. Afr. J. Root Tuber Crops 2010, 8, 1–17. [Google Scholar]
- Kolombia, Y.A.; Fabiyi, O.A. Nematode problems in tuber crops and their sustainable management. In Nematode Diseases of Crops and Their Sustainable Management; Academic Press: New York, NY, USA, 2023; pp. 251–278. [Google Scholar]
- Nzeako, S.O.; Barisuka, G.A.; Torle, M.E.; Imafidor, H.O.; Eze, C.N. Plant parasitic nematodes status of seed yams in Khana LGA, Rivers State, Nigeria. Int. J. Sci. Res. Environ. Sci. 2015, 3, 266. [Google Scholar]
- Coyne, D.L.; Tchabi, A.; Baimey, H.; Labuschagne, N.; Rotifa, I. Distribution and prevalence of nematodes (Scutellonema bradys and Meloidogyne spp.) on marketed yam (Dioscorea spp.) in West Africa. Field Crops Res. 2006, 96, 142–150. [Google Scholar] [CrossRef]
- Mignouna, B.D.; Kumar, P.L.; Coyne, D.; Bandyopadhyay, R.; Ortega-Beltran, A.; Bhattacharjee, R.; De Koeyer, D. Identifying and managing plant health risks for key African crops: Yam, taro and cocoyam. In Critical Issues in Plant Health; Burleigh Dodds Science Publishing: Chambridge, UK, 2019; pp. 213–228. [Google Scholar]
- Green, K.R.; Florini, D.A. Pests and pathogens of yams in storage. Workshop Rep. Afr. J. Root Tuber Crops 1996, 1, 38–42. [Google Scholar]
- Ntui, V.O.; Uyoh, E.A.; Ita, E.E.; Markson, A.A.A.; Tripathi, J.N.; Okon, N.I.; Tripathi, L. Strategies to combat the problem of yam anthracnose disease: Status and prospects. Mol. Plant Pathol. 2021, 22, 1302–1314. [Google Scholar] [CrossRef] [PubMed]
- Claudius-Cole, A. Importance and integrated nematode management of the yam nematode (Scutellonema bradys) in yam cropping systems of West Africa. In Integrated Nematode Management: State-of-the-Art and Visions for the Future; CAB International: Wallingford, UK, 2022; pp. 374–380. [Google Scholar]
- Ackah, D.; Agboyi, M.R.; Gyamfi, L.A. An investigation of yam ingestion customs in Ghanaian urban communities, Ghana. Int. J. Sci. Basic Appl. Res. 2014, 17, 411. [Google Scholar]
- Acheampong, P.P.; Owusu Danquah, E.; Dissanayake, H.G.; Hayford, P.; Weebadde, C. A socioeconomic study of transition zone yam farmers addressing constraints and exploring opportunities for integrating pigeonpea into yam cropping systems. Sustainability 2019, 11, 717. [Google Scholar] [CrossRef]
