Development and Valuation of Novel PLA-Based Biodegradable Packaging Materials Complemented with Food Waste of Plant and Animal Origin for Shelf-Life Extension of Selected Foods: Trends and Challenges
Abstract
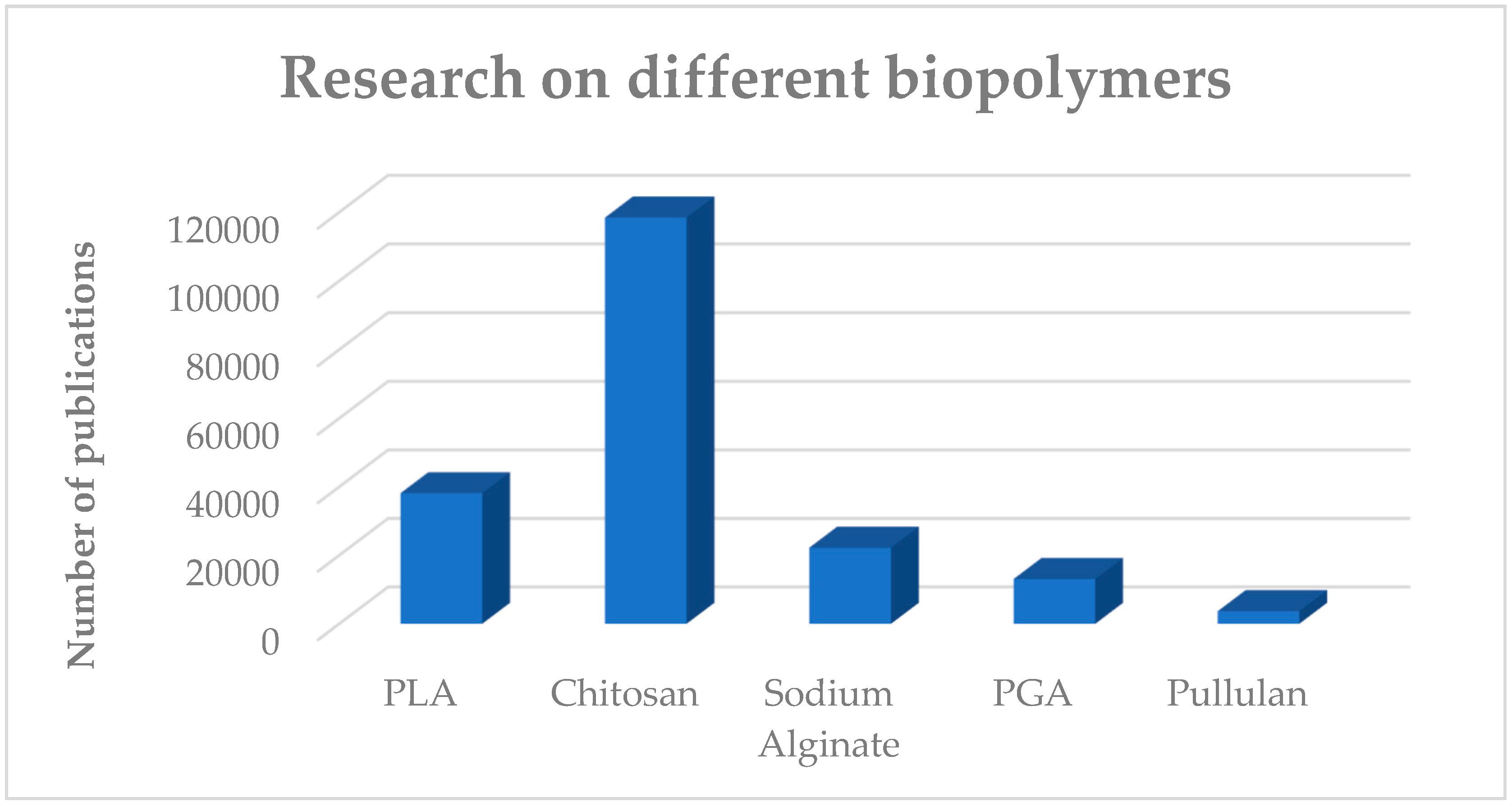
1. Introduction
2. Food Waste By-Products of Plant Origin
2.1. Onion By-Products
2.2. Winery By-Products
2.3. Prickly Pear Cladodes
3. Food Waste By-Products of Animal Origin
3.1. Whey Protein
3.2. Fishery By-Products
3.2.1. Collagen
3.2.2. Gelatin
4. Discussion
5. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, H.; Sablani, S. Biodegradable Packaging Reinforced with Plant-Based Food Waste and By-Products. Curr. Opin. Food Sci. 2021, 42, 61–68. [Google Scholar] [CrossRef]
- Dilucia, F.; Lacivita, V.; Conte, A.; Del Nobile, M.A. Sustainable Use of Fruit and Vegetable By-Products to Enhance Food Packaging Performance. Foods 2020, 9, 857. [Google Scholar] [CrossRef]
- Rodsamran, P.; Sothornvit, R. Lime Peel Pectin Integrated with Coconut Water and Lime Peel Extract as a New Bioactive Film Sachet to Retard Soybean Oil Oxidation. Food Hydrocoll. 2019, 97, 105173. [Google Scholar] [CrossRef]
- Munir, S.; Hu, Y.; Liu, Y.; Xiong, S. Enhanced Properties of Silver Carp Surimi-Based Edible Films Incorporated with Pomegranate Peel and Grape Seed Extracts under Acidic Condition. Food Packag. Shelf Life 2019, 19, 114–120. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Yong, H.; Qin, Y.; Liu, J.; Liu, J. Development of Multifunctional Food Packaging Films Based on Chitosan, TiO2 Nanoparticles and Anthocyanin-Rich Black Plum Peel Extract. Food Hydrocoll. 2019, 94, 80–92. [Google Scholar] [CrossRef]
- Shankar, S.; Rhim, J.-W. Antimicrobial Wrapping Paper Coated with a Ternary Blend of Carbohydrates (Alginate, Carboxymethyl Cellulose, Carrageenan) and Grapefruit Seed Extract. Carbohydr. Polym. 2018, 196, 92–101. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Fortunati, E.; Dominici, F.; Rayón, E.; López, J.; Kenny, J.M. PLA-PHB/Cellulose Based Films: Mechanical, Barrier and Disintegration Properties. Polym. Degrad. Stab. 2014, 107, 139–149. [Google Scholar] [CrossRef]
- Ferrer-Gallego, R.; Silva, P. The Wine Industry By-Products: Applications for Food Industry and Health Benefits. Antioxidants 2022, 11, 2025. [Google Scholar] [CrossRef] [PubMed]
- Lazaridis, D.G.; Kitsios, A.-P.; Koutoulis, A.S.; Malisova, O.; Karabagias, I.K. Fruits, Spices and Honey Phenolic Compounds: A Comprehensive Review on Their Origin, Methods of Extraction and Beneficial Health Properties. Antioxidants 2024, 13, 1335. [Google Scholar] [CrossRef] [PubMed]
- Nanni, A.; Parisi, M.; Colonna, M. Wine By-Products as Raw Materials for the Production of Biopolymers and of Natural Reinforcing Fillers: A Critical Review. Polymers 2021, 13, 381. [Google Scholar] [CrossRef] [PubMed]
- Panou, A.; Karabagias, I.K. Biodegradable Packaging Materials for Foods Preservation: Sources, Advantages, Limitations, and Future Perspectives. Coatings 2023, 13, 1176. [Google Scholar] [CrossRef]
- Thakur, S.; Chaudhary, J.; Singh, P.; Alsanie, W.F.; Grammatikos, S.A.; Thakur, V.K. Synthesis of Bio-Based Monomers and Polymers Using Microbes for a Sustainable Bioeconomy. Bioresour. Technol. 2022, 344, 126156. [Google Scholar] [CrossRef]
