Exploring the Potential of Licuri (Syagrus coronata) Using Sustainable Techniques and Solvents for Extracting Bioactive Compounds
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Pre-Treatment of the Biomass
2.3. Experimental Design Using Full Factorial Design (FFD)
2.4. Phenolic Compound Determination
2.5. Total Flavonoid Determination
3. Results and Discussion
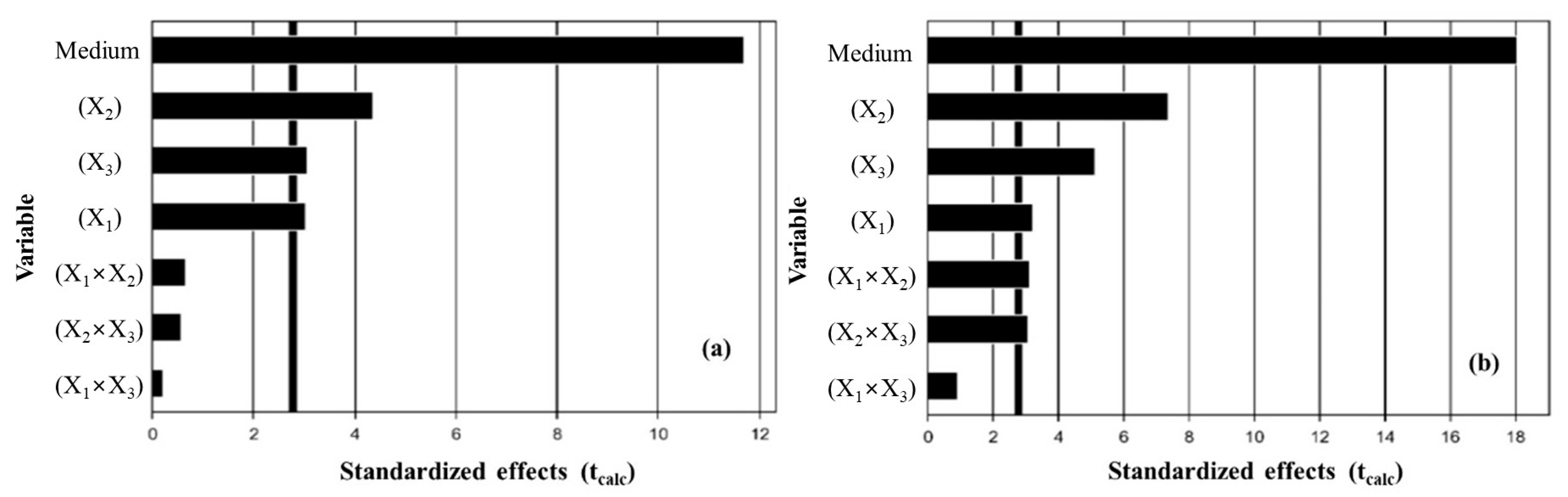
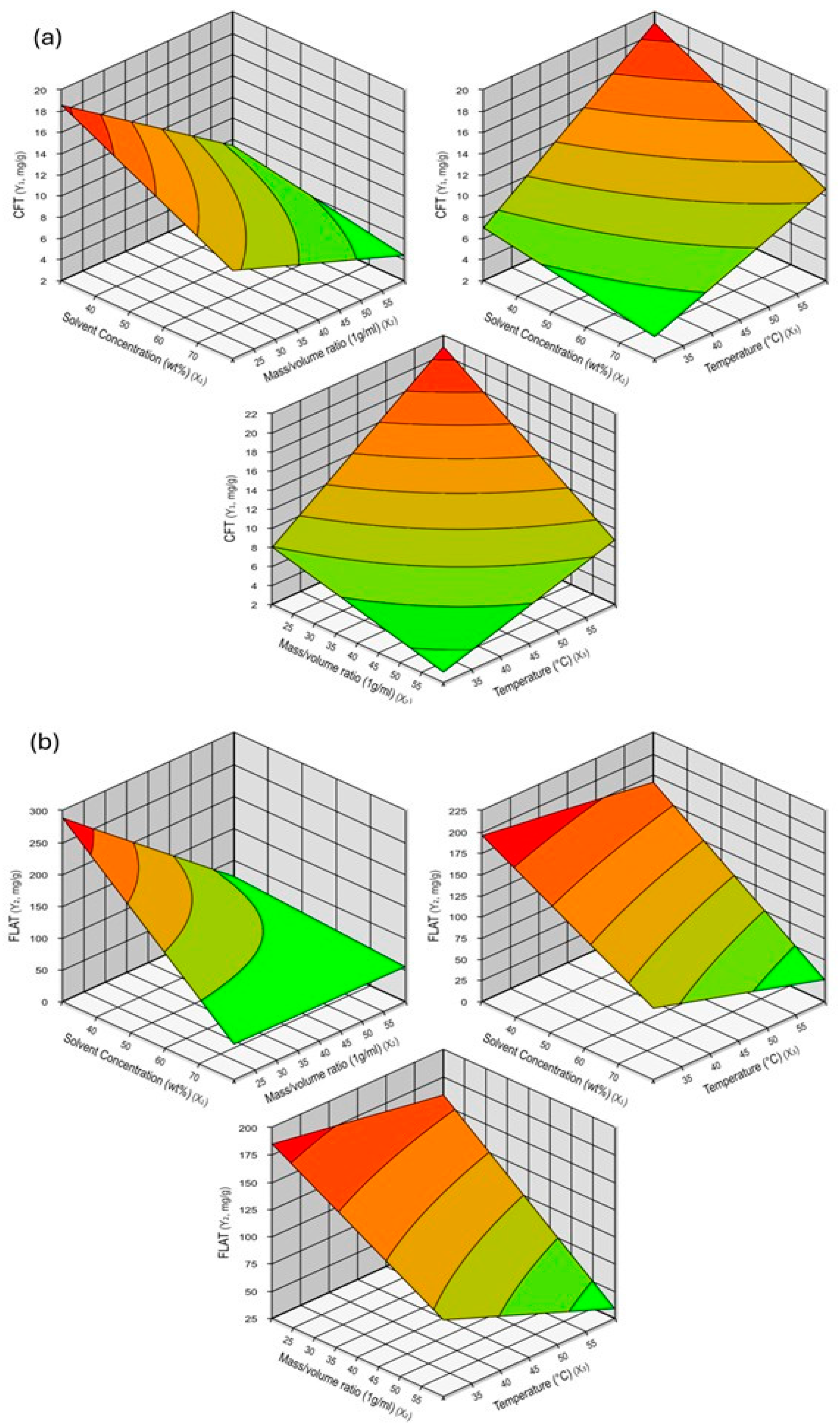
3.1. Extraction by Full Factorial Design (FFD)
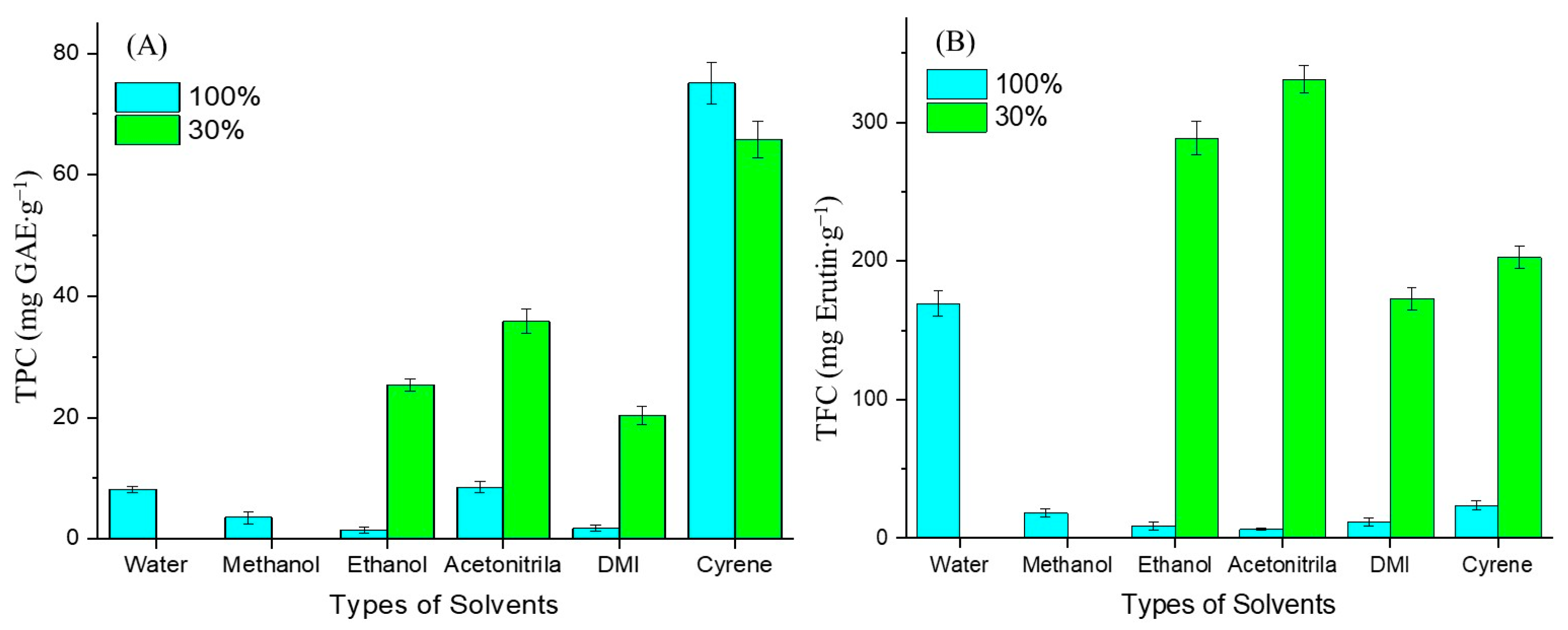
3.2. Effect of Solvent Type and Polarity
3.3. Comparative Study of Extraction Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lisboa, M.C.; Wiltshire, F.M.S.; Fricks, A.T.; Dariva, C.; Carrière, F.; Lima, Á.S.; Soares, C.M.F. Oleochemistry potential from Brazil northeastern exotic plants. Biochimie 2020, 178, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.A.; Barbosa, M.S.; Santos, J.C.B.; Lisboa, M.C.; Souza, R.L.; Pereira, M.M.; Lima, A.S.; Soares, C.M.F. Computational and experimental analysis on the preferential selectivity of lipases for triglycerides in Licuri oil. Bio. Biosyst. Eng. 2021, 44, 2141–2151. [Google Scholar] [CrossRef] [PubMed]
- Bagaldo, A.R.; Miranda, G.S.; Soares Júnior, M.S.F.; de Araújo, F.L.; Matoso, R.V.M.; Chizzotti, M.L.; Bezerra, L.R.; Oliveira, R.L. Effect of Licuri cake supplementation on performance, digestibility, ingestive behavior, carcass traits and meat quality of grazing lambs. Small Rumin. Res. 2019, 177, 18–24. [Google Scholar] [CrossRef]
- Silva, L.F.; Barbosa, A.M.; da Silva Júnior, J.M.; Oliveira, V.d.S.; Gouvêia, A.A.L.; Silva, T.M.; Lima, A.G.V.d.O.; Nascimento, T.V.C.; Bezerra, L.R.; Oliveira, R.L. Growth, physicochemical properties, fatty acid composition and sensorial attributes from longissumus lumborum of young bulls fed diets with containing licuri cake: Meat quality of bulls fed licuri cake. Livest. Sci. 2022, 255, 104775. [Google Scholar] [CrossRef]
- Belkacemi, L.; Belalia, M.; Djendara, A.; Bouhadda, Y. Antioxidant and antibacterial activities and identification of bioactive compounds of various extracts of Caulerpa racemosa from Algerian coast. Asian Pac. J. Trop. Biomed. 2020, 10, 87–94. [Google Scholar] [CrossRef]
