Binary Supplementary Cementitious Material from Expanded Clay Production Dust and Opoka
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparations
2.3. Methods
3. Results
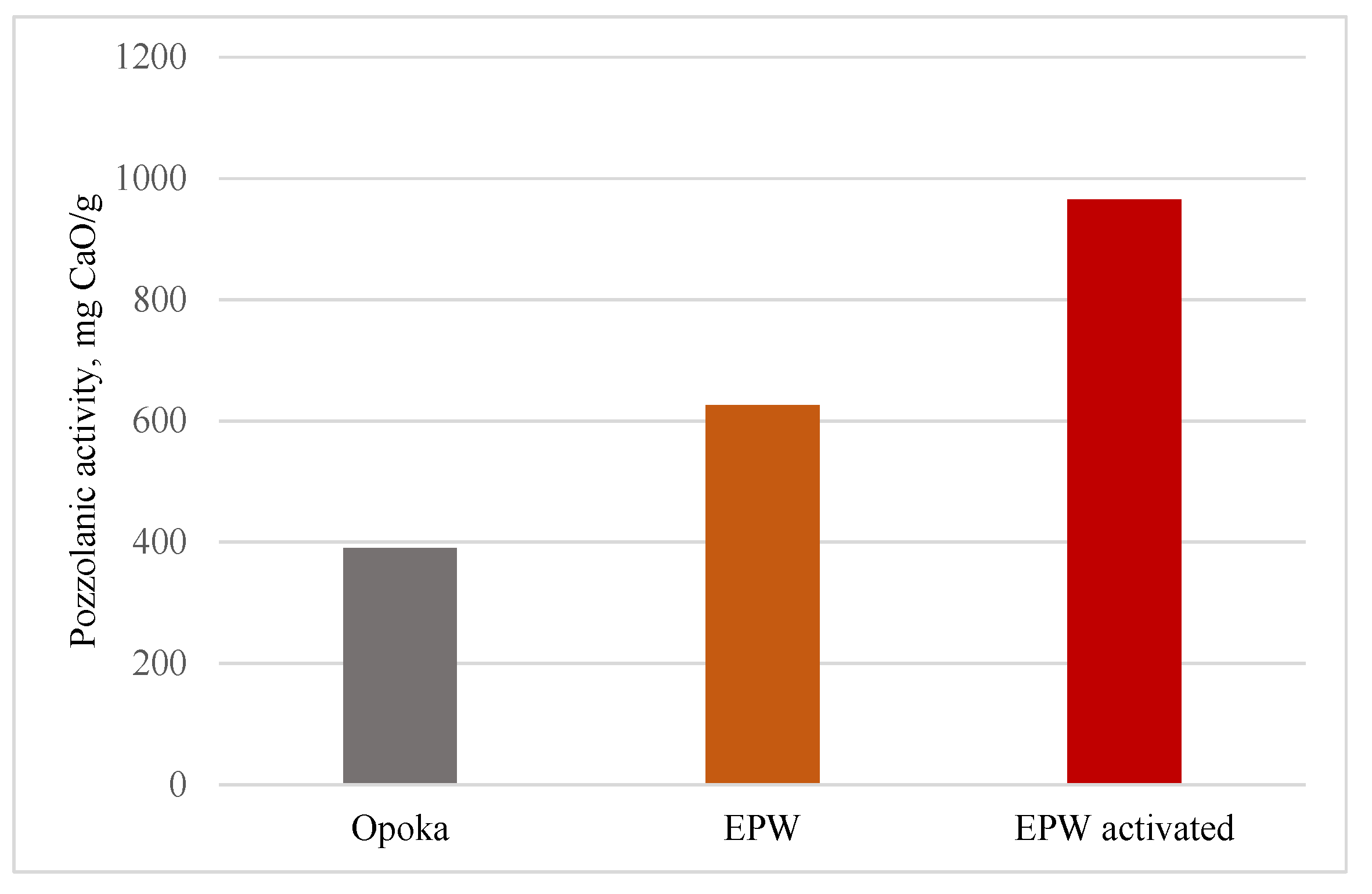
3.1. Pozzolanic Activity
3.2. The Influence of Additives on the Cement Paste
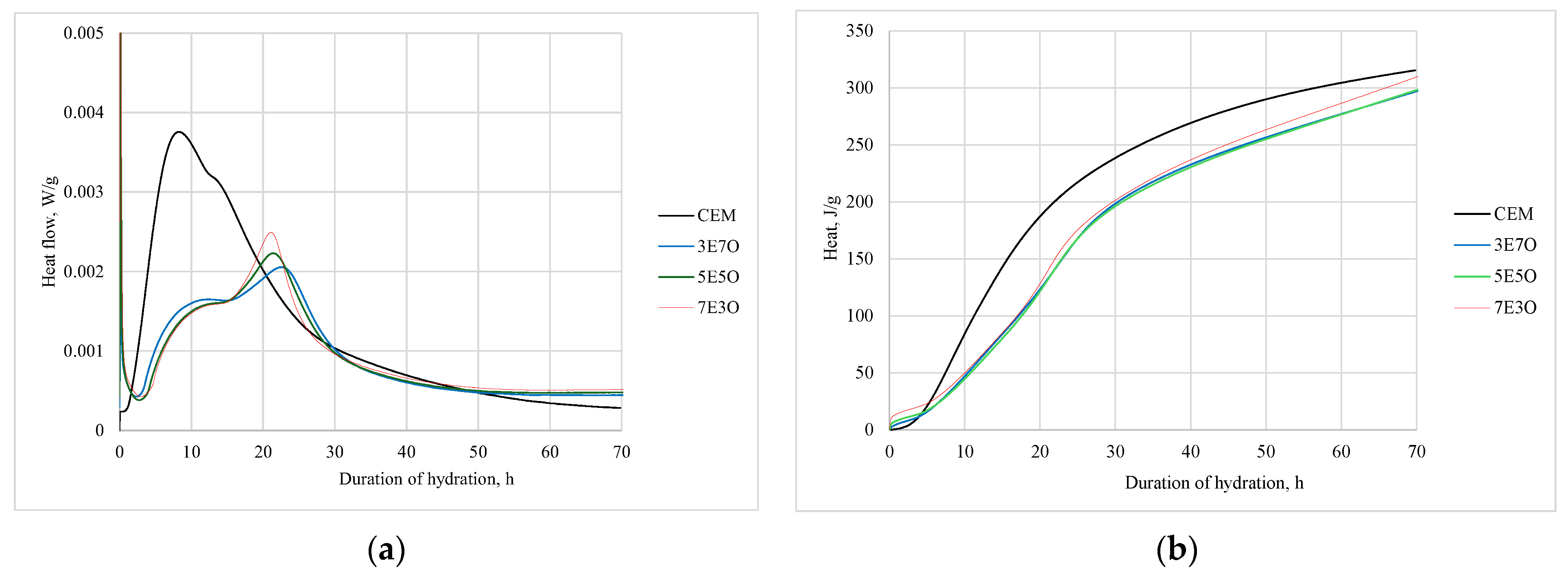
3.3. The Influence of Additives on the Early Hydration of Portland Cement
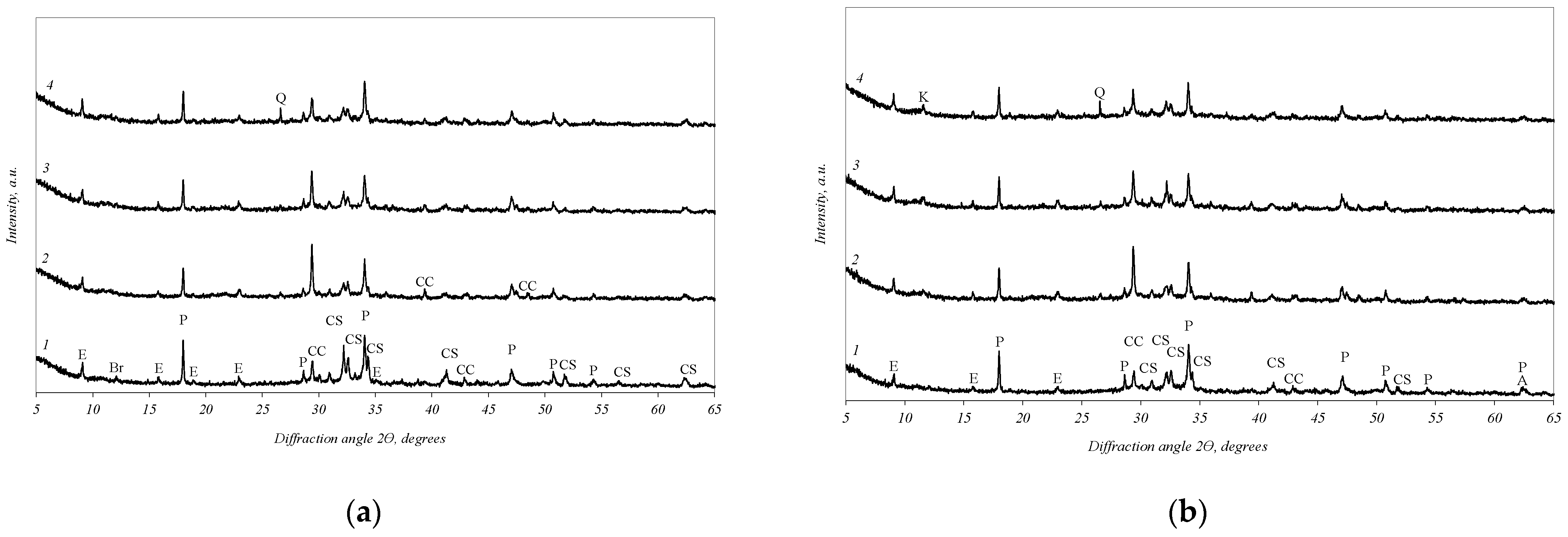
3.4. The Influence of Additives on the Portland Cement Compressive Strength and Hydration Course
4. Conclusions
- Opoka is composed of SiO2 strains, quartz, tridymite, cristobalite, and calcium carbonate, while expanded clay furnace dust consists of a mixture of different clay minerals (muscovite, anorthite, illite, and kaolinite) with impurities of quartz, calcite, etc. In the mineral composition of thermally activated dust, kaolinite transforms into metakaolinite, while other minerals remain. The pozzolanic activity of additional thermally activated expanded clay production waste (965 mg CaO/g) is almost three times that of opoka (390 mg CaO/g).
- The two-component additive has a complex influence on the hydration of Portland cement: due to the high activity of activated expanded clay furnace dust, a pozzolanic reaction takes place; meanwhile, the opoka component promotes the formation of monocarboaluminates.
