An Analysis of BTEX Occurrence in Stored Rainwater and Rainwater Runoff in Urban Environment
Abstract
:1. Introduction
2. BTEX: Characteristics and Occurrence
2.1. Benzene
2.2. Toluene
2.3. Ethylbenzene
2.4. Xylene
2.5. The Occurrence of BTEX in Rainwater and Stormwater—Previous Studies
2.6. BTEX in Urban Rainwater and Stormwater—Sources and Causes of Occurrence
3. Materials and Methods
- -
- Sheet metal (including sheet metal with bituminous elements used for repairs);
- -
- Terraces made of terracotta;
- -
- Roofs made of ceramic tiles;
- -
- Corrugated polyester boards reinforced with glass fibre;
- -
- Extensive green roofs (after passing through the green roof layers).
- -
- In the city centre;
- -
- In the district located further from the city centre;
- -
- On the outskirts of the city but close to the approach path of airplanes to the airport.
- -
- Via a tank tap (in the case of large closed tanks equipped with a tap);
- -
- By submerging a bottle (or a beaker in the case of small container/tank) under the water table (in the case of open tanks).
- -
- Mass balance for each substance of BTEX was calculated based on the general mass balance equations [69], and for this study, it is as follows:
- -
- Absorption equilibrium equation (Henry’s law [69]):
4. Results
5. Analysis and Discussion of the Results
6. Summary and Conclusions
- -
- It has been shown that with the methodology used, it is possible to confirm the presence of BTEX in stored rainwater/rainwater runoff from the roof/terrace surfaces in the city in various locations (from the city centre to the outskirts);
- -
- In some locations in the city, it is possible that BTEX compounds may occur in stored rainwater/rainwater runoff;
- -
- The recorded TEX (without benzene) concentrations are relatively low compared to the values of acceptable limits for BTEX in drinking water;
- -
- The subject of future research on the occurrence of BTEX compounds in stored rainwater/rainwater runoff should be in particular the factors on which residents have a measurable impact;
- -
- If possible, it is advisable to use rainwater tanks with a drinking water approval certificate;
- -
- Due to the possibility of benzene presence, a GAC filter should be provided if the water is intended for, e.g., hydroponics or aquaponics.
- -
- Studies of the possible impact of the tank material (or factors related to the installation of the tank);
- -
- Deepening the current research on the impact of the tank’s exposure (and runoff surface’s) to a gas station, airports, traffic routes and other facilities that may affect the content of BTEX;
- -
- Investigation of the role of components acting as a shield (the height of surrounding buildings/fencing walls/green belts should be taken into account);
- -
- Further research on the quality of water from the green roof;
- -
- Confirmation of obtaining BTEX concentration after treatment in the adsorption process on GAC below the normative values for drinking water.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shepherd, N. Making Sense of “Day Zero”: Slow Catastrophes, Anthropocene Futures, and the Story of Cape Town’s Water Crisis. Water 2019, 11, 1744. [Google Scholar] [CrossRef]
- Bąk, J. The Use of Precipitation in the Cities of the Future—Problems, Barriers and Challenges. Sustainability 2023, 15, 14381. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, Y.; Xu, H.; Wang, S.; Long, T.; Zhao, Q. Evaluation of Precipitation Frequency and Intensity as Estimated by the GPM IMERG Precipitation Product at Daily and Hourly Scales over the Tibetan Plateau. Atmosphere 2023, 14, 1653. [Google Scholar] [CrossRef]
- Polkowska, Ż. Precipitation and atmospheric deposits: Problems and challenges. Ed. 609. In Zeszyty Naukowe Politechnika Gdańska; Wydawnictwo Politechniki Gdańskiej: Gdańsk, Poland, 2008. [Google Scholar]
- Wojciechowska, E. The Use of Green Infrastructure to Reduce Surface Water Pollution in the Urban Catchment; Monografie Komitetu Inżynierii Środowiska Polskiej Akademii Nauk; Polska Akademia Nauk: Gdańsk, Poland, 2018; Volume 145. (In Polish) [Google Scholar]
