Biomass Hydrochar: A Critical Review of Process Chemistry, Synthesis Methodology, and Applications
Abstract
1. Introduction
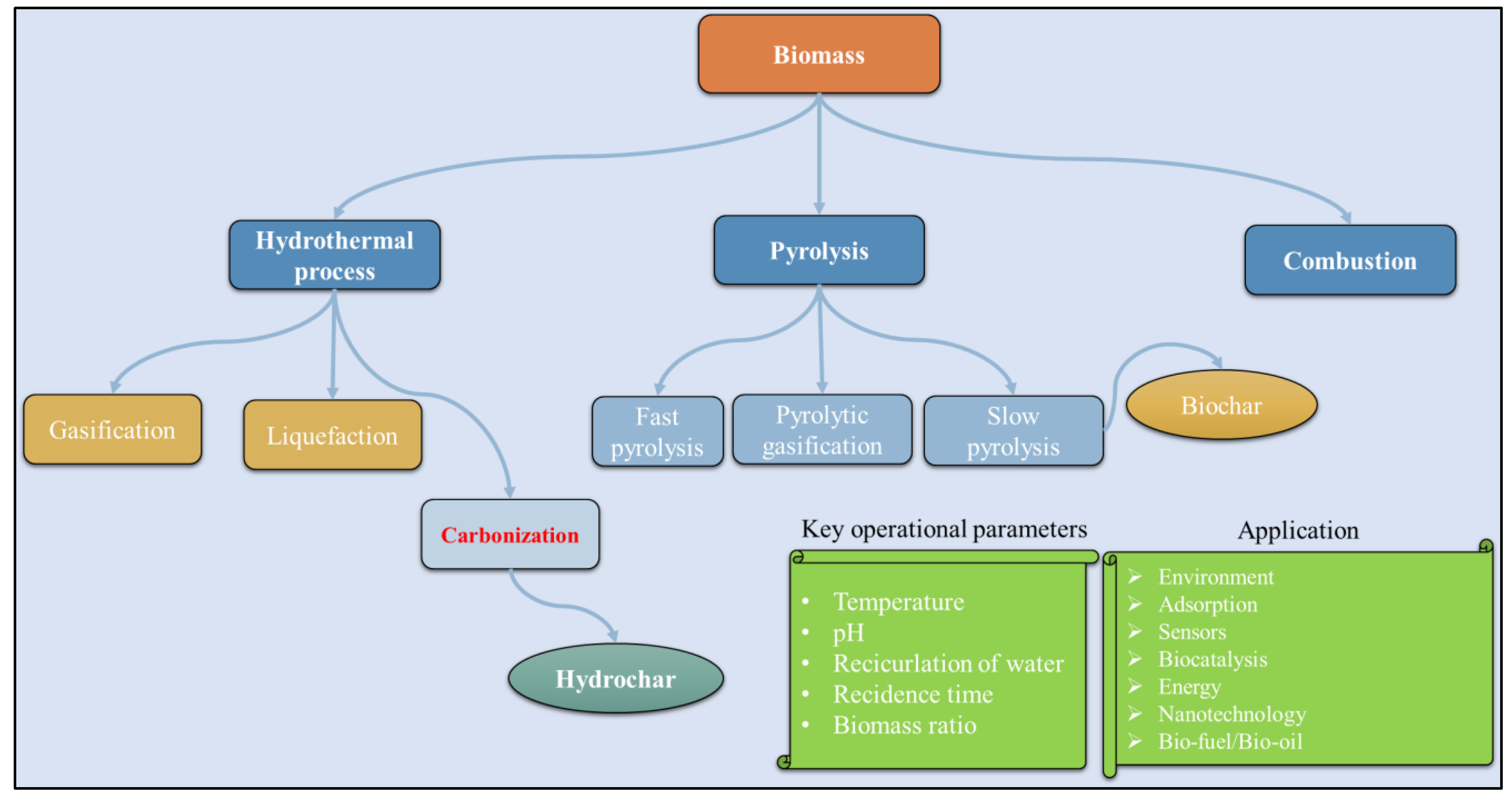
2. Hydrothermal Carbonization
2.1. Solid Yield and Chemical Composition Characterization of Biochar and Hydrochar
2.2. Hydrothermal Carbonization: Water
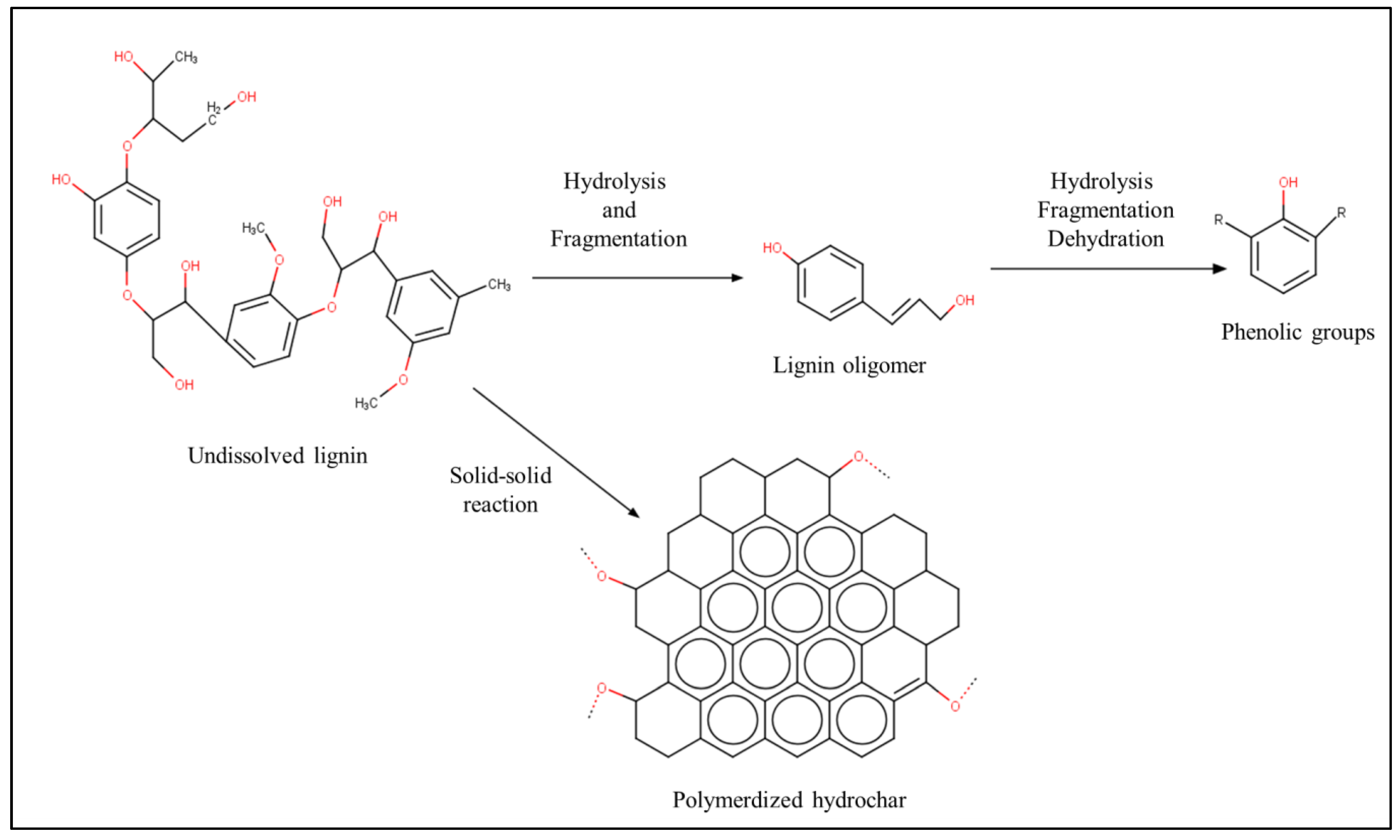
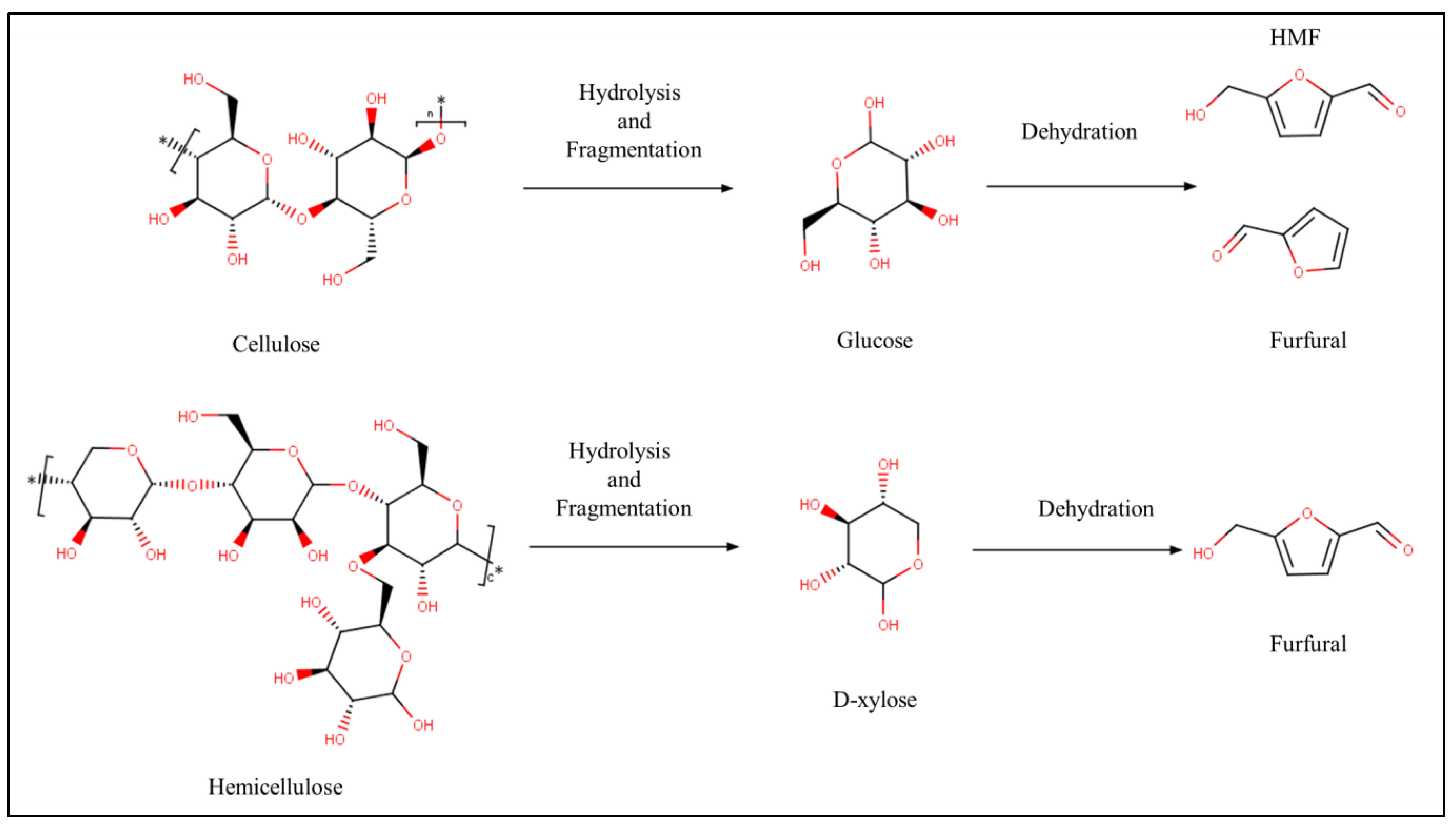
2.3. Chemistry of Hydrothermal Carbonization
2.4. Chemical Route for Biomass Conversion
3. Experimental Conditions Influencing the Thermal Carbonization Process
3.1. Chemical and Physical Composition of Biomass
Co-Hydrothermal Carbonization
3.2. pH and Temperature
3.3. Residence Time
3.4. Water and Biomass Ratios Used
3.5. Pyrolysis and Gasification
4. Large-Scale Hydrothermal Carbonization Reactors
5. Hydrochar Manufacturing Methods
6. Possible Areas of Application of Hydrochar
6.1. Energy
6.2. Application as an Adsorbent in Water Decontamination and Its Differences to Biochar
6.3. Magnetic Hydrochar
6.4. Sensors
6.5. Biocatalysts
7. Future Perspectives
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Georgin, J.; Stracke, D.; Franco, P.; Sher, F.; Franco, D.S.P.; Ramos, C.G.; Piccilli, D.G.A.; Lima, E.C.; Sher, F.; Stracke, D.; et al. A Review of the Antibiotic Ofloxacin: Current Status of Ecotoxicology and Scientific Advances in Its Removal from Aqueous Systems by Adsorption Technology. Chem. Eng. Res. Des. 2023, 193, 99–120. [Google Scholar] [CrossRef]
- Georgin, J.; Franco, D.S.P.; da Boit Martinello, K.; Lima, E.C.; Silva, L.F.O. A Review of the Toxicology Presence and Removal of Ketoprofen through Adsorption Technology. J. Environ. Chem. Eng. 2022, 10, 107798. [Google Scholar] [CrossRef]
- Ewuzie, U.; Saliu, O.D.; Dulta, K.; Ogunniyi, S.; Bajeh, A.O.; Iwuozor, K.O.; Ighalo, J.O. A Review on Treatment Technologies for Printing and Dyeing Wastewater (PDW). J. Water Process Eng. 2022, 50, 103273. [Google Scholar] [CrossRef]
- Nandan, A.; Siddiqui, N.A.; Singh, C.; Aeri, A.; Gwenzi, W.; Ighalo, J.O.; Nagliate, P.C.; Meili, L.; Singh, P.; Chaukura, N.; et al. COVID-19 Pandemic in Uttarakhand, India: Environmental Recovery or Degradation? J. Environ. Chem. Eng. 2021, 9, 106595. [Google Scholar] [CrossRef]
- Nanda, S.; Mohammad, J.; Reddy, S.N.; Kozinski, J.A.; Dalai, A.K.; Kannan, S.; Burelle, I.; Orsat, V.; Vijaya Raghavan, G.S.; Oliveira, I.; et al. Waste-to-Wealth: Biowaste Valorization into Valuable Bio(Nano)Materials. Chem. Soc. Rev. 2019, 48, 4791–4822. [Google Scholar] [CrossRef]
- Ighalo, J.O.; Iwuchukwu, F.U.; Eyankware, O.E.; Iwuozor, K.O.; Olotu, K.; Bright, C.E.; Igwegbe, C.A. Flash Pyrolysis of Biomass: A Review of Recent Advances. Clean. Technol. Environ. Policy 2022, 24, 2349–2363. [Google Scholar] [CrossRef]
- Azzaz, A.A.; Khiari, B.; Jellali, S.; Ghimbeu, C.M.; Jeguirim, M. Hydrochars Production, Characterization and Application for Wastewater Treatment: A Review. Renew. Sustain. Energy Rev. 2020, 127, 109882. [Google Scholar] [CrossRef]
- Román, S.; Libra, J.; Berge, N.; Sabio, E.; Ro, K.; Li, L.; Ledesma, B.; Álvarez, A.; Bae, S. Hydrothermal Carbonization: Modeling, Final Properties Design and Applications: A Review. Energies 2018, 11, 216. [Google Scholar] [CrossRef]
- Cavali, M.; Libardi Junior, N.; de Sena, J.D.; Woiciechowski, A.L.; Soccol, C.R.; Belli Filho, P.; Bayard, R.; Benbelkacem, H.; de Castilhos Junior, A.B. A Review on Hydrothermal Carbonization of Potential Biomass Wastes, Characterization and Environmental Applications of Hydrochar, and Biorefinery Perspectives of the Process. Sci. Total Environ. 2023, 857, 159627. [Google Scholar] [CrossRef]
- Petrović, J.; Ercegović, M.; Simić, M.; Koprivica, M.; Dimitrijević, J.; Jovanović, A.; Janković Pantić, J. Hydrothermal Carbonization of Waste Biomass: A Review of Hydrochar Preparation and Environmental Application. Processes 2024, 12, 207. [Google Scholar] [CrossRef]
- Hu, L.; Yu, J.; Luo, H.; Wang, H.; Xu, P.; Zhang, Y. Simultaneous Recovery of Ammonium, Potassium and Magnesium from Produced Water by Struvite Precipitation. Chem. Eng. J. 2020, 382, 123001. [Google Scholar] [CrossRef]
- Fang, J.; Zhan, L.; Ok, Y.S.; Gao, B. Minireview of Potential Applications of Hydrochar Derived from Hydrothermal Carbonization of Biomass. J. Ind. Eng. Chem. 2018, 57, 15–21. [Google Scholar] [CrossRef]
- Wu, S.; Wang, Q.; Fang, M.; Wu, D.; Cui, D.; Pan, S.; Bai, J.; Xu, F.; Wang, Z. Hydrothermal Carbonization of Food Waste for Sustainable Biofuel Production: Advancements, Challenges, and Future Prospects. Sci. Total Environ. 2023, 897, 165327. [Google Scholar] [CrossRef]
- Wu, S.; Wang, Q.; Cui, D.; Wang, X.; Wu, D.; Bai, J.; Xu, F.; Wang, Z.; Zhang, J. Analysis of Fuel Properties of Hydrochar Derived from Food Waste and Biomass: Evaluating Varied Mixing Techniques Pre/Post-Hydrothermal Carbonization. J. Clean. Prod. 2023, 430, 139660. [Google Scholar] [CrossRef]
- Ning, X.; Teng, H.; Wang, G.; Zhang, J.; Zhang, N.; Huang, C.; Wang, C. Physiochemical, Structural and Combustion Properties of Hydrochar Obtained by Hydrothermal Carbonization of Waste Polyvinyl Chloride. Fuel 2020, 270, 117526. [Google Scholar] [CrossRef]
- Ighalo, J.O.; Rangabhashiyam, S.; Dulta, K.; Umeh, C.T.; Iwuozor, K.O.; Aniagor, C.O.; Eshiemogie, S.O.; Iwuchukwu, F.U.; Igwegbe, C.A. Recent Advances in Hydrochar Application for the Adsorptive Removal of Wastewater Pollutants. Chem. Eng. Res. Des. 2022, 184, 419–456. [Google Scholar] [CrossRef]
- Chen, Z.; Guo, Y.; Luo, L.; Liu, Z.; Miao, W.; Xia, Y. A Critical Review of Hydrochar Based Photocatalysts by Hydrothermal Carbonization: Synthesis, Mechanisms, and Applications. Biochar 2024, 6, 74. [Google Scholar] [CrossRef]
- Ge, Q.; Dong, C.; Wang, G.; Zhang, J.; Hou, R. Production, Characterization and Environmental Remediation Application of Emerging Phosphorus-Rich Biochar/Hydrochar: A Comprehensive Review. RSC Adv. 2024, 14, 33649–33665. [Google Scholar] [CrossRef]
- Masoumi, S.; Borugadda, V.B.; Nanda, S.; Dalai, A.K. Hydrochar: A Review on Its Production Technologies and Applications. Catalysts 2021, 11, 939. [Google Scholar] [CrossRef]
- Jalilian, M.; Bissessur, R.; Ahmed, M.; Hsiao, A.; He, Q.S.; Hu, Y. A Review: Hydrochar as Potential Adsorbents for Wastewater Treatment and CO2 Adsorption. Sci. Total Environ. 2024, 914, 169823. [Google Scholar] [CrossRef]
- Oumabady, S.; Paul Sebastian, P.S.; Kamaludeen, S.P.B.; Ramasamy, M.; Kalaiselvi, P.; Parameswari, E. Preparation and Characterization of Optimized Hydrochar from Paper Board Mill Sludge. Sci. Rep. 2020, 10, 773. [Google Scholar] [CrossRef]
- Ojewumi, M.E.; Chen, G. Hydrochar Production by Hydrothermal Carbonization: Microwave versus Supercritical Water Treatment. Biomass 2024, 4, 574–598. [Google Scholar] [CrossRef]
- Huang, J.; Feng, Y.; Xie, H.; Wu, P.; Wang, M.; Wang, B.; Zhang, Q.; Zhang, S.; Liu, Z. A Bibliographic Study Reviewing the Last Decade of Hydrochar in Environmental Application: History, Status Quo, and Trending Research Paths. Biochar 2023, 5, 12. [Google Scholar] [CrossRef]
- Sharma, R.; Jasrotia, K.; Singh, N.; Ghosh, P.; Srivastava, S.; Sharma, N.R.; Singh, J.; Kanwar, R.; Kumar, A. A Comprehensive Review on Hydrothermal Carbonization of Biomass and Its Applications. Chem. Afr. 2020, 3, 1–19. [Google Scholar] [CrossRef]