- Jabeen, N.; Majid, I.; Nayik, G.A. Bioplastics and Food Packaging: A Review. Cogent Food Agric. 2015, 1, 1. [Google Scholar] [CrossRef]
- DiGregorio, B.E. Biobased Performance Bioplastic: Mirel. Chem. Biol. 2009, 16, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Voznyak, Y.; Morawiec, J.; Galeski, A. Ductility of Polylactide Composites Reinforced with Poly(Butylene Succinate) Nanofibers. Compos. Part A Appl. Sci. Manuf. 2016, 90, 218–224. [Google Scholar] [CrossRef]
- Karabagias, V.K.; Giannakas, A.E.; Andritsos, N.D.; Leontiou, A.A.; Moschovas, D.; Karydis-Messinis, A.; Avgeropoulos, A.; Zafeiropoulos, N.E.; Proestos, C.; Salmas, C.E. Shelf Life of Minced Pork in Vacuum-Adsorbed Carvacrol@Natural Zeolite Nanohybrids and Poly-Lactic Acid/Triethyl Citrate/Carvacrol@Natural Zeolite Self-Healable Active Packaging Films. Antioxidants 2024, 13, 776. [Google Scholar] [CrossRef]
- Karabagias, V.K.; Giannakas, A.E.; Andritsos, N.D.; Moschovas, D.; Karydis-Messinis, A.; Leontiou, A.; Avgeropoulos, A.; Zafeiropoulos, N.E.; Proestos, C.; Salmas, C.E. Novel Polylactic Acid/Tetraethyl Citrate Self-Healable Active Packaging Films Applied to Pork Fillets’ Shelf-Life Extension. Polymers 2024, 16, 1130. [Google Scholar] [CrossRef]
- Swetha, T.A.; Bora, A.; Mohanrasu, K.; Balaji, P.; Raja, R.; Ponnuchamy, K.; Muthusamy, G.; Arun, A. A Comprehensive Review on Polylactic Acid (PLA)—Synthesis, Processing and Application in Food Packaging. Int. J. Biol. Macromol. 2023, 234, 123715. [Google Scholar] [CrossRef] [PubMed]
- Dorgan, J.R.; Lehermeier, H.; Mang, M. Thermal and Rheological Properties of Commercial-Grade Poly(Lactic Acid)s. J. Polym. Environ. 2000, 8, 1–9. [Google Scholar] [CrossRef]
- Conn, R.E.; Kolstad, J.J.; Borzelleca, J.F.; Dixler, D.S.; Filer, L.J.; Ladu, B.N.; Pariza, M.W. Safety Assessment of Polylactide (PLA) for Use as a Food-Contact Polymer. Food Chem. Toxicol. 1995, 33, 273–283. [Google Scholar] [CrossRef] [PubMed]
- European Bioplastics. Bioplastics; European Bioplastics: Berlin, Germany, 2020. [Google Scholar]
- Nilsen-Nygaard, J.; Fernández, E.N.; Radusin, T.; Rotabakk, B.T.; Sarfraz, J.; Sharmin, N.; Sivertsvik, M.; Sone, I.; Pettersen, M.K. Current Status of Biobased and Biodegradable Food Packaging Materials: Impact on Food Quality and Effect of Innovative Processing Technologies. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1333–1380. [Google Scholar] [CrossRef] [PubMed]
- European Commision. Available online: https://environment.ec.europa.eu/topics/plastics/single-use-plastics_en#related-links (accessed on 20 November 2024).
- Khatri, M.; Al-Juboori, R.A.; Khanzada, N.K.; Hilal, N. Recycled PET Conversion to High Efficiency Catalyst for 4-NP Reduction with Extended Reusability: Insights into Reduction Products and Kinetics. Chem. Eng. J. 2025, 505, 159304. [Google Scholar] [CrossRef]
- Wellenreuther, C.; Wolf, A.; Zander, N. Cost Competitiveness of Sustainable Bioplastic Feedstocks—A Monte Carlo Analysis for Polylactic Acid. Clean. Eng. Technol. 2022, 6, 100411. [Google Scholar] [CrossRef]
- Packaging Dive. Available online: https://www.packagingdive.com/news/polylactic-acid-pla-bioplastic-compostable-packaging/728875/ (accessed on 10 January 2025).