- The new binary SCMs from natural raw materials and waste are suitable for industrial applications because they allow for rational and sustainable use of existing cement production resources. This two-component material can replace a significant amount (up to 25% by weight) of Portland cement without reducing the compressive strength of the cement.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Madlool, N.A.; Saidur, R.; Hossain, M.S.; Rahim, N.A. A critical review on energy use and savings in the cement industries. Renew. Sustain. Energy Rev. 2011, 15, 2042–2060. [Google Scholar] [CrossRef]
- Mokhtar, A.; Nasooti, M. A decision support tool for cement industry to select energy efficiency measures. Energy Strateg. Rev. 2020, 28, 100458. [Google Scholar] [CrossRef]
- Khaiyum, M.Z.; Sarker, S.; Kabir, G. Evaluation of Carbon Emission Factors in the Cement Industry: An Emerging Economy Context. Sustainability 2023, 15, 15407. [Google Scholar] [CrossRef]
- Supriya; Chaudhury, R.; Sharma, U.; Thapliyal, P.C.; Singh, L.P. Low-CO2 emission strategies to achieve net zero target in cement sector. J. Clean. Prod. 2023, 417, 137466. [Google Scholar] [CrossRef]
- Knight, A.K.; Cunningham, P.R.; Miller, A.S. Optimizing supplementary cementitious material replacement to minimize the environmental impacts of concrete. Cem. Concr. Compos. 2023, 139, 105049. [Google Scholar] [CrossRef]
- Snellings, R.; Suraneni, P.; Skibsted, J. Future and emerging supplementary cementitious materials. Cem. Concr. Res. 2023, 171, 107199. [Google Scholar] [CrossRef]
- Juenger, M.C.G.; Snellings, R.; Bernal, S.A. Supplementary cementitious materials: New sources, characterization, and performance insights. Cem. Concr. Res. 2019, 122, 257–273. [Google Scholar] [CrossRef]