- Palinkas, L.A.; Horwitz, S.M.; Green, C.A.; Wisdom, J.P.; Duan, N.; Hoagwood, K. Purposeful sampling for qualitative data collection and analysis in mixed method implementation research. Adm. Policy Ment. Health Ment. Health Serv. Res. 2015, 42, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, W.R. A rapid centrifugal flotation technique for separating nematodes from soil. Plant Dis. Report. 1964, 48, 692. [Google Scholar]
- Whitehead, A.G.; Hemming, J.R. A comparison of some quantitative methods of extracting small vermiform nematodes from soil. Ann. Appl. Biol. 1965, 55, 25–38. [Google Scholar] [CrossRef]
- Siddiqi, M.R. Tylenchida Parasites of Plants and Insects, 2nd ed.; CABI Publishing: Wallingford, UK, 2000. [Google Scholar]
- Adam, H.; Zakaria, H.; Abujaja, A.M. Sociocultural implications on innovation adoption: The case of adoption of yam minisett technology among farmers in Northern Region. J. Agric. Ext. Rural Dev. 2014, 1, 105–113. [Google Scholar]
- Zakaria, H.; Salifu, W.Y.; Abujaja, A.M.; Adam, H. Socioeconomics analysis of smallholder yam production in north eastern corridors of northern Ghana: Does type of planting material used makes any difference. Int. J. Agric. Innov. Res. 2014, 3, 1043–1051. [Google Scholar]
- Mignouna, D.B.; Abdoulaye, T.; Akinola, A.A.; Alene, A.; Nweke, F. Factors influencing the use of selected inputs in yam production in Nigeria and Ghana. J. Agric. Rural Dev. Trop. Subtrop. 2015, 116, 131–142. [Google Scholar]
- Obidiegwu, J.E.; Akpabio, E.M. The geography of yam cultivation in southern Nigeria: Exploring its social meanings and cultural functions. J. Ethnic Foods 2017, 4, 28–35. [Google Scholar] [CrossRef]
- Kawarazuka, N.; Damtew, E.; Mayanja, S.; Okonya, J.S.; Rietveld, A.; Slavchevska, V.; Teeken, B. A gender perspective on pest and disease management from the cases of roots, tubers and bananas in Asia and sub-Saharan Africa. Front. Agron. 2020, 2, 7. [Google Scholar] [CrossRef]
- Kerubo Rogena, B. Analysis of coping mechanisms by emancipated women to combat household food security in Kalagala sub-County of Luweero, Uganda. Am. Based Res. J. 2017, 6, 12. [Google Scholar]
- Ufondu, H.E.; Maziya-Dixon, B.; Okonkwo, T.M. Yam production in some South East and North Central zones of Nigeria beyond COVID-19 for acceleration towards inclusive sustainable development. Agro-Science 2021, 20, 1–7. [Google Scholar] [CrossRef]
- Leavy, J.; Hossain, N. Who wants to farm? Youth aspirations, opportunities and rising food prices. IDS Work. Pap. 2014, 439, 1–44. [Google Scholar] [CrossRef]