- Kambo, H.S.; Dutta, A. A Comparative Review of Biochar and Hydrochar in Terms of Production, Physico-Chemical Properties and Applications. Renew. Sustain. Energy Rev. 2015, 45, 359–378. [Google Scholar] [CrossRef]
- Munir, M.T.; Soheil, S.; Udugama, I.A.; Baroutian, S.; Gernaey, K.V.; Young, B.R. Resource Recovery from Organic Solid Waste Using Hydrothermal Processing: Opportunities and Challenges. Renew. Sustain. Energy Rev. 2018, 96, 64–75. [Google Scholar] [CrossRef]
- Usman, M.; Chen, H.; Chen, K.; Ren, S.; Clark, J.H.; Fan, J.; Luo, G.; Zhang, S. Characterization and Utilization of Aqueous Products from Hydrothermal Conversion of Biomass for Bio-Oil and Hydro-Char Production: A Review. Green Chem. 2019, 21, 1553–1572. [Google Scholar] [CrossRef]
- Kannan, S.; Burelle, I.; Orsat, V.; Vijaya Raghavan, G.S. Characterization of Bio-Crude Liquor and Bio-Oil Produced by Hydrothermal Carbonization of Seafood Waste. Waste Biomass Valorization 2020, 11, 3553–3565. [Google Scholar] [CrossRef]
- Sun, Y.; Gao, B.; Yao, Y.; Fang, J.; Zhang, M.; Zhou, Y.; Chen, H.; Yang, L. Effects of Feedstock Type, Production Method, and Pyrolysis Temperature on Biochar and Hydrochar Properties. Chem. Eng. J. 2014, 240, 574–578. [Google Scholar] [CrossRef]
- Zhang, X.; Ardasheva, Y.; Austin, B.W. Self-Efficacy and English Public Speaking Performance: A Mixed Method Approach. Engl. Specif. Purp. 2020, 59, 1–16. [Google Scholar] [CrossRef]
- Moreno, J.; Espada, J.J. Chapter 10—Environmental and Techno-Economic Assessment of Thermochemical Treatment Systems for Sludge. In Wastewater Treatment Residues as Resources for Biorefinery Products and Biofuels; Olivares, J.A., Puyol, D., Melero, J.A., Dufour, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 201–223. ISBN 978-0-12-816204-0. [Google Scholar]
- Akmach, D.; Bathla, S.; Tran, C.-C.; Kaliaguine, S.; Mushrif, S.H. Transition Metal Carbide Catalysts for Upgrading Lignocellulosic Biomass-Derived Oxygenates: A Review of the Experimental and Computational Investigations into Structure-Property Relationships. Catal. Today 2023, 423, 114285. [Google Scholar] [CrossRef]
- Cypres, R.; Planchon, D.; Braekman-Danheux, C. Evolution of Crystallite Size, Gas Composition and Reaction Heat during Pyrolysis and Hydropyrolysis of a Bituminous Belgian Coal. Fuel 1988, 67, 341–344. [Google Scholar] [CrossRef]
- Cognigni, P.; Leonelli, C.; Berrettoni, M. A Bibliographic Study of Biochar and Hydrochar: Differences and Similarities. J. Anal. Appl. Pyrolysis 2025, 187, 106985. [Google Scholar] [CrossRef]
- Cao, X.; Peng, X.; Sun, S.; Zhong, L.; Sun, R. Hydrothermal Conversion of Bamboo: Identification and Distribution of the Components in Solid Residue, Water-Soluble and Acetone-Soluble Fractions. J. Agric. Food Chem. 2014, 62, 12360–12365. [Google Scholar] [CrossRef] [PubMed]
- Colin, J.; Sarrion, A.; Diaz, E.; de la Rubia, M.A.; Mohedano, A.F. Ecotoxicity Assessment of Hydrochar from Hydrothermal Carbonization of Biomass Waste. Sustain. Chem. Pharm. 2025, 44, 101909. [Google Scholar] [CrossRef]
- Xiao, L.-P.; Shi, Z.-J.; Xu, F.; Sun, R.-C. Hydrothermal Carbonization of Lignocellulosic Biomass. Bioresour. Technol. 2012, 118, 619–623. [Google Scholar] [CrossRef]
- Gascó, G.; Paz-Ferreiro, J.; Álvarez, M.L.; Saa, A.; Méndez, A. Biochars and Hydrochars Prepared by Pyrolysis and Hydrothermal Carbonisation of Pig Manure. Waste Manag. 2018, 79, 395–403. [Google Scholar] [CrossRef]
- Singh, A.; Nanda, S.; Guayaquil-Sosa, J.F.; Berruti, F. Pyrolysis of Miscanthus and Characterization of Value-added Bio-oil and Biochar Products. Can. J. Chem. Eng. 2021, 99, S55–S68. [Google Scholar] [CrossRef]
- Schimmelpfennig, S.; Glaser, B. One Step Forward toward Characterization: Some Important Material Properties to Distinguish Biochars. J. Environ. Qual. 2012, 41, 1001–1013. [Google Scholar] [CrossRef]
- Baxter, L.L.; Miles, T.R.; Miles, T.R.; Jenkins, B.M.; Milne, T.; Dayton, D.; Bryers, R.W.; Oden, L.L. The Behavior of Inorganic Material in Biomass-Fired Power Boilers: Field and Laboratory Experiences. Fuel Process. Technol. 1998, 54, 47–78. [Google Scholar] [CrossRef]
- Qiu, B.; Shao, Q.; Shi, J.; Yang, C.; Chu, H. Application of Biochar for the Adsorption of Organic Pollutants from Wastewater: Modification Strategies, Mechanisms and Challenges. Sep. Purif. Technol. 2022, 300, 121925. [Google Scholar] [CrossRef]
- Hodgson, E.M.; Nowakowski, D.J.; Shield, I.; Riche, A.; Bridgwater, A.V.; Clifton-Brown, J.C.; Donnison, I.S. Variation in Miscanthus Chemical Composition and Implications for Conversion by Pyrolysis and Thermo-Chemical Bio-Refining for Fuels and Chemicals. Bioresour. Technol. 2011, 102, 3411–3418. [Google Scholar] [CrossRef] [PubMed]
- Demirbaş, A. Estimating of Structural Composition of Wood and Non-Wood Biomass Samples. Energy Sources 2005, 27, 761–767. [Google Scholar] [CrossRef]
- Yan, W.; Acharjee, T.C.; Coronella, C.J.; Vásquez, V.R. Thermal Pretreatment of Lignocellulosic Biomass. Environ. Prog. Sustain. Energy 2009, 28, 435–440. [Google Scholar] [CrossRef]
- Mäkelä, M.; Benavente, V.; Fullana, A. Hydrothermal Carbonization of Lignocellulosic Biomass: Effect of Process Conditions on Hydrochar Properties. Appl. Energy 2015, 155, 576–584. [Google Scholar] [CrossRef]
- Liu, Z.; Quek, A.; Kent Hoekman, S.; Balasubramanian, R. Production of Solid Biochar Fuel from Waste Biomass by Hydrothermal Carbonization. Fuel 2013, 103, 943–949. [Google Scholar] [CrossRef]
- Shen, Y. Biomass and Bioenergy A Review on Hydrothermal Carbonization of Biomass and Plastic Wastes to Energy Products. Biomass Bioenergy 2020, 134, 105479. [Google Scholar] [CrossRef]
- Khan, M.A.; Alqadami, A.A.; Otero, M.; Siddiqui, M.R.; Alothman, Z.A.; Alsohaimi, I.; Rafatullah, M.; Hamedelniel, A.E. Heteroatom-Doped Magnetic Hydrochar to Remove Post-Transition and Transition Metals from Water: Synthesis, Characterization, and Adsorption Studies. Chemosphere 2019, 218, 1089–1099. [Google Scholar] [CrossRef]
- Reza, M.T.; Andert, J.; Wirth, B.; Busch, D.; Pielert, J.; Lynam, J.G.; Mumme, J. Hydrothermal Carbonization of Biomass for Energy and Crop Production. Appl. Bioenergy 2014, 1, 11–29. [Google Scholar] [CrossRef]
- Reza, M.T.; Lynam, J.G.; Uddin, M.H.; Coronella, C.J. Hydrothermal Carbonization: Fate of Inorganics. Biomass Bioenergy 2013, 49, 86–94. [Google Scholar] [CrossRef]
- Song, Y.; Li, Z.; Zhu, Q.; Huang, Z.; Cheng, Z. Precipitation Behavior of Salts in Supercritical Water: Experiments and Molecular Dynamics Simulations. Processes 2022, 10, 423. [Google Scholar] [CrossRef]
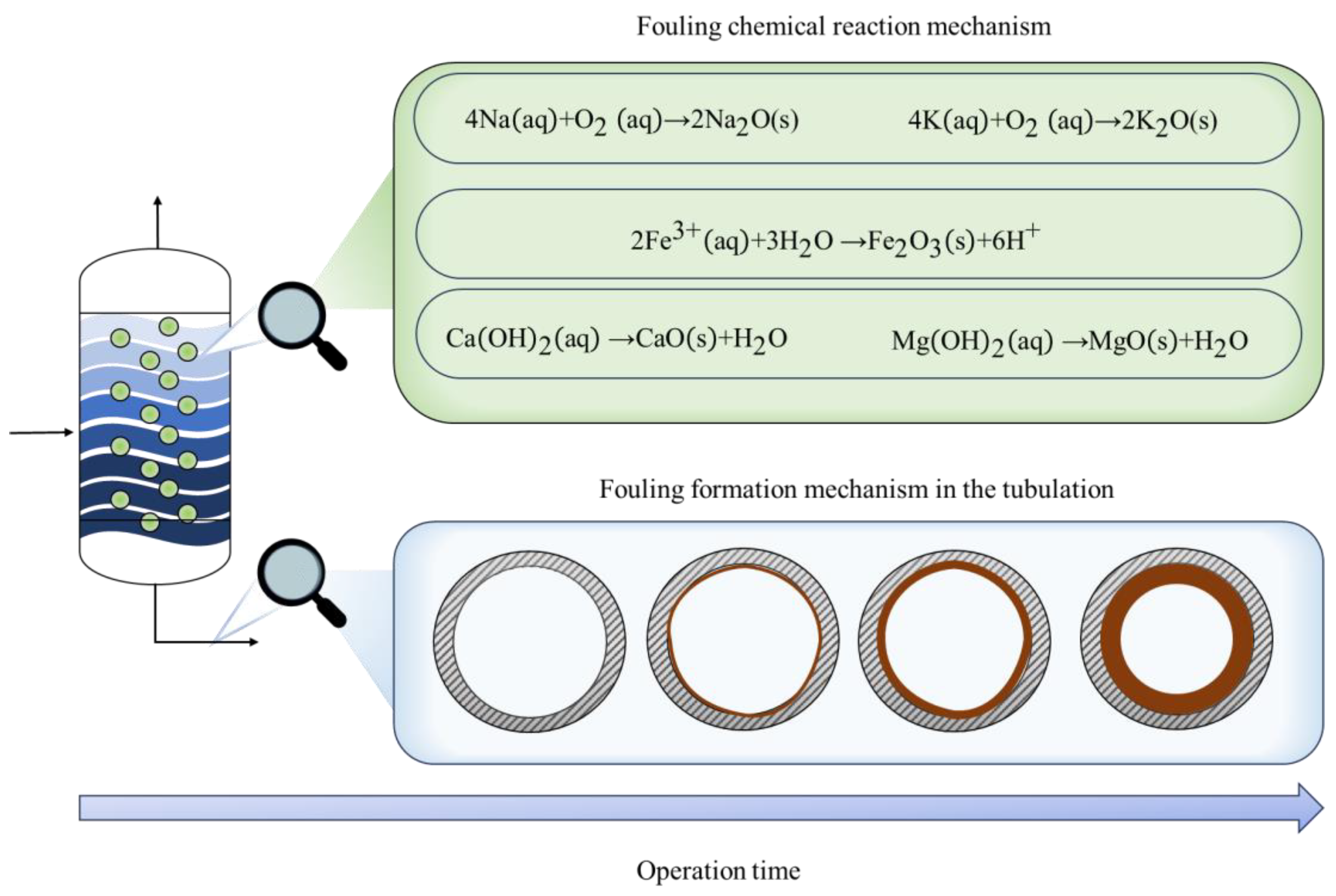
- Park, J.C.; Namkung, H.; Yoon, S.-P.; Seo, H.S.; Xu, L.-H.; Kim, H.-T. Influence of Phosphorus on Ash Fouling Deposition of Hydrothermal Carbonization Sewage Sludge Fuel via Drop Tube Furnace Combustion Experiments. J. Energy Inst. 2020, 93, 2399–2408. [Google Scholar] [CrossRef]
- Berce, J.; Zupančič, M.; Može, M.; Golobič, I. A Review of Crystallization Fouling in Heat Exchangers. Processes 2021, 9, 1356. [Google Scholar] [CrossRef]
- Barroso, J.; Ballester, J.; Ferrer, L.M.; Jiménez, S. Study of Coal Ash Deposition in an Entrained Flow Reactor: Influence of Coal Type, Blend Composition and Operating Conditions. Fuel Process. Technol. 2006, 87, 737–752. [Google Scholar] [CrossRef]
- Yang, M.; Xie, Q.; Wang, X.; Dong, H.; Zhang, H.; Li, C. Lowering Ash Slagging and Fouling Tendency of High-Alkali Coal by Hydrothermal Pretreatment. Int. J. Min. Sci. Technol. 2019, 29, 521–525. [Google Scholar] [CrossRef]