- Oliveira, V.M.; Souza, F.; Cruz, R.T.; Py, L.G.; Kirchheim, A.P.; Bragança, S.R. Valorization of non-beneficiated clays as supplementary cementitious materials in the production of cement-based mortar. J. Build. Eng. 2021, 42, 102474. [Google Scholar] [CrossRef]
- Lorentz, B.; Shanahan, N.; Stetsko, Y.P.; Zayed, A. Characterization of Florida kaolin clays using multiple-technique approach. Appl. Clay Sci. 2018, 161, 326–333. [Google Scholar] [CrossRef]
- Rakhimova, C.N. Montmorillonite clays in Portland clinker-reduced, non-clinker cements, and cement composites: A review. Constr. Build. Mater. 2024, 411, 134678. [Google Scholar] [CrossRef]
- Pacewska, B.; Wilińska, I. Usage of supplementary cementitious materials: Advantages and limitations. J. Therm. Anal. Calorim. 2020, 142, 371–393. [Google Scholar] [CrossRef]
- Abdul-Wahab, S.A.; Al-Dhamri, H.; Ram, G.; Chatterjee, V.P. An overview of alternative raw materials used in cement and clinker manufacturing. Int. J. Sustain. Eng. 2020, 14, 743–760. [Google Scholar] [CrossRef]
- Kaminskas, R.; Barauskas, I.; Kazlauskas, E. Supplementary Cementitious Materials from Different Wastes. ACI Mater. J. 2023, 120, 73–79. [Google Scholar]
- Kaminskas, R.; Savickaite, B. Expanded Clay Production Waste as Supplementary Cementitious Material. Sustainability 2023, 15, 11850. [Google Scholar] [CrossRef]
- Shayan, A.K.; Fazal, H.; Hassan, A.; Rao, A.K. A scientometric review of the synthesis and application of expanded clay aggregate in cementitious composites. Constr. Build. Mater. 2024, 437, 136654. [Google Scholar]