- Midega, C.A.; Murage, A.W.; Pittchar, J.O.; Khan, Z.R. Managing storage pests of maize: Farmers’ knowledge, perceptions and practices in western Kenya. Crop Prot. 2016, 90, 142–149. [Google Scholar] [CrossRef]
- Reddy, P.P. Crop Rotation. In Agro-Ecological Approaches to Pest Management for Sustainable Agriculture; Springer: Singapore, 2017. [Google Scholar] [CrossRef]
- Claudius-Cole, A.O.; Fawole, B.; Asiedu, R. Population changes of plant-parasitic nematodes associated with cover crops following a yam (Dioscorea rotundata) crop. Trop. Plant Pathol. 2015, 40, 193–199. [Google Scholar] [CrossRef]
- Uyoh, E.A.; Ita, E.E.; Ntui, V.O. Strategies for the production of disease-free yam planting materials: Implications for improved yam production in Nigeria. In Agricultural Biotechnology, Biodiversity and Bioresources Conservation and Utilization; CRC Press: Boca Raton, FL, USA, 2022; pp. 101–126. [Google Scholar]
- Bamire, A.S.; Amujoyegbe, B.J. Economic analysis of land improvement techniques in smallholder yam-based production systems in the agro-ecological zones of Southwestern Nigeria. J. Hum. Ecol. 2005, 18, 1–12. [Google Scholar] [CrossRef]
- Jamani, Y.; Nyaku, S.T.; Lutuf, H.; Cornelius, E. Plant-parasitic nematodes associated with yam in the two agro-ecological zones of Ghana. Niger. J. Nematol. 2016, 3, 1–12. [Google Scholar]
- Neina, D. Ecological and edaphic drivers of yam production in West Africa. Appl. Environ. Soil Sci. 2021, 1, 5019481. [Google Scholar] [CrossRef]
- Wumbei, A.; Gautier, S.K.N.; Kwodaga, J.K.; Joseph, D.F.; Galani, Y.J.H. State of the Art of Yam Production; CCCU Research Space Repository: Canterbury, UK, 2022. [Google Scholar]
- Enchill, A.; Nyaku, S.T.; Osabutey, S.; Lutuf, H.; Cornelius, E. Prevalence of yam nematodes in diverse soil communities, and their morphometrics in the Krachi-Nchumuru District of Ghana. Afr. Crop Sci. J. 2023, 31, 319–336. [Google Scholar] [CrossRef]
- Amponsah, S.K.; Akowuah, J.O.; Adu-Kwarteng, E.; Bessah, E. Design and construction of improved yam storage structure using locally-available materials. Int. J. Res. 2015, 1, 1–11. [Google Scholar]
- Nwakonobi, T.U.; Obetta, S.E.; Iorwtsav, H. Evaluation of ventilated underground pit structures for yam (Dioscorea spp.) storage. Res. J. Appl. Sci. Eng. Technol. 2012, 4, 393–397. [Google Scholar]
