Integrated Risk Framework (IRF)—Interconnection of the Ishikawa Diagram with the Enhanced HACCP System in Risk Assessment for the Sustainable Food Industry
Abstract
1. Introduction
2. Materials and Methods
2.1. Fundamental Principles, Basic Concepts, and Specific Implementation Steps
2.2. The Tools and Techniques Used for the Integrated Risk Framework (IRF) Methodology
- Identification and analysis of hazards;
- Determining the CCPs;
- Establishing critical limits for each CCP;
- Monitoring and applying corrective actions when necessary.
- Materials—quality of raw materials and ingredients used;
- Machines—performance of equipment and its maintenance;
- Manpower—level of training, competencies, and work practices of staff;
- Methods—procedures and working methods implemented in the technological process;
- Environment—environmental conditions such as temperature, humidity, and cleanliness.
2.3. Advantages and Outcomes of the Proposed Methodology Compared to Classical HACCP
- (a)
- Detailed and structured identification of causes
- (b)
- Evaluation of synergistic and cumulative risks
- (c)
- Real-time risk control and high adaptability
- (d)
- Improved coordination between departments
- (e)
- Potential for extrapolation to other industries
- (f)
- Improved product quality and customer satisfaction
- (g)
- Reduction of risk-related costs
3. Application of the Integrated Risk Framework (IRF) Methodology: Case Study
3.1. Implementation of the Classical HACCP System
3.2. Implementation of the Integrated Risk Framework (IRF) System
- Allergens are substances or foods to which the body has an allergic reaction of an immunological nature. The products that can cause food allergic reactions in the human body are the following 14 categories: cereals containing gluten, crustaceans and derived products, eggs and egg-based products, fish and derived products, peanuts and derived products, soy and soy-based products, milk and dairy products, nuts (walnuts, pistachios, etc.), hazelnuts, celery and derived products, sesame and sesame-based products, mustard and mustard-based products, SO2 and sulphite content > 10 mg/kg, lupin, and molluscs [50];
- Fraud/sabotage is represented by treatments to modify the properties or defraud food products, in order to obtain financial benefits, increase the shelf life, improve sensory or physico-chemical properties, improve microbiological properties, etc. Fraud is represented by treatments to modify the properties of or falsify food products, and sabotage is represented by preventing, stopping, or altering an activity, produced in order to obtain benefits or for revenge, ill will, etc. Products, machinery and equipment, and working conditions can be sabotaged by unit operators, drivers, visiting personnel, etc. [51];
- Irradiation/radioactivity/GMOs are represented by genetically modified products or ingredients derived from GMOs or by products treated with ionizing radiation in order to preserve them or improve their properties. Products treated with ionizing radiation are vegetable food products, which can be treated with a maximum of 10 kGy (total average absorbed radiation dose) [52]. The use of irradiated products must be indicated to the consumer by their appropriate labeling. Failure to comply with this provision constitutes a violation of European requirements, which leads to the withdrawal and destruction of the entire irradiated quantity.Another risk is represented by radioactive contamination of food products. Radioactive contamination can occur following a nuclear accident or other radiological emergency. Thus, in areas with the risks described above, plants and feed can be contaminated, leading to the accumulation of radioactive isotopes in them.The European Union has established maximum levels of radioactive contamination of food products. Products and feed that exceed the legal limit will be withdrawn and neutralized.GMOs: This category includes organisms whose genetic material (DNA) has been modified in an unnatural way using DNA recombination technologies. The main types of products that can be genetically modified are the following: corn and its derivatives, soy and its derivatives, potato and its derivatives. The use of approved GMOs must be indicated to the consumer by appropriate labeling. Failure to comply with this provision constitutes a violation of European requirements, which leads to the withdrawal and destruction of the products in question [53];
- Kosher/Halal are food standards with religious roots according to which some food products are prohibited for consumption by Muslims or Jews. This also includes products that may come into contact with Kosher/Halal food or may be contaminated/may contaminate products permitted for consumption by the two communities. There are dangers for the Jewish and Muslim communities that may affect base materials, goods, intermediate products, and end products, in the case of products sold to them. These dangers (pork products and derivatives, carmine, alcohol, etc.) can arise from the use of products prohibited for consumption by the Jewish or Muslim community, from contamination during transport, handling, storage, and processing [54];
- PAHs are represented by plant products that can be contaminated with these hydrocarbons from crops (source of origin soil, water, etc.). They are hydrocarbons that can appear in plants, from the soil in which they were grown or the irrigation water [55];
- RASFF products are represented by non-compliant products reported on the European Rapid Alert System. They are non-compliant products that can enter the establishment or that can be processed in the establishment [56];
- Swine fever/peste of small ruminants are represented by products from animals sick with the swine fever/peste of small ruminants virus and which may have an impact on food safety. They are products derived from pigs/goats and sheep (collagen protein, gelatin, hemoglobin, etc.) which are contaminated with the swine fever virus and cause swine flu. To control this risk, specific measures are taken for the supply, storage of goods, and marketing. This risk is not allowed to occur in the unit [57];
- Other risks are represented by the risks specific to the activity carried out by each department in the unit related to the business process, understanding the context in which it operates, and understanding the needs and expectations of stakeholders.