- Dondi, M.; Cappelletti, P.; D’Amore, M.; de Gennaro, R.; Graziano, S.F.; Langella, A.; Raimondo, M.; Zanelli, C. Lightweight aggregates from waste materials: Reappraisal of expansion behavior and prediction schemes for bloating. Constr. Build. Mater. 2016, 127, 394–409. [Google Scholar] [CrossRef]
- Barauskas, I.; Kaminskas, R.; Stanaitis, A.; Vilkaite, I. Influence of Opoka Additive on Composite Cement Hydration. Solid State Phenom. 2015, 244, 3–11. [Google Scholar] [CrossRef]
- Perez, A.; Favier, A.; Martirena, F.; Scrivener, K. Influence of the manufacturing process on the performance of low clinker, calcined clay-limestone Portland cement. In Calcined Clays for Sustainable Concrete; RILEM Bookseries 10; Springer: Berlin/Heidelberg, Germany, 2015; pp. 283–289. [Google Scholar]
- Kaminskas, R.; Barauskas, I.; Drapanauskaite, D. Influence of carbonated pozzolana on sulphate attack of cement stone at low temperatures. Adv. Cem. Res. 2014, 26, 85–92. [Google Scholar] [CrossRef]
- Gineika, A.; Siauciunas, R.; Baltakys, K. Synthesis of wollastonite from AlF3-rich silica gel and its hardening in the CO2 atmosphere. Sci. Rep. 2019, 9, 18063. [Google Scholar] [CrossRef] [PubMed]
- Standard EN 196-3:2016; Methods of Testing Cement—Part 3: Determination of Setting Times and Soundness. Academia: Cambridge, MA, USA, 2016.
- Standard EN 196-1:2016; Methods of Testing Cement—Part 1: Determination of Strength. Academia: Cambridge, MA, USA, 2016.
- NF P18-513:2012; Metakaolin. Pozzolanic Addition for Concrete. Definitions, Specifications and Conformity Criteria. Association Française de Normalisation: La Plaine Saint-Denis, France, 2012.