- Nwaigwe, K.N.; Okafor, V.C.; Asonye, G.U.; Nwokocha, J.C. Analysis of tuber storage techniques in Africa: A review. In Proceedings of the 2015 ASABE Annual International Meeting, Detroit, MI, USA, 29 July–1 August 2015; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2015; p. 1. [Google Scholar]
- Amusa, N.A.; Adigbite, A.A.; Muhammed, S.; Baiyewu, R.A. Yam diseases and its management in Nigeria. Afr. J. Biotechnol. 2003, 2, 497–502. [Google Scholar] [CrossRef]
- Kwoseh, C.K.; Plowright, R.A.; Bridge, J.; Asiedu, R. Yam-based farm practices and nematode problems in stored yams (Dioscorea spp.) in Ghana. J. Sci. Technol. 2005, 25, 35–43. [Google Scholar] [CrossRef][Green Version]
- Obuezie, C.B.; Ikpeze, O.O. Parasitic Nematodes of maize in farms at Oba, Idemili south Local Government Area of Anambra state Nigeria. J. Occup. Saf. Environ. Health 2012, 1, 73–78. [Google Scholar]
- Aighewi, B.A.; Asiedu, R.; Maroya, N.; Balogun, M. Improved propagation methods to raise the productivity of yam (Dioscorea rotundata Poir.). Food Secur. 2015, 7, 823–834. [Google Scholar] [CrossRef]
- Kayode, O.; Claudius-Cole, A. Host efficiency of Scutellonema bradys in yam companion crops in Nigeria. Int. J. Plant Soil Sci. 2017, 15, 1–10. [Google Scholar] [CrossRef]
- Adegbite, A.A.; Saka, J.O.; Agbaje, G.O.; Owolade, O.F.; Olaifa, G.O.; Lawal, A.; Ojo, S.T. Survey of plant-parasitic nematodes associated with yams in Edo, Ekiti and Oyo states of Nigeria. Afr. J. Agric. Res. 2006, 1, 125–130. [Google Scholar]
- Adegbite, A.; Saka, J.; Agbaje, G.; Osuloye, F. Survey of plant-parasitic nematodes associated with yams in Ogun and Osun states of Nigeria. J. Plant Prot. Res. 2008, 48, 421–428. [Google Scholar] [CrossRef]
- Adam, M.; Heuer, H.; Ramadan, E.M.; Hussein, M.A.; Hallmann, J. Occurrence of plant-parasitic nematodes in organic farming in Egypt. Int. J. Nematol. 2013, 23, 82–90. [Google Scholar]
- Vincent, C.D.; Nwankwo, E.N.; Ogbonna, C.U.; Onyido, A.E.; Adewuyi, O. A survey of plant-parasitic nematodes of yam farms in Awka-North local government area, Anambra state, Nigeria. J. Appl. Biosci. 2015, 95, 8950–8957. [Google Scholar]
- Kingsley, E. Investigation of Plant-parasitic nematodes of yam in selected yam farms in two local Government areas of Rivers State. Trop. Agrobiodivers. 2021, 2, 67–71. [Google Scholar] [CrossRef]




| Agro-Ecological Zone | Location | Global Positioning System |
|---|---|---|
| Semi-Deciduous Rainforest | Kpando Gbefi | 6°59′44.05″ N 0°22′40.85″ E |
| Semi-Deciduous Rainforest | Grubi | 8°18′41.4″ N 0°17′17.4″ E |