- Silva, A.R.C.; Soares, L.R.C.; Lima, A.S.; Soares, C.M.F.; Souza, R.L. Strategies to Reuse of Biocatalysts in the Hydrolysis and Esterification Reactions of Licuri (Syagrus coronata (Mart.) Becc.) Oil. ChemCatChem 2022, 14, e202200448. [Google Scholar] [CrossRef]
- Belviso, S.; Ghirardello, D.; Giordano, M.; Sousa Ribeiro, G.; de Souza Alves, J.; Parodi, S.; Risso, S.; Zeppa, G. Phenolic composition, antioxidant capacity and volatile compounds of licuri (Syagrus coronata (Martius) Beccari) fruits as affected by the traditional roasting process. Food Res. Int. 2013, 51, 39–45. [Google Scholar] [CrossRef]
- Ferreira, W.A.; Aguiar, G.S.; Pessoa, H.R.; Costa, D.C.F.; Zago, L. Potencial antitumoral dos compostos fenólicos de produtos da oliveira (Olea europaea L.): Uma revisão integrativa da literatura. Res. Soc. Dev. 2021, 10, e22101320733. [Google Scholar] [CrossRef]
- Habibi, F.; Ramezanian, A. Vacuum infiltration of putrescine enhances bioactive compounds and maintains quality of blood orange during cold storage. Food Chem. 2017, 227, 1–8. [Google Scholar] [CrossRef]
- Souza, J.V.d.; Oliveira, A.P.D.d.; Ferrari, I.d.S.; Miyasato, I.F.; Carrijo, K.d.F.; Schwan, R.F.; Dias, F.S. Autochthonous and commercial cultures with functional properties in goat milk supplemented with licuri fruit. Food Biosci. 2020, 35, 100585. [Google Scholar] [CrossRef]
- Rispail, N.; Phillip, M.; Judith, W. Phenolic compounds: Extraction and analysis. In Lotus Japonicus Handbook; Springer: Berlin/Heidelberg, Germany, 2005; pp. 349–354. [Google Scholar]
- Alamu, E.O.; Maziya-Dixon, B.; Menkir, A.; Irondi, E.A.; Olaofe, O. Bioactive Composition and Free Radical Scavenging Activity of Fresh Orange Maize Hybrids: Impacts of Genotype, Maturity Stages, and Processing Methods. Front. Nutr. 2021, 8, 640563. [Google Scholar] [CrossRef]
- Almeida, C.L.F.; Brito, S.A.; De Santana, T.I.; Costa, H.B.A.; De Carvalho Júnior, C.H.R.; Da Silva, M.V.; De Almeida, L.L.; Rolim, L.A.; Dos Santos, V.L.; Wanderley, A.G.; et al. Spondias purpurea L. (Anacardiaceae): Antioxidant and antiulcer activities of the leaf hexane extract. Oxidative Med. Cell. Longev. 2017, 2017, 6593073. [Google Scholar] [CrossRef] [PubMed]
