Research on Hydroponic Cultivation Substrates Made from Rice Straw
Abstract
1. Introduction
2. Materials and Methods
2.1. Basic Principle
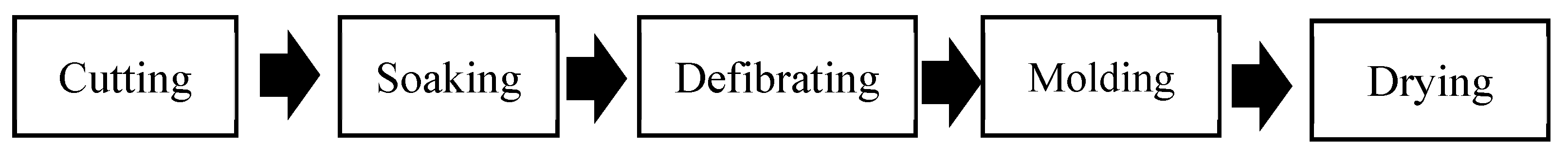
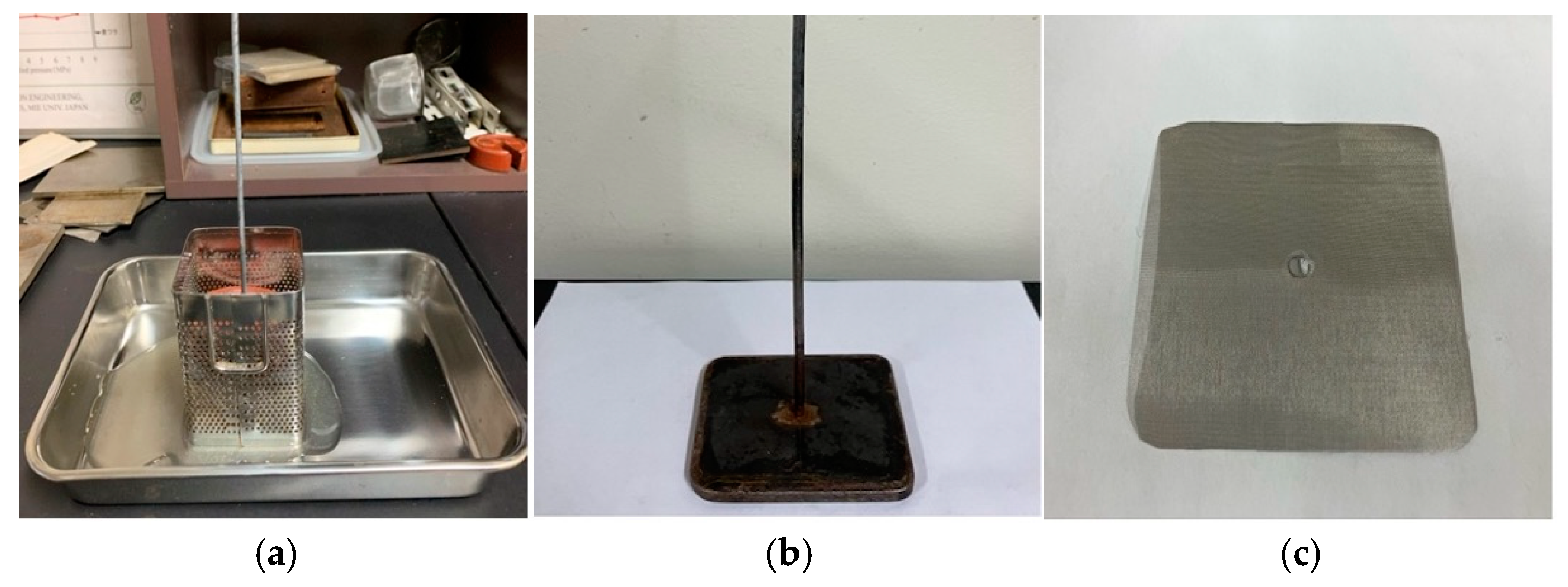
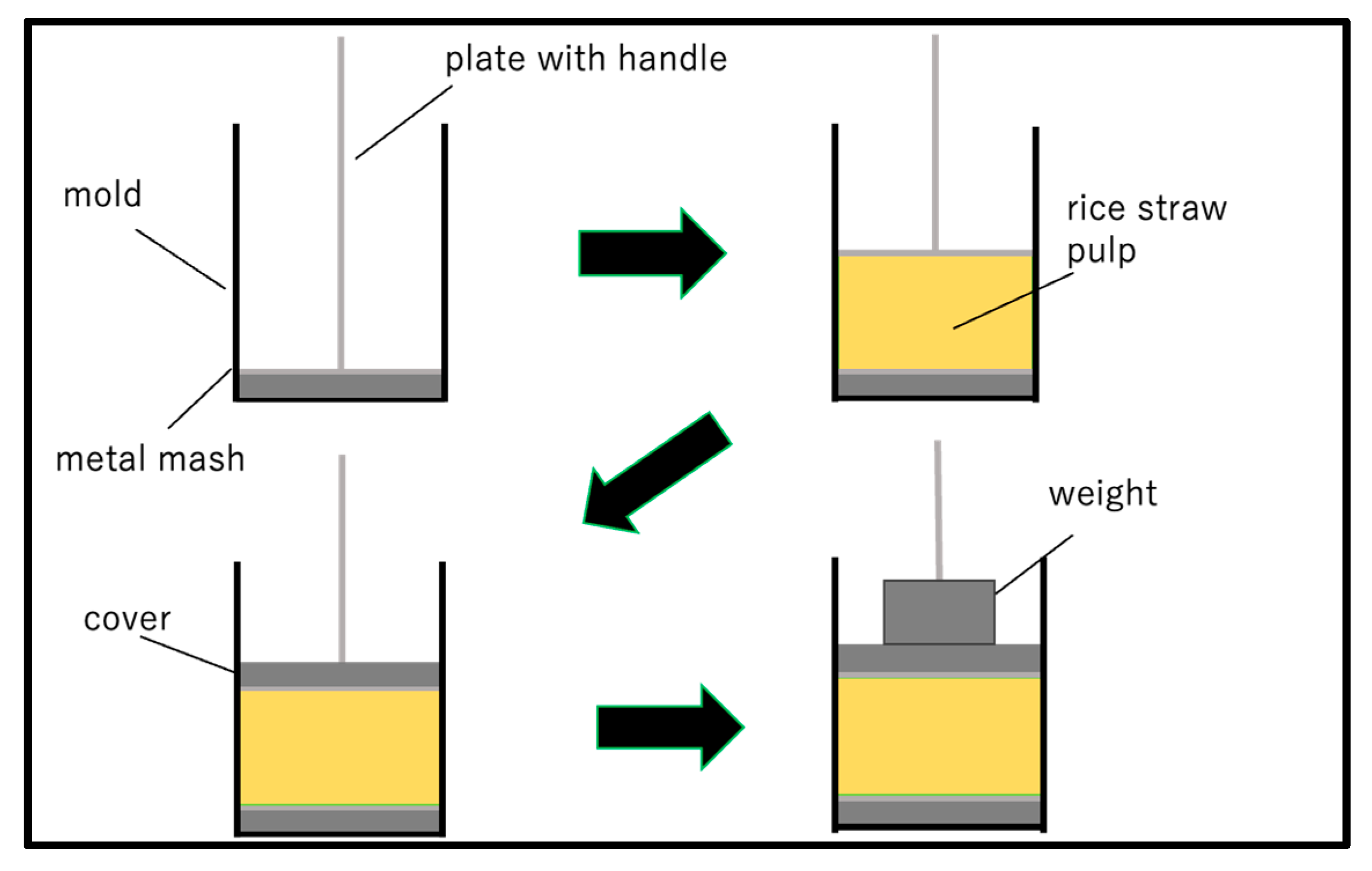
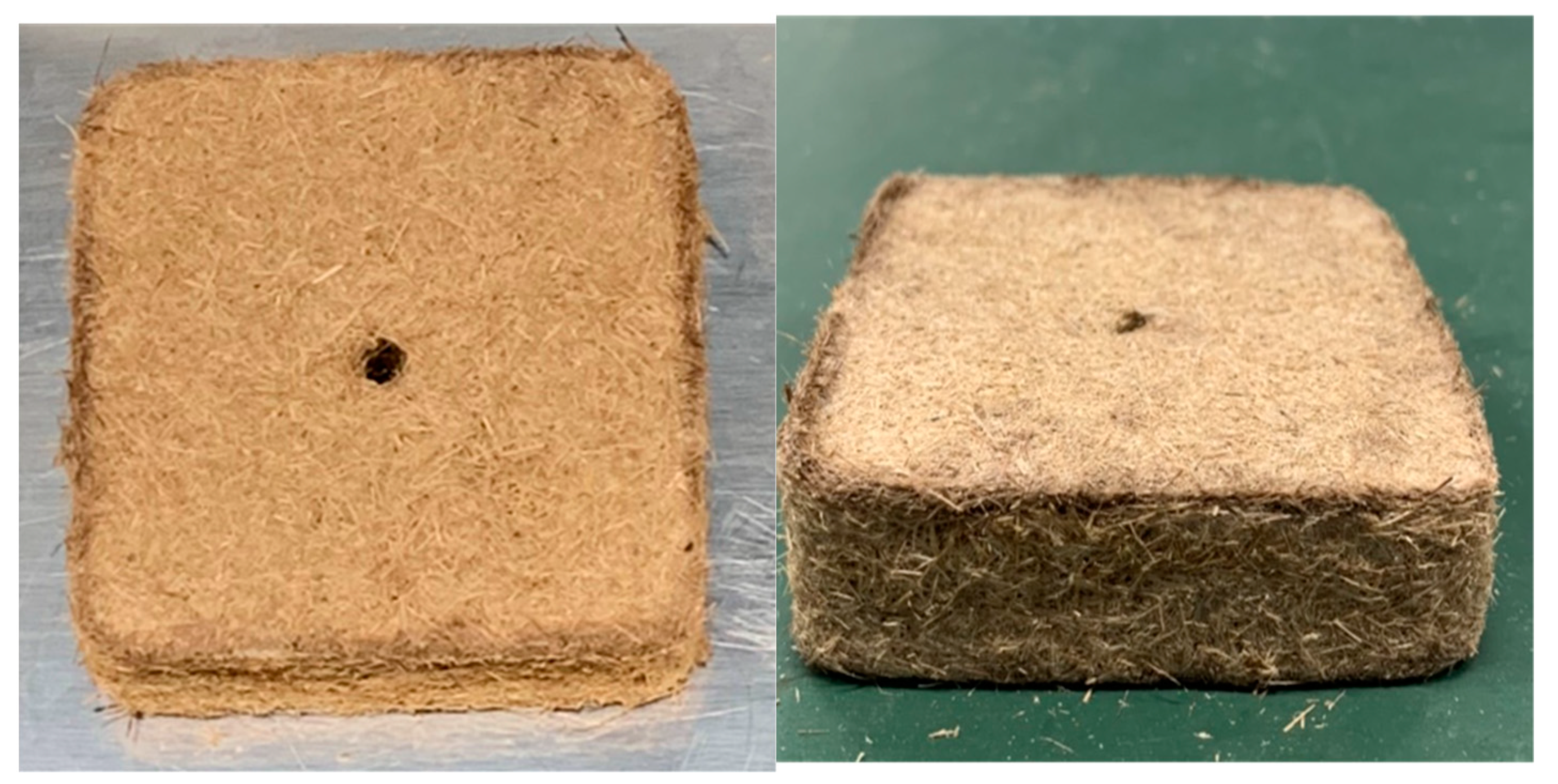
2.2. Manufacture of Substrates
- Pretreatment
- Cutting
- Soaking
- Defibrating
- Molding
2.3. Density and Porosity
- ;
- : volume (cm3);
- : solid volume (cm3);
- : fiber weight (g);