- Polkowska, Ż. Pesticides in precipitation-a review of data from 1975 to 2001. Chem. I Inżynieria Ekol. 2006, 13, 1331–1343. [Google Scholar]
- Lode, O.; Eklo, O.M.; Holen, B.; Svensen, A.; Johnsen, A.M. Pesticides in precipitation in Norway. Sci. Total Environ. 1995, 160–161, 421–431. [Google Scholar] [CrossRef]
- Sakson, G.; Zawilski, M.; Badowska, E.; Brzezińska, A. Zanieczyszczenie ścieków opadowych jako podstawa wyboru sposobu ich zagospodarowania. J. Civ. Eng. Environ. Archit. 2014, XXXI, 253–264. [Google Scholar]
- Nawrot, N.; Wojciechowska, E. Assessment of trace metals leaching during rainfall events from building rooftops with different types of coverage-Case study. J. Ecol. Eng. 2018, 19, 45–51. [Google Scholar] [CrossRef]
- Zdeb, M.; Zamorska, J.; Papciak, D.; Słyś, D. The Quality of Rainwater Collected from Roofs and the Possibility of Its Economic Use. Resources 2020, 9, 12. [Google Scholar] [CrossRef]
- Bąk, J.; Mucha, Z.; Rybicki, S.M. Identification of potential water sources for water sharing in multi-family buildings in cities for hydroponics-opportunities, challenges and barriers, manuscript, under review process. Sustainability 2025. under review. [Google Scholar]
- Gromaire-Mertz, M.C.; Garnaud, S.; Gonzalez, A.; Chebbo, G. Characterization of urban runoff pollution in Paris. Water Sci. Technol. 1999, 2, 1–8. [Google Scholar] [CrossRef]
- Gnecco, I.; Beretta, C.; Lanza, L.G.; La Barbera, P. Storm water pollution in the urban environment of Genoa, Italy. Atmos. Res. 2005, 77, 60–73. [Google Scholar] [CrossRef]
- Fitobór, K. Purification of Rainwater in Terms of Their Economic Use. Ph.D. Thesis, Politechnika Gdańska, Wydział Inżynierii Lądowej i Środowiska, Gdańsk, Poland, 2018. (In Polish). [Google Scholar]
- Hamilton, K.; Reyneke, B.; Waso, M.; Clements, T.; Ndlovu, T.; Khan, W.; DiGiovanni, K.; Rakestraw, E.; Montalto, F.; Haas, C.N.; et al. A global review of the microbiological quality and potential health risks associated with roof-harvested rainwater tanks. NPJ Clean. Water 2019, 2, 7. [Google Scholar] [CrossRef]
- Hong, N.; Liu, A.; Zhu, P.; Zhao, X.; Guan, Y.; Yang, M.; Wang, H. Modelling benzene series pollutants (BTEX) build-up loads on urban roads and their human health risk: Implications for stormwater reuse safety. Ecotoxicol. Environ. Saf. 2018, 164, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Bian, Y.; Zhou, Y.; Zhou, Y.; Feng, X.-S. BTEX in the environment: An update of sources, fate, distribution, pretreatment, analysis, and removal techniques. Chem. Eng. J. 2022, 435, 134825. [Google Scholar] [CrossRef]
- Seńczuk, W. (Ed.) Toxicology. A Textbook for Pharmacy Students; Wydawnictwo Lekarskie PZWL: Warsaw, Poland, 1994. (In Polish) [Google Scholar]
- Piotrowski, J.K. (Ed.) Fundamentals of Toxicology: A Compendium for Higher Education Students; Wydawnictwo WNT: Warsaw, Poland, 2017. (In Polish) [Google Scholar]
- CDC, Facts About Benzene. Available online: https://emergency.cdc.gov/agent/benzene/basics/facts.asp (accessed on 1 February 2024).
- CDC, Benzene, Chemical Fact Sheet. Available online: https://www.cdc.gov/chemical-emergencies/chemical-fact-sheets/benzene.html (accessed on 21 December 2024).
- Health Council of The Netherlands. Benzene-Health-Based Recommended Occupational Exposure Limit; Health Council of The Netherlands: The Hague, The Netherlands, 2014; Publication No. 2014/03. [Google Scholar]
- Szymańska, J.; Frydrych, B.; Brauchajzer, E.; Benzene. Documentation of Proposed Values of Occupational Exposure Limits (OELs); Podstawy i Metody Oceny Środowiska Pracy: Warsaw, Poland, 2022; no. 3(113); pp. 21–117. (In Polish) [Google Scholar]
- Seńczuk, W. (Ed.) Contemporary Toxicology; PZWL: Warsaw, Poland, 2020. (In Polish) [Google Scholar]
- Bogdanik, T. (Ed.) Clinical Toxicology; PZWL: Warsaw, Poland, 1988. [Google Scholar]
- Directive (EU) 2022/431 of the European Parliament and of the Council of 9 March 2022 Amending Directive 2004/37/EC on the Protection of Workers from the Risks Related to Exposure to Carcinogens or Mutagens at Work, OJ L 88, 16.3.2022. pp. 1–14. Available online: https://data.europa.eu/eli/dir/2022/431/oj (accessed on 19 December 2024).