- Sameh, S.; Al-Sayed, E.; Labib, R.M.; Singab, A.N. Genus Spondias: A Phytochemical and Pharmacological Review. Evid. -Based Complement. Altern. Med. 2018, 12, 5382904–5382917. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Rombaut, N.; Sicaire, A.G.; Meullemiestre, A.; Fabiano-Tixier, A.S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochemistry 2017, 34, 540–560. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.; Gomes, P.G.C.; Buarque, F.S.; Soares, C.M.F.; Bjerk, T.R.; Lima, Á.S. Novel strategy for extraction and partitioning of phenol compounds from industrial residue of seriguela (Spondia purpurea L. ) using aqueous two-phase systems. Food Bioprod. Process. 2023, 141, 219–229. [Google Scholar] [CrossRef]
- Đurović, S.; Nikolić, B.; Luković, N.; Jovanović, J.; Stefanović, A.; Šekuljica, N.; Mijin, D.; Knežević-Jugović, Z. The impact of high-power ultrasound and microwave on the phenolic acid profile and antioxidant activity of the extract from yellow soybean seeds. Ind. Crops Prod. 2018, 122, 223–231. [Google Scholar] [CrossRef]
- Benítez, V.; Esteban, R.M.; Moniz, E.; Casado, N.; Aguilera, Y.; Mollá, E. Breads fortified with wholegrain cereals and seeds as source of antioxidant dietary fibre and other bioactive compounds. J. Cereal Sci. 2018, 82, 113–120. [Google Scholar] [CrossRef]
- Gil-Martín, E.; Forbes-Hernández, T.; Romero, A.; Cianciosi, D.; Giampieri, F.; Battino, M. Influence of the extraction method on the recovery of bioactive phenolic compounds from food industry by-products. Food Chem. 2022, 378, 131918. [Google Scholar] [CrossRef]
- Frauholz, J.; Roth, H.; Wessling, M. Seawater desalination by forward-osmosis-assisted temperature swing solvent extraction. Desalination 2023, 564, 116697. [Google Scholar] [CrossRef]
- Santos, A.G.; Buarque, F.S.; Ribeiro, B.D.; Coelho, M.A.Z. Extractive fermentation for the production and partitioning of lipase and citric acid by Yarrowia lipolytica. Process Biochem. 2022, 122, 374–385. [Google Scholar] [CrossRef]
- Buarque, F.S.; Guimarães, D.E.; Soares, C.M.; Souza, R.L.; Pereira, M.M.; Lima, Á.S. Ethanolic two-phase system formed by polypropylene glycol, ethylene glycol and/or ionic liquid (phase-forming or adjuvant) as a platform to phase separation and partitioning study. J. Mol. Liq. 2021, 344, 117702. [Google Scholar] [CrossRef]