- (g/cm3).
2.4. Observation by Scanning Electron Microscopy (SEM)
2.5. Constant Head Permeameter Test
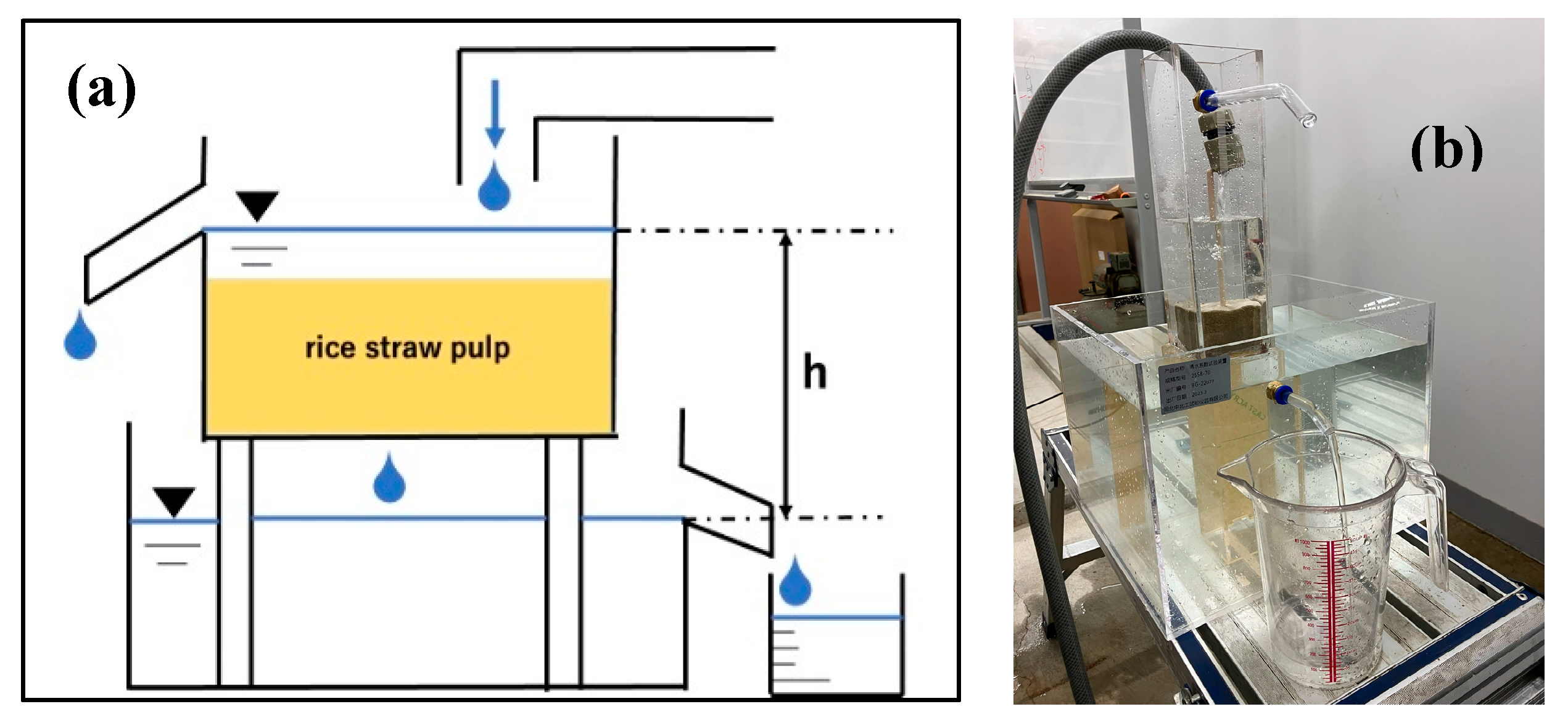
2.5.1. Assembled Permeameter Apparatus
2.5.2. Calculation of the Saturated Permeability
- : saturated permeability (cm/s);
- : flow rate of water passing through the substrate per unit time (cm3/s);
- : length of the flow path (cm);
- A: cross-sectional area of the substrate (cm2);
- : hydraulic head difference across the substrate (cm).