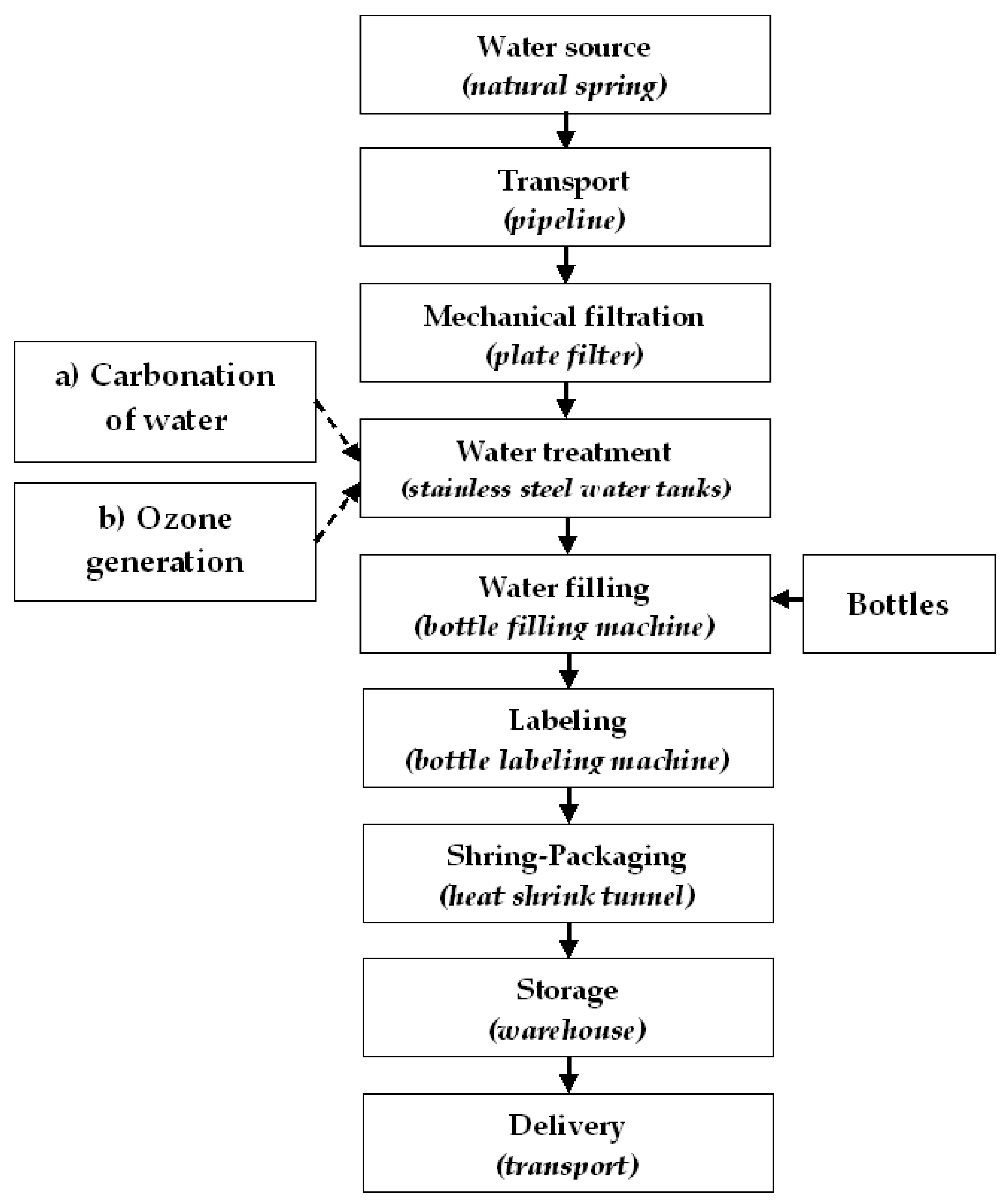
3.3. Applying the Integrated Risk Framework (IRF) Methodology to the Spring Water Bottling Flow
3.3.1. Risk Assessment for Each Hazard
3.3.2. Identification of Control Measures
3.3.3. Control Plan
3.4. Discussions
3.5. A Prospective Outlook: Future Directions and Objectives
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Financial Reporting Standards (IFRS) Foundation. IFRS S1 General Requirements for Disclosure of Sustainability-Related Financial Information. 2023. Available online: https://www.ifrs.org/issued-standards/ifrs-sustainability-standards-navigator/ifrs-s1-general-requirements/ (accessed on 29 October 2024).
- International Financial Reporting Standards (IFRS) Foundation. IFRS S2 Climate-Related Disclosures. 2023. Available online: https://www.ifrs.org/issued-standards/ifrs-sustainability-standards-navigator/ifrs-s2-climate-related-disclosures/ (accessed on 29 October 2024).
- United Nations Global Compact. Guide to Corporate Sustainability: Shaping a Sustainable Future. 2015. Available online: https://unglobalcompact.org/library/1151 (accessed on 2 November 2024).
- Global Reporting Initiative (GRI). GRI Standards. Global Reporting Initiative. 2020. Available online: https://www.globalreporting.org (accessed on 5 November 2024).
- Task Force on Climate-related Financial Disclosures (TCFD). Final Report: Recommendations of the Task Force on Climate-Related Financial Disclosures. 2017. Available online: https://www.fsb-tcfd.org (accessed on 21 October 2024).
- Yamina, F.; Noureddine, B.; Mebarek, D. Food Risk Management and Sustainable Development. J. Serv. Manag. 2014, 7, 182–188. [Google Scholar] [CrossRef][Green Version]
- Rotaru, O.; Mihaiu, M. Veterinary Hygiene of Food Products; Todesco Publishing House: Cluj Napoca, Romania, 2007; p. 177. (In Romanian) [Google Scholar]
- Bernstein, P.L. Against the Gods: The Remarkable Story of Risk; Wiley & Sons: New York, NY, USA, 1996; pp. 126–127. [Google Scholar]
- Aven, T. Risk assessment and risk management: Review of recent advances on their foundation. Eur. J. Oper. Res. 2016, 253, 1–13. [Google Scholar] [CrossRef]
- Aven, T. Risk Analysis: Assessing Uncertainties Beyond Expected Values and Probabilities; Wiley & Sons: New York, NY, USA, 2015; pp. 77–79. [Google Scholar]
- ISO 31000:2018; Risk Management—Guidelines. International Organization for Standardization: Geneva, Switzerland, 2018.