- Directive 2004/37/EC of the European Parliament and of the Council of 29 April 2004 on the Protection of Workers from the Risks Related to Exposure to Carcinogens or Mutagens at Work (Sixth Individual Directive Within the Meaning of Article 16(1) of Council Directive 89/391/EEC) (Codified Version) (Text with EEA Relevance), OJ L 158, 30.4.2004. pp. 50–76. Available online: https://data.europa.eu/eli/dir/2004/37/oj (accessed on 19 December 2024).
- Rappaport, S.M.; Kim, S.; Lan, Q.; Vermeulen, R.; Waidyanatha, S.; Zhang, L.; Li, G.; Yin, S.; Hayes, R.B.; Rothman, N.; et al. Evidence That Humans Metabolize Benzene via Two Pathways. Environ. Health Perspect. 2009, 117, 946–952. [Google Scholar] [CrossRef]
- Rappaport, S.M.; Kim, S.; Lan, Q.; Li, G.; Vermeulen, R.; Waidyanatha, S.; Zhang, L.; Yin, S.; Smith, M.T.; Rothman, N. Human benzene metabolism following occupational and environmental exposures. Chem.-Biol. Interact. 2010, 184, 189–195. [Google Scholar] [CrossRef]
- Available online: https://wwwn.cdc.gov/TSP/substances/ToxSubstance.aspx?toxid=29 (accessed on 19 December 2024).
- Wang, A.; Dangleben, N.L.; Dodge, D.E.; Silva, R.M. Toluene Reference Exposure Levels Technical Support Document for the Derivation of Noncancer Reference Exposure Levels Appendix D1; Office of Environmental Health Hazard Assessment: Sacramento, CA, USA, 2020. [Google Scholar]
- National Health and Medical Research Council; National Resource Management Ministerial Council. Australian Drinking Water Guidelines 6, 2011; National Water Quality Management Strategy, Commonwealth of Australia: Canberra, Australia, 2022. [Google Scholar]
- U.S. Department of Health & Human Services. Toxicological Profile for Total Xylenes; Prepared by Clement Associates, Inc. Under Contract No. 205-88-0608, Prepared for Agency for Toxic Substances and Disease Registry; U.S. Public Health Service: Atlanta, GA, USA, 1990. [Google Scholar]
- U.S. Environmental Protection Agency. Locating and Estimating Air Emissions from Sources of Xylene; EPA-454/R-93-048; Emission Inventory Branch, Technical Support Division, Office of Air Quality Planning and Standards: Research Triangle Park, USA, 1994. [Google Scholar]
- Sikora, J.; Niemiec, M.; Szeląg–Sikora, A.; Kuboń, M. The Content of Heavy Metals in Rainwater Flowing from Roofs with Different Coatings. Annu. Set. Environ. Prot. 2018, 20, 1079–1094. [Google Scholar]
- Beecham, S.; Razzaghmanesh, M. Water quality and quantity investigation of green roofs in a dry climate. Water Res. 2015, 70, 370–384. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraghavan, K.; Joshi, U.M. Can green roof act as a sink for contaminants? A methodological study to evaluate runoff quality from green roofs. Environ. Pollut. 2014, 194, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.; McBroom, M.W.; Beasley, R.S. Roofing as a source of nonpoint water pollution. J. Environ. Manag. 2004, 73, 307–315. [Google Scholar] [CrossRef]
- Pęczkowski, G.; Szawernoga, K.; Kowalczyk, T.; Orzepowski, W.; Pokładek, R. Runoff and Water Quality in the Aspect of Environmental Impact Assessment of Experi-mental Area of Green Roofs in Lower Silesia. Sustainability 2020, 12, 4793. [Google Scholar] [CrossRef]
- Olivella, M.A. Polycyclic aromatic hydrocarbons in rainwater and surface waters of Lake Maggiore, a subalpine lake in Northern Italy. Chemosphere 2006, 63, 116–131. [Google Scholar] [CrossRef] [PubMed]
- Konan, B.R.; Yoboue, V.; Adiaffi, B.; Diaby, M.; Oga, Y.M.S.; Bakayoko, A.; Gnamien, S.; Keita, S.; Bahino, J.; Ossohou, M. Source of polycyclic aromatic hydrocarbons (PAHs) in rainwater and effect on the health of the population: The case of the District of Abidjan in the South of Ivory Coast. J. Water Health 2022, 20, 985–1004. [Google Scholar] [CrossRef] [PubMed]