- Stini, N.A.; Gkizis, P.L.; Kokotos, C.G. Cyrene: A bio-based novel and sustainable solvent for organic synthesis. Green Chem. 2022, 24, 6435–6449. [Google Scholar] [CrossRef]
- Brouwer, T.; Schuur, B. Dihydrolevoglucosenone (Cyrene), a Biobased Solvent for Liquid-Liquid Extraction Applications. ACS Sustain. Chem. Eng. 2020, 8, 14807–14817. [Google Scholar] [CrossRef]
- Kong, D.; Dolzhenko, A.V. Cyrene: A bio-based sustainable solvent for organic synthesis. Sustain. Chem. Pharm. 2022, 25, 100591. [Google Scholar] [CrossRef]
- Sherwood, J.; De bruyn, M.; Constantinou, A.; Moity, L.; McElroy, C.R.; Farmer, T.J.; Duncan, T.; Raverty, W.; Hunt, A.J.; Clark, J.H. Dihydrolevoglucosenone (Cyrene) as a bio-based alternative for dipolar aprotic solvents. Chem. Commun. 2014, 50, 9650–9652. [Google Scholar] [CrossRef] [PubMed]
- Russo, F.; Galiano, F.; Pedace, F.; Aricò, F.; Figoli, A. Dimethyl Isosorbide As a Green Solvent for Sustainable Ultrafiltration and Microfiltration Membrane Preparation. ACS Sustain. Chem. Eng. 2020, 8, 659–668. [Google Scholar] [CrossRef]
- Yang, S.; Yang, X.; Meng, X.; Wang, L. Efficient pretreatment using dimethyl isosorbide as a biobased solvent for potential complete biomass valorization. Green Chem. 2020, 24, 4082–4094. [Google Scholar] [CrossRef]
- Hou, Q.; Li, W.; Ju, M.; Liu, L.; Chen, Y.; Yang, Q.; Wang, J. Separation of polysaccharides from rice husk and wheat bran using solvent system consisting of BMIMOAc and DMI. Carbohydr. Polym. 2015, 133, 517–523. [Google Scholar] [CrossRef]
- Nemes, S.M.; Orsat, V. Screening the experimental domain for the microwave-assisted extraction of secoisolariciresinol diglucoside from flaxseed prior to optimization procedures. Food Bioproc. Tech. 2010, 3, 300–307. [Google Scholar] [CrossRef]
- Suktham, K.; Daisuk, P.; Shotipruk, A. Microwave-assisted extraction of antioxidative anthraquinones from roots of Morinda citrifolia L. (Rubiaceae): Errata and review of technological development and prospects. Sep. Purif. Technol. 2021, 256, 117844. [Google Scholar] [CrossRef]
- Mahindrakar, K.V.; Rathod, V.K. Ultrasonic assisted aqueous extraction of catechin and gallic acid from Syzygium cumini seed kernel and evaluation of total phenolic, flavonoid contents and antioxidant activity. Chem. Eng. Process. Process Intensif. 2020, 149, 107841. [Google Scholar] [CrossRef]