- Lachman, J.; Baláš, M.; Lisý, M.; Lisá, H.; Milčák, P.; Elbl, P. An Overview of Slagging and Fouling Indicators and Their Applicability to Biomass Fuels. Fuel Process. Technol. 2021, 217, 106804. [Google Scholar] [CrossRef]
- Antony, A.; Low, J.H.; Gray, S.; Childress, A.E.; Le-Clech, P.; Leslie, G. Scale Formation and Control in High Pressure Membrane Water Treatment Systems: A Review. J. Memb. Sci. 2011, 383, 1–16. [Google Scholar] [CrossRef]
- Yu, W.; Song, D.; Chen, W.; Yang, H. Antiscalants in RO Membrane Scaling Control. Water Res. 2020, 183, 115985. [Google Scholar] [CrossRef]
- Ruigómez, I.; González, E.; Rodríguez-Gómez, L.; Vera, L. Fouling Control Strategies for Direct Membrane Ultrafiltration: Physical Cleanings Assisted by Membrane Rotational Movement. Chem. Eng. J. 2022, 436, 135161. [Google Scholar] [CrossRef]
- Jain, A.; Balasubramanian, R.; Srinivasan, M.P. Hydrothermal Conversion of Biomass Waste to Activated Carbon with High Porosity: A Review. Chem. Eng. J. 2016, 283, 789–805. [Google Scholar] [CrossRef]
- Wang, T.; Zhai, Y.; Zhu, Y.; Li, C.; Zeng, G. A Review of the Hydrothermal Carbonization of Biomass Waste for Hydrochar Formation: Process Conditions, Fundamentals, and Physicochemical Properties. Renew. Sustain. Energy Rev. 2018, 90, 223–247. [Google Scholar] [CrossRef]
- Zhuang, X.; Song, Y.; Zhan, H.; Bi, X.T.; Yin, X.; Wu, C. Pyrolytic Conversion of Biowaste-Derived Hydrochar: Decomposition Mechanism of Specific Components. Fuel 2020, 266, 117106. [Google Scholar] [CrossRef]
- Higgins, L.J.R.; Brown, A.P.; Harrington, J.P.; Ross, A.B.; Kaulich, B.; Mishra, B. Evidence for a Core-Shell Structure of Hydrothermal Carbon. Carbon N. Y. 2020, 161, 423–431. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. Chemical and Structural Properties of Carbonaceous Products Obtained by Hydrothermal Carbonization of Saccharides. Chem.–Eur. J. 2009, 15, 4195–4203. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yang, H.; Liu, Y.; Sun, X.; Zhang, D.; Xue, D. Hydrophobic Precipitation of Carbonaceous Spheres from Fructose by a Hydrothermal Process. Carbon N. Y. 2012, 50, 2155–2161. [Google Scholar] [CrossRef]
- Cherinka, B.; Andrews, B.H.; Sánchez-Gallego, J.; Brownstein, J.; Argudo-Fernández, M.; Blanton, M.; Bundy, K.; Jones, A.; Masters, K.; Law, D.R.; et al. Marvin: A Tool Kit for Streamlined Access and Visualization of the SDSS-IV MaNGA Data Set. Astron. J. 2019, 158, 74. [Google Scholar] [CrossRef]
- Khan, T.A.; Saud, A.S.; Jamari, S.S.; Ab Rahim, M.H.; Park, J.-W.; Kim, H.-J. Hydrothermal Carbonization of Lignocellulosic Biomass for Carbon Rich Material Preparation: A Review. Biomass Bioenergy 2019, 130, 105384. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, Y.-J.; Hao, X.; Peng, P.; Shi, J.-Y.; Peng, F.; Sun, R.-C. Hydrothermal Synthesis and Applications of Advanced Carbonaceous Materials from Biomass: A Review. Adv. Compos. Hybrid Mater. 2020, 3, 267–284. [Google Scholar] [CrossRef]
- Elaigwu, S.E.; Greenway, G.M. Chemical, Structural and Energy Properties of Hydrochars from Microwave-Assisted Hydrothermal Carbonization of Glucose. Int. J. Ind. Chem. 2016, 7, 449–456. [Google Scholar] [CrossRef]
- Dang, H.; Xu, R.; Zhang, J.; Wang, M.; Xu, K. Hydrothermal Carbonization of Waste Furniture for Clean Blast Furnace Fuel Production: Physicochemical, Gasification Characteristics and Conversion Mechanism Investigation. Chem. Eng. J. 2023, 469, 143980. [Google Scholar] [CrossRef]
- Dinjus, E.; Kruse, A.; Troeger, N. Hydrothermal Carbonization: 1. Influence of Lignin in Lignocelluloses. Chem. Ing. Tech. 2011, 83, 1734–1741. [Google Scholar] [CrossRef]
- Li, S.; Lyons-Hart, J.; Banyasz, J.; Shafer, K. Real-Time Evolved Gas Analysis by FTIR Method: An Experimental Study of Cellulose Pyrolysis. Fuel 2001, 80, 1809–1817. [Google Scholar] [CrossRef]
- Lu, X.; Berge, N.D. Influence of Feedstock Chemical Composition on Product Formation and Characteristics Derived from the Hydrothermal Carbonization of Mixed Feedstocks. Bioresour. Technol. 2014, 166, 120–131. [Google Scholar] [CrossRef]
- Zheng, X.; Jiang, Z.; Ying, Z.; Song, J.; Chen, W.; Wang, B. Role of Feedstock Properties and Hydrothermal Carbonization Conditions on Fuel Properties of Sewage Sludge-Derived Hydrochar Using Multiple Linear Regression Technique. Fuel 2020, 271, 117609. [Google Scholar] [CrossRef]
- Li, L.; Wang, Y.; Xu, J.; Flora, J.R.V.; Hoque, S.; Berge, N.D. Quantifying the Sensitivity of Feedstock Properties and Process Conditions on Hydrochar Yield, Carbon Content, and Energy Content. Bioresour. Technol. 2018, 262, 284–293. [Google Scholar] [CrossRef]
- Kalderis, D.; Kotti, M.S.; Méndez, A.; Gascó, G. Characterization of Hydrochars Produced by Hydrothermal Carbonization of Rice Husk. Solid Earth 2014, 5, 477–483. [Google Scholar] [CrossRef]
- Sermyagina, E.; Saari, J.; Kaikko, J.; Vakkilainen, E. Hydrothermal Carbonization of Coniferous Biomass: Effect of Process Parameters on Mass and Energy Yields. J. Anal. Appl. Pyrolysis 2015, 113, 551–556. [Google Scholar] [CrossRef]
- Kang, S.; Li, X.; Fan, J.; Chang, J. Characterization of Hydrochars Produced by Hydrothermal Carbonization of Lignin, Cellulose, d-Xylose, and Wood Meal. Ind. Eng. Chem. Res. 2012, 51, 9023–9031. [Google Scholar] [CrossRef]
- Saha, N.; Saba, A.; Reza, M.T. Effect of Hydrothermal Carbonization Temperature on PH, Dissociation Constants, and Acidic Functional Groups on Hydrochar from Cellulose and Wood. J. Anal. Appl. Pyrolysis 2019, 137, 138–145. [Google Scholar] [CrossRef]
- Ahmed Khan, T.; Kim, H.-J.; Gupta, A.; Jamari, S.S.; Jose, R. Synthesis and Characterization of Carbon Microspheres from Rubber Wood by Hydrothermal Carbonization. J. Chem. Technol. Biotechnol. 2019, 94, 1374–1383. [Google Scholar] [CrossRef]
- Mendoza Martinez, C.L.; Sermyagina, E.; Saari, J.; Silva de Jesus, M.; Cardoso, M.; Matheus de Almeida, G.; Vakkilainen, E. Hydrothermal Carbonization of Lignocellulosic Agro-Forest Based Biomass Residues. Biomass Bioenergy 2021, 147, 106004. [Google Scholar] [CrossRef]
- Galindo, J.; Climent, H.; de la Morena, J.; González-Domínguez, D.; Guilain, S. Assessment of Air Management Strategies to Improve the Transient Response of Advanced Gasoline Engines Operating under High EGR Conditions. Energy 2023, 262, 125586. [Google Scholar] [CrossRef]
- Chen, X.; Ma, X.; Peng, X.; Lin, Y.; Yao, Z. Conversion of Sweet Potato Waste to Solid Fuel via Hydrothermal Carbonization. Bioresour. Technol. 2018, 249, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Iryani, D.A.; Kumagai, S.; Nonaka, M.; Sasaki, K.; Hirajima, T. Characterization and Production of Solid Biofuel from Sugarcane Bagasse by Hydrothermal Carbonization. Waste Biomass Valorization 2017, 8, 1941–1951. [Google Scholar] [CrossRef]
- Masoumi, S.; Dalai, A.K. Optimized Production and Characterization of Highly Porous Activated Carbon from Algal-Derived Hydrochar. J. Clean. Prod. 2020, 263, 121427. [Google Scholar] [CrossRef]
- Lai, F.; Chang, Y.; Huang, H.; Wu, G.; Xiong, J.; Pan, Z.; Zhou, C. Liquefaction of Sewage Sludge in Ethanol-Water Mixed Solvents for Bio-Oil and Biochar Products. Energy 2018, 148, 629–641. [Google Scholar] [CrossRef]
- Masoumi, S.; Boahene, P.E.; Dalai, A.K. Biocrude Oil and Hydrochar Production and Characterization Obtained from Hydrothermal Liquefaction of Microalgae in Methanol-Water System. Energy 2021, 217, 119344. [Google Scholar] [CrossRef]