| Guinea Savanna | Buya | 8°20′51.67″ N 0°3′55.28″ E |
| Forest Savanna Transition | Dotobaa | 77°31′53.3″ N 1°48′21.5″ W |
| Forest Savanna Transition | Botokrom | 7°31′12.39″ N 2°45′7.15″ W |
| Forest Savanna Transition | Abirikasu | 7°29′52.51″ N 2°49′40.63″ W |
| Guinea Savanna | Sabari | 9°16′47.14″ N 0°15′32.18″ E |
| Variables | Frequency | Percentage |
|---|---|---|
| Sex | ||
| Male | 135 | 90.00 |
| Female | 15 | 10.00 |
| Age (years) | ||
| ≤20 | 1 | 0.67 |
| 21–30 | 23 | 15.33 |
| 31–40 | 42 | 28.00 |
| 41–50 | 40 | 26.67 |
| >50 | 44 | 29.33 |
| Education | ||
| None | 47 | 31.33 |
| Primary | 32 | 21.33 |
| Middle school | 45 | 30.00 |
| Secondary | 21 | 14.00 |
| Tertiary | 5 | 3.33 |
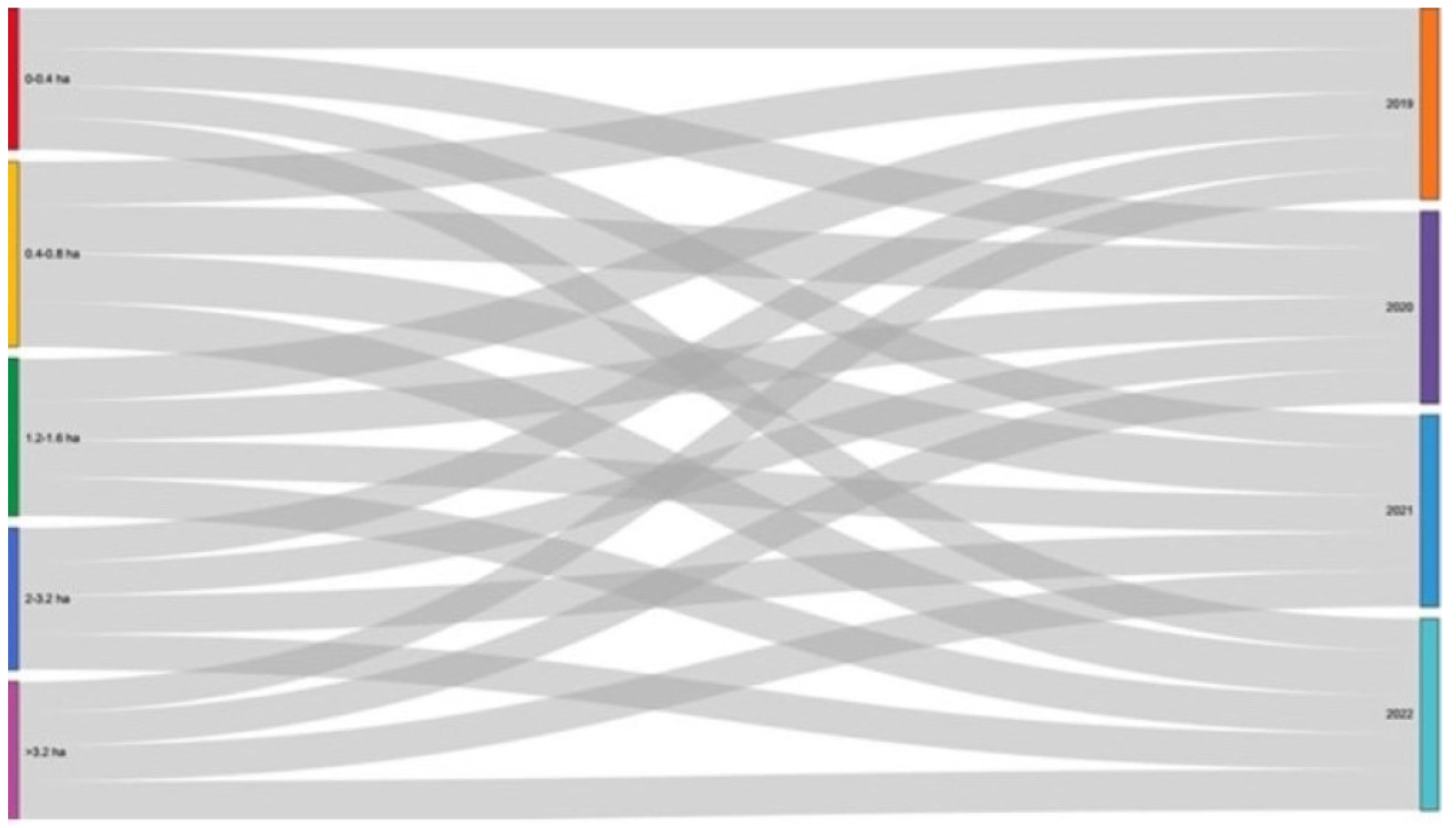
| Farm Size (Hectares) | 2019 | 2020 | 2021 | 2022 | ||||
|---|---|---|---|---|---|---|---|---|
| Male (n = 135) | Female (n = 15) | Male (n = 135) | Female (n = 15) | Male (n = 135) | Female (n = 15) | Male (n = 135) | Female (n = 15) | |
| 0–0.4 | 17.78 | 53.33 | 16.30 | 46.67 | 13.33 | 46.67 | 12.59 | 53.33 |
| 0.4–0.8 | 22.22 | 26.67 | 23.70 | 40.00 | 24.44 | 33.33 | 22.96 | 26.67 |
| 1.2–1.6 | 23.70 | 6.67 | 22.96 | 21.48 | 21.48 | 6.67 | ||
| 2–3.2 | 18.52 | 13.33 | 17.78 | 13.33 | 20.00 | 20.00 | 19.26 | 13.33 |
| >3.2 | 17.78 | 19.26 | 20.74 | 23.70 | ||||
| Variables | Frequency | Percentage |
|---|---|---|
| Farming experience (years) | ||
| 1–5 | 17 | 11.33 |
| 6–10 | 31 | 20.67 |
| 11–15 | 18 | 12.00 |
| >15 | 84 | 56.00 |
| Reason for yam cultivation | ||
| Food | 9 | 6.00 |
| Income | 141 | 94.00 |
| Cultivation of other crops on land (years) | ||
| 1–3 | 26 | 17.33 |
| 4–6 | 51 | 34.00 |
| 7–10 | 25 | 16.67 |
| 11–15 | 8 | 5.33 |
| >15 | 40 | 26.67 |
| Period of yam cultivation (years) | ||
| 1–3 | 43 | 28.67 |
| 4–6 | 51 | 34.00 |
| 7–10 | 15 | 10.00 |
| >10 | 41 | 27.33 |
| Rotation of yam crops | ||
| Yes | 121 | 80.67 |
| No | 29 | 19.33 |
| Period of crop rotation (years) | ||
| None | 29 | 19.33 |
| 1–2 | 100 | 66.67 |
| 3–5 | 18 | 12.00 |
| >5 | 3 | 2.00 |
| Land fallow practice | ||
| Yes | 113 | 75.33 |
| No | 37 | 24.67 |
| Period of land fallow (years) | ||
| None | 33 | 22.00 |
| 1 | 53 | 35.33 |
| 2–4 | 48 | 32.00 |
| 5–10 | 16 | 10.67 |
| Variables | Frequency | Percentage |
|---|---|---|
| Pests and diseases experience | ||
| Yes | 146 | 97.99 |
| No | 3 | 2.01 |
| No answer | 1 | 0.67 |
| Knowledge of nematodes | ||
| Yes | 34 | 22.67 |
| No | 116 | 77.33 |