- Chiarakorn, S.; Permpoonwiwat, C.K.; Nanthachatchavankul, P. Financial and Economic Viability of Bioplastic Production in Thailand; Economy and Environment Program for Southeast Asia (EEPSEA): Ho Chi Minh City, Vietnam, 2016. [Google Scholar]
- Kwan, T.H.; Hu, Y.; Lin, C.S.K. Techno-Economic Analysis of a Food Waste Valorisation Process for Lactic Acid, Lactide and Poly(Lactic Acid) Production. J. Clean. Prod. 2018, 181, 72–87. [Google Scholar] [CrossRef]
- Sanaei, S.; Stuart, P.R. Systematic Assessment of Triticale-Based Biorefinery Strategies: Techno-Economic Analysis to Identify Investment Opportunities. Biofuels Bioprod. Biorefin. 2018, 12, S46–S59. [Google Scholar] [CrossRef]
- Manandhar, A.; Shah, A. Techno-Economic Analysis of Bio-Based Lactic Acid Production Utilizing Corn Grain as Feedstock. Processes 2020, 8, 199. [Google Scholar] [CrossRef]
- Aroura, A. The Business Case for Commercial Production of Bioplastics in Main; Jim Lunt & Associates; Maine Technological Institute: Portland, ME, USA, 2010. [Google Scholar]
- Salunkhe, S.; Chaudhary, B.U.; Tewari, S.; Meshram, R.; Kale, R.D. Utilization of Agricultural Waste as an Alternative for Packaging Films. Ind. Crops Prod. 2022, 188, 115685. [Google Scholar] [CrossRef]
- Thivya, P.; Bhanu Prakash Reddy, N.; Bhosale Yuvraj, K.; Sinija, V.R. Recent Advances and Perspectives for Effective Utilization of Onion Solid Waste in Food Packaging: A Critical Review. Rev Environ. Sci Biotechnol 2023, 22, 29–53. [Google Scholar] [CrossRef]
- Samota, M.K.; Sharma, M.; Kaur, K.; Sarita; Yadav, D.K.; Pandey, A.K.; Tak, Y.; Rawat, M.; Thakur, J.; Rani, H. Onion Anthocyanins: Extraction, Stability, Bioavailability, Dietary Effect, and Health Implications. Front. Nutr. 2022, 9, 917617. [Google Scholar] [CrossRef] [PubMed]
- Bagade, S.B.; Patil, M. Recent Advances in Microwave Assisted Extraction of Bioactive Compounds from Complex Herbal Samples: A Review. Crit. Rev. Anal. Chem. 2021, 51, 138–149. [Google Scholar] [CrossRef]
- Kumar, M.; Barbhai, M.D.; Hasan, M.; Punia, S.; Dhumal, S.; Radha; Rais, N.; Chandran, D.; Pandiselvam, R.; Kothakota, A.; et al. Onion (Allium cepa L.) Peels: A Review on Bioactive Compounds and Biomedical Activities. Biomed. Pharmacother. 2022, 146, 112498. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.M.; Estevinho, B.N.; Rocha, F. Preparation and Incorporation of Functional Ingredients in Edible Films and Coatings. Food Bioprocess Technol 2021, 14, 209–231. [Google Scholar] [CrossRef]
- Kola, V.; Carvalho, I.S. Plant Extracts as Additives in Biodegradable Films and Coatings in Active Food Packaging. Food Biosci. 2023, 54, 102860. [Google Scholar] [CrossRef]
- Trivedi, A.K.; Gupta, M.K.; Singh, H. PLA Based Biocomposites for Sustainable Products: A Review. Adv. Ind. Eng. Polym. Res. 2023, 6, 382–395. [Google Scholar] [CrossRef]
- Pirsa, S.; Bener, M.; Şen, F.B. Biodegradable Film of Carboxymethyl Cellulose Modified with Red Onion Peel Powder Waste and Boron Nitride Nanoparticles: Investigation of Physicochemical Properties and Release of Active Substances. Food Chem. 2024, 445, 138721. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.G.; Silva, G.F.A.; Gomes, B.M.; Martins, V.G. A Novel Sodium Alginate Active Films Functionalized with Purple Onion Peel Extract (Allium cepa). Biocatal. Agric. Biotechnol. 2021, 35, 102096. [Google Scholar] [CrossRef]
- Bruna, J.E.; Castillo, M.; López de Dicastillo, C.; Muñoz-Shugulí, C.; Lira, M.; Guarda, A.; Rodríguez-Mercado, F.J.; Galotto, M.J. Development of Active Biocomposite Films Based on Poly(Lactic Acid) and Wine by-Product: Effect of Grape Pomace Content and Extrusion Temperature. J. Appl. Polym. Sci. 2023, 140, e54425. [Google Scholar] [CrossRef]
- Verano-Naranjo, L.; Cejudo-Bastante, C.; Casas, L.; Martínez de la Ossa, E.; Mantell, C. Use of Winemaking By-Products for the Functionalization of Polylactic Acid for Biomedical Applications. Antioxidants 2023, 12, 1416. [Google Scholar] [CrossRef]
- Gómez, M.M.C. Development of Bio-Based Active Packaging Material Evaluating Grapefruit Seed and Grape Seed Extract as Antimicrobials. Bachelor’s Thesis, BSc, Food Science and Technology-Zamorano, San Antonio de Oriente, Honduras, 2020. [Google Scholar]
- Díaz-Galindo, E.P.; Nesic, A.; Cabrera-Barjas, G.; Dublan-García, O.; Ventura-Aguilar, R.I.; Vázquez-Armenta, F.J.; Aguilar-Montes de Oca, S.; Mardones, C.; Ayala-Zavala, J.F. Physico-Chemical and Antiadhesive Properties of Poly(Lactic Acid)/Grapevine Cane Extract Films against Food Pathogenic Microorganisms. Polymers 2020, 12, 2967. [Google Scholar] [CrossRef]
- Christopoulos, M.V.; Gkatzos, D.; Kafkaletou, M.; Bai, J.; Fanourakis, D.; Tsaniklidis, G.; Tsantili, E. Edible Coatings from Opuntia Ficus-Indica Cladodes Alongside Chitosan on Quality and Antioxidants in Cherries during Storage. Foods 2022, 11, 699. [Google Scholar] [CrossRef]
- Phupoksakul, T.; Leuangsukrerk, M.; Numpiboonmarn, P.; Somwangthanaroj, A.; Janjarasskul, T. Properties of Poly(Lactide)-Whey Protein Isolate Laminated Films. J. Sci. Food Agric. 2014, 95, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Etxabide, A.; Arregi, M.; Cabezudo, S.; Guerrero, P.; de la Caba, K. Whey Protein Films for Sustainable Food Packaging: Effect of Incorporated Ascorbic Acid and Environmental Assessment. Polymers 2023, 15, 387. [Google Scholar] [CrossRef]
- Fitriani, F.; Aprilia, S.; Arahman, N.; Bilad, M.R.; Suhaimi, H.; Huda, N. Properties of Biocomposite Film Based on Whey Protein Isolate Filled with Nanocrystalline Cellulose from Pineapple Crown Leaf. Polymers 2021, 13, 4278. [Google Scholar] [CrossRef] [PubMed]
- Bhuimbar, M.V.; Bhagwat, P.K.; Dandge, P.B. Extraction and Characterization of Acid Soluble Collagen from Fish Waste: Development of Collagen-Chitosan Blend as Food Packaging Film. J. Environ. Chem. Eng. 2019, 7, 102983. [Google Scholar] [CrossRef]
- Marangoni Júnior, L.; Rodrigues, P.R.; da Silva, R.G.; Vieira, R.P.; Alves, R.M.V. Sustainable Packaging Films Composed of Sodium Alginate and Hydrolyzed Collagen: Preparation and Characterization. Food Bioprocess Technol. 2021, 14, 2336–2346. [Google Scholar] [CrossRef]
- Nilsuwan, K.; Benjakul, S.; Prodpran, T. Physical/Thermal Properties and Heat Seal Ability of Bilayer Films Based on Fish Gelatin and Poly(Lactic Acid). Food Hydrocoll. 2018, 77, 248–256. [Google Scholar] [CrossRef]
- Chakka, A.K.; Babu, A.S. Bioactive Compounds of Winery By-Products: Extraction Techniques and Their Potential Health Benefits. Appl. Food Res. 2022, 2, 100058. [Google Scholar] [CrossRef]
- USDA. Available online: https://fdc.nal.usda.gov/food-details/1102665/nutrients (accessed on 23 October 2024).
- Virgin Wines. Available online: https://www.virginwines.co.uk/hub/wine-guide/winemaking/how-is-red-wine-made/ (accessed on 23 October 2024).