- Yu, J.; Tang, T.; Cheng, F.; Huang, D.; Martin, J.L.; Brewer, C.E.; Grimm, R.L.; Zhou, M.; Luo, H. Waste-to-Wealth Application of Wastewater Treatment Algae-Derived Hydrochar for Pb(II) Adsorption. MethodsX 2021, 8, 101263. [Google Scholar] [CrossRef]
- Saber, M.; Takahashi, F.; Yoshikawa, K. Characterization and Application of Microalgae Hydrochar as a Low-Cost Adsorbent for Cu(II) Ion Removal from Aqueous Solutions. Environ. Sci. Pollut. Res. 2018, 25, 32721–32734. [Google Scholar] [CrossRef]
- Prasannamedha, G.; Kumar, P.S.; Mehala, R.; Sharumitha, T.J.; Surendhar, D. Enhanced Adsorptive Removal of Sulfamethoxazole from Water Using Biochar Derived from Hydrothermal Carbonization of Sugarcane Bagasse. J. Hazard. Mater. 2021, 407, 124825. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Perez, S.; Sheng, K. Upgrading Fuel Quality of Moso Bamboo via Low Temperature Thermochemical Treatments: Dry Torrefaction and Hydrothermal Carbonization. Fuel 2017, 196, 473–480. [Google Scholar] [CrossRef]
- Shao, Y.; Tan, H.; Shen, D.; Zhou, Y.; Jin, Z.; Zhou, D.; Lu, W.; Long, Y. Synthesis of Improved Hydrochar by Microwave Hydrothermal Carbonization of Green Waste. Fuel 2020, 266, 117146. [Google Scholar] [CrossRef]
- Zhu, G.; Yang, L.; Gao, Y.; Xu, J.; Chen, H.; Zhu, Y.; Wang, Y.; Liao, C.; Lu, C.; Zhu, C. Characterization and Pelletization of Cotton Stalk Hydrochar from HTC and Combustion Kinetics of Hydrochar Pellets by TGA. Fuel 2019, 244, 479–491. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, S.; Wang, B.; Wang, Q.; Yang, G.; Chen, J. Effect of Residence Time on Hydrothermal Carbonization of Corn Cob Residual. Bioresources 2015, 10, 3979–3986. [Google Scholar] [CrossRef]
- Guo, S.; Dong, X.; Zhu, C.; Han, Y.; Ma, F.; Wu, T. Pyrolysis Behaviors and Thermodynamics Properties of Hydrochar from Bamboo (Phyllostachys heterocycla Cv. Pubescens) Shoot Shell. Bioresour. Technol. 2017, 233, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Salaudeen, S.A.; Acharya, B.; Dutta, A. Steam Gasification of Hydrochar Derived from Hydrothermal Carbonization of Fruit Wastes. Renew. Energy 2021, 171, 582–591. [Google Scholar] [CrossRef]
- Yao, Z.; Ma, X. Hydrothermal Carbonization of Chinese Fan Palm. Bioresour. Technol. 2019, 282, 28–36. [Google Scholar] [CrossRef]
- Bhakta, H.; Sarmah, A.K.; Dubey, B. Hydrothermal Carbonization of Renewable Waste Biomass for Solid Biofuel Production: A Discussion on Process Mechanism, the Influence of Process Parameters, Environmental Performance and Fuel Properties of Hydrochar. Renew. Sustain. Energy Rev. 2020, 123, 109761. [Google Scholar] [CrossRef]
- Cui, D.; Zhang, B.; Wu, S.; Xu, X.; Liu, B.; Wang, Q.; Zhang, X.; Zhang, J. From Sewage Sludge and Lignocellulose to Hydrochar by Co-Hydrothermal Carbonization: Mechanism and Combustion Characteristics. Energy 2024, 305, 132414. [Google Scholar] [CrossRef]
- Lang, Q.; Guo, Y.; Zheng, Q.; Liu, Z.; Gai, C. Co-Hydrothermal Carbonization of Lignocellulosic Biomass and Swine Manure: Hydrochar Properties and Heavy Metal Transformation Behavior. Bioresour. Technol. 2018, 266, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhan, H.; Zhuang, X.; Yin, X.; Wu, C. Synergistic Characteristics and Capabilities of Co-Hydrothermal Carbonization of Sewage Sludge/Lignite Mixtures. Energy Fuels 2019, 33, 8735–8745. [Google Scholar] [CrossRef]
- Zhang, S.; Pi, M.; Su, Y.; Xu, D.; Xiong, Y.; Zhang, H. Biomass and Bioenergy Physiochemical Properties and Pyrolysis Behavior Evaluations of Hydrochar from Co-Hydrothermal Treatment of Rice Straw and Sewage Sludge. Biomass Bioenergy 2020, 140, 105664. [Google Scholar] [CrossRef]
- Chen, F.; Zhou, C.; Li, G.; Peng, F. Thermodynamics and Kinetics of Glyphosate Adsorption on Resin D301. Arab. J. Chem. 2016, 9, S1665–S1669. [Google Scholar] [CrossRef]
- Shen, Y.; Yu, S.; Ge, S.; Chen, X.; Ge, X.; Chen, M. Hydrothermal Carbonization of Medical Wastes and Lignocellulosic Biomass for Solid Fuel Production from Lab-Scale to Pilot-Scale. Energy 2017, 118, 312–323. [Google Scholar] [CrossRef]
- Zhang, C.; Ma, X.; Huang, T.; Zhou, Y.; Tian, Y. Co-Hydrothermal Carbonization of Water Hyacinth and Polyvinyl Chloride: Optimization of Process Parameters and Characterization of Hydrochar. Bioresour. Technol. 2020, 314, 123676. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Ma, X. Characteristics of Co-Hydrothermal Carbonization on Polyvinyl Chloride Wastes with Bamboo. Bioresour. Technol. 2018, 247, 302–309. [Google Scholar] [CrossRef]
- Lu, X.; Ma, X.; Chen, X.; Yao, Z.; Zhang, C. Co-Hydrothermal Carbonization of Polyvinyl Chloride and Corncob for Clean Solid Fuel Production. Bioresour. Technol. 2020, 301, 122763. [Google Scholar] [CrossRef]
- Leng, S.; Li, W.; Han, C.; Chen, L.; Chen, J.; Fan, L.; Lu, Q.; Li, J.; Leng, L.; Zhou, W. Aqueous Phase Recirculation during Hydrothermal Carbonization of Microalgae and Soybean Straw: A Comparison Study. Bioresour. Technol. 2020, 298, 122502. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Pan, L.; Suvarna, M.; Tong, Y.W.; Wang, X. Fuel Properties of Hydrochar and Pyrochar: Prediction and Exploration with Machine Learning. Appl. Energy 2020, 269, 115166. [Google Scholar] [CrossRef]
- Petrović, J.; Perišić, N.; Maksimović, J.D.; Maksimović, V.; Kragović, M.; Stojanović, M.; Laušević, M.; Mihajlović, M. Hydrothermal Conversion of Grape Pomace: Detailed Characterization of Obtained Hydrochar and Liquid Phase. J. Anal. Appl. Pyrolysis 2016, 118, 267–277. [Google Scholar] [CrossRef]
- Zhuang, X.; Zhan, H.; Song, Y.; He, C.; Huang, Y.; Yin, X.; Wu, C. Insights into the Evolution of Chemical Structures in Lignocellulose and Non-Lignocellulose Biowastes during Hydrothermal Carbonization (HTC). Fuel 2019, 236, 960–974. [Google Scholar] [CrossRef]
- Lu, X.; Pellechia, P.J.; Flora, J.R.V.; Berge, N.D. Influence of Reaction Time and Temperature on Product Formation and Characteristics Associated with the Hydrothermal Carbonization of Cellulose. Bioresour. Technol. 2013, 138, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Dehmani, Y.; Franco, D.S.P.; Georgin, J.; Lamhasni, T.; Brahmi, Y.; Oukhrib, R.; Mustapha, B.; Moussout, H.; Ouallal, H.; Sadik, A. Comparison of Phenol Adsorption Property and Mechanism onto Different Moroccan Clays. Water 2023, 15, 1881. [Google Scholar] [CrossRef]
- Franco, D.S.P.; Georgin, J.; Ramos, C.G.; Eljaiek, S.M.; Badillo, D.R.; de Oliveira, A.H.P.; Allasia, D.; Meili, L. The Synthesis and Evaluation of Porous Carbon Material from Corozo Fruit (Bactris guineensis) for Efficient Propranolol Hydrochloride Adsorption. Molecules 2023, 28, 5232. [Google Scholar] [CrossRef] [PubMed]
- Manzar, M.S.; Ahmad, T.; Zubair, M.; Ullah, N.; Alqahtani, H.A.; da Gama, B.M.V.; Georgin, J.; Nasir, M.; Mu’azu, N.D.; Al Ghamdi, J.M.; et al. Comparative Adsorption of Tetracycline onto Unmodified and NaOH-Modified Silicomanganese Fumes: Kinetic and Process Modeling. Chem. Eng. Res. Des. 2023, 192, 521–533. [Google Scholar] [CrossRef]
- Reza, M.T.; Rottler, E.; Herklotz, L.; Wirth, B. Hydrothermal Carbonization (HTC) of Wheat Straw: Influence of Feedwater PH Prepared by Acetic Acid and Potassium Hydroxide. Bioresour. Technol. 2015, 182, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Capobianco, L.; Di Caprio, F.; Altimari, P.; Astolfi, M.L.; Pagnanelli, F. Production of an Iron-Coated Adsorbent for Arsenic Removal by Hydrothermal Carbonization of Olive Pomace: Effect of the Feedwater PH. J. Environ. Manag. 2020, 273, 111164. [Google Scholar] [CrossRef] [PubMed]