3. Results and Discussion
3.1. Density and Porosity of the Rice Straw Substrate
3.2. Microstructural Properties of RS
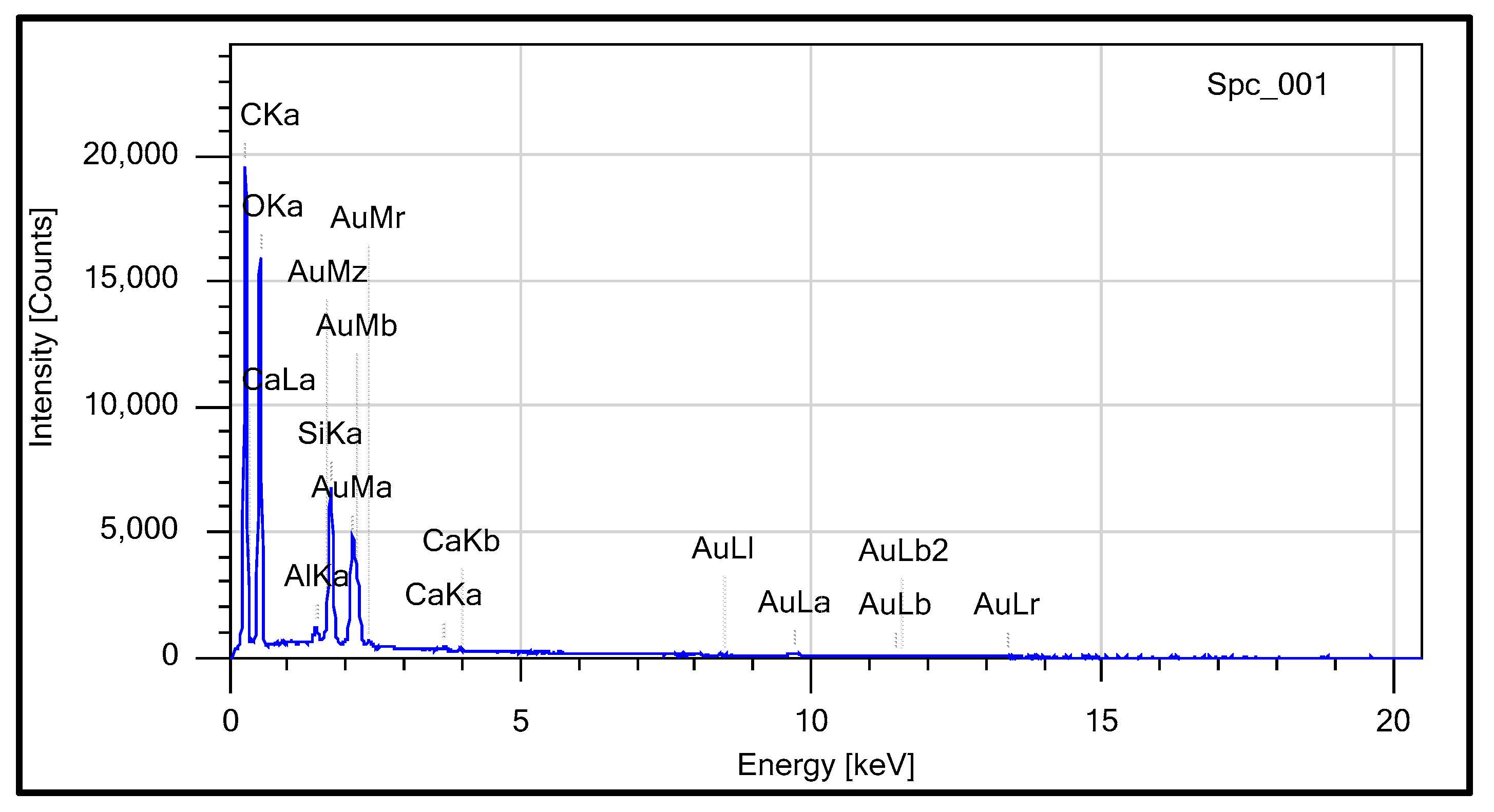
3.2.1. Elemental Composition of RS
3.2.2. The X-Ray Spectrum Analysis
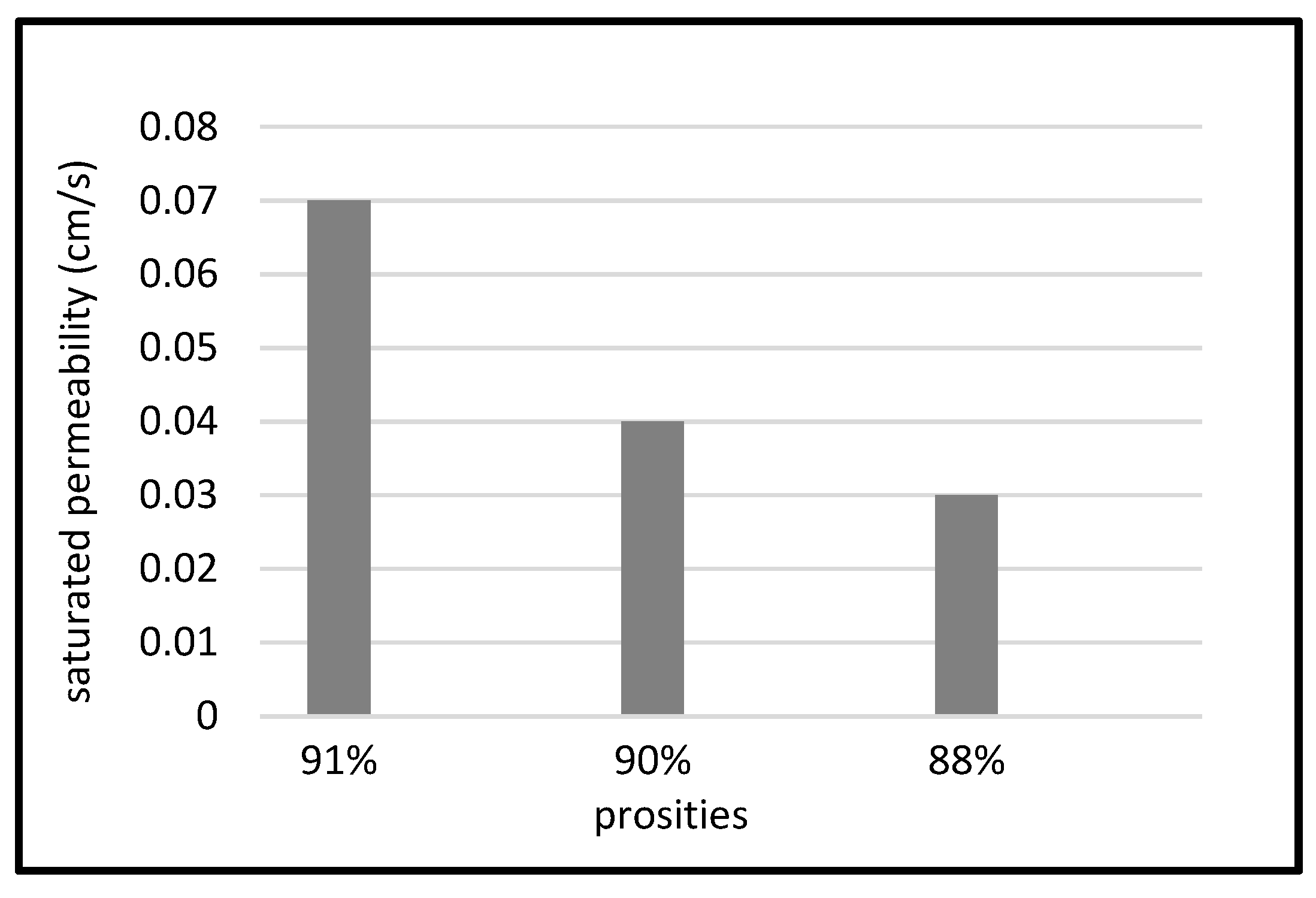
3.3. Constant Head Permeameter Test
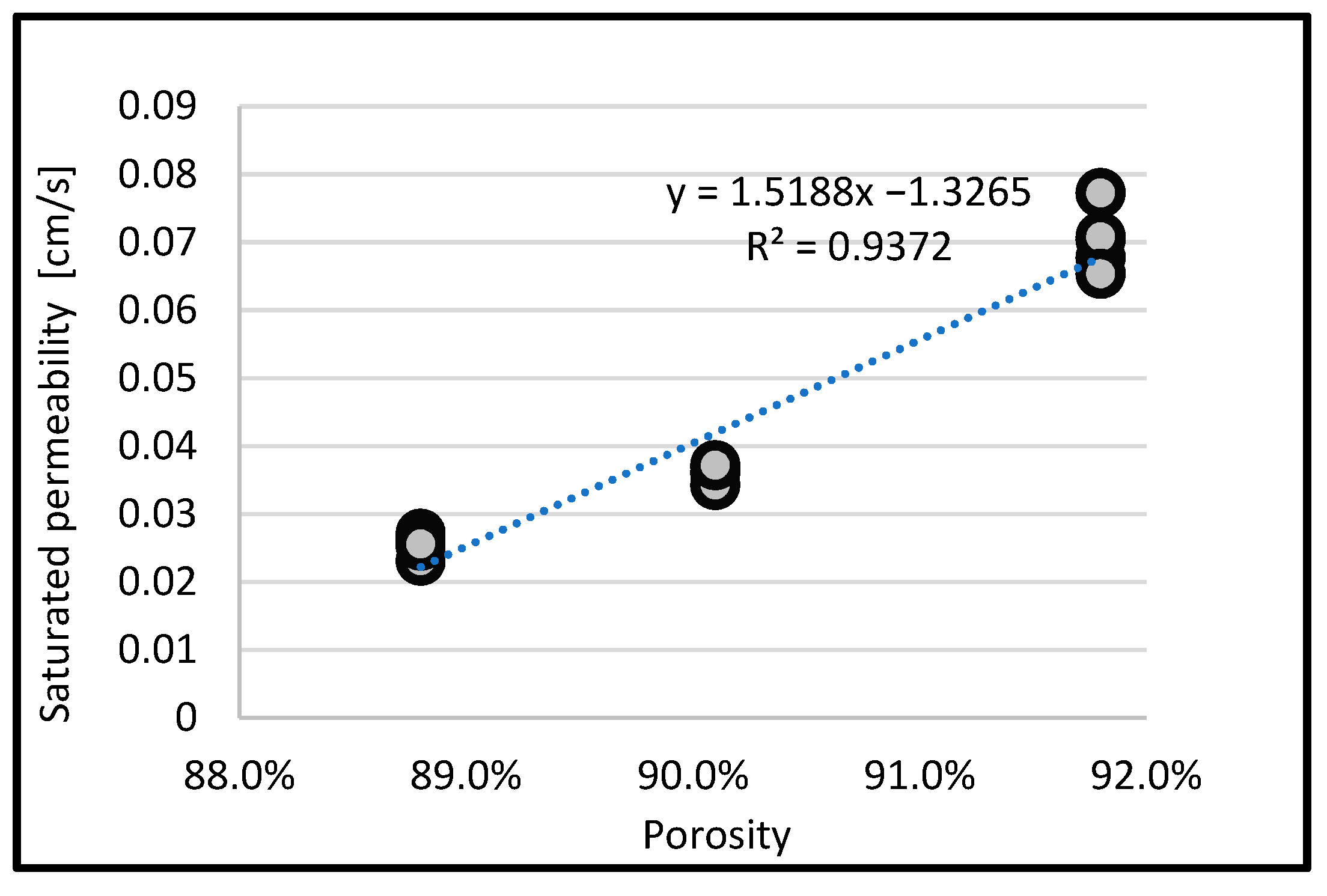
3.4. Relationship Between Porosity and Saturated Permeability
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gericke, W.F. Hydroponics—Crop Production