- Arvanitoyannis, I.S.; Ladas, D.; Mavromatis, A. Potential Uses and Applications of Treated Wine Waste: A Review. Int. J. Food Sci. Technol. 2006, 41, 475–487. [Google Scholar] [CrossRef]
- Peixoto, C.M.; Dias, M.I.; Alves, M.J.; Calhelha, R.C.; Barros, L.; Pinho, S.P.; Ferreira, I.C.F.R. Grape Pomace as a Source of Phenolic Compounds and Diverse Bioactive Properties. Food Chem. 2018, 253, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Ahmedna, M. Functional Components of Grape Pomace: Their Composition, Biological Properties and Potential Applications. Int. J. Food Sci. Technol. 2013, 48, 221–237. [Google Scholar] [CrossRef]
- Montealegre, R.R.; Peces, R.R.; Vozmediano, J.L.C.; Gascueña, J.M.; Romero, E.G. Phenolic Compounds in Skins and Seeds of Ten Grape Vitis Vinifera Varieties Grown in a Warm Climate. J. Food Compos. Anal. 2006, 19, 687–693. [Google Scholar] [CrossRef]
- Ivanov, Y.; Godjevargova, T. Antimicrobial Polymer Films with Grape Seed and Skin Extracts for Food Packaging. Microorganisms 2024, 12, 1378. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Fang, Y.; Wang, H.; Li, H.; Zhang, Z. Free-Radical Scavenging Properties and Reducing Power of Grape Cane Extracts from 11 Selected Grape Cultivars Widely Grown in China. Molecules 2011, 16, 10104–10122. [Google Scholar] [CrossRef] [PubMed]
- Piñeiro, Z.; Marrufo-Curtido, A.; Serrano, M.J.; Palma, M. Ultrasound-Assisted Extraction of Stilbenes from Grape Canes. Molecules 2016, 21, 784. [Google Scholar] [CrossRef] [PubMed]
- Maiuolo, J.; Nucera, S.; Serra, M.; Caminiti, R.; Oppedisano, F.; Macrì, R.; Scarano, F.; Ragusa, S.; Muscoli, C.; Palma, E.; et al. Cladodes of Opuntia ficus-indica (L.) Mill. Possess Important Beneficial Properties Dependent on Their Different Stages of Maturity. Plants 2024, 13, 1365. [Google Scholar] [CrossRef]
- Karabagias, V.K.; Karabagias, I.K.; Gatzias, I.; Riganakos, K.A. Characterization of Prickly Pear Juice by Means of Shelf Life, Sensory Notes, Physicochemical Parameters and Bio-Functional Properties. J Food Sci Technol 2019, 56, 3646–3659. [Google Scholar] [CrossRef] [PubMed]
- Giraldo-Silva, L.; Ferreira, B.; Rosa, E.; Dias, A.C.P. Opuntia ficus-indica Fruit: A Systematic Review of Its Phytochemicals and Pharmacological Activities. Plants 2023, 12, 543. [Google Scholar] [CrossRef]
- Karabagias, V.K.; Karabagias, I.K.; Louppis, A.; Badeka, A.; Kontominas, M.G.; Papastephanou, C. Valorization of Prickly Pear Juice Geographical Origin Based on Mineral and Volatile Compound Contents Using LDA. Foods 2019, 8, 123. [Google Scholar] [CrossRef] [PubMed]
- Karabagias, V.K.; Karabagias, I.K.; Prodromiti, M.; Gatzias, I.; Badeka, A. Bio-Functional Alcoholic Beverage Preparation Using Prickly Pear Juice and Its Pulp in Combination with Sugar and Blossom Honey. Food Biosci. 2020, 35, 100591. [Google Scholar] [CrossRef]
- Bensadón, S.; Hervert-Hernández, D.; Sáyago-Ayerdi, S.G.; Goñi, I. By-Products of Opuntia ficus-indica as a Source of Antioxidant Dietary Fiber. Plant Foods Hum. Nutr. 2010, 65, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Galati, E.M.; Monforte, M.T.; Miceli, N.; Mondello, M.R.; Taviano, M.F.; Galluzzo, M.; Tripodo, M.M. Opuntia ficus indica (L.) Mill. Mucilages Show Cytoprotective Effect on Gastric Mucosa in Rat. Phytother. Res. 2007, 21, 344–346. [Google Scholar] [CrossRef] [PubMed]
- Perucini-Avendaño, M.; Nicolás-García, M.; Jiménez-Martínez, C.; Perea-Flores, M.d.J.; Gómez-Patiño, M.B.; Arrieta-Báez, D.; Dávila-Ortiz, G. Cladodes: Chemical and Structural Properties, Biological Activity, and Polyphenols Profile. Food Sci. Nutr. 2021, 9, 4007–4017. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, F.; Silipo, A.; Molinaro, A.; Parrilli, M.; Schiraldi, C.; D’Agostino, A.; Izzo, E.; Rizza, L.; Bonina, A.; Bonina, F.; et al. The Polysaccharide and Low Molecular Weight Components of Opuntia ficus indica Cladodes: Structure and Skin Repairing Properties. Carbohydr. Polym. 2017, 157, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Medina-Torres, L.; García-Cruz, E.E.; Calderas, F.; González Laredo, R.F.; Sánchez-Olivares, G.; Gallegos-Infante, J.A.; Rocha-Guzmán, N.E.; Rodríguez-Ramírez, J. Microencapsulation by Spray Drying of Gallic Acid with Nopal Mucilage (Opuntia ficus indica). LWT—Food Sci. Technol. 2013, 50, 642–650. [Google Scholar] [CrossRef]
- Madera-Santana, T.J.; Vargas-Rodríguez, L.; Núñez-Colín, C.A.; González-García, G.; Peña-Caballero, V.; Núñez-Gastélum, J.A.; Gallegos-Vázquez, C.; Rodríguez-Núñez, J.R. Mucilage from Cladodes of Opuntia spinulifera Salm-Dyck: Chemical, Morphological, Structural and Thermal Characterization. Cyta-J. Food 2018, 16, 650–657. [Google Scholar] [CrossRef]
- Lefsih, K.; Delattre, C.; Pierre, G.; Michaud, P.; Aminabhavi, T.M.; Dahmoune, F.; Madani, K. Extraction, Characterization and Gelling Behavior Enhancement of Pectins from the Cladodes of Opuntia ficus indica. Int. J. Biol. Macromol. 2016, 82, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Souza, V.G.L.; Mello, I.P.; Khalid, O.; Pires, J.R.A.; Rodrigues, C.; Alves, M.M.; Santos, C.; Fernando, A.L.; Coelhoso, I. Strategies to Improve the Barrier and Mechanical Properties of Pectin Films for Food Packaging: Comparing Nanocomposites with Bilayers. Coatings 2022, 12, 108. [Google Scholar] [CrossRef]
- Rodrigues, C.; Paula, C.D.d.; Lahbouki, S.; Meddich, A.; Outzourhit, A.; Rashad, M.; Pari, L.; Coelhoso, I.; Fernando, A.L.; Souza, V.G.L. Opuntia spp.: An Overview of the Bioactive Profile and Food Applications of This Versatile Crop Adapted to Arid Lands. Foods 2023, 12, 1465. [Google Scholar] [CrossRef]
- Del-Valle, V.; Hernández-Muñoz, P.; Guarda, A.; Galotto, M.J. Development of a Cactus-Mucilage Edible Coating (Opuntia ficus indica) and Its Application to Extend Strawberry (Fragaria ananassa) Shelf-Life. Food Chem. 2005, 91, 751–756. [Google Scholar] [CrossRef]
- Allegra, A.; Inglese, P.; Sortino, G.; Settanni, L.; Todaro, A.; Liguori, G. The Influence of Opuntia ficus-indica Mucilage Edible Coating on the Quality of ‘Hayward’ Kiwifruit Slices. Postharvest Biol. Technol. 2016, 120, 45–51. [Google Scholar] [CrossRef]
- Allegra, A.; Sortino, G.; Inglese, P.; Settanni, L.; Todaro, A.; Gallotta, A. The Effectiveness of Opuntia ficus-indica Mucilage Edible Coating on Post-Harvest Maintenance of ‘Dottato’ Fig (Ficus carica L.) Fruit. Food Packag. Shelf Life 2017, 12, 135–141. [Google Scholar] [CrossRef]
- Koutoulis, A.S.; Giannakas, A.E.; Lazaridis, D.G.; Kitsios, A.-P.; Karabagias, V.K.; Giannakas, A.E.; Ladavos, A.; Karabagias, I.K. Preparation and Characterization of PLA-Based Films Fabricated with Different Citrus Species Peel Powder. Coatings 2024, 14, 1311. [Google Scholar] [CrossRef]
- Kosikowski, F.V. Whey Utilization and Whey Products. J. Dairy Sci. 1979, 62, 1149–1160. [Google Scholar] [CrossRef]
- USDA. Available online: https://fdc.nal.usda.gov/food-details/171282/nutrients (accessed on 9 November 2024).