- Barbosa, A.M.; Santos, K.S.; Borges, G.R.; Muniz, A.V.C.S.; Mendonça, F.M.R.; Pinheiro, M.S.; Franceschi, E.; Dariva, C.; Padilha, F.F. Separation of antibacterial biocompounds from Hancornia speciosa leaves by a sequential process of pressurized liquid extraction. Sep. Purif. Technol. 2019, 222, 390–395. [Google Scholar] [CrossRef]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Mané, C.; Souquet, J.M.; Ollé, D.; Verriés, C.; Véran, F.; Mazerolles, G.; Cheynier, V.; Fulcrand, H. Optimization of simultaneous flavanol, phenolic acid, and anthocyanin extraction from grapes using an experimental design: Application to the characterization of champagne grape varieties. J. Agric. Food Chem. 2007, 55, 7224–7233. [Google Scholar] [CrossRef]
- Xu, B.J.; Chang, S.K.C. A comparative study on phenolic profiles and antioxidant activities of legumes as affected by extraction solvents. J. Food Sci. 2007, 72, 159–166. [Google Scholar] [CrossRef]
- Gomes, S.; Torres, A.G. Optimized extraction of polyphenolic antioxidant compounds from Brazil nut (Bertholletia excelsa) cake and evaluation of the polyphenol profile by HPLC. J. Sci. Food Agric. 2016, 96, 2805–2814. [Google Scholar] [CrossRef]
- Santos, M.H.O.; Simionato, J.I.; Gualberto, S.A.; Santana, R.F.; Silva, M.H.S. Quantificação de compostos bioativos da farinha de licuri: Fenólicos totais e fibras. Agrar. Acad. 2014, 1, 150–157. [Google Scholar] [CrossRef]
- Aires, G.C.M.; de Carvalho Junior, R.N. Potential of Supercritical Acrocomia aculeata Oil and Its Technology Trends. Appl. Sci. 2023, 13, 8594–8606. [Google Scholar] [CrossRef]
- Meireles, B.R.L.d.A.; Alcantara, M.A.; Brito, I.d.L.; Lima, R.P.; Sousa, A.S.B.d.; Cordeiro, A.M.T.M. Aspectos físico-químicos e qualidade nutricional do coco catolé (Syagrus cearensis). Res. Soc. Dev. 2020, 9, 133973822. [Google Scholar] [CrossRef]
- Castro-Puyana, M.; Marina, M.L.; Plaza, M. Water as green extraction solvent: Principles and reasons for its use. Curr. Opin. Green Sustain. Chem. 2017, 5, 31–36. [Google Scholar] [CrossRef]
- ChemSpider at. Available online: https://www.chemspider.com/ (accessed on 23 June 2023).