- Hillson, D.; Simon, P. Practical Project Risk Management: The ATOM Methodology, 3rd ed.; Berrett-Koehler Publisher: Oakland, CA, SUA, 2020; pp. 33–35. [Google Scholar]
- ISO 9001:2015; Quality Management Systems—Requirements. International Organization for Standardization: Geneva, Switzerland, 2015.
- ISO 31010:2019; Risk Management—Risk Assessment Techniques. International Organization for Standardization: Geneva, Switzerland, 2019.
- Frigo, M.L.; Anderson, R.J. Strategic risk management: A foundation for improving enterprise risk management and governance. J Corp. Account. Financ. 2011, 92, 81–88. [Google Scholar] [CrossRef]
- Hazard Analysis and Critical Control Point (HACCP) System and Guidelines for Its Application, Codex Alimentarius—Food Hygiene—Basic Texts—Second Edition, Annex to CAC/RCP 1-1969, Rev. 3. 1997. Available online: https://www.fao.org/4/y1579e/y1579e03.htm (accessed on 19 October 2024).
- Weinroth, M.D.; Belk, A.D.; Belk, K.E. History, development, and current status of food safety systems worldwide. Anim. Front. 2018, 8, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Vica, M.L.; Popa, D.; Glevitzky, M.; Siserman, C.; Matei, H.V. Quality of Drinkable Water Springs in Two Alba County Regions–Comparative Study. J. Environ. Prot. Ecol. 2017, 18, 1389–1397. [Google Scholar]
- Popa, M.; Glevitzky, M.; Popa, D.M.; Dumitrel, G.A. Study Regarding the Water Contamination and the Negative Effects on the Population from the Blaj Area, Romania. J. Environ. Prot. Ecol. 2014, 15, 1543–1554. [Google Scholar]
- Fortin, N.D. Chapter 35—HACCP and Other Regulatory Approaches to Prevention of Foodborne Diseases. In Food Science and Technology, Foodborne Infections and Intoxications, 4th ed.; Morris, J.G., Potter, M.E., Eds.; Academic Press: Cambridge, MA, USA, 2013; pp. 497–510. [Google Scholar] [CrossRef]
- Dina Al-Kandari, D.; Jukes, D. Incorporating HACCP into national food control systems—Analyzing progress in the United Arab Emirates. Food Control 2011, 22, 851–861. [Google Scholar] [CrossRef]
- Regulation (EC) No 852/2004 of the European Parliament and of the Council of 29 April 2004 on the Hygiene of Foodstuffs, L 139. In Official Journal of the European Union; European Union: Brussels, Belgium, 2004; pp. 1–54.
- Verbytskyi, S. HACCP System and Traceability of Raw Materials and Products of Meat Industry. In Proceedings of the Agrobiotechnology-2021, Moscow, Russia, 24–25 November 2021. [Google Scholar]
- Glevitzky, M.; Bogdan, I.; Calisevici, M.N.; Brusturean, G.A.; Perju, D.M. Analiza punctelor critice cu risc major în reducerea termenului de valabilitate a băuturilor răcoritoare utilizând metoda ASLD. Buletinul AGIR 2008, 1–2, 54–59. (In Romanian) [Google Scholar]
- Popa, M.; Glevitzky, I.; Popa, D.; Glevitzky, M. Water Quality Assessments Through the Application of Cause-and-Effect Diagrams in Conjunction with HACCP and Risk Assessment for “Roua Apusenilor” Spring Water Bottling Process. Sci. Pap.-Ser.-E-Land. R. 2021, 10, 158–165. [Google Scholar]
- Mee, C. Sustainable Food Safety Management System (S.F.S.M.S.)—Sustainable HACCP. Available online: https://occupli.com/sustainable-food-safety-management-system-s-f-s-m-s-sustainable-haccp/ (accessed on 12 November 2024).
- Kumah, A.; Nwogu, C.N.; Issah, A.R.; Obot, E.; Kanamitie, D.T.; Sifa, J.S.; Aidoo, L.A. Cause-and-Effect (Fishbone) Diagram: A Tool for Generating and Organizing Quality Improvement Ideas. Glob. J. Qual. Saf. Healthc. 2024, 7, 85–87. [Google Scholar] [CrossRef]
- Watson, G. The Legacy of Ishikawa. Qual. Prog. 2004, 37, 54–67. [Google Scholar]
- Ciocoiu, C.N.; Ilie, G. Application Of Fishbone Diagram To Determine The Risk Of An Event With Multiple Causes. Knowl. Man. Res. Pract. 2010, 2, 1–20. [Google Scholar]
- ISO 22000:2019; Food Safety Management Systems—Requirements for Any Organization in the Food Chain. International Organization for Standardization with Technical Committee: Geneva, Switzerland, 2019.