- Li, D.-C.; Jiang, H. The Thermochemical Conversion of Non-Lignocellulosic Biomass to Form Biochar: A Review on Characterizations and Mechanism Elucidation. Bioresour. Technol. 2017, 246, 57–68. [Google Scholar] [CrossRef]
- Zhai, Y.; Liu, X.; Zhu, Y.; Peng, C.; Wang, T.; Zhu, L.; Li, C.; Zeng, G. Hydrothermal Carbonization of Sewage Sludge: The Effect of Feed-Water PH on Fate and Risk of Heavy Metals in Hydrochars. Bioresour. Technol. 2016, 218, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Wei, N.; Dong, Y.T.; Deng, J.; Wang, Y.; Qi, X.L.; Yu, W.F.; Xiao, Y.; Zhou, J.J.; Guan, Z.Z.; Chioca, L.R.; et al. Assessment of Potential Health Risk of Fluoride Consumption through Rice, Pulses, and Vegetables in Addition to Consumption of Fluoride-Contaminated Drinking Water of West Bengal, India. Environ. Geol. 2018, 33, 20300–20314. [Google Scholar] [CrossRef]
- Hasanzadeh, V.; Rahmanian, O.; Heidari, M. Cefixime Adsorption onto Activated Carbon Prepared by Dry Thermochemical Activation of Date Fruit Residues. Microchem. J. 2020, 152, 104261. [Google Scholar] [CrossRef]
- Pauline, A.L.; Joseph, K. Hydrothermal Carbonization of Organic Wastes to Carbonaceous Solid Fuel—A Review of Mechanisms and Process Parameters. Fuel 2020, 279, 118472. [Google Scholar] [CrossRef]
- Simsir, H.; Eltugral, N.; Karagoz, S. Hydrothermal Carbonization for the Preparation of Hydrochars from Glucose, Cellulose, Chitin, Chitosan and Wood Chips via Low-Temperature and Their Characterization. Bioresour. Technol. 2017, 246, 82–87. [Google Scholar] [CrossRef]
- Inada, M.; Enomoto, N.; Hojo, J.; Hayashi, K. Structural Analysis and Capacitive Properties of Carbon Spheres Prepared by Hydrothermal Carbonization. Adv. Powder Technol. 2017, 28, 884–889. [Google Scholar] [CrossRef]
- Zhao, Y.; Tian, Y.; Zhou, H.; Tian, Y. Hydrothermal Conversion of Black Liquor to Phenolics and Hydrochar: Characterization, Application and Comparison with Lignin. Fuel 2020, 280, 118651. [Google Scholar] [CrossRef]
- Álvarez-Murillo, A.; Román, S.; Ledesma, B.; Sabio, E. Study of Variables in Energy Densification of Olive Stone by Hydrothermal Carbonization. J. Anal. Appl. Pyrolysis 2015, 113, 307–314. [Google Scholar] [CrossRef]
- Volpe, M.; Goldfarb, J.L.; Fiori, L. Hydrothermal Carbonization of Opuntia Ficus-Indica Cladodes: Role of Process Parameters on Hydrochar Properties. Bioresour. Technol. 2018, 247, 310–318. [Google Scholar] [CrossRef]
- Stemann, J.; Putschew, A.; Ziegler, F. Hydrothermal Carbonization: Process Water Characterization and Effects of Water Recirculation. Bioresour. Technol. 2013, 143, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Addadi, L.; Joester, D.; Nudelman, F.; Weiner, S. Mollusk Shell Formation: A Source of New Concepts for Understanding Biomineralization Processes. Chem. Eur. J. 2006, 12, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Pang, S. Advances in Thermochemical Conversion of Woody Biomass to Energy, Fuels and Chemicals. Biotechnol. Adv. 2019, 37, 589–597. [Google Scholar] [CrossRef]
- Yu, Q.; Wang, Y.; Van Le, Q.; Yang, H.; Hosseinzadeh-Bandbafha, H.; Yang, Y.; Sonne, C.; Tabatabaei, M.; Lam, S.S.; Peng, W. An Overview on the Conversion of Forest Biomass into Bioenergy. Front. Energy Res. 2021, 9, 684234. [Google Scholar] [CrossRef]
- Sansaniwal, S.K.; Rosen, M.A.; Tyagi, S.K. Global Challenges in the Sustainable Development of Biomass Gasification: An Overview. Renew. Sustain. Energy Rev. 2017, 80, 23–43. [Google Scholar] [CrossRef]
- Butterman, H.C.; Castaldi, M.J. CO2 as a Carbon Neutral Fuel Source via Enhanced Biomass Gasification. Environ. Sci. Technol. 2009, 43, 9030–9037. [Google Scholar] [CrossRef]
- Roncancio, R.; Gore, J.P. CO2 Char Gasification: A Systematic Review from 2014 to 2020. Energy Convers. Manag. X 2021, 10, 100060. [Google Scholar] [CrossRef]
- Cai, J.; Xu, D.; Dong, Z.; Yu, X.; Yang, Y.; Banks, S.W.; Bridgwater, A. V Processing Thermogravimetric Analysis Data for Isoconversional Kinetic Analysis of Lignocellulosic Biomass Pyrolysis: Case Study of Corn Stalk. Renew. Sustain. Energy Rev. 2018, 82, 2705–2715. [Google Scholar] [CrossRef]
- Van de Velden, M.; Baeyens, J.; Brems, A.; Janssens, B.; Dewil, R. Fundamentals, Kinetics and Endothermicity of the Biomass Pyrolysis Reaction. Renew. Energy 2010, 35, 232–242. [Google Scholar] [CrossRef]
- Sánchez-Jiménez, P.E.; Pérez-Maqueda, L.A.; Perejón, A.; Criado, J.M. Limitations of Model-Fitting Methods for Kinetic Analysis: Polystyrene Thermal Degradation. Resour. Conserv. Recycl. 2013, 74, 75–81. [Google Scholar] [CrossRef]
- Preciado-Hernandez, J.; Zhang, J.; Jones, I.; Zhu, M.; Zhang, Z.; Zhang, D. An Experimental Study of Gasification Kinetics during Steam Activation of a Spent Tyre Pyrolysis Char. J. Environ. Chem. Eng. 2021, 9, 105306. [Google Scholar] [CrossRef]
- Zaini, I.N.; López, C.G.; Pretz, T.; Yang, W.; Jönsson, P.G. Characterization of Pyrolysis Products of High-Ash Excavated-Waste and Its Char Gasification Reactivity and Kinetics under a Steam Atmosphere. Waste Manag. 2019, 97, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Nowicki, L.; Siuta, D.; Markowski, M. Carbon Dioxide Gasification Kinetics of Char from Rapeseed Oil Press Cake. Energies 2020, 13, 2318. [Google Scholar] [CrossRef]
- Tian, H.; Hu, Q.; Wang, J.; Liu, L.; Yang, Y.; Bridgwater, A. V Steam Gasification of Miscanthus Derived Char: The Reaction Kinetics and Reactivity with Correlation to the Material Composition and Microstructure. Energy Convers. Manag. 2020, 219, 113026. [Google Scholar] [CrossRef]
- Liu, M.; He, Q.; Bai, J.; Yu, J.; Kong, L.; Bai, Z.; Li, H.; He, C.; Cao, X.; Ge, Z. Char Reactivity and Kinetics Based on the Dynamic Char Structure during Gasification by CO2. Fuel Process. Technol. 2021, 211, 106583. [Google Scholar] [CrossRef]
- Gomez, A.; Silbermann, R.; Mahinpey, N. A Comprehensive Experimental Procedure for CO2 Coal Gasification: Is There Really a Maximum Reaction Rate? Appl. Energy 2014, 124, 73–81. [Google Scholar] [CrossRef]
- Wang, B.; Xu, F.; Wang, X.; Li, J.; Song, Y.; Qiao, Y.; Tian, Y. Comparative Study on Pyrolysis and Gasification within CO2 Atmosphere of Typical Forestry Biomass in Northeast Asia: Thermal Behavior and Kinetic Analysis. Fuel 2022, 324, 124540. [Google Scholar] [CrossRef]
- Hoekman, S.K.; Broch, A.; Robbins, C.; Purcell, R.; Zielinska, B.; Felix, L.; Irvin, J. Process Development Unit (PDU) for Hydrothermal Carbonization (HTC) of Lignocellulosic Biomass. Waste Biomass Valorization 2014, 5, 669–678. [Google Scholar] [CrossRef]
- Hoekman, S.K.; Broch, A.; Felix, L.; Farthing, W. Hydrothermal Carbonization (HTC) of Loblolly Pine Using a Continuous, Reactive Twin-Screw Extruder. Energy Convers. Manag. 2017, 134, 247–259. [Google Scholar] [CrossRef]
- Liu, X.; Hoekman, S.K.; Farthing, W.; Felix, L. TC2015: Life Cycle Analysis of Co-formed Coal Fines and Hydrochar Produced in Twin-screw Extruder (TSE). Environ. Prog. Sustain. Energy 2017, 36, 668–676. [Google Scholar] [CrossRef]
- Oliveira, I.; Blöhse, D.; Ramke, H.G. Hydrothermal Carbonization of Agricultural Residues. Bioresour. Technol. 2013, 142, 138–146. [Google Scholar] [CrossRef]