- Quarcioni, Y.; Chotoli, V.A.F.F.; Coelho, A.C.V.; Cincotto, M.A. Indirect and direct Chapelle’s methods for the determination of lime consumption in pozzolanic materials. Rev. IBR. Estrut. Mat. 2015, 8, 1–7. [Google Scholar] [CrossRef]
- Luzu, B.; Trauchessec, R.; Lecomte, A. Packing density of limestone calcined clay binder. Powder Technol. 2022, 408, 117702. [Google Scholar] [CrossRef]
- Babako, M.; Apeh, J.A. Setting time and standard consistency of Portland cement binders blended with rice husk ash, calcium carbide and metakaolin admixtures. IOP Conf. Ser. Mater. Sci. Eng. 2020, 805, 012031. [Google Scholar] [CrossRef]
- Schöler, A.; Lothenbach, B.; Winnefeld, F.; Haha, M.B.; Zajac, M.; Ludwig, H.M. Early hydration of SCM-blended Portland cements: A pore solution and isothermal calorimetry study. Cem. Concr. Res. 2017, 93, 71–82. [Google Scholar] [CrossRef]
- Hesse, F.; Götz-Neunhoeffer, C.; Neubauer, J. A new approach in quantitative in-situ XRD of cement pastes: Correlation of heat flow curves with early hydration reactions. Cem. Concr. Res. 2011, 41, 123–128. [Google Scholar] [CrossRef]
- El-Diadamony, H.; Amer, A.A.; Sokkary, T.M.; El-Hoseny, S. Hydration and characteristics of metakaolin pozzolanic cement pastes. HBRC J. 2018, 14, 150–158. [Google Scholar] [CrossRef]






| Component (wt.%) | EPW | Portland Cement (OPC) | Opoka |
|---|---|---|---|
| SiO2 | 44.90 | 19.51 | 36.60 |
| Al2O3 | 25.30 | 5.04 | 2.39 |
| Fe2O3 | 9.78 | 3.06 | 1.02 |
| CaO | 4.84 | 61.38 | 31.35 |
| MgO | 1.32 | 3.94 | 0.96 |
| K2O | 1.47 | 1.05 | 0.56 |
| Na2O | 0.27 | 0.12 | 0.06 |
| SO3 | 0.67 | 2.5 | 0.65 |
| P2O5 | 0.35 | - | - |
| TiO2 | 1.06 | - | - |
| Other | 0.10 | 3.4 | 0.11 |
| LOI | 9.84 | - | 26.8 |
| Specific surface area (m2/kg) | 360 | 350 | 360 |
| Component (wt.%) | Abbreviation | ||
|---|---|---|---|
| Portland Cement (OPC) | EPW | Opoka | |
| 100 | - | - | CEM |
| 75 | 7.5 | 17.5 | 3E7O |
| 75 | 12.5 | 12.5 | 5E5O |
| 75 | 17.5 | 7.5 | 7E3O |
| Sample | Water-to-Cement (W/C) Ratio | Setting Time (min) | |
|---|---|---|---|
| Initial | Final | ||
| CEM | 0.273 | 100 | 145 |
| 3E7O | 0.327 | 130 | 190 |
| 5E5O | 0.336 | 135 | 195 |
| 7E3O | 0.342 | 150 | 215 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaminskas, R.; Barauskas, I.; Uselis, S.; Savickaite, B. Binary Supplementary Cementitious Material from Expanded Clay Production Dust and Opoka. Sustainability 2025, 17, 794. https://doi.org/10.3390/su17020794
Kaminskas R, Barauskas I, Uselis S, Savickaite B. Binary Supplementary Cementitious Material from Expanded Clay Production Dust and Opoka. Sustainability. 2025; 17(2):794. https://doi.org/10.3390/su17020794
Chicago/Turabian StyleKaminskas, Rimvydas, Irmantas Barauskas, Skomantas Uselis, and Brigita Savickaite. 2025. "Binary Supplementary Cementitious Material from Expanded Clay Production Dust and Opoka" Sustainability 17, no. 2: 794. https://doi.org/10.3390/su17020794
APA StyleKaminskas, R., Barauskas, I., Uselis, S., & Savickaite, B. (2025). Binary Supplementary Cementitious Material from Expanded Clay Production Dust and Opoka. Sustainability, 17(2), 794. https://doi.org/10.3390/su17020794