- Ayeni, O.; Olagoke-Komolafe, O.E. Innovative risk management techniques in food safety: A review of best practices and emerging trends. Int. J. Appl. Res. Soc. Sci. 2024, 6, 2241–2257. [Google Scholar] [CrossRef]
- Varzakas, T.H. HACCP and ISO22000: Risk Assessment in Conjunction with Other Food Safety Tools Such as FMEA, Ishikawa Diagrams and Pareto. In Encyclopedia of Food and Health, 1st ed.; Caballero, B., Finglas, P., Toldra, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 295–302. [Google Scholar] [CrossRef]
- Awuchi, C.G. HACCP, quality, and food safety management in food and agricultural systems. Cogent Food Agric. 2023, 9, 2176280. [Google Scholar] [CrossRef]
- Kasyk, L.; Wolnowska, A.E.; Pleskacz, K.; Kapuściński, T. The Analysis of Social and Situational Systems as Components of Human Errors Resulting in Navigational Accidents. Appl. Sci. 2023, 13, 6780. [Google Scholar] [CrossRef]
- Stoyanova, A.; Marinova, V.; Stoilov, D.; Kirechev, D. Food Safety Management System (FSMS) Model with Application of the PDCA Cycle and Risk Assessment as Requirements of the ISO 22000:2018 Standard. Standards 2022, 2, 329–351. [Google Scholar] [CrossRef]
- Surareungchai, S.; Borompichaichartkul, C.; Rachtanapun, C.; Pongprasert, N.; Jitareerat, P.; Srilaong, V. Comparison of Risk Assessment Schemes in GHPs and HACCP, FSMA Preventive Controls for Human Food, ISO 22000, and GFSI Recognized Standards with Risk Scoring Guidance in General Use with Fresh Produce. Horticulturae 2022, 8, 181. [Google Scholar] [CrossRef]
- Kosasih, O.; Hidayat, K.; Hutahayan, B.; Sunarti. Achieving Sustainable Customer Loyalty in the Petrochemical Industry: The Effect of Service Innovation, Product Quality, and Corporate Image with Customer Satisfaction as a Mediator. Sustainability 2024, 16, 7111. [Google Scholar] [CrossRef]
- Sharma, A.; Bhaduri, S. Selection and adoption of water purification technologies in the bottled water industry in India. Cleaner Water 2024, 2, 100039. [Google Scholar] [CrossRef]
- Popa, M.; Glevitzky, I.; Dumitrel, G.-A.; Popa, D.; Virsta, A.; Glevitzky, M. Qualitative Analysis and Statistical Models Between Spring Water Quality Indicators in Alba County, Romania. Sci. Pap.-Ser.-E-Land. R. 2022, 11, 358–366. [Google Scholar]
- Popa, M.; Glevitzky, I.; Glevitzky, M.; Popa, M.; Todoran, A. Statistical Analysis Used in Evaluation of Water Quality from Wells in Alba County, Romania. Sci. Pap.-Ser.-E-Land. R. 2020, 9, 147–153. [Google Scholar]
- Glevitzky, I.; Sârb, A.; Popa, M. Study Regarding the Improvement of Bottling Process for Spring Waters, through the Implementation of the Occupational Health and Food Safety Requirements. Safety 2019, 5, 32. [Google Scholar] [CrossRef]
- Popa, M.; Glevitzky, M.; Dumitrel, G.-A.; Popa, D.; Virsta, A. Improving the System of Logistics Management and Signaling, Identification, Classification of Noncompliance in the Water Bottling Industry. Sci. Pap.-Ser.-E-Land. R. 2023, 12, 251–257. [Google Scholar]
- Zemanova, Z.; Krocova, S.; Sirotiak, P. Risk Management in the Water Industry. Eng. Proc. 2023, 57, 20. [Google Scholar] [CrossRef]
- Popa, M.; Glevitzky, I.; Dumitrel, G.A.; Glevitzky, M.; Popa, D. Impact of Environmental Factors on the Quality of Spring Water from Abrud-Câmpeni Area, Alba County, Romania. AgroLife Sci. J. 2019, 8, 220–226. [Google Scholar]
- Chira, A.; Chira, C.L. Quality and Safety Management of Agri-Food Products; Ex Terra Aurum Publishing House: București, Romania, 2023; pp. 147–148. (In Romanian) [Google Scholar]
- Mitrea, I.S.; Petcu, C.D.; Savu, G. Food Safety Through the Application of the HACCP System; Bogdana Publishing House: Bucureşti, Romania, 2003; pp. 18–19. (In Romanian) [Google Scholar]
- Djekic, I.; Jankovic, D.; Rajkovic, A. Analysis of foreign bodies present in European food using data from Rapid Alert System for Food and Feed (RASFF). Food Control 2017, 79, 143–149. [Google Scholar] [CrossRef]
- Marin, D.V.; Vultur, T. National Guide to Good Practices for Food Safety—HACCP Food Safety System; Uranus Publishing House: București, Romania, 2007; pp. 187–189. (In Romanian) [Google Scholar]
- Order No. 35 of March 30, 2016 on the Approval of the Methodological Norms for the Application of the Program of Actions for the Surveillance, Prevention, Control and Eradication of Animal Diseases, Those Transmissible from Animals to Humans, Animal Protection and Environmental Protection, Identification and Registration of Cattle, Pigs, Sheep, Goats and Equidae, as well as the Methodological Norms for the Application of the Program of Surveillance and Control in the Field of Food Safety, ISSUER: National Sanitary Veterinary and Food Safety Authority. Official Gazette No. 303, 20 April 2016. Available online: https://legislatie.just.ro/Public/DetaliiDocument/177719 (accessed on 7 November 2024).
- Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the Provision of Food Information to Consumers, OJ L 304, 22.11.2011. In Official Journal of the European Union; European Union: Brussels, Belgium, 2011; pp. 18–63.