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to whey protein and increase in satiety leading to a reduction in energy intake (ID 425), contribution to the maintenance or achievement of a normal body weight (ID 1683), growth and maintenance of muscle mass (ID 418, 419, 423, 426, 427, 429, 4307), increase in lean body mass during energy restriction and resistance training (ID 421), reduction of body fat mass during energy restriction and resistance training (ID 420, 421), increase in muscle strength (ID 422, 429), increase in endurance capacity during the subsequent exercise bout after strenuous exercise (ID 428), skeletal muscle tissue repair (ID 428) and faster recovery from muscle fatigue after exercise (ID 423, 428, 431), pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2010, 8, 1818. [Google Scholar] [CrossRef][Green Version]
- Naclerio, F.; Larumbe-Zabala, E. Effects of Whey Protein Alone or as Part of a Multi-Ingredient Formulation on Strength, Fat-Free Mass, or Lean Body Mass in Resistance-Trained Individuals: A Meta-Analysis. Sports Med. 2015, 46, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Master, P.B.Z.; Macedo, R.C.O. Effects of Dietary Supplementation in Sport and Exercise: A Review of Evidence on Milk Proteins and Amino Acids. Crit. Rev. Food Sci. Nutr. 2021, 61, 1225–1239. [Google Scholar] [CrossRef]
- Cava, E.; Padua, E.; Campaci, D.; Bernardi, M.; Muthanna, F.M.S.; Caprio, M.; Lombardo, M. Investigating the Health Implications of Whey Protein Consumption: A Narrative Review of Risks, Adverse Effects, and Associated Health Issues. Healthcare 2024, 12, 246. [Google Scholar] [CrossRef] [PubMed]
- Marshall, K. Therapeutic applications of whey protein. Altern. Med. Rev. 2004, 9, 136–156. [Google Scholar] [PubMed]
- Power, O.; Fernández, A.; Norris, R.; Riera, F.A.; FitzGerald, R.J. Selective Enrichment of Bioactive Properties during Ultrafiltration of a Tryptic Digest of β-Lactoglobulin. J. Funct. Foods 2014, 9, 38–47. [Google Scholar] [CrossRef]
- Patel, S. Functional Food Relevance of Whey Protein: A Review of Recent Findings and Scopes Ahead. J. Funct. Foods 2015, 19, 308–319. [Google Scholar] [CrossRef]
- Rieu, I.; Balage, M.; Sornet, C.; Debras, E.; Ripes, S.; Rochon-Bonhomme, C.; Pouyet, C.; Grizard, J.; Dardevet, D. Increased Availability of Leucine with Leucine-Rich Whey Proteins Improves Postprandial Muscle Protein Synthesis in Aging Rats. Nutrition 2007, 23, 323–331. [Google Scholar] [CrossRef]
- Micke, P.; Beeh, K.M.; Buhl, R. Effects of Long-Term Supplementation with Whey Proteins on Plasma Glutathione Levels of HIV-Infected Patients. Eur. J. Nutr. 2002, 41, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Elias, R.J.; Kellerby, S.S.; Decker, E.A. Antioxidant Activity of Proteins and Peptides. Crit. Rev. Food Sci. Nutr. 2008, 48, 430–441. [Google Scholar] [CrossRef]
- Cinelli, P.; Schmid, M.; Bugnicourt, E.; Wildner, J.; Bazzichi, A.; Anguillesi, I.; Lazzeri, A. Whey Protein Layer Applied on Biodegradable Packaging Film to Improve Barrier Properties While Maintaining Biodegradability. Polym. Degrad. Stab. 2014, 108, 151–157. [Google Scholar] [CrossRef]
- Chiellini, E.; Cinelli, P.; Corti, A.; Kenawy, E.R.; Grillo Fernandes, E.; Solaro, R. Environmentally Sound Blends and Composites Based on Water Soluble Polymer Matrices. Macromol. Symp. 2000, 152, 1. [Google Scholar] [CrossRef]
- Di Gioia, L.; Guilbert, S. Corn Protein-Based Thermoplastic Resins: Effect of Some Polar and Amphiphilic Plasticizers. J. Agric. Food Chem. 1999, 47, 1254–1261. [Google Scholar] [CrossRef] [PubMed]
- Mo, X.; Sun, X. Plasticization of Soy Protein Polymer by Polyol-Based Plasticizers. J. Am. Oil Chem. Soc. 2002, 79, 197–202. [Google Scholar] [CrossRef]
- Chiellini, E.; Cinelli, P.; Corti, A.; Kenawy, E.R. Composite Films Based on Waste Gelatin: Thermal–Mechanical Properties and Biodegradation Testing. Polym. Degrad. Stab. 2001, 73, 549–555. [Google Scholar] [CrossRef]
- Le Tien, C.; Letendre, M.; Ispas-Szabo, P.; Mateescu, M.A.; Delmas-Patterson, G.; Yu, H.-L.; Lacroix, M. Development of Biodegradable Films from Whey Proteins by Cross-Linking and Entrapment in Cellulose. J. Agric. Food Chem. 2000, 48, 5566–5575. [Google Scholar] [CrossRef] [PubMed]
- González, A.; Strumia, M.C.; Alvarez Igarzabal, C.I. Cross-Linked Soy Protein as Material for Biodegradable Films: Synthesis, Characterization and Biodegradation. J. Food Eng. 2011, 106, 331–338. [Google Scholar] [CrossRef]
- Vroman, I.; Tighzert, L. Biodegradable Polymers. Materials 2009, 2, 307–344. [Google Scholar] [CrossRef]
- Alexy, P.; Bakoš, D.; Hanzelová, S.; Kukolíková, L.; Kupec, J.; Charvátová, K.; Chiellini, E.; Cinelli, P. Poly(Vinyl Alcohol)–Collagen Hydrolysate Thermoplastic Blends: I. Experimental Design Optimisation and Biodegradation Behaviour. Polym. Test. 2003, 22, 801–809. [Google Scholar] [CrossRef]
- Kamali, S.; Yavarmanesh, M.; Habibi Najafi, M.B.; Koocheki, A. Poly (Lactic Acid) and Whey Protein/Pullulan Composite Bilayer Film Containing Phage A511 as an Anti-Listerial Packaging for Chicken Breast at Refrigerated Temperatures. LWT 2022, 170, 114085. [Google Scholar] [CrossRef]