- Shao, Y.; Long, Y.; Wang, H.; Liu, D.; Shen, D.; Chen, T. Hydrochar Derived from Green Waste by Microwave Hydrothermal Carbonization. Renew. Energy 2019, 135, 1327–1334. [Google Scholar] [CrossRef]
- Afolabi, O.O.D.; Sohail, M. Comparative Evaluation of Conventional and Microwave Hydrothermal Carbonization of Human Biowaste for Value Recovery. Water Sci. Technol. 2017, 75, 2852–2863. [Google Scholar] [CrossRef]
- Amonette, J.E.; Joseph, S. Characteristics of Biochar: Microchemical Properties. In Biochar for Environmental Management; Routledge: London, UK, 2012; pp. 65–84. [Google Scholar]
- Libra, J.A.; Ro, K.S.; Kammann, C.; Funke, A.; Berge, N.D.; Neubauer, Y.; Titirici, M.-M.; Fühner, C.; Bens, O.; Kern, J. Hydrothermal Carbonization of Biomass Residuals: A Comparative Review of the Chemistry, Processes and Applications of Wet and Dry Pyrolysis. Biofuels 2011, 2, 71–106. [Google Scholar] [CrossRef]
- Sharma, H.B.; Panigrahi, S.; Dubey, B.K. Hydrothermal Carbonization of Yard Waste for Solid Bio-Fuel Production: Study on Combustion Kinetic, Energy Properties, Grindability and Flowability of Hydrochar. Waste Manag. 2019, 91, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Hong, J.; Jang, D.; Park, K.Y. Hydrothermal Carbonization of Waste from Leather Processing and Feasibility of Produced Hydrochar as an Alternative Solid Fuel. J. Environ. Manag. 2019, 247, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Olszewski, M.P.; Arauzo, P.J.; Wądrzyk, M.; Kruse, A. Py-GC-MS of Hydrochars Produced from Brewer’s Spent Grains. J. Anal. Appl. Pyrolysis 2019, 140, 255–263. [Google Scholar] [CrossRef]
- Wang, Z.; Zhai, Y.; Wang, T.; Wang, B.; Peng, C.; Li, C. Pelletizing of Hydrochar Biofuels with Organic Binders. Fuel 2020, 280, 118659. [Google Scholar] [CrossRef]
- Zhou, J.Y.; Luo, Z.Y.; Yin, M.J.; Wang, N.; Qin, Z.; Lee, K.R.; An, Q.F. A Comprehensive Study on Phase Inversion Behavior of a Novel Polysulfate Membrane for High-Performance Ultrafiltration Applications. J. Memb. Sci. 2020, 610, 118404. [Google Scholar] [CrossRef]
- Chong, C.T.; Ng, J. Chapter 4-Combustion Performance of Biojet Fuels. Biojet Fuel Aviat. Appl. 2021, 175–230. [Google Scholar]
- Fakudze, S.; Chen, J. A Critical Review on Co-Hydrothermal Carbonization of Biomass and Fossil-Based Feedstocks for Cleaner Solid Fuel Production: Synergistic Effects and Environmental Benefits. Chem. Eng. J. 2023, 457, 141004. [Google Scholar] [CrossRef]
- Qian, C.; Li, Q.; Zhang, Z.; Wang, X.; Hu, J.; Cao, W. Prediction of Higher Heating Values of Biochar from Proximate and Ultimate Analysis. Fuel 2020, 265, 116925. [Google Scholar] [CrossRef]
- Parnthong, J.; Nualyai, S.; Kraithong, W.; Jiratanachotikul, A.; Khemthong, P.; Faungnawakij, K.; Kuboon, S. Higher Heating Value Prediction of Hydrochar from Sugarcane Leaf and Giant Leucaena Wood during Hydrothermal Carbonization Process. J. Environ. Chem. Eng. 2022, 10, 108529. [Google Scholar] [CrossRef]
- Fassinou, W.F. Higher Heating Value (HHV) of Vegetable Oils, Fats and Biodiesels Evaluation Based on Their Pure Fatty Acids’ HHV. Energy 2012, 45, 798–805. [Google Scholar] [CrossRef]
- Rosa, J.S.; Lorenzini, G.; Altafini, C.R.; Wander, P.R.; Telli, G.D.; Rocha, L.A.O. Performance Effects and Economic Viability of High-Hydrated Ethanol Fumigation and Diesel Direct Injection in a Small Compression Ignition Engine. Math. Model. Eng. Probl. 2019, 6, 1–9. [Google Scholar] [CrossRef]
- Nguyen, D.; Zhao, W.; Mäkelä, M.; Alwahabi, Z.T.; Kwong, C.W. Effect of Hydrothermal Carbonisation Temperature on the Ignition Properties of Grape Marc Hydrochar Fuels. Fuel 2022, 313, 122668. [Google Scholar] [CrossRef]
- An, Q.; Wang, Q.; Zhai, J. Hydrothermal Carbonization of Corncob for Hydrochar Production and Its Combustion Reactivity in a Blast Furnace. Environ. Sci. Pollut. Res. 2024, 31, 16653–16666. [Google Scholar] [CrossRef]
- Poomsawat, S.; Poomsawat, W. Analysis of Hydrochar Fuel Characterization and Combustion Behavior Derived from Aquatic Biomass via Hydrothermal Carbonization Process. Case Stud. Therm. Eng. 2021, 27, 101255. [Google Scholar] [CrossRef]
- Bergthorson, J.M.; Thomson, M.J. A Review of the Combustion and Emissions Properties of Advanced Transportation Biofuels and Their Impact on Existing and Future Engines. Renew. Sustain. Energy Rev. 2015, 42, 1393–1417. [Google Scholar] [CrossRef]
- Ahmad, T.; Manzar, M.S.; Khan, S.; Al-Sharafi, M.A.; Georgin, J.; Franco, D.S.P.; Ullah, N. Enhanced Adsorption of Bisphenol-A from Water through the Application of Isocyanurate Based Hyper Crosslinked Resin. J. Mol. Liq. 2024, 395, 123861. [Google Scholar] [CrossRef]
- Grassi, P.; Georgin, J.; Franco, D.S.P.; Sá, Í.M.G.L.; Lins, P.V.S.; Foletto, E.L.; Jahn, S.L.; Meili, L.; Lins, P.V.S.; Foletto, E.L.; et al. Removal of Dyes from Water Using Citrullus Lanatus Seed Powder in Continuous and Discontinuous Systems. Int. J. Phytoremediation 2023, 26, 82–97. [Google Scholar] [CrossRef]
- Yanan, C.; Srour, Z.; Ali, J.; Guo, S.; Taamalli, S.; Fèvre-Nollet, V.; da Boit Martinello, K.; Georgin, J.; Franco, D.S.P.; Silva, L.F.O.; et al. Adsorption of Paracetamol and Ketoprofenon Activated Charcoal Prepared from the Residue of the Fruit of Butiacapitate: Experiments and Theoretical Interpretations. Chem. Eng. J. 2023, 454, 139943. [Google Scholar] [CrossRef]
- Jiang, Q.; Xie, W.; Han, S.; Wang, Y.; Zhang, Y. Enhanced Adsorption of Pb(II) onto Modified Hydrochar by Polyethyleneimine or H3PO4: An Analysis of Surface Property and Interface Mechanism. Colloids Surf. A Physicochem. Eng. Asp. 2019, 583, 123962. [Google Scholar] [CrossRef]
- Nguyen, D.H.; Tran, H.N.; Chao, H.-P.; Lin, C. Effect of Nitric Acid Oxidation on the Surface of Hydrochars to Sorb Methylene Blue: An Adsorption Mechanism Comparison. Adsorpt. Sci. Technol. 2019, 37, 607–622. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, T.; Ren, H.; Kruse, A.; Cui, R. Polyethylene Imine Modified Hydrochar Adsorption for Chromium (VI) and Nickel (II) Removal from Aqueous Solution. Bioresour. Technol. 2018, 247, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Lv, J.-Q.; Guo, J.-Z.; Fu, S.-Y.; Guo, M.; Yang, P. The Polyaminocarboxylated Modified Hydrochar for Efficient Capturing Methylene Blue and Cu (II) from Water. Bioresour. Technol. 2019, 275, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xiang, W.; Wang, B.; Fang, J.; Zou, W.; He, F.; Li, Y.; Tsang, D.C.W.; Ok, Y.S.; Gao, B. Adsorption of Acetone and Cyclohexane onto CO2 Activated Hydrochars. Chemosphere 2020, 245, 125664. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Guo, J.; Lv, K.; Fan, J. Adsorption of Methylene Blue and Cd(II) onto Maleylated Modified Hydrochar from Water. Environ. Pollut. 2019, 254, 113014. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Gao, B.; Fang, J.; Zou, W.; Dong, L.; Cao, C.; Zhang, J.; Li, Y.; Wang, H. Chemically Activated Hydrochar as an Effective Adsorbent for Volatile Organic Compounds (VOCs). Chemosphere 2019, 218, 680–686. [Google Scholar] [CrossRef]
- Yu, X.; Liu, S.; Lin, G.; Yang, Y.; Zhang, S.; Zhao, H.; Zheng, C. KOH-Activated Hydrochar with Engineered Porosity as Sustainable Adsorbent for Volatile Organic Compounds. Colloids Surf. A 2020, 588, 124372. [Google Scholar] [CrossRef]