- Watts, A.W.; Ballestero, T.P.; Roseen, R.M.; Houle, J.P. Polycyclic Aromatic Hydrocarbons in Stormwater Runoff from Sealcoated Pavements. Environ. Sci. Technol. 2010, 44, 8849–8854. [Google Scholar] [CrossRef]
- Rodrigo-Ilarri, J.; Rodrigo-Clavero, M.-E.; Capilla, J.E.; Romero-Ballesteros, L. Environmental Assessment of Soil and Groundwater Pollution by BTEX Leaching in Valencia Region (Spain). Water 2023, 15, 3279. [Google Scholar] [CrossRef]
- Davis, G.B.; Barber, C.; Power, T.R.; Thierrin, J.; Patterson, B.M.; Rayner, J.L.; Wu, O. The variability and intrinsic remediation of a BTEX plume in anaerobic sulphate-rich groundwater. J. Contam. Hydrol. 1999, 36, 265–290. [Google Scholar] [CrossRef]
- Asejeje, G.I.; Ipeaiyeda, A.R.; Onianwa, P.C. Occurrence of BTEX from petroleum hydrocarbons in surface water, sediment, and biota from Ubeji Creek of Delta State, Nigeria. Environ. Sci. Pollut. Res. 2021, 28, 15361–15379. [Google Scholar] [CrossRef]
- Kerchich, Y.; Kerbachi, R. Measurement of BTEX (benzene, toluene, ethybenzene, and xylene) levels at urban and semirural areas of Algiers City using passive air samplers. J. Air Waste Manag. Assoc. 2012, 62, 1370–1379. [Google Scholar] [CrossRef]
- Rad, H.D.; Babaei, A.A.; Goudarzi, G.; Angali, K.A.; Ramezani, Z.; Mohammadi, M.M. Levels and sources of BTEX in ambient air of Ahvaz metropolitan city. Air Qual. Atmos. Health 2014, 7, 515–524. [Google Scholar] [CrossRef]
- Abtahi, M.; Fakhri, Y.; Oliveri Conti, G.; Ferrante, M.; Taghavi, M.; Tavakoli, J.; Heshmati, A.; Keramati, H.; Moradi, B.; Amanidaz, N.; et al. The Concentration of BTEX in the Air of Tehran: A Systematic Review-Meta Analysis and Risk Assessment. Int. J. Environ. Res. Public Health 2018, 15, 1837. [Google Scholar] [CrossRef] [PubMed]
- Correa, S.M.; Arbilla, G.; Marques, M.R.C.; Oliveira, K.M.P.G. The impact of BTEX emissions from gas stations into the atmosphere. Atmos. Pollut. Res. 2012, 3, 163–169. [Google Scholar] [CrossRef]
- Mahbub, P.; Goonetilleke, A.; Ayoko, G. Prediction model of the buildup of volatile organic compounds on urban roads. Environ. Sci. Technol. 2011, 45, 4453–4459. [Google Scholar] [CrossRef]
- Mahbub, P.; Goonetilleke, A.; Ayoko, G.; Egodawatta, P.; Tan, Y. Analysis of build-up of heavy metals and volatile organics on urban roads in Gold Coast, Australia. Water Sci. Technol. 2011, 63, 2077–2085. [Google Scholar] [CrossRef]
- Mahbub, P.; Goonetilleke, A.; Ayoko, G.A.; Egodawatta, P. Effects of climate change on the wash-off of volatile organic compounds from urban roads. Sci. Total Environ. 2011, 409, 3934–3942. [Google Scholar] [CrossRef]
- Liu, A.; Hong, N.; Zhong, J.; Ou, G.; Yang, B. Temporal and spatial distributions of benzene series pollutants build-up on urban road surfaces. J. Shenzhen Univ. Sci. Eng. 2018, 35, 590–596. [Google Scholar] [CrossRef]
- Šoštarić, A.; Stanišić Stojić, S.; Vuković, G.; Mijić, Z.; Stojić, A.; Gržetić, I. Rainwater capacities for BTEX scavenging from ambient air. Atmos. Environ. 2017, 168, 46–54. [Google Scholar] [CrossRef]
- Janowska, B.; Szymański, K. Examples for elimination of selected groups of petroleum-derived compounds from rainwater and meltwater. Desalination Water Treat. 2014, 52, 3993–3999. [Google Scholar] [CrossRef]
- Zahed, M.A.; Pardakhti, A.; Mohajeri, L.; Bateni, F. Wet deposition of hydrocarbons in the city of Tehran-Iran. Air Qual. Atmos. Health 2010, 3, 77–82. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yu, S.; Lee, P.K.; Yun, S.T.; Hwang, S.-I.; Chae, G. Comparison of volatile organic compounds in stormwater and groundwater in Seoul metropolitan city, South Korea. Environ. Earth Sci. 2017, 76, 338. [Google Scholar] [CrossRef]