in Liquid Culture Media. Science 1937, 85, 177–178. [Google Scholar] [CrossRef]
- Beibel, J.P. “Hydroponics”—The Science of Growing Crops Without Soil. Int. J. Agric. Crop Sci. 1960, 180, 833–842. [Google Scholar]
- Jan, S.; Rashid, Z.; Ahngar, T.A.; Iqbal, S.; Naikoo, M.A.; Majeed, S.; Bhat, T.A.; Gul, R.; Nazir, I. Hydroponics—A Review. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 2319–7706. [Google Scholar] [CrossRef]
- Butler, J.D.; Oebker, N.F. Hydroponics as a Hobby—Growing Plants Without Soil. Circular 1962, 844, 3–16. [Google Scholar]
- Tilman, D.; Cassman, K.G.; Matson, P.A.; Naylor, R.; Polasky, S. Agricultural sustainability and intensive production practices. Nature 2002, 418, 671–677. [Google Scholar] [CrossRef]
- Menon, S.; Naranje, V.G. Experimental Investigation of Recycling of Rock-Wool Insulation as Insulator in Concrete Blocks. Int. J. Eng. Appl. Sci. 2017, 4, 71–74. [Google Scholar]
- Harahap, M.A.; Harahap, F. The Effect of Ab mix Nutrient on growth and Yield of Pak choi (Brassica chinesis L.) Plants under Hydroponic Wick System Condition. J. Phys. Conf. Ser. 2020, 1485, 0122028. [Google Scholar]
- Yang, T.; Altland, J.E.; Samarakoon, U.C. Evaluation of substrates for cucumber production in the Dutch bucket hydroponic system. Sci. Hortic. 2023, 308, 111578. [Google Scholar] [CrossRef]
- FAO. FAOSTAT Food Balance Sheets. 2020. Available online: http://www.fao.org/faostat/en/#data/FBS (accessed on 24 April 2020).