- Li, Y.; Tsend, N.; Li, T.K.; Liu, H.; Yang, R.; Gai, X.; Wang, H.; Shan, S. Microwave Assisted Hydrothermal Preparation of Rice Straw Hydrochars for Adsorption of Organics and Heavy Metals. Bioresour. Technol. 2019, 273, 136–143. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Ngo, H.H.; Guo, W.; Wen, H.; Zhang, D.; Li, C.; Qi, L. Characterization and Sulfonamide Antibiotics Adsorption Capacity of Spent Coffee Grounds Based Biochar and Hydrochar. Sci. Total Environ. 2020, 716, 137015. [Google Scholar] [CrossRef]
- Hua, Y.; Zheng, X.; Xue, L.; Han, L.; He, S.; Mishra, T.; Feng, Y.; Yang, L.; Xing, B. Microbial Aging of Hydrochar as a Way to Increase Cadmium Ion Adsorption Capacity: Process and Mechanism. Bioresour. Technol. 2020, 300, 122708. [Google Scholar] [CrossRef]
- Dehbi, A.; Dehmani, Y.; Franco, D.S.P.; Omari, H.; Georgin, J.; Brahmi, Y.; Elazhari, K.; Messaoudi, M.; Aadnan, I.; Lamhasni, T. A Statistical Physics Approach to Understanding the Adsorption of Methylene Blue onto Cobalt Oxide Nanoparticles. Molecules 2024, 29, 412. [Google Scholar] [CrossRef]
- Georgin, J.; Franco, D.S.P.; Meili, L.; Bonilla-Petriciolet, A.; Kurniawan, T.A.; Imanova, G.; Demir, E.; Ali, I. Environmental Remediation of the Norfloxacin in Water by Adsorption: Advances, Current Status and Prospects. Adv. Colloid Interface Sci. 2024, 324, 103096. [Google Scholar] [CrossRef] [PubMed]
- Gai, C.; Chen, M.; Liu, T.; Peng, N.; Liu, Z. Gasification Characteristics of Hydrochar and Pyrochar Derived from Sewage Sludge. Energy 2016, 113, 957–965. [Google Scholar] [CrossRef]
- Li, L.; Flora, J.R.V.; Berge, N.D. Predictions of Energy Recovery from Hydrochar Generated from the Hydrothermal Carbonization of Organic Wastes. Renew. Energy 2020, 145, 1883–1889. [Google Scholar] [CrossRef]
- Franco, D.S.P.; Georgin, J.; Ramos, C.G.; Netto, M.S.; Ojeda, N.J.; Vega, N.A.; Meili, L.; Lima, E.C.; Naushad, M. The Production of Activated Biochar Using Calophyllum Inophyllum Waste Biomass and Use as an Adsorbent for Removal of Diuron from the Water in Batch and Fixed Bed Column. Environ. Sci. Pollut. Res. 2023, 30, 52498–52513. [Google Scholar] [CrossRef] [PubMed]
- Georgin, J.; Franco, D.S.P.; Netto, M.S.; Gama, B.M.V.; Fernandes, D.P.; Sepúlveda, P.; Silva, L.F.O.; Meili, L. Effective Adsorption of Harmful Herbicide Diuron onto Novel Activated Carbon from Hovenia Dulcis. Colloids Surf. A Physicochem. Eng. Asp. 2022, 654, 129900. [Google Scholar] [CrossRef]
- Langmuir, I. Adsorption of Gases on Glass, Mica and Platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-Second Order Model for Sorption Processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Duy Nguyen, H.; Nguyen Tran, H.; Chao, H.-P.; Lin, C.-C. Activated Carbons Derived from Teak Sawdust-Hydrochars for Efficient Removal of Methylene Blue, Copper, and Cadmium from Aqueous Solution. Water 2019, 11, 2581. [Google Scholar] [CrossRef]
- Ramutshatsha-Makhwedzha, D.; Mavhungu, A.; Moropeng, M.L.; Mbaya, R. Activated Carbon Derived from Waste Orange and Lemon Peels for the Adsorption of Methyl Orange and Methylene Blue Dyes from Wastewater. Heliyon 2022, 8, e09930. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.-Z.; Li, B.; Liu, L.; Lv, K. Removal of Methylene Blue from Aqueous Solutions by Chemically Modified Bamboo. Chemosphere 2014, 111, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Franco, D.S.P.; Georgin, J.; Lima, E.C.; Silva, L.F.O. Advances Made in Removing Paraquat Herbicide by Adsorption Technology: A Review. J. Water Process Eng. 2022, 49, 102988. [Google Scholar] [CrossRef]
- Dhaouadi, F.; Sellaoui, L.; Taamalli, S.; Louis, F.; El Bakali, A.; Badawi, M.; Georgin, J.; Franco, D.S.P.; Silva, L.F.O.; Bonilla-Petriciolet, A. Enhanced Adsorption of Ketoprofen and 2, 4-Dichlorophenoxyactic Acid on Physalis Peruviana Fruit Residue Functionalized with H2SO4: Adsorption Properties and Statistical Physics Modeling. Chem. Eng. J. 2022, 445, 136773. [Google Scholar] [CrossRef]
- Ojukwu, V.E.; Akaeme, F.C.; Ighalo, J.O. Microplastic Pollution in the Changing Climate. In Microplastics in African and Asian Environments: The Influencers, Challenges, and Solutions; Springer: Berlin/Heidelberg, Germany, 2024; pp. 219–232. [Google Scholar]
- Adeyanju, C.A.; Ogunniyi, S.; Rangabhashiyam, S.; Oniye, M.M.; Ajala, O.J.; Adeniyi, A.G.; Igwegbe, C.A.; Ighalo, J.O. Recent Advances on the Aqueous Phase Adsorption of Carbamazepine. ChemBioEng Rev. 2022, 9, 231–247. [Google Scholar] [CrossRef]
- Ghosh, S.; Malloum, A.; Igwegbe, C.A.; Ighalo, J.O.; Ahmadi, S.; Deghani, M.H.; Othmani, A.; Gokkus, O.; Mubarak, N.M. A Review on New Generation Adsorbent for Remediation of Fluoride from Water and Wastewater. J. Mol. Liq. 2021, 346, 118257. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.J.; Chao, H.P. Insight into Adsorption Mechanism of Cationic Dye onto Agricultural Residues-Derived Hydrochars: Negligible Role of π-π Interaction. Korean J. Chem. Eng. 2017, 34, 1708–1720. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, Y.; Zhou, C.; Luo, G.; Zhang, S.; Chen, J. A Novel Porous Carbon Derived from Hydrothermal Carbon for Efficient Adsorption of Tetracycline. Carbon N. Y. 2014, 77, 627–636. [Google Scholar] [CrossRef]
- Parshetti, G.K.; Chowdhury, S.; Balasubramanian, R. Hydrothermal Conversion of Urban Food Waste to Chars for Removal of Textile Dyes from Contaminated Waters. Bioresour. Technol. 2014, 161, 310–319. [Google Scholar] [CrossRef]
- Ruan, X.; Liu, Y.; Wang, G.; Frost, R.L.; Qian, G.; Tsang, D.C.W. Transformation of Functional Groups and Environmentally Persistent Free Radicals in Hydrothermal Carbonisation of Lignin. Bioresour. Technol. 2018, 270, 223–229. [Google Scholar] [CrossRef]
- Islam, M.A.; Ahmed, M.J.; Khanday, W.A.; Asif, M.; Hameed, B.H. Mesoporous Activated Carbon Prepared from NaOH Activation of Rattan (Lacosperma secundiflorum) Hydrochar for Methylene Blue Removal. Ecotoxicol. Environ. Saf. 2017, 138, 279–285. [Google Scholar] [CrossRef]
- He, C.; Giannis, A.; Wang, J.-Y. Conversion of Sewage Sludge to Clean Solid Fuel Using Hydrothermal Carbonization: Hydrochar Fuel Characteristics and Combustion Behavior. Appl. Energy 2013, 111, 257–266. [Google Scholar] [CrossRef]
- Li, R.; Zhang, Y.; Deng, H.; Zhang, Z.; Wang, J.J.; Shaheen, S.M.; Xiao, R.; Rinklebe, J.; Xi, B.; He, X. Removing Tetracycline and Hg (II) with Ball-Milled Magnetic Nanobiochar and Its Potential on Polluted Irrigation Water Reclamation. J. Hazard. Mater. 2020, 384, 121095. [Google Scholar] [CrossRef]
- Franzreb, M. New Classes of Selective Separations Exploiting Magnetic Adsorbents. Curr. Opin. Colloid 2020, 46, 65–76. [Google Scholar] [CrossRef]
- Reynel-Ávila, H.E.; Camacho-Aguilar, K.I.; Bonilla-Petriciolet, A.; Mendoza-Castillo, D.I.; González-Ponce, H.A.; Trejo-Valencia, R. Engineered Magnetic Carbon-Based Adsorbents for the Removal of Water Priority Pollutants: An Overview. Adsorpt. Sci. Technol. 2021, 2021, 9917444. [Google Scholar] [CrossRef]
- Rudakov, G.A.; Tsiberkin, K.B.; Ponomarev, R.S.; Henner, V.K.; Ziolkowska, D.A.; Jasinski, J.B.; Sumanasekera, G. Magnetic Properties of Transition Metal Nanoparticles Enclosed in Carbon Nanocages. J. Magn. Magn. Mater. 2019, 472, 34–39. [Google Scholar] [CrossRef]
- Siddiqui, M.T.H.; Nizamuddin, S.; Baloch, H.A.; Mubarak, N.M.; Al-Ali, M.; Mazari, S.A.; Bhutto, A.W.; Abro, R.; Srinivasan, M.; Griffin, G. Fabrication of Advance Magnetic Carbon Nano-Materials and Their Potential Applications: A Review. J. Environ. Chem. Eng. 2019, 7, 102812. [Google Scholar] [CrossRef]