- Wijesiri, B.; Liu, A.; Goonetilleke, A. Uncertainties in the assessment of volatile hydrocarbon pollution of urban stormwater. In Proceedings of the E-Proceedings of the 38th IAHR World Congress. International Association for Hydro-Environment Engineering and Research (IAHR), Panama City, Panama, 1–6 September 2019; pp. 4571–4582. [Google Scholar]
- Mullaugh, K.M.; Hamilton, J.M.; Avery, G.B.; Felix, J.D.; Mead, R.N.; Willey, J.D.; Kieber, R.J. Temporal and spatial variability of trace volatile organic compounds in rainwater. Chemosphere 2015, 134, 203–209. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Liu, F.; Tong, L.; Li, K.; Yang, H.; Zhang, L. Volatile organic compounds in stormwater from a community of Beijing, China. Environ. Pollut. 2018, 239, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Borden, R.C.; Black, D.C.; McBlief, K.V. MTBE and aromatic hydrocarbons in North Carolina stormwater runoff. Environ. Pollut. 2002, 118, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Okochi, H.; Sugimoto, D.; Igawa, M. The enhanced dissolution of some chlorinated hydrocarbons and monocyclic aromatic hydrocarbons in rainwater collected in Yokohama, Japan. Atmos. Environ. 2004, 38, 4403–4414. [Google Scholar] [CrossRef]
- Buczynska, A.J.; Krata, A.; Stranger, M.; Locateli Godoi, A.F.; Kontozova-Deutsch, V.; Bencs, L.; Naveau, I.; Roekens, E.; Van Grieken, R. Atmospheric BTEX-concentrations in an area with intensive street traffic. Atmos. Environ. 2009, 43, 311–318. [Google Scholar] [CrossRef]
- US EPA. United States National Primary Drinking Water Standards; US EPA: Washington, DC, USA, 2003. [Google Scholar]
- Aziz, S.Q.; Alrawi, R.A.A.; Dizayee, K.K.; Khalil, C.; Shaheed, I.M.; Mustafa, J.S. Investigation of rainwater quality in Erbil Province and the feasibility of various uses. Environ. Prot. Res. 2023, 240–262. [Google Scholar] [CrossRef]
- Šoštarić, A.; Stojić, A.; Stanišić Stojić, S.; Gržetić, I. Quantification and mechanisms of BTEX distribution between aqueos and gaseous phase in a dynamic system. Chemosphere 2016, 144, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Statistical Database. Area and Population in the Territorial Profile in 2023; Statistics Poland: Warsaw, Poland, 2023. [Google Scholar]
- Pala, K. (Ed.) USAGE Urban Stormwater Aquaponics Garden Environment—Full Proposal; NCBiR: Warsaw, Poland, 2020. [Google Scholar]
- Coulson, J.M.; Richardson, J.F. Coulson and Richardson’s Chemical Engineering; Butterworth-Heinemann: Oxford, UK, 2009; Volume 1–2. [Google Scholar]
- Liu, A.; Hong, N.; Zhu, P.; Guan, Y. Understanding benzene series (BTEX) pollutant load characteristics in the urban environment. Sci. Total Environ. 2018, 619–620, 938–945. [Google Scholar] [CrossRef]
- Fayemiwo, O.M.; Daramola, M.O.; Moothi, K. Review: BTEX compounds in water–future trends and directions for water treatment. Water 2017, 43, 602–613. [Google Scholar] [CrossRef]
- NHMRC; NRMMC. Australian Drinking Water Guidelines Paper 6 National Water Quality Management Strategy; National Health and Medical Research Council, National Resource Management Ministerial Council, Commonwealth of Australia: Canberra, Australia, 2011. [Google Scholar]
- World Health Organization. Drinking Water Guidelines; WHO: Geneva, Switzerland, 2008. [Google Scholar]
- Ikem, J.O. The removal of benzene toluene, ethyl benzene and p-xylene (BTEX) from aqueous solution by granular activated carbon. Int. J. Water Resour. Environ. Eng. 2024, in press.
- Rineksa, G.; Bustomy, A.; Don-Hee, P.; Misri Gozan, M. Isotherm Coefficient for Benzene and Toluene Adsorption on Granular Activated Carbon for Preparation of Biobarrier. IOP Conf. Ser. Earth Environ. Sci. 2022, 1111, 1–6. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First and Second Addenda; WHO: Geneva, Switzerland, 2022; Available online: https://www.who.int/publications/i/item/9789240045064 (accessed on 19 December 2024).