- Alves, D.; Marques, A.; Milho, C.; Costa, M.J.; Pastrana, L.M.; Cerqueira, M.A.; Sillankorva, S.M. Bacteriophage ϕIBB-PF7A Loaded on Sodium Alginate-Based Films to Prevent Microbial Meat Spoilage. Int. J. Food Microbiol. 2019, 291, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Gouvêa, D.M.; Mendonça, R.C.S.; Soto, M.L.; Cruz, R.S. Acetate Cellulose Film with Bacteriophages for Potential Antimicrobial Use in Food Packaging. LWT—Food Sci. Technol. 2015, 63, 85–91. [Google Scholar] [CrossRef]
- Radford, D.; Guild, B.; Strange, P.; Ahmed, R.; Lim, L.-T.; Balamurugan, S. Characterization of Antimicrobial Properties of Salmonella Phage Felix O1 and Listeria Phage A511 Embedded in Xanthan Coatings on Poly(Lactic Acid) Films. Food Microbiol. 2017, 66, 117–128. [Google Scholar] [CrossRef]
- Leung, V.; Szewczyk, A.; Chau, J.; Hosseinidoust, Z.; Groves, L.; Hawsawi, H.; Anany, H.; Griffiths, M.W.; Ali, M.M.; Filipe, C.D.M. Long-Term Preservation of Bacteriophage Antimicrobials Using Sugar Glasses. ACS Biomater. Sci. Eng. 2018, 4, 3802–3808. [Google Scholar] [CrossRef] [PubMed]
- López de Dicastillo, C.; Settier-Ramírez, L.; Gavara, R.; Hernández-Muñoz, P.; López Carballo, G. Development of Biodegradable Films Loaded with Phages with Antilisterial Properties. Polymers 2021, 13, 327. [Google Scholar] [CrossRef]
- Tomat, D.; Soazo, M.; Verdini, R.; Casabonne, C.; Aquili, V.; Balagué, C.; Quiberoni, A. Evaluation of an WPC Edible Film Added with a Cocktail of Six Lytic Phages against Foodborne Pathogens Such as Enteropathogenic and Shigatoxigenic Escherichia Coli. LWT 2019, 113, 108316. [Google Scholar] [CrossRef]
- Vonasek, E.; Le, P.; Nitin, N. Encapsulation of Bacteriophages in Whey Protein Films for Extended Storage and Release. Food Hydrocoll. 2014, 37, 7–13. [Google Scholar] [CrossRef]
- González, A.; Igarzabal, C.I.A. Soy Protein—Poly (Lactic Acid) Bilayer Films as Biodegradable Material for Active Food Packaging. Food Hydrocoll. 2013, 33, 289–296. [Google Scholar] [CrossRef]
- Ramos, Ó.L.; Reinas, I.; Silva, S.I.; Fernandes, J.C.; Cerqueira, M.A.; Pereira, R.N.; Vicente, A.A.; Poças, M.F.; Pintado, M.E.; Malcata, F.X. Effect of Whey Protein Purity and Glycerol Content upon Physical Properties of Edible Films Manufactured Therefrom. Food Hydrocoll. 2013, 30, 110–122. [Google Scholar] [CrossRef]
- Giannakas, A.E.; Karabagias, V.K.; Ndreka, A.; Dimitrakou, A.; Leontiou, A.A.; Katerinopoulou, K.; Karakassides, M.A.; Proestos, C.; Salmas, C.E. Effect of Corona Treatment Method to Carvacrol Nanocoating Process for Carvacrol/Halloysite-Nanotube/Low-Density-Polyethylene Active Packaging Films Development. Nanomanufacturing 2024, 4, 138–158. [Google Scholar] [CrossRef]
- Sothornvit, R.; Krochta, J.M. Oxygen Permeability and Mechanical Properties of Films from Hydrolyzed Whey Protein. J. Agric. Food Chem. 2000, 48, 3913–3916. [Google Scholar] [CrossRef] [PubMed]
- McHugh, T.H.; Krochta, J.M. Sorbitol- vs Glycerol-Plasticized Whey Protein Edible Films: Integrated Oxygen Permeability and Tensile Property Evaluation. J. Agric. Food Chem. 1994, 42, 841–845. [Google Scholar] [CrossRef]
- Shaw, N.b.; Monahan, F.j.; O’Riordan, E.d.; O’Sullivan, M. Physical Properties of WPI Films Plasticized with Glycerol, Xylitol, or Sorbitol. J. Food Sci. 2002, 67, 164–167. [Google Scholar] [CrossRef]
- Coupland, J.N.; Shaw, N.B.; Monahan, F.J.; Dolores O’Riordan, E.; O’Sullivan, M. Modeling the Effect of Glycerol on the Moisture Sorption Behavior of Whey Protein Edible Films. J. Food Eng. 2000, 43, 25–30. [Google Scholar] [CrossRef]
- Agudelo-Cuartas, C.; Granda-Restrepo, D.; Sobral, P.J.A.; Hernandez, H.; Castro, W. Characterization of Whey Protein-Based Films Incorporated with Natamycin and Nanoemulsion of α-Tocopherol. Heliyon 2020, 6, e03809. [Google Scholar] [CrossRef] [PubMed]
- Papadaki, A.; Manikas, A.C.; Papazoglou, E.; Kachrimanidou, V.; Lappa, I.; Galiotis, C.; Mandala, I.; Kopsahelis, N. Whey Protein Films Reinforced with Bacterial Cellulose Nanowhiskers: Improving Edible Film Properties via a Circular Economy Approach. Food Chem. 2022, 385, 132604. [Google Scholar] [CrossRef] [PubMed]
- Chollakup, R.; Pongburoos, S.; Boonsong, W.; Khanoonkon, N.; Kongsin, K.; Sothornvit, R.; Sukyai, P.; Sukatta, U.; Harnkarnsujarit, N. Antioxidant and Antibacterial Activities of Cassava Starch and Whey Protein Blend Films Containing Rambutan Peel Extract and Cinnamon Oil for Active Packaging. LWT 2020, 130, 109573. [Google Scholar] [CrossRef]
- Roohi; Srivastava, P.; Bano, K.; Zaheer, M.R.; Kuddus, M. Biodegradable Smart Biopolymers for Food Packaging: Sustainable Approach Toward Green Environment. In Bio-Based Materials for Food Packaging: Green and Sustainable Advanced Packaging Materials; Ahmed, S., Ed.; Springer: Singapore, 2018; pp. 197–216. ISBN 9789811319099. [Google Scholar]
- Zafar, R.; Zia, K.M.; Tabasum, S.; Jabeen, F.; Noreen, A.; Zuber, M. Polysaccharide Based Bionanocomposites, Properties and Applications: A Review. Int. J. Biol. Macromol. 2016, 92, 1012–1024. [Google Scholar] [CrossRef]