- Yang, Y.; Du, Y.; Gupta, V.K.; Ahmad, F.; Amiri, H.; Pan, J.; Aghbashlo, M.; Tabatabaei, M.; Rajaei, A. Exploring blockchain and artificial intelligence in intelligent packaging to combat food fraud: A comprehensive review. Food Packag. Shelf Life 2024, 43, 101287. [Google Scholar] [CrossRef]
- Maherani, B.; Hossain, F.; Criado, P.; Ben-Fadhel, Y.; Salmieri, S.; Lacroix, M. World Market Development and Consumer Acceptance of Irradiation Technology. Foods 2016, 5, 79. [Google Scholar] [CrossRef] [PubMed]
- Oliver, M.J. Why we need GMO crops in agriculture. Mo. Med. 2014, 111, 492–507. [Google Scholar]
- Regenstein, J.M.; Chaudry, M.M.; Regenstein, C.E. The Kosher and Halal Food Laws. Compr. Rev. Food Sci. Food Saf. 2003, 2, 111–127. [Google Scholar] [CrossRef]
- Patel, A.B.; Shaikh, S.; Jain, K.R.; Desai, C.; Madamwar, D. Polycyclic Aromatic Hydrocarbons: Sources, Toxicity, and Remediation Approaches. Front. Microbiol. 2020, 11, 562813. [Google Scholar] [CrossRef] [PubMed]
- Kleter, G.A.; Prandini, A.; Filippi, L.; Marvin, H.J. Identification of potentially emerging food safety issues by analysis of reports published by the European Community’s Rapid Alert System for Food and Feed (RASFF) during a four-year period. Food Chem. Toxicol. 2009, 47, 932–950. [Google Scholar] [CrossRef] [PubMed]
- Bellini, S.; Casadei, G.; De Lorenzi, G.; Tamba, M. A Review of Risk Factors of African Swine Fever Incursion in Pig Farming within the European Union Scenario. Pathogens 2021, 10, 84. [Google Scholar] [CrossRef] [PubMed]

| Severity [G] | Frequency [F] | ||
|---|---|---|---|
| Low (the Event Is Unlikely to Happen) | Medium (an Event with a Low Chance of Occurring) | High (the Event Is Likely to Happen) | |
| Low (low risk and no significant impact) | 1 | 2 | 3 |
| Medium (it may affect the process/product) | 2 | 3 | 4 |
| High (it affects the organization, process, or product on a large scale) | 3 | 4 | 4 |
| Step | Hazard | Hazard Description | G | F | RC | Q1 | Q2 | Q3 | Q4 | CCP |
|---|---|---|---|---|---|---|---|---|---|---|
| Mechanical filtration | Physical | Impurities (sand, soil, sediment, sludge, rust, or suspended particles) | Low | High | 3 | Yes | Yes | - | - | CCP1 |
| CO2 impregnation | Biological | Pathogenic agents: E. coli, Clostridium spp. L. monocytogenes, Salmonella spp., Staphyloccocus spp., Y. enterocolitica, C. jejuni, P. aeruginosa, Shigella spp., Streptococcus Faecalis, Legionella spp., etc.; parasites | High | Low | 3 | Yes | Yes | - | - | CCP2 |
| Step | CCP | Control Measures | Critical Limits | Monitoring | Corrective Actions and Measures | Records, Documents | Responsibilities |
|---|---|---|---|---|---|---|---|
| Mechanical filtration | CCP1 | - Δp monitoring at the plate filter, - Calibrating the pressure gauges. | maximum 4 bar | Every hour | - Filter replacement, - Filter washing, - Filter inspection, - Staff training. | - Operational control sheet, - Input water quality, monitoring register. | - Operator, - Quality Control Laboratory, - Maintenance Manager. |
| Carbonation | CCP2 | - Determining the CO2 content in the product | Minimum 2500 mg/L CO2 | For each batch | - CO2 cylinder replacement, - CO2 flow adjustment, - Staff training. | - Operational control sheet, - Finished product quality, monitoring register. | - Operator, - Quality Control Laboratory, - Maintenance Manager. |
| Step | Hazard | Hazard Description | G | F | RC | Q1 | Q2 | Q3 | Q4 | CCP |
|---|---|---|---|---|---|---|---|---|---|---|
| Mechanical filtration | Physical | Impurities (sand, soil, sediment, mud, rust or suspended particles) | Low | High | 3 | Yes | Yes | - | - | CCP1 |