- Shahbandeh, M. Total Global Rice Consumption 2008/09–2023/24. 2024. Available online: https://www.statista.com/statistics/255977/total-global-rice-consumption/?utm_source=chatgpt.com (accessed on 5 January 2025).
- MAFF. A Review of Rice Straw Application Situation. 2021. Available online: https://www.maff.go.jp/j/chikusan/sinko/lin/l_siryo/attach/pdf/inawara-19.pdf (accessed on 22 November 2024).
- Nguyen, M.N. Thermal induced changes of rice straw phytolith in relation to arsenic release: A perspective of rice straw arsenic under open burning. J. Environ. Manag. 2022, 304, 114294. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Gummert, M. Rice Straw Overview: Availability, Properties, and Management Practices. In Sustainable Rice Straw Management; Gummert, M., Chivenge, P., Eds.; Springer Nature: Cham, Switzerland, 2020; pp. 1–13. [Google Scholar]
- Pauline, C.; Francis, R.; Duong, V.C. Rice Straw Incorporation Influences Nutrient Cycling and Soil Organic Matter. In Sustainable Rice Straw Management; Gummert, M., Chivenge, P., Eds.; Springer Nature: Cham, Switzerland, 2020; pp. 131–144. [Google Scholar]
- Koppmann, R.; Reid, J.S.; Eeeuterio, D.P. A review of biomass burning emissions part II: Intensive physical properties of biomass burning particles. Atmos. Chem. Phys. 2005, 5, 700–825. [Google Scholar]
- Zha, S.; Zhang, S.; Cheng, T.; Chen, J.; Huang, G.; Li, X.; Wang, Q. Agricultural Fires and Their Potential Impacts on Regional Air Quality over China. Aerosol Air Qual. Res. 2013, 13, 992–1001. [Google Scholar] [CrossRef]
- Zhang, L.B.; Liu, Y.Q.; Hao, L. Contributions of open crop straw burning emissions to PM2.5 concentrations in China. Environ. Res. Lett. 2016, 11, 14014. [Google Scholar] [CrossRef]
- Enishi, O.; Shijimaya, K.; Outa, H. Varietal Differences in Chemical Composition and in vitro Dry Matter Digestibility of Rice (Oryza sativa L.) Straw. Grassl. Sci. 1995, 41, 152–155. [Google Scholar]
- Kaur, D.; Bhardwaj, N.K.; Lohchab, R.K. Prospects of rice straw as a raw material for paper making. Waste Manag. 2017, 60, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Hubbe, M. Bonding between cellulosic fibers in the absence and presence of dry-strength agents—A review. BioResources 2006, 1, 281–318. [Google Scholar] [CrossRef]
- Wu, T.T.; Wang, X.L.; Kito, K. Effects of pressures on the mechanical properties of corn straw bio-board. Eng. Agric. Environ. Food 2015, 8, 123–129. [Google Scholar] [CrossRef][Green Version]
- Igafurusato, J.A. Cultivation Calendar. Available online: https://www.jaiga.or.jp/?p=694 (accessed on 27 November 2024).