- Zolfi, M.; Khodaiyan, F.; Mousavi, M.; Hashemi, M. Development and Characterization of the Kefiran-Whey Protein Isolate-TiO2 Nanocomposite Films. Int. J. Biol. Macromol. 2014, 65, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhou, J.; Zhang, Q.; Zhao, G.; Heng, L.; Chen, D.; Liu, D. Preparation of Cellulose Nanocrystals from Humulus Japonicus Stem and the Influence of High Temperature Pretreatment. Carbohydr. Polym. 2017, 164, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh-Sani, M.; Khezerlou, A.; Ehsani, A. Fabrication and Characterization of the Bionanocomposite Film Based on Whey Protein Biopolymer Loaded with TiO2 Nanoparticles, Cellulose Nanofibers and Rosemary Essential Oil. Ind. Crops Prod. 2018, 124, 300–315. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, C.; Chang, P.R.; Anderson, D.P.; Huneault, M.A. Pea Starch-Based Composite Films with Pea Hull Fibers and Pea Hull Fiber-Derived Nanowhiskers. Polym. Eng. Sci. 2009, 49, 369–378. [Google Scholar] [CrossRef]
- Samarajeewa, U. Safety, Processing, and Utilization of Fishery Products. Fishes 2024, 9, 146. [Google Scholar] [CrossRef]
- Siscovick, D.S.; Barringer, T.A.; Fretts, A.M.; Wu, J.H.Y.; Lichtenstein, A.H.; Costello, R.B.; Kris-Etherton, P.M.; Jacobson, T.A.; Engler, M.B.; Alger, H.M.; et al. Omega-3 Polyunsaturated Fatty Acid (Fish Oil) Supplementation and the Prevention of Clinical Cardiovascular Disease. Circulation 2017, 135, e867–e884. [Google Scholar] [CrossRef]
- Jayasekara, C.; Mendis, E.; Kim, S.-K. Seafood in the Human Diet for Better Nutrition and Health. In Encyclopedia of Marine Biotechnology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2020; pp. 2939–2959. ISBN 978-1-119-14380-2. [Google Scholar]
- Thirukumaran, R.; Anu Priya, V.K.; Krishnamoorthy, S.; Ramakrishnan, P.; Moses, J.A.; Anandharamakrishnan, C. Resource Recovery from Fish Waste: Prospects and the Usage of Intensified Extraction Technologies. Chemosphere 2022, 299, 134361. [Google Scholar] [CrossRef] [PubMed]
- FAO. The State of World Fisheries and Aquaculture. Towards Blue Transformation, Food and Agriculture Organization of the United Nations. Rome: Food and Agriculture Organization of the United Nations. Available online: https://policycommons.net/artifacts/8945635/the-state-of-world-fisheries-and-aquaculture/9788497/ (accessed on 10 December 2024).
- Mutalipassi, M.; Esposito, R.; Ruocco, N.; Viel, T.; Costantini, M.; Zupo, V. Bioactive Compounds of Nutraceutical Value from Fishery and Aquaculture Discards. Foods 2021, 10, 1495. [Google Scholar] [CrossRef]
- Nurilmala, M.; Suryamarevita, H.; Husein Hizbullah, H.; Jacoeb, A.M.; Ochiai, Y. Fish Skin as a Biomaterial for Halal Collagen and Gelatin. Saudi J. Biol. Sci. 2021, 29, 1100–1110. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, J.A.; Hermida-Merino, C.; Hermida-Merino, D.; Piñeiro, M.M.; Johansen, J.; Sotelo, C.G.; Pérez-Martín, R.I.; Valcarcel, J. Characterization of Gelatin and Hydrolysates from Valorization of Farmed Salmon Skin By-Products. Polymers 2021, 13, 2828. [Google Scholar] [CrossRef] [PubMed]
- Geahchan, S.; Baharlouei, P.; Rahman, A. Marine Collagen: A Promising Biomaterial for Wound Healing, Skin Anti-Aging, and Bone Regeneration. Mar. Drugs 2022, 20, 61. [Google Scholar] [CrossRef]
- Chotphruethipong, L.; Binlateh, T.; Hutamekalin, P.; Aluko, R.E.; Tepaamorndech, S.; Zhang, B.; Benjakul, S. Impact of Hydrolyzed Collagen from Defatted Sea Bass Skin on Proliferation and Differentiation of Preosteoblast MC3T3-E1 Cells. Foods 2021, 10, 1476. [Google Scholar] [CrossRef] [PubMed]
- Haq, M.; Ahmed, R.; Cho, Y.-J.; Chun, B.-S. Quality Properties and Bio-Potentiality of Edible Oils from Atlantic Salmon By-Products Extracted by Supercritial Carbon Dioxide and Conventional Methods. Waste Biomass Valorization 2016, 8, 1953–1967. [Google Scholar] [CrossRef]
- Tacias-Pascacio, V.G.; Castañeda-Valbuena, D.; Morellon-Sterling, R.; Tavano, O.; Berenguer-Murcia, Á.; Vela-Gutiérrez, G.; Rather, I.A.; Fernandez-Lafuente, R. Bioactive Peptides from Fisheries Residues: A Review of Use of Papain in Proteolysis Reactions. Int. J. Biol. Macromol. 2021, 184, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Stenzel, K.H.; Miyata, T.; Rubin, A.L. Collagen: A Biological Plastic. In Polymers in Medicine and Surgery; Kronenthal, R.L., Oser, Z., Martin, E., Eds.; Springer: Boston, MA, USA, 1975. [Google Scholar] [CrossRef]
- Maliha, M.; Rashid, T.U.; Rahman, M.M. Fabrication of Collagen-Sodium Alginate Based Antibacterial and Edible Packaging Material: Performance Evaluation Using Entropy-Combined Compromise Solution (CoCoSo). Carbohydr. Polym. Technol. Appl. 2024, 8, 100582. [Google Scholar] [CrossRef]
- Benedetti, G.; Zabini, F.; Tagliavento, L.; Meneguzzo, F.; Calderone, V.; Testai, L. An Overview of the Health Benefits, Extraction Methods and Improving the Properties of Pomegranate. Antioxidants 2023, 12, 1351. [Google Scholar] [CrossRef]
- Gaikwad, S.; Kim, M.J. Fish By-Product Collagen Extraction Using Different Methods and Their Application. Mar. Drugs 2024, 22, 60. [Google Scholar] [CrossRef]
- El-Ghazali, S.; Kobayashi, H.; Khatri, M.; Phan, D.-N.; Khatri, Z.; Mahar, S.K.; Kobayashi, S.; Kim, I.-S. Preparation of a Cage-Type Polyglycolic Acid/Collagen Nanofiber Blend with Improved Surface Wettability and Handling Properties for Po-tential Biomedical Applications. Polymers 2021, 13, 3458. [Google Scholar] [CrossRef]
- Bakry, N.F.; Isa, M.I.N.; Sarbon, N.M. Effect of sorbitol at different concentrations on the functional properties of gelatin/carboxymethyl cellulose (CMC)/chitosan composite films. Int. Food Res. J. 2017, 24, 1753–1762. [Google Scholar]