- Nourmoradi, H.; Khiadani, M.; Nikaeen, M. Multi-Component Adsorption of Benzene, Toluene, Ethylbenzene, and Xylene from Aqueous Solutions by Montmorillonite Modified with Tetradecyl Trimethyl Ammonium Bromide. J. Chem. 2013, 2013, 589354. [Google Scholar] [CrossRef]
- Aivalioti, M.; Pothoulaki, D.; Papoulias, P.; Gidarakos, E. Removal of BTEX, MTBE and TAME from aqueous solutions by adsorption onto raw and thermally treated lignite. J. Hazard. Mater. 2012, 207–208, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Daifullah, A.A.M.; Girgis, B.S. Impact of surface characteristics of activated carbon on adsorption of BTEX. Colloids Surf. A 2003, 214, 181–193. [Google Scholar] [CrossRef]




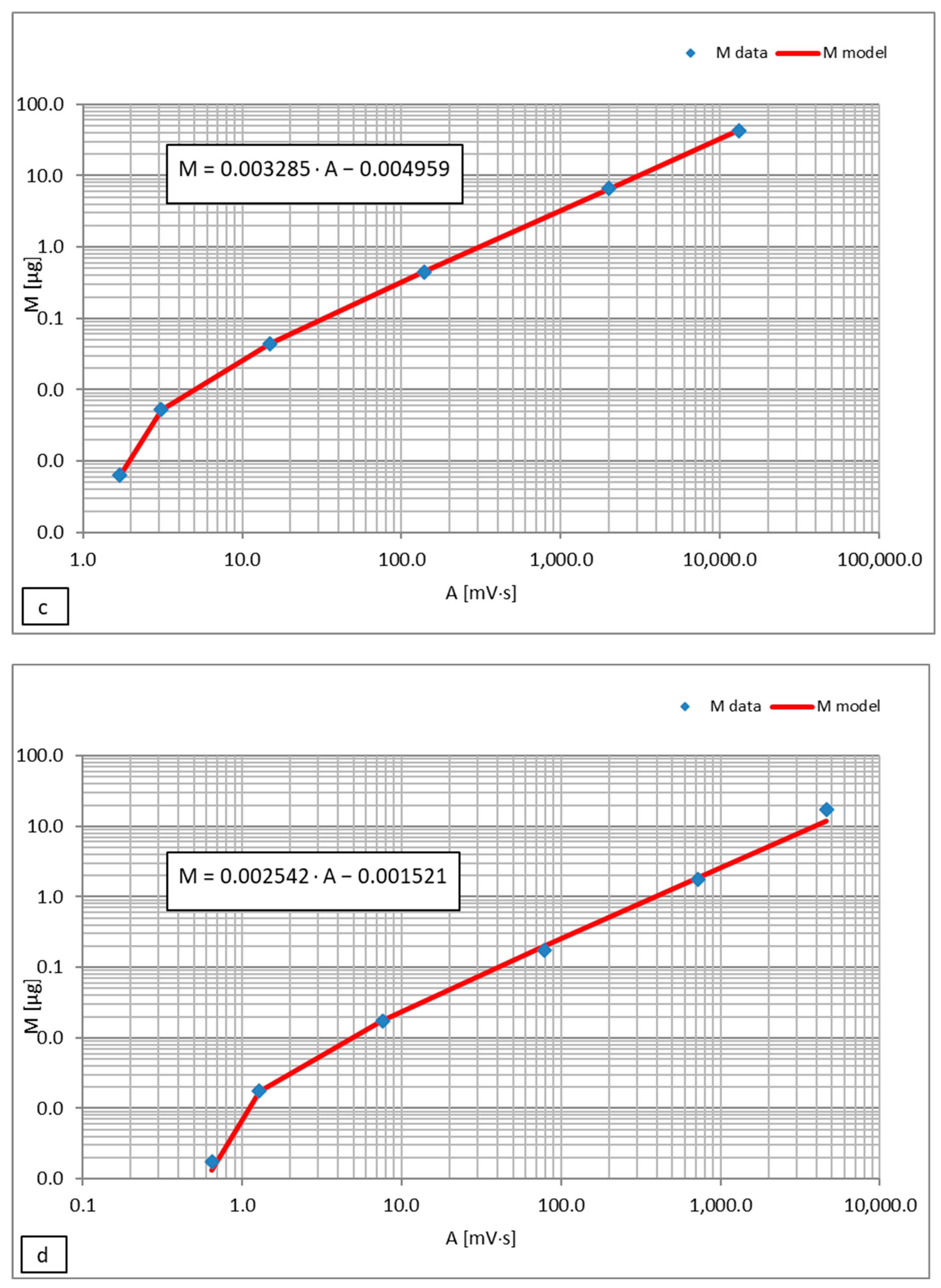

| Substance | C0 [μg/mL] | A [mV·s] | M [μg] | Cm.ch. [μg/mL] | CW (Equation (2)) [μg/mL] | KH (Equation (4)) |
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| Benzene (78.1 g/mol) | 0.17530 | 1.979 | M = 0.001933 · A −1.5725·10−5 = 0.003810 | 0.3810 | 0.02295 | 16.6 |
| Toluene (92.1 g/mol) | 0.17246 | 2.120 | M = 0.002213 · A −0.001021 = 0.003671 | 0.3671 | 0.02567 | 14.3 |
| Ethylbenzene + m-xylene + p-xylene (106 g/mol) (mean value of KH) | 0.51666 | 4.407 | M = 0.003285 · A −0.004959 = 0.009518 | 0.9518 | 0.1360 | 7.0 |
| o-xylene (106 g/mol) | 0.17510 | 2.127 | M = 0.002542 · A −0.001521 = 0.003886 | 0.3886 | 0.01963 | 19.8 |
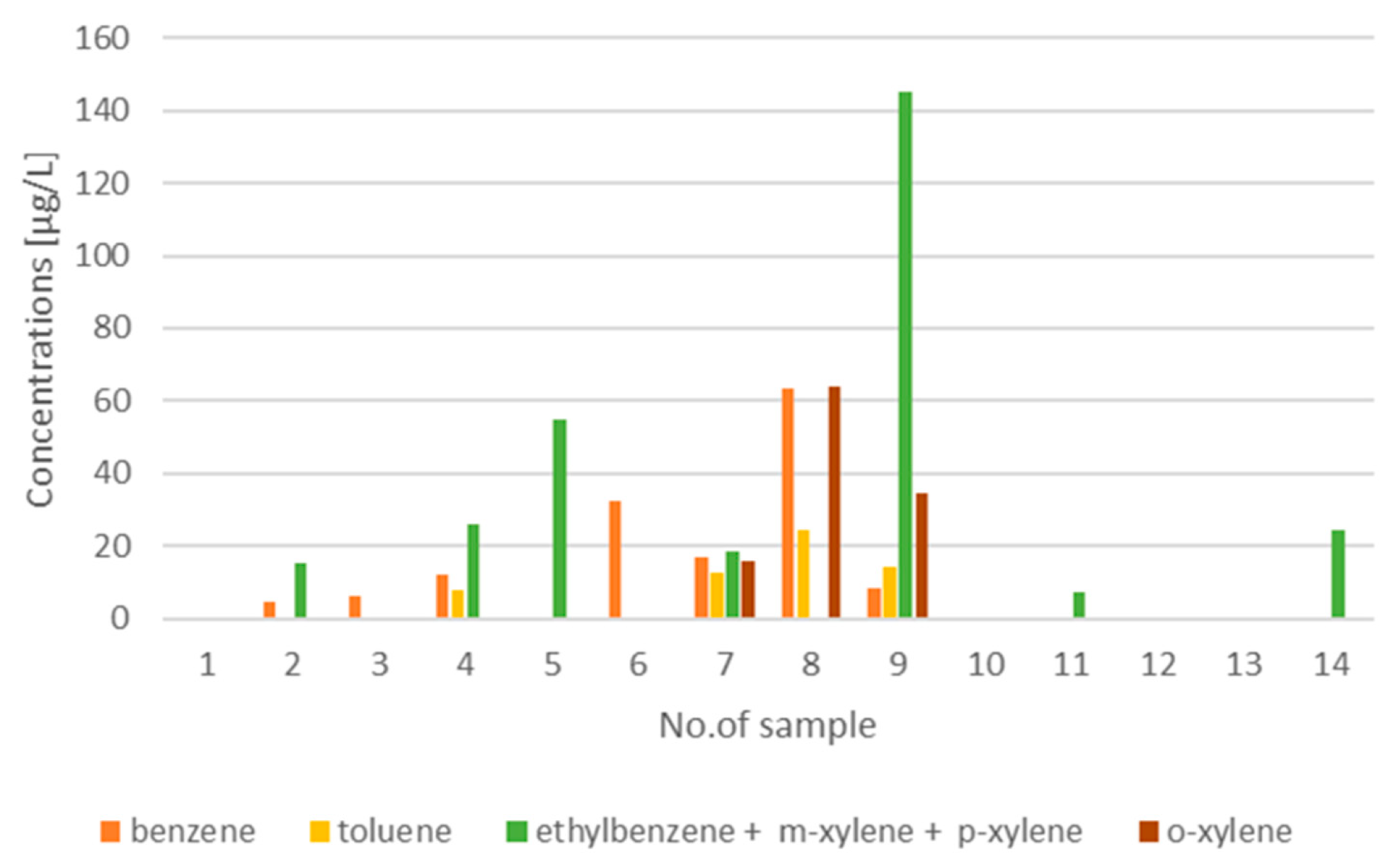
| Number of Sample | Characteristic of Water Sample Collection Point | Benzene | Toluene | Ethylbenzene + m-xylene + p-xylene | o-xylene |
|---|---|---|---|---|---|
| μg/L | |||||