- Wang, H.; Duan, R.; Ding, L.; Tian, L.; Liu, Y.; Zhang, Y.; Xu, R. Magnetic Hydrochar Derived from Waste Lignin for Thallium Removal from Wastewater: Performance and Mechanisms. Bioresour. Technol. 2023, 374, 128736. [Google Scholar] [CrossRef]
- Staroń, P.; Kuciakowski, J.; Chwastowski, J. Biocomposite of Hydrochar and Lindnera Jadinii with Magnetic Properties for Adsorptive Removal of Cadmium Ions. J. Environ. Chem. Eng. 2023, 11, 110270. [Google Scholar] [CrossRef]
- Kazak, O.; Tor, A. In Situ Preparation of Magnetic Hydrochar by Co-Hydrothermal Treatment of Waste Vinasse with Red Mud and Its Adsorption Property for Pb (II) in Aqueous Solution. J. Hazard. Mater. 2020, 393, 122391. [Google Scholar] [CrossRef] [PubMed]
- Algethami, J.S.; Alhamami, M.A.M.; Alqadami, A.A.; Melhi, S.; Seliem, A.F. Adsorptive Performance of a New Magnetic Hydrochar Nanocomposite for Highly Efficient Removal of Cadmium Ions from Water: Mechanism, Modeling, and Reusability Studies. Environ. Technol. Innov. 2023, 32, 103404. [Google Scholar] [CrossRef]
- Murthy, T.P.K.; Gowrishankar, B.S.; Krishna, R.H.; Chandraprabha, M.N.; Mathew, B.B. Magnetic Modification of Coffee Husk Hydrochar for Adsorptive Removal of Methylene Blue: Isotherms, Kinetics and Thermodynamic Studies. Environ. Chem. Ecotoxicol. 2020, 2, 205–212. [Google Scholar] [CrossRef]
- Liu, T.; Chen, Z.; Li, Z.; Fu, H.; Chen, G.; Feng, T.; Chen, Z. Preparation of Magnetic Hydrochar Derived from Iron-Rich Phytolacca Acinosa Roxb. for Cd Removal. Sci. Total Environ. 2021, 769, 145159. [Google Scholar] [CrossRef] [PubMed]
- Espro, C.; Satira, A.; Mauriello, F.; Anajafi, Z.; Moulaee, K.; Iannazzo, D.; Neri, G. Orange Peels-Derived Hydrochar for Chemical Sensing Applications. Sens. Actuators B Chem. 2021, 341, 130016. [Google Scholar] [CrossRef]
- Han, M.; Kim, J.K.; Lee, J.; An, H.K.; Yun, J.P.; Kang, S.-W.; Jung, D. Room-Temperature Hydrogen-Gas Sensor Based on Carbon Nanotube Yarn. J. Nanosci. Nanotechnol. 2020, 20, 4011–4014. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.; Liu, R.; Lei, C.; Wang, K.; Li, Z.; Li, Y. Preparation and Test of NH3 Gas Sensor Based on Single-Layer Graphene Film. Micromachines 2020, 11, 965. [Google Scholar] [CrossRef]
- Babeker, T.M.A.; Chen, Q. Heavy Metal Removal from Wastewater by Adsorption with Hydrochar Derived from Biomass: Current Applications and Research Trends. Curr. Pollut. Rep. 2021, 7, 54–71. [Google Scholar] [CrossRef]
- Torrinha, Á.; Oliveira, T.M.B.F.; Ribeiro, F.W.P.; Correia, A.N.; Lima-Neto, P.; Morais, S. Application of Nanostructured Carbon-Based Electrochemical (Bio) Sensors for Screening of Emerging Pharmaceutical Pollutants in Waters and Aquatic Species: A Review. Nanomaterials 2020, 10, 1268. [Google Scholar] [CrossRef] [PubMed]
- Barreto, F.C.; Ito, E.Y.; Mounienguet, N.K.; Dal’Evedove Soares, L.; Yang, J.; He, Q.; Cesarino, I. Electrochemical Sensor Based on Spent Coffee Grounds Hydrochar and Metal Nanoparticles for Simultaneous Detection of Emerging Contaminants in Natural Water. Chemosensors 2023, 11, 562. [Google Scholar] [CrossRef]
- Hung, C.M.; Van Duy, N.; Van Quang, V.; Van Toan, N.; Van Hieu, N.; Hoa, N.D. Facile Synthesis of Ultrafine RGO/WO3 Nanowire Nanocomposites for Highly Sensitive Toxic NH3 Gas Sensors. Mater. Res. Bull. 2020, 125, 110810. [Google Scholar] [CrossRef]
- Shen, R.; Lu, J.; Yao, Z.; Zhao, L.; Wu, Y. The Hydrochar Activation and Biocrude Upgrading from Hydrothermal Treatment of Lignocellulosic Biomass. Bioresour. Technol. 2021, 342, 125914. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.O.S.; Granja, H.S.; dos Santos, J.F.; Freitas, L.S.; Sussuchi, E.M. Biochar and Hydrochar in the Development and Application of Electrochemical Devices in the Sensing and Degradation of Target Compounds: A Mini-Review of the Recent Contributions of 2020-2023. J Braz Chem Soc 2024, 35, e-20230143. [Google Scholar] [CrossRef]
- de Oliveira, S.; Silva, J.; Sant’Anna, M.V.S.; Gevaerd, A.; Lima, J.B.S.; Monteiro, M.D.S.; Carvalho, S.W.M.M.; Midori Sussuchi, E. A Novel Carbon Nitride Nanosheets-based Electrochemical Sensor for Determination of Hydroxychloroquine in Pharmaceutical Formulation and Synthetic Urine Samples. Electroanalysis 2021, 33, 2152–2160. [Google Scholar] [CrossRef]
- Durai, L.; Gopalakrishnan, A.; Badhulika, S. Facile Synthesis of Biomass-Derived Sulfonated Carbon Microspheres and Nanosheets for the Electrochemical Detection of Glutathione in Biological Samples. Mater. Lett. 2021, 282, 128683. [Google Scholar] [CrossRef]
- Ma, F.; Li, X.; Li, Y.; Feng, Y.; Ye, B.-C. High Current Flux Electrochemical Sensor Based on Nickel-Iron Bimetal Pyrolytic Carbon Material of Paper Waste Pulp for Clenbuterol Detection. Talanta 2022, 250, 123756. [Google Scholar] [CrossRef]
- Ferlazzo, A.; Bressi, V.; Espro, C.; Iannazzo, D.; Piperopoulos, E.; Neri, G. Electrochemical Determination of Nitrites and Sulfites by Using Waste-Derived Nanobiochar. J. Electroanal. Chem. 2023, 928, 117071. [Google Scholar] [CrossRef]
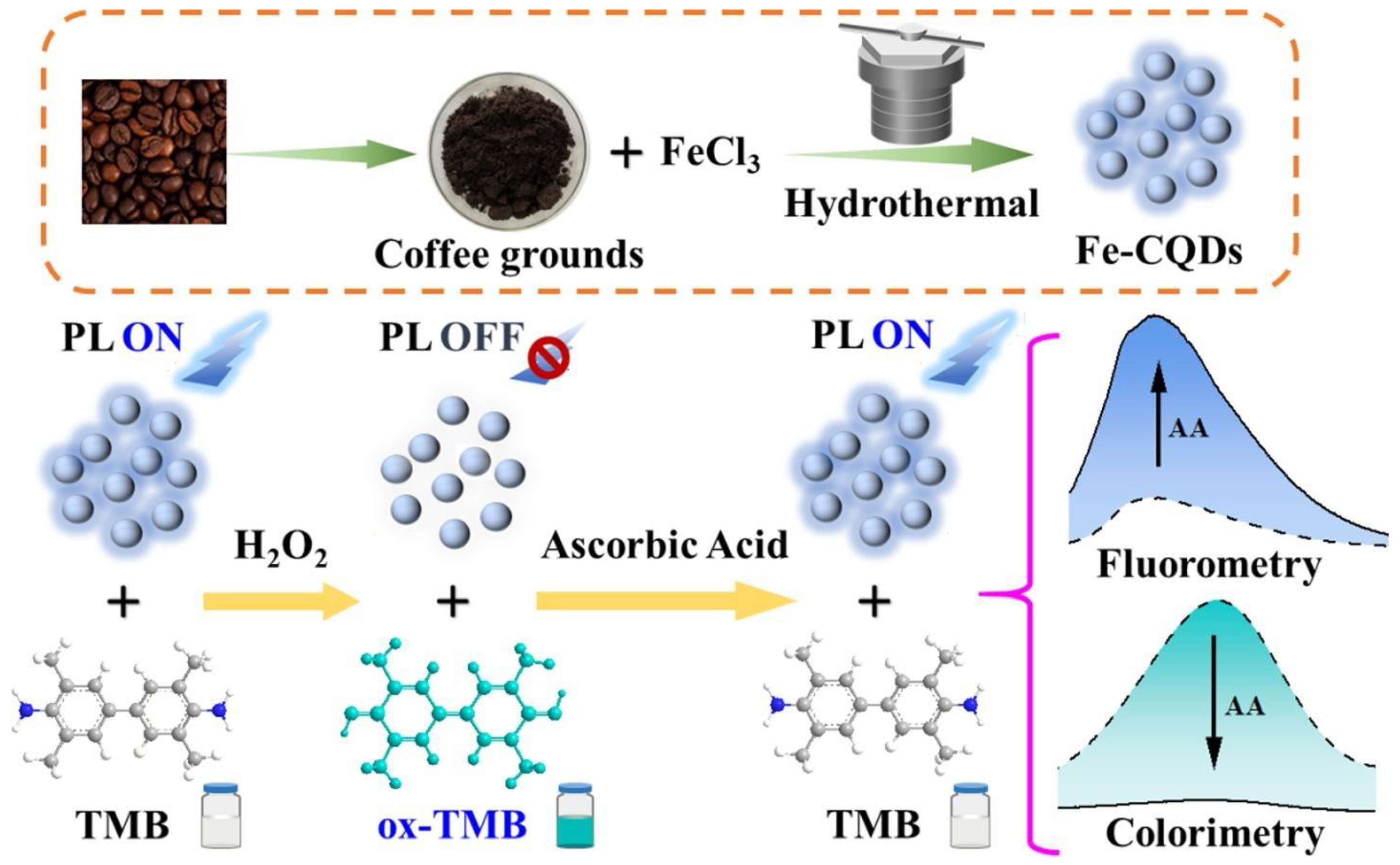
- Zhu, Y.; Deng, X.; Chen, J.; Hu, Z.; Wu, F. Coffee Grounds-Derived Carbon Quantum Dots as Peroxidase Mimetics for Colorimetric and Fluorometric Detection of Ascorbic Acid. Food Chem. 2023, 429, 136957. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.O.; de Santiago, M.Q.; Nascimento, K.S.; Sousa Cavada, B.; de Castro Miguel, E.; de Paula, A.J.; Ferreira, O.P. Hydrochar as Protein Support: Preservation of Biomolecule Properties with Non-Covalent Immobilization. J. Mater. Sci. 2017, 52, 13378–13389. [Google Scholar] [CrossRef]
- Da Silva, R.B.P.; Campos, M.C.C.; Silva, L.S.; de Brito Filho, E.G.; de Lima, A.F.L.; Pinheiro, E.N.; Cunha, J.M. Concentration of Heavy Metals in Soils under Cemetery Occupation in Amazonas, Brazil. Soil. Sediment. Contam. Int. J. 2020, 29, 192–208. [Google Scholar] [CrossRef]
- Lonappan, L.; Liu, Y.; Rouissi, T.; Pourcel, F.; Kaur, S.; Verma, M.; Surampalli, R.Y. Covalent Immobilization of Laccase on Citric Acid Functionalized Micro- Biochars Derived from Di Ff Erent Feedstock and Removal of Diclofenac. Chem. Eng. J. 2018, 351, 985–994. [Google Scholar] [CrossRef]
- Primožič, M.; Podrepšek, G.H.; Pavlovič, I.; Škerget, M.; Knez, Ž.; Leitgeb, M. Enzyme Immobilization onto Biochar Produced by the Hydrothermal Carbonization of Biomass. Acta Chim. Slov. 2019, 66, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Lucian, M.; Fiori, L. Hydrothermal Carbonization of Waste Biomass: Process Design, Modeling, Energy Efficiency and Cost Analysis. Energies 2017, 10, 211. [Google Scholar] [CrossRef]
- Akbari, M.; Oyedun, A.O.; Kumar, A. Comparative Energy and Techno-Economic Analyses of Two Different Configurations for Hydrothermal Carbonization of Yard Waste. Bioresour. Technol. Rep. 2019, 7, 100210. [Google Scholar] [CrossRef]
- Saba, A.; McGaughy, K.; Reza, M.T. Techno-Economic Assessment of Co-Hydrothermal Carbonization of a Coal-Miscanthus Blend. Energies 2019, 12, 630. [Google Scholar] [CrossRef]
- Medina-Martos, E.; Istrate, I.-R.; Villamil, J.A.; Gálvez-Martos, J.-L.; Dufour, J.; Mohedano, Á.F. Techno-Economic and Life Cycle Assessment of an Integrated Hydrothermal Carbonization System for Sewage Sludge. J. Clean. Prod. 2020, 277, 122930. [Google Scholar] [CrossRef]




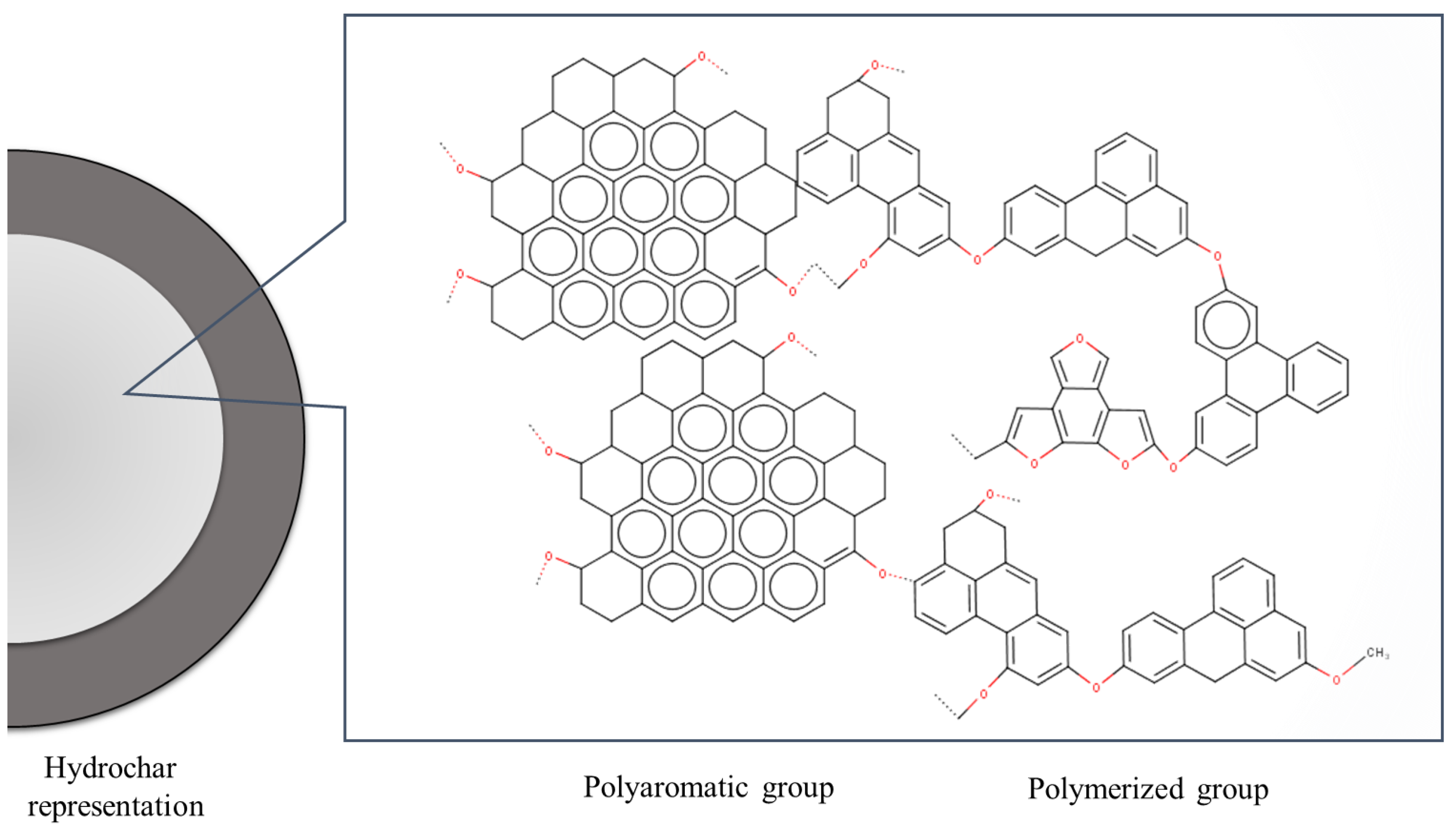