| Ozone treatment | Biological | Pathogens (E. coli, Clostridium spp., Listeria monocytogenes, Salmonella spp., Staphyloccocus spp., Yersinia enterocolitica, Campylobacter jejuni, Pseudomonas aeruginosa, Shigella spp., Streptococcus Faecalis, Legionella spp., etc.) Parasites | High | Low | 3 | Yes | Yes | - | - | CCP2 |
| Step | CCP | Control Measures | Critical Limits | Monitoring | Corrective Measures and Actions | Records | Responsibilities |
|---|---|---|---|---|---|---|---|
| Mechanical filtration | CCP1 | -Δp monitoring on the plate filter; -Calibration of pressure gauges. | Maximum 4 bar | Every one hour | -Change of filter cartridges; -Filter washing; -Plate filter overhaul; -Staff training. | -Operational checklist; -Water monitoring register. | -Operators; -Quality control laboratory; -Maintenance coordinator. |
| Ozone treatment | CCP2 | -Monitoring pressure of ozonated air; -Analysis of residual O3 in the product. | 0.05 mg/L O3 residual | Every batch | -O3 generator maintenance; -O3 generator air flow adjustment; -Operator training. | -Operational control sheet; -Finished product quality monitoring register. | -Operator; -Quality control laboratory; -Maintenance manager. |
| Step | Risk | Type M | ||||
|---|---|---|---|---|---|---|
| Environment (M1) | Man (M2) | Method (M3) | Materials (M4) | Machines (M5) | ||
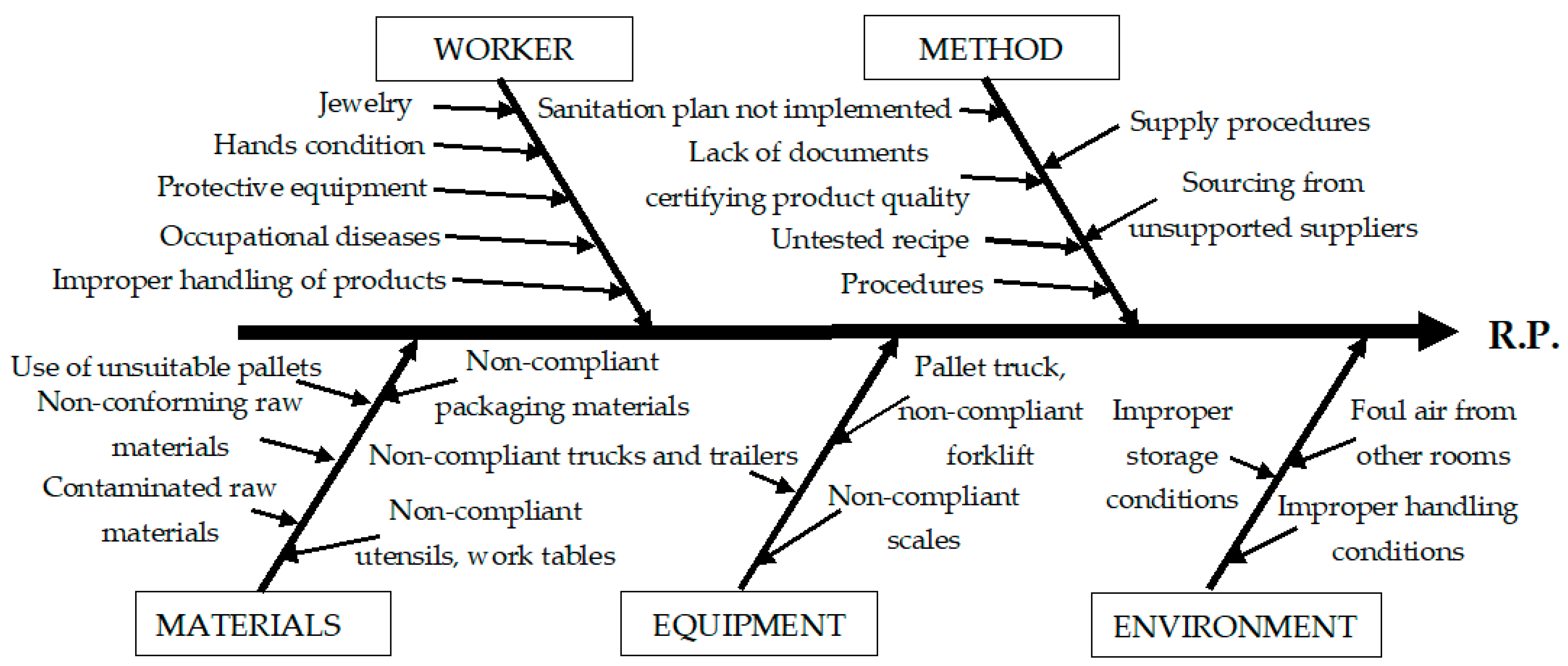
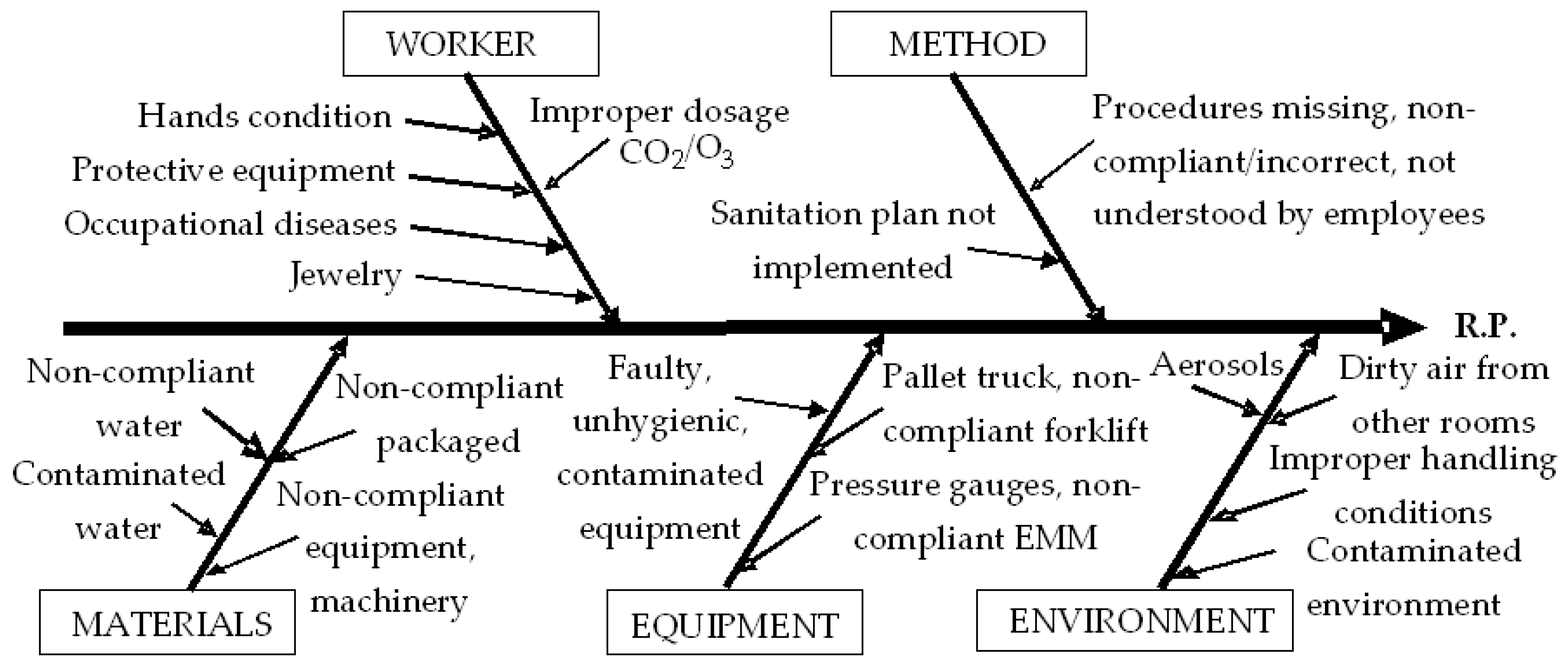
| BOTTLING | Physical | -Presence of foreign contaminants from the workplace; -Cross-contamination between handled products; -Contamination from PET bottles; -Contamination from airborne dust. | -Foreign objects from workers and their clothing; -Contamination due to the handling of raw materials and packaging; -Contamination due to storage of opened bottles for filling. | -Ignorance of work and sanitation standards; -Failure to comply with work and sanitation standards; -Unreviewed work and sanitation standards. | -Cross-contamination between containers (PET bottles); -Contamination from water with impurities. | -Contamination from transport equipment, machinery and equipment, working papers, foils, or labels; -Contamination from defective pallets. |
| Chemical | -Contamination from chemicals (including sanitizing substances) handled in the same space. | -Contamination due to workers and working equipment; -CO2/O3 overdose. | -Ignorance or non-compliance with work and sanitation standards; -Unrevised work and sanitation standards. | -Contamination with chemicals (including sanitizing substances) handled or stored in the area; -Use of impure CO2/O3. | -Contamination from faulty machinery. | |
| Biological | -Microbial pollutants from the workplace; -Proliferation of microorganisms due to improper sanitation. | -Contamination from ill workers, those with sick pets, or dirty work equipment. | -Ignorance or non-compliance with work and sanitation standards; -Unrevised work and sanitation standards. | -Contamination from water, or microbiologically contaminated packaging. | -Contamination from unsanitized machinery, pallets, or shelves. | |
| Allergens | -Handling of allergenic foods inside the factory; -There is no delimitation of the dining room; -Contamination from air contaminated with allergens; -Inadequate operation of the ventilation/air filtration system; -Contamination from an environment that is not properly sanitized. | -Contamination from operators and their equipment; -Contamination from operators or other people passing through the area; -Contamination during dry cleaning. | -Ignorance of work and sanitation standards; -Failure to comply with work and sanitation standards; -Unrevised work and sanitation standards. | -Contamination between allergenic foods and containers (PET bottles) stored and handled in the same space. | -Contamination from transport equipment, shelves, pallets, or contaminated floors. | |