| Necrotic streaks or root lesions | ||
| Yes | 140 | 93.33 |
| No | 10 | 6.67 |
| Internal rotting and discoloration of vegetative organs | ||
| Yes | 145 | 96.67 |
| No | 5 | 3.33 |
| Dwarfing of yam plant | ||
| Yes | 85 | 56.67 |
| No | 65 | 43.33 |
| Stunting and resetting | ||
| Yes | 82 | 54.67 |
| No | 66 | 44.00 |
| No answer | 2 | 1.33 |
| Soil condition during disease prevalence | ||
| Well drained soil | 2 | 1.33 |
| Sandy clayey | 29 | 19.33 |
| Sandy soil | 12 | 8.00 |
| Loamy soil | 98 | 65.33 |
| Clayey soil | 9 | 6.00 |
| Yam storage after harvest | ||
| In a pit at the farm | 9 | 6.00 |
| Yam barn at the farm | 136 | 90.67 |
| In a store at home | 5 | 3.33 |
| Storage losses due to pests and diseases | ||
| Yes | 95 | 63.33 |
| No | 55 | 36.67 |
| Hardened dried tissues | ||
| Yes | 95 | 63.33 |
| No | 55 | 36.67 |
| External cracks on skin of tubers | ||
| Yes | 91 | 60.67 |
| No | 59 | 39.33 |
| Significant weight loss in tubers | ||
| Yes | 89 | 59.73 |
| No | 60 | 40.27 |
| No answer | 1 | 0.67 |
| Light yellow lesions below the outer skin of tuber | ||
| Yes | 93 | 62.00 |
| No | 57 | 38.00 |
| Rotting of the outer 1–2 cm layer | ||
| Yes | 88 | 58.67 |
| No | 62 | 41.33 |
| Flaking off of external coverings | ||
| Yes | 83 | 55.33 |
| No | 66 | 44.00 |
| No answer | 1 | 0.67 |
| General decay of tubers | ||
| Yes | 91 | 60.67 |
| No | 59 | 39.33 |
| Method of nematode spread | ||
| Infected tubers | 9 | 6.00 |
| Soil | 135 | 90.00 |
| Heat | 4 | 2.67 |
| Prolonged drought | 1 | 0.67 |
| Heavy rainfall | 1 | 0.67 |
| Variables | Frequency | Percent |
|---|---|---|
| Able to manage or control nematode infestation | ||
| No | 108 | 72.00 |
| Yes | 42 | 28.00 |
| Reason for not being able control nematode infestation | ||
| No reason | 2 | 1.33 |
| I do not know how to control them | 106 | 70.67 |
| Controlled | 42 | 28.00 |
| Management practices used on yam farm? | ||
| Crop rotation | ||
| Yes | 40 | 26.67 |
| No | 110 | 73.33 |
| Nematicides | ||
| Yes | 2.67 | |
| No | 146 | 97.33 |
| Land rotation | ||
| Yes | 39 | 26.00 |
| No | 111 | 74.00 |
| Use of organic or inorganic fertilizer | ||
| Yes | 3 | 2.00 |
| No | 147 | 98.00 |
| Variable | Management of Nematode Infection (Frequency, %) | χ2/Fisher’s Exact Test | |
|---|---|---|---|
| Yes | No | ||
| Yam storage after harvest | 0.056 * | ||
| In a pit at the farm | 0 (0.00) | 9 (8.33) | |
| Yam barn at the farm | 42 (100) | 94 (87.04) | |