- Zhang, J. Development of Biodegradable Biomass Board and Its Mechanical Properties Using Rice Straw. Ph.D. Thesis, Graduate School of Bioresources Mie University, Tsu, Japan, 2016; pp. 50–52. [Google Scholar]
- Chen, H.Z.; Liu, Z.H. Multilevel composition fractionation process for high-value utilization of wheat straw cellulose. Biotechnol. Biofuels 2014, 7, 137. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Yu, H.; Cheng, J. Enhancing Enzymatic Hydrolysis of Rice Straw by Acid-Assisted Mechanocatalytic Depolymerization Pretreatment. Agronomy 2024, 14, 2550. [Google Scholar] [CrossRef]
- EI-Kassas, A.M.; Elsheikh, A.H. A new eco-friendly mechanical technique for production of rice straw fibers for medium density fiberboards manufacturing. Int. J. Environ. Sci. Technol. 2021, 18, 979–988. [Google Scholar] [CrossRef]
- Knauf Insolation, Green Solutions, Hortiscape Core Products. Available online: https://www.knaufinsulation-horticulture.com/wp-content/uploads/2021/03/KI-HORTISCAPE-A4-PROSPEKT-Hydroponic-plant-growing-system-for-advanced-users.pdf (accessed on 22 November 2024).
- Gliñski, J.; Horabik, J.; Lipiec, J. (Eds.) Encyclopedia of Agrophysics; Springer: Dordrecht, the Netherlands, 2011; pp. 364–367. [Google Scholar]
- Patil, S.T.; Kadam, U.S.; Mane, M.S.; Mahale, D.M.; Dhekale, J.S. Hydroponic Growth Media (Substrate): A Review. Int. Res. J. Pure Appl. Chem. 2020, 21, 106–113. [Google Scholar] [CrossRef]
- Chirans, H.S.; Pavan Kumar, H.R.; Sachin, R.; Yathod, K.M. A review on optimum substrate surface roughness to create better wetting. Mater. Open Res. 2024, 3, 5. [Google Scholar]
- Feng, Z.; Zheng, X.; Li, X. Soil Surface Roughness Characteristics Under Different Agricultural Tillage Practices—A Case Study in the Black Soil Region of Northeast China. J. Sel. Top. Appl. Earth Obs. Remote Sens. 2024, 7, 10781–10791. [Google Scholar] [CrossRef]
- Fortin, J.P.; Nemati, R.; Vetanovetz, R. Peat, Coir or Rock Wool? 2019. Available online: https://www.growertalks.com/Article/?articleid=24344 (accessed on 27 November 2024).
- Hata, T.; Tabuchi, T.; Nakano, K. Guide to the Engineering and Physical Properties of Soils. In Soil Engineering and Physics Experimental Guidebook Editorial Committee; Agricultural and Rural Development Society: Tokyo, Japan, 1983; pp. 102–106. [Google Scholar]
- Yamanaka, S. Physical Properties of Rockwool Propagation Media—Moisture Characteristics. Sci. Bull. Fac. Agric. Kyushu Univ. 1991, 46, 1–7. [Google Scholar]





| Samples No. | Pressure (Pa) | Rice Straw Fiber Length (mm) | Drying Temperature (°C) | Drying Duration (h) |
|---|---|---|---|---|
| RS-A1, RS-A2 | 416 | 0~5.6 | 110 | 24 |
| RS-B1, RS-B2 | 1216 | 0~5.6 | 110 | 24 |
| RS-C1, RS-C2 | 2016 | 0~5.6 | 110 | 24 |
| Samples | Pressure (Pa) | Porosity (%) |
|---|---|---|
| Rockwool | - | 95 ± 5 [23] |
| Coir | - | 91 ± 5 [23] |
| RS-A1 | 416 | 91 |
| RS-A2 | 416 | 92 |
| RS-B1 | 1216 | 91 |
| RS-B2 | 1216 | 90 |
| RS-C1 | 2016 | 88 |
| RS-C2 | 2016 | 89 |
| C | O | Al | Si | Ca | Au | Total | |
|---|---|---|---|---|---|---|---|
| Spc_001 | 42.93 | 34.8 | 0.48 | 4.67 | 0.34 | 16.77 | 100 |
| Atom/Mol % | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, T.; Wang, X.; Inada, T. Research on Hydroponic Cultivation Substrates Made from Rice Straw. Sustainability 2025, 17, 772. https://doi.org/10.3390/su17020772
Wu T, Wang X, Inada T. Research on Hydroponic Cultivation Substrates Made from Rice Straw. Sustainability. 2025; 17(2):772. https://doi.org/10.3390/su17020772
Chicago/Turabian StyleWu, Tingting, Xiulun Wang, and Tomoki Inada. 2025. "Research on Hydroponic Cultivation Substrates Made from Rice Straw" Sustainability 17, no. 2: 772. https://doi.org/10.3390/su17020772
APA StyleWu, T., Wang, X., & Inada, T. (2025). Research on Hydroponic Cultivation Substrates Made from Rice Straw. Sustainability, 17(2), 772. https://doi.org/10.3390/su17020772