| Fraud | - | -Malicious actions by operators. | - | -Contamination from counterfeit, manipulated products. | -Sabotage of machinery and equipment. | |
| Kosher/ Halal | -Contamination from an environment that is not properly sanitized. | -Contamination with prohibited foods from operators and their equipment; -Contamination during handling and storage. | -Ignorance of work and sanitation standards; -Failure to comply with work and sanitation standards. | - | -Contamination from machinery, pallets, shelves, or contaminated with prohibited products. | |
| RASFF | - | -Contamination of products from operators who consumed batches of products notified to RASFF. | - | -Cross-contamination between products that have been reported to RASFF. | -Contamination from machinery, pallets, or contaminated with products that have been reported to RASFF. | |
| Other | - | -Failure to comply with EMM verification procedures or prescription/dosage. | - | - | -Defective, uncalibrated EMM. | |
| Step | Risk | M1 | M2 | M3 | M4 | M5 | RC | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G | F | I | G | F | I | G | F | I | G | F | I | G | F | I | |||
| BOTTLING | Physical | 3 | 1 | 2 | 3 | 1 | 2 | 2 | 1 | 1.5 | 2 | 1 | 1.5 | 3 | 1 | 2.5 | 1.9 |
| Chemical | 3 | 1 | 2 | 3 | 1 | 2 | 2 | 1 | 1.5 | 3 | 1 | 2 | 4 | 1 | 2.5 | 2 | |
| Biological | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 2 | 2.5 | 3 | 1 | 2 | 3 | 2 | 2.5 | 2.2 | |
| Allergens | 1 | 0 | 0.5 | 2 | 1 | 1.5 | 1 | 0 | 0.5 | 2 | 1 | 1.5 | 1 | 1 | 1 | 1 | |
| Fraud | 0 | 0 | 0 | 4 | 1 | 2.5 | 1 | 1 | 1 | 2 | 1 | 1.5 | 1 | 1 | 1 | 1.2 | |
| Kosher/Halal | 0 | 0 | 0 | 2 | 1 | 1.5 | 2 | 1 | 1.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0.6 | |
| RASFF | 0 | 0 | 0 | 2 | 1 | 1.5 | 0 | 0 | 0 | 2 | 1 | 1.5 | 1 | 1 | 1 | 0.8 | |
| Other | 0 | 0 | 0 | 2 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 1.5 | 0.5 | |
| M Type | Risk | Measures | Responsibilities/ Period |
|---|---|---|---|
| M1 | -Contamination from chemicals (including sanitizing substances) used within the same area. | -Sanitation check through pH tests; -Elimination of chemicals from the area; -Sanitization and disinfection of the area. | -Flow control/annually; -Operations coordinator/daily. |
| M2 | -Contamination from staff, visitors’ or gowns, caps, etc.; -CO2 or O3 overdose. | -Control of personnel health, hygiene, and compliance of protective equipment; -Monitoring the dosage through rapid testing. | -Operations coordinator/daily; -Laboratory assistant/daily. |
| M3 | -Negligence or failure to adhere to work and sanitation standards, as well as preventive measures; -Unrevised work and sanitation procedures, risk prevention protocols. | -Regular training for production personnel; -Regular and systematic review of protocols. | -Process operators/upon hiring, quarterly, and annually. |
| M4 | -Contamination from chemicals (including sanitizing agents) used or just kept in the immediate vicinity; -Utilization of contaminated, impure, or technical CO2 or O3. | -Handling detergents only when sanitizing; -Periodic monitoring of CO2 or O3 purity. | -Production coordinator/sanitation tests/internal control plan. |
| M5 | -Contamination from defective or improperly sanitized equipment; -Failure to calibrate DMM. | -Equipment maintenance; -Adherence to hygiene and protocols; -DMM regular calibration. | -Operational staff/according to schedule; -Production coordinator/for sanitation; -Technical manager/according to schedule. |
| M Type | Risk | Procedures | CCP/PRPO Determination | Corrections/Corrective Actions | Responsibility | Registration | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Type | ||||||