| In a store at home | 0 (0.00) | 5 (4.63) | |
| Pest and disease occurrence | |||
| Yes | 41 (97.62) | 105 (98.13) | 1.00 |
| No | 1 (2.38) | 2 (1.87) | |
| Knowledge of nematode | 0.136 | ||
| Yes | 13 (30.95) | 21 (19.44) | |
| No | 29 (69.05) | 87 (80.56) | |
| Education | |||
| No education | 11 (26.19) | 21 (19.44) | 0.247 |
| Formal education | 31 (73.81) | 87 (80.56) | |
| Years in yam farming | |||
| 1–5 years | 5 (11.90) | 12 (11.11) | |
| 6–10 years | 8 (19.05) | 23 (21.30) | 0.685 |
| 11–15 | 3 (7.14) | 15(13.89) | |
| >15 years | 26 (61.90) | 58 (53.70) | |
| Community | Scu | Mel | Hel | Pra | Mes | Tyl | Het | Lon | Ape | Par | Dit | Xip | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dotobaa | 2 | 0 | 57 | 28 | 10 | 19 | 25 | 35 | 14 | 2 | 0 | 0 | 192 |
| Gbefi | 23 | 85 | 1 | 27 | 0 | 145 | 0 | 25 | 11 | 6 | 2 | 0 | 325 |
| Grubi | 44 | 141 | 99 | 13 | 0 | 248 | 0 | 45 | 0 | 10 | 2 | 0 | 602 |
| Buya | 47 | 146 | 66 | 9 | 1 | 183 | 10 | 75 | 7 | 0 | 0 | 10 | 554 |
| Sabari | 7 | 126 | 85 | 7 | 0 | 231 | 0 | 49 | 2 | 2 | 0 | 102 | 611 |
| Botokrom | 5 | 60 | 28 | 0 | 0 | 64 | 0 | 20 | 1 | 29 | 0 | 22 | 229 |
| Jaman South | 4 | 103 | 18 | 4 | 0 | 99 | 1 | 22 | 0 | 19 | 0 | 13 | 283 |
| Total | 132 | 661 | 354 | 88 | 11 | 989 | 36 | 271 | 35 | 68 | 4 | 147 | 2796 |
| Nematode Genera | Order | Population/200CC Soil | Frequency of Occurrence | % Frequency Rating * | Relative Abundance (%) ** |
|---|---|---|---|---|---|
| Scutellonema bradys | Rabditida | 132 | 19 | 54 | 4.7 |
| Meloidogyne spp. | Rabditida | 661 | 30 | 86 | 23.7 |
| Helicotylenchus spp. | Rabditida | 353 | 29 | 83 | 12.7 |
| Pratylenchus spp. | Rabditida | 88 | 20 | 57 | 3.2 |
| Mesocriconema spp. | Rabditida | 11 | 4 | 11 | 0.4 |
| Tylenchus spp. | Rabditida | 989 | 35 | 100 | 35.5 |
| Heterodera spp. | Rabditida | 36 | 5 | 14 | 1.3 |
| Paratylenchus spp. | Rabditida | 68 | 18 | 51 | 2.4 |
| Aphelenchus spp. | Rabditida | 35 | 8 | 23 | 1.3 |
| Ditylenchus spp. | Rabditida | 4 | 3 | 9 | 0.1 |
| Longidorus spp. | Dorylaimida | 271 | 34 | 97 | 9.7 |
| Xiphinema spp. | Dorylaimida | 137 | 14 | 40 | 4.9 |
| Community | Scu | Mel | Pra | Het | Hel | Tyl | Ape | Mes | Lon | Dit | Par | Xip | Hop | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dotobaa | 347 | 129 | 39 | 16 | 7 | 72 | 37 | 2 | 6 | 43 | 2 | 0 | 0 | 700 |
| Gbefi | 3290 | 43 | 8 | 0 | 0 | 4 | 33 | 0 | 4 | 0 | 3 | 2 | 0 | 3387 |