- Silva, S.S.; Justi, M.; Chagnoleau, J.B.; Papaiconomou, N.; Fernandez, X.; Santos, S.A.O.; Passos, H.; Ferreira, A.M.; Coutinho, J.A.P. Using biobased solvents for the extraction of phenolic compounds from kiwifruit industry waste. Sep. Purif. Technol. 2023, 304, 122344. [Google Scholar] [CrossRef]
- Moura Filho, J.M.; Nascimento, L.C.S.; Nagai, L.Y.N.; Cavalcante Neto, A.A.; Penna, A.L.B. Determinação do solvente ótimo para extração dos compostos fenólicos do fruto de buriti. Braz. J. Food Res. 2017, 8, 22–28. [Google Scholar]
- Beltran, L.B.; Souza, A.C.; Meloni, C.E.P.; Magiero, P.E.; Bergamasco, R.; Vieira, A.M.S. Evaluation of antioxidant capacity and technology properties of fruit flour of Palm Aiphanes Aculeata. Braz. J. Dev. 2021, 7, 36868–36884. [Google Scholar] [CrossRef]
- Flores-Jiménez, N.T.; Ulloa, J.A.; Silvas, J.E.U.; Ramírez, J.C.R.; Ulloa, P.R.; Rosales, P.U.B.; Carrillo, Y.S.; Leyva, R.G. Effect of high-intensity ultrasound on the compositional, physicochemical, biochemical, functional and structural properties of canola (Brassica napus L.) protein isolate. Food Res. Int. 2019, 121, 947–956. [Google Scholar] [CrossRef]
- Ismail, B.B.; Yusuf, H.L.; Pu, Y.; Zhao, H.; Guo, M.; Liu, D. Ultrasound-assisted adsorption/desorption for the enrichment and purification of flavonoids from baobab (Adansonia digitata) fruit pulp. Ultrason. Sonochemistry 2020, 65, 104980. [Google Scholar] [CrossRef]
- Priego-Capote, F.; Luque De Castro, M.D. Analytical uses of ultrasound I. Sample preparation. TrAC—Trends Anal. Chem. 2004, 23, 644–653. [Google Scholar] [CrossRef]
- Veillet, S.; Tomao, V.; Chemat, F. Ultrasound assisted maceration: An original procedure for direct aromatisation of olive oil with basil. Food Chem. 2010, 123, 905–911. [Google Scholar] [CrossRef]



| Independent Variables | Levels | |||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| Solvent concentration (wt%) | X1 | 30 | 55 | 80 |
| Mass/volume ratio (1 g.mL−1) | X2 | 20 | 40 | 60 |
| Temperature (°C) | X3 | 30 | 45 | 60 |
| Entry | Conditions | Response Variables (mg·g) | |||
|---|---|---|---|---|---|
| ACN (wt%) | Biomass/Solvent Ratio | T (°C) | TPC | TFC | |
| 1 | (−1) 30 | (−1) 20 | (−1) 30 | 25.00 | 304.87 |
| 2 | (1) 80 | (−1) 20 | (−1) 30 | 12.50 | 54.83 |
| 3 | (−1) 30 | (1) 60 | (−1) 30 | 9.36 | 57.53 |
| 4 | (1) 80 | (1) 60 | (−1) 30 | 3.89 | 20.71 |
| 5 | (−1) 30 | (−1) 20 | (1) 60 | 35.84 | 331.10 |