| M1 | -Contamination from substances (such as sanitizing substances) used within the same area. | PRP | yes | no | no | - | N/A | Product separation, remediation/destruction | Quality manager | Sanitation sheets |
| M2 | -Contamination caused by workers and equipment; -CO2/O3 overdose. | PRP | yes | no | no | - | N/A | Product separation, remediation /destruction | Quality manager | Personnel control procedure/ form |
| M3 | -Lack of awareness or failure to comply with work and sanitation guidelines and safety measures; -Unrevised work, sanitation standards, and prevention measures. | PRP, OP, work standards and prevention | yes | no | no | - | N/A | Testing, retraining | Quality manager | Training report |
| M4 | -Contamination with substances (such as sanitizing agents) handled or stored in the area; -Use of impure or technical CO2/O3. | PRP, self-control program | yes | no | no | - | N/A | Product separation/destruction | Quality manager | Sanitation sheets, maintenance sheets |
| M5 | -Contamination resulting from improperly maintained or sanitized equipment; -Failure to calibrate EMM. | PRP, EMM verification program | yes | no | no | - | N/A | Product separation, remediation /destruction | Quality manager | Sanitation sheets, maintenance sheets |
| Method | Classical HACCP | IRF | |
|---|---|---|---|
| Factor | |||
| Risk identification | Basic: physical, chemical, biological | -Basic: physical, chemical, and biological risks; -Auxiliary, supporting the risk identification process: allergens, fraud/sabotage, Kosher/Halal, RASFF alerts; -Specific, related to particular conditions imposed by the ingredients and specific technological processes: irradiation, radioactivity, GMOs, PAHs, ASF, PPR, etc. | |
| Risk analysis | Based on severity and the likelihood of occurrence of the hazard; | -Based on impact for each risk (as arithmetic mean of frequency and severity). | |
| Risk level | each identified risk is assessed on three severity levels: low, medium, and high | -Each identified risk is assessed on four severity levels: low, medium, high, and critical. | |
| Identifying the causes | Process flow analysis | -Ishikawa diagram is employed to systematically identify potential causes of a risk, categorized under the 5M framework: Man, Machine, Material, Method, and Environment. | |
| Risk class calculation | RC = G × F (matrix from Table 1) | I = G + F/2; RC = (IMan +IMachine + IMaterial, +IMethod + IMedium)/5. | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Glevitzky, M.; Glevitzky, I.; Mucea-Ștef, P.; Popa, M.; Dumitrel, G.-A.; Vică, M.L. Integrated Risk Framework (IRF)—Interconnection of the Ishikawa Diagram with the Enhanced HACCP System in Risk Assessment for the Sustainable Food Industry. Sustainability 2025, 17, 536. https://doi.org/10.3390/su17020536
Glevitzky M, Glevitzky I, Mucea-Ștef P, Popa M, Dumitrel G-A, Vică ML. Integrated Risk Framework (IRF)—Interconnection of the Ishikawa Diagram with the Enhanced HACCP System in Risk Assessment for the Sustainable Food Industry. Sustainability. 2025; 17(2):536. https://doi.org/10.3390/su17020536
Chicago/Turabian StyleGlevitzky, Mirel, Ioana Glevitzky, Paul Mucea-Ștef, Maria Popa, Gabriela-Alina Dumitrel, and Mihaela Laura Vică. 2025. "Integrated Risk Framework (IRF)—Interconnection of the Ishikawa Diagram with the Enhanced HACCP System in Risk Assessment for the Sustainable Food Industry" Sustainability 17, no. 2: 536. https://doi.org/10.3390/su17020536
APA StyleGlevitzky, M., Glevitzky, I., Mucea-Ștef, P., Popa, M., Dumitrel, G.-A., & Vică, M. L. (2025). Integrated Risk Framework (IRF)—Interconnection of the Ishikawa Diagram with the Enhanced HACCP System in Risk Assessment for the Sustainable Food Industry. Sustainability, 17(2), 536. https://doi.org/10.3390/su17020536