| 1 | the centre of Cracow city, runoff from the roof, closed plastic tank | N.D. | N.D. | N.D. | N.D. |
| 2 | runoff from the building’s roof surface (roof made of ceramic tiles, terracotta), exposure to communication route, PVC gutter system, open tank | 4.53 | N.D. | 15.45 | N.D. |
| 3 | direct rainfall, open tank | 6.00 | N.D. | N.D. | N.D. |
| 4 | partly direct rainfall, partly runoff from the roof, runoff from the roof made of glass fibre-reinforced polyester, used for about 20 years, covered with a layer of pollution originated from Cracow’s air(especially smog), roof exposure to a nearby gas station, open tank | 11.87 | 7.87 | 26.16 | N.D. |
| 5 | the suburb of Cracow city, metal roof, airport nearby, closed plastic tank | N.D. | N.D. | 55.05 | N.D. |
| 6 | the centre of Cracow city, open black plastic container | 32.55 | N.D. | N.D. | N.D. |
| 7 | runoff from the building’s roof surface (roof made of ceramic tiles, terracotta), exposure to communication route, PVC gutter system, open tank | 16.76 | 12.45 | 18.49 | 15.88 |
| 8 | direct rainfall, open tank | 63.43 | 24.51 | N.D. | 63.70 |
| 9 | partly direct rainfall, partly runoff from the roof, runoff from the roof made of glass fibre-reinforced polyester, used for about 20 years, covered with a layer of pollution originated from Cracow’s air (especially smog), roof exposure to a nearby gas station, open tank | 8.53 | 14.16 | 145.11 | 34.49 |
| 10 | the suburb of Cracow city, airport nearby, open black plastic container | N.D. | N.D. | N.D. | N.D. |
| 11 | the suburb of Cracow city, airport nearby, open white plastic container | N.D. | N.D. | 7.16 | N.D. |
| 12 | the suburb of Cracow city, airport nearby, open glass container | N.D. | N.D. | N.D. | N.D. |