| Grubi | 2556 | 224 | 0 | 0 | 0 | 3 | 117 | 0 | 0 | 0 | 27 | 0 | 0 | 2927 |
| Buya | 4840 | 41 | 10 | 0 | 0 | 7 | 33 | 0 | 0 | 0 | 0 | 0 | 0 | 4931 |
| Sabari | 6299 | 117 | 5 | 0 | 0 | 0 | 78 | 0 | 0 | 0 | 11 | 23 | 0 | 6533 |
| Botokrom | 374 | 138 | 2 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 515 |
| Jaman South | 600 | 104 | 0 | 0 | 0 | 15 | 2 | 0 | 1 | 0 | 16 | 1 | 1 | 740 |
| Total | 18,306 | 796 | 64 | 16 | 7 | 102 | 300 | 2 | 11 | 43 | 59 | 26 | 1 | 19,733 |
| Nematode Genera | Order | Population/50 g Yam Peel | Frequency of Occurrence | % Frequency Rating * | Relative Abundance (%) ** |
|---|---|---|---|---|---|
| Scutellonema bradys | Rabditida | 18,306 | 35 | 100 | 92.8 |
| Meloidogyne spp. | Rabditida | 796 | 33 | 94 | 4.0 |
| Pratylenchus spp. | Rabditida | 64 | 12 | 34 | 0.3 |
| Heterodera spp. | Rabditida | 16 | 2 | 6 | 0.1 |
| Helicotylenchus spp. | Rabditida | 7 | 4 | 11 | 0.0 |
| Tylenchus spp. | Rabditida | 102 | 18 | 51 | 0.5 |
| Aphelenchus spp. | Rabditida | 300 | 26 | 74 | 1.5 |
| Mesocriconema spp. | Rabditida | 2 | 1 | 3 | 0.0 |
| Ditylenchus spp. | Rabditida | 43 | 4 | 11 | 0.2 |
| Paratylenchus spp. | Rabditida | 59 | 15 | 43 | 0.3 |
| Hoplolaimus spp. | Rabditida | 1 | 1 | 3 | 0.0 |
| Longidorus spp. | Dorylaimida | 11 | 5 | 14 | 0.1 |
| Xiphinema spp. | Dorylaimida | 26 | 5 | 14 | 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Whedie, B.O.; Yami, G.M.; Nyaku, S.T.; Brentu, C.F.; Ankrah, D.; Dzidzienyo, D.; Bhattacharjee, R. Yam Nematodes as Production Constraints in Ghana: A Socio-Economic Perspective. Sustainability 2025, 17, 482. https://doi.org/10.3390/su17020482
Whedie BO, Yami GM, Nyaku ST, Brentu CF, Ankrah D, Dzidzienyo D, Bhattacharjee R. Yam Nematodes as Production Constraints in Ghana: A Socio-Economic Perspective. Sustainability. 2025; 17(2):482. https://doi.org/10.3390/su17020482
Chicago/Turabian StyleWhedie, Boafo Osei, Gurmu Mesay Yami, Seloame Tatu Nyaku, Collison Francis Brentu, Daniel Ankrah, Daniel Dzidzienyo, and Ranjana Bhattacharjee. 2025. "Yam Nematodes as Production Constraints in Ghana: A Socio-Economic Perspective" Sustainability 17, no. 2: 482. https://doi.org/10.3390/su17020482
APA StyleWhedie, B. O., Yami, G. M., Nyaku, S. T., Brentu, C. F., Ankrah, D., Dzidzienyo, D., & Bhattacharjee, R. (2025). Yam Nematodes as Production Constraints in Ghana: A Socio-Economic Perspective. Sustainability, 17(2), 482. https://doi.org/10.3390/su17020482