- Suderman, N.; Isa, M.I.N.; Sarbon, N.M. The Effect of Plasticizers on the Functional Properties of Biodegradable Gelatin-Based Film: A Review. Food Biosci. 2018, 24, 111–119. [Google Scholar] [CrossRef]
- Gómez-Guillén, M.C.; Pérez-Mateos, M.; Gómez-Estaca, J.; López-Caballero, E.; Giménez, B.; Montero, P. Fish Gelatin: A Renewable Material for Developing Active Biodegradable Films. Trends Food Sci. Technol. 2009, 20, 3–16. [Google Scholar] [CrossRef]
- Nilsuwan, K.; Guerrero, P.; de la Caba, K.; Benjakul, S.; Prodpran, T. Properties and Application of Bilayer Films Based on Poly (Lactic Acid) and Fish Gelatin Containing Epigallocatechin Gallate Fabricated by Thermo-Compression Molding. Food Hydrocoll. 2020, 105, 105792. [Google Scholar] [CrossRef]
- Maqsood, S.; Benjakul, S. Comparative Studies of Four Different Phenolic Compounds on In Vitro Antioxidative Activity and the Preventive Effect on Lipid Oxidation of Fish Oil Emulsion and Fish Mince. Food Chem. 2010, 119, 123–132. [Google Scholar] [CrossRef]
- Reygaert, W.C. Green Tea Catechins: Their Use in Treating and Preventing Infectious Diseases. BioMed Res. Int. 2018, 2018, 9105261. [Google Scholar] [CrossRef] [PubMed]
- Bikiaris, N.D.; Koumentakou, I.; Samiotaki, C.; Meimaroglou, D.; Varytimidou, D.; Karatza, A.; Kalantzis, Z.; Roussou, M.; Bikiaris, R.D.; Papageorgiou, G.Z. Recent Advances in the Investigation of Poly(lactic acid) (PLA) Nanocomposites: Incorpo-ration of Various Nanofillers and their Properties and Applications. Polymers 2023, 15, 1196. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Liu, X.; Pu, X.; Shen, Z.; Xu, W.; Yang, J. Preparation Method and Application of Porous Poly(lactic acid) Membranes: A Review. Polymers 2024, 16, 1846. [Google Scholar] [CrossRef]
- Özdoğan, R.; Çelebi, M. Enhanced Flexibility and Thermal Characteristics of Poly(Lactic Acid) Based Biocomposites with Poly(Ethylene Glycol) and Montmorillonite Clay by Solvent Casting Method. J. Innov. Eng. Nat. Sci. 2021, 1, 33–40. [Google Scholar] [CrossRef]
- Jahangir, M.A.; Rumi, T.M.; Wahab, M.A.; Khan, M.I.; Sayed, Z.B. Poly Lactic Acid (PLA) Fibres: Different Solvent Systems and Their Effect on Fibre Morphology and Diameter. Am. J. Chem. 2017, 7, 177–186. [Google Scholar] [CrossRef]

| Matrix | Food Waste | Mechanical Properties | Biochemical/Antibacterial/Sensory Properties | References |
|---|---|---|---|---|
| PLA | Onion peel | TS ↑ | AA ↑ antibacterial | [32] |
| Carboxymethyl cellulose | Onion peel | Water barrier ↓ moisture and solubility ↓ | Antioxidant activity ↑ | [40] |
| Sodium alginate | Onion peel extract | TS ↓ EB ↓ water barrier ↑ solubility ↓ | TPC ↑ antioxidant activity ↑ no antibacterial properties (E. coli and S. Aureus) | [41] |
| PLA | Red grape pomace extract | TS ↓ elastic modulus ↓ | Antioxidant activity ↑ antibacterial | [42] |
| PLA | Tempranillo grape pomace extract | - | Antioxidant activity ↑ | [43] |
| PLA | Grape seed extract | EB ↑ | Antibacterial | [44] |
| PLA | Grapevine cane extract | Elastic modulus ↓TS ↓ Water barrier ↑ | Antifungal | [45] |
| Chitosan | Prickly pear cladode gel | - | Cherry shelf-life extension, organoleptic ↑ | [46] |
| PLA | Whey | EB ↓ elastic modulus ↑ oxygen barrier ↑ water barrier ↓ | - | [47] |
| Whey | Whey with ascorbic acid | UV barrier ↑ water barrier ↓ elastic modulus ↓ TS ↓ EB ↑ | - | [48] |
| Whey | Pineapple crown leaf cellulose | Solubility ↓ TS ↑EB ↓ Moisture absorption ↓ | - | [49] |
| Chitosan | Collagen | Solubility ↓ | Antifungal and antibacterial | [50] |
| Sodium alginate | Collagen | Water barrier ↑ | - | [51] |
| PLA | Gelatin | TS ↓ EB ↑ water barrier ↓ oxygen barrier ↑ | - | [52] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazaridis, D.G.; Andritsos, N.D.; Giannakas, A.E.; Karabagias, I.K. Development and Valuation of Novel PLA-Based Biodegradable Packaging Materials Complemented with Food Waste of Plant and Animal Origin for Shelf-Life Extension of Selected Foods: Trends and Challenges. Sustainability 2025, 17, 720. https://doi.org/10.3390/su17020720
Lazaridis DG, Andritsos ND, Giannakas AE, Karabagias IK. Development and Valuation of Novel PLA-Based Biodegradable Packaging Materials Complemented with Food Waste of Plant and Animal Origin for Shelf-Life Extension of Selected Foods: Trends and Challenges. Sustainability. 2025; 17(2):720. https://doi.org/10.3390/su17020720
Chicago/Turabian StyleLazaridis, Dimitrios G., Nikolaos D. Andritsos, Aris E. Giannakas, and Ioannis K. Karabagias. 2025. "Development and Valuation of Novel PLA-Based Biodegradable Packaging Materials Complemented with Food Waste of Plant and Animal Origin for Shelf-Life Extension of Selected Foods: Trends and Challenges" Sustainability 17, no. 2: 720. https://doi.org/10.3390/su17020720
APA StyleLazaridis, D. G., Andritsos, N. D., Giannakas, A. E., & Karabagias, I. K. (2025). Development and Valuation of Novel PLA-Based Biodegradable Packaging Materials Complemented with Food Waste of Plant and Animal Origin for Shelf-Life Extension of Selected Foods: Trends and Challenges. Sustainability, 17(2), 720. https://doi.org/10.3390/su17020720