| 13 | rainwater collected by a PVC gutter system, infiltrated through the green roof, closed tank with drinking water approval | N.D. | N.D. | N.D. | N.D. |
| 14 | runoff from the roof made of sheet metal, gutters made of galvanized sheet metal, presence of communication routes near sampling point, open tank | N.D. | N.D. | 24.46 | N.D. |
| Mean μg/L | Median μg/L | Sample Standard Deviation μg/L | Maximum μg/L | |
|---|---|---|---|---|
| benzene | 20.5 | 11.9 | 21.2 | 63.4 |
| toluene | 14.7 | 13.3 | 7.0 | 24.5 |
| ethylbenzene + m-xylene + p-xylene | 41.7 | 24.5 | 48.0 | 145.1 |
| o-xylene | 38.0 | 34.5 | 24.1 | 63.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bąk, J.; Bielski, A.; Meland, S.; Pala, K.; Wassilkowska, A. An Analysis of BTEX Occurrence in Stored Rainwater and Rainwater Runoff in Urban Environment. Sustainability 2025, 17, 1607. https://doi.org/10.3390/su17041607
Bąk J, Bielski A, Meland S, Pala K, Wassilkowska A. An Analysis of BTEX Occurrence in Stored Rainwater and Rainwater Runoff in Urban Environment. Sustainability. 2025; 17(4):1607. https://doi.org/10.3390/su17041607
Chicago/Turabian StyleBąk, Joanna, Andrzej Bielski, Sondre Meland, Katarzyna Pala, and Anna Wassilkowska. 2025. "An Analysis of BTEX Occurrence in Stored Rainwater and Rainwater Runoff in Urban Environment" Sustainability 17, no. 4: 1607. https://doi.org/10.3390/su17041607
APA StyleBąk, J., Bielski, A., Meland, S., Pala, K., & Wassilkowska, A. (2025). An Analysis of BTEX Occurrence in Stored Rainwater and Rainwater Runoff in Urban Environment. Sustainability, 17(4), 1607. https://doi.org/10.3390/su17041607