| 6 | (1) 80 | (−1) 20 | (1) 60 | 24.71 | 117.53 |
| 7 | (−1) 30 | (1) 60 | (1) 60 | 19.48 | 298.75 |
| 8 | (1) 80 | (1) 60 | (1) 60 | 9.73 | 144.48 |
| 9 | (0) 55 | (0) 40 | (0) 45 | 11.60 | 190.52 |
| 10 | (0) 55 | (0) 40 | (0) 45 | 11.61 | 191.43 |
| 11 | (0) 55 | (0) 40 | (0) 45 | 11.60 | 172.85 |
| Entry | Conditions | Response Variables (mg/g) | |||
|---|---|---|---|---|---|
| EtOH (wt%) | Biomass/Solvent Ratio | T (° C) | TPC | TFC | |
| 1 | (−1) 30 | (−1) 20 | (−1) 30 | 9.19 | 282.77 |
| 2 | (1) 80 | (−1) 20 | (−1) 30 | 4.25 | 82.49 |
| 3 | (−1) 30 | (1) 60 | (−1) 30 | 2.24 | 104.16 |
| 4 | (1) 80 | (1) 60 | (−1) 30 | 1.33 | 81.38 |
| 5 | (−1) 30 | (−1) 20 | (1) 60 | 25.37 | 288.72 |
| 6 | (1) 80 | (−1) 20 | (1) 60 | 13.80 | 23.76 |
| 7 | (−1) 30 | (1) 60 | (1) 60 | 10.02 | 39.91 |
| 8 | (1) 80 | (1) 60 | (1) 60 | 4.90 | 53.52 |
| 9 | (0) 55 | (0) 40 | (0) 45 | 13.50 | 123.52 |
| 10 | (0) 55 | (0) 40 | (0) 45 | 13.50 | 123.50 |
| 11 | (0) 55 | (0) 40 | (0) 45 | 13.50 | 123.49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inocêncio, E.S.; Buarque, F.S.; Ferreira, L.F.R.; Soares, C.M.F.; Lima, Á.S.; Souza, R.L.d. Exploring the Potential of Licuri (Syagrus coronata) Using Sustainable Techniques and Solvents for Extracting Bioactive Compounds. Sustainability 2025, 17, 1507. https://doi.org/10.3390/su17041507
Inocêncio ES, Buarque FS, Ferreira LFR, Soares CMF, Lima ÁS, Souza RLd. Exploring the Potential of Licuri (Syagrus coronata) Using Sustainable Techniques and Solvents for Extracting Bioactive Compounds. Sustainability. 2025; 17(4):1507. https://doi.org/10.3390/su17041507
Chicago/Turabian StyleInocêncio, Emília Silva, Filipe Smith Buarque, Luiz F. R. Ferreira, Cleide M. F. Soares, Álvaro S. Lima, and Ranyere Lucena de Souza. 2025. "Exploring the Potential of Licuri (Syagrus coronata) Using Sustainable Techniques and Solvents for Extracting Bioactive Compounds" Sustainability 17, no. 4: 1507. https://doi.org/10.3390/su17041507
APA StyleInocêncio, E. S., Buarque, F. S., Ferreira, L. F. R., Soares, C. M. F., Lima, Á. S., & Souza, R. L. d. (2025). Exploring the Potential of Licuri (Syagrus coronata) Using Sustainable Techniques and Solvents for Extracting Bioactive Compounds. Sustainability, 17(4), 1507. https://doi.org/10.3390/su17041507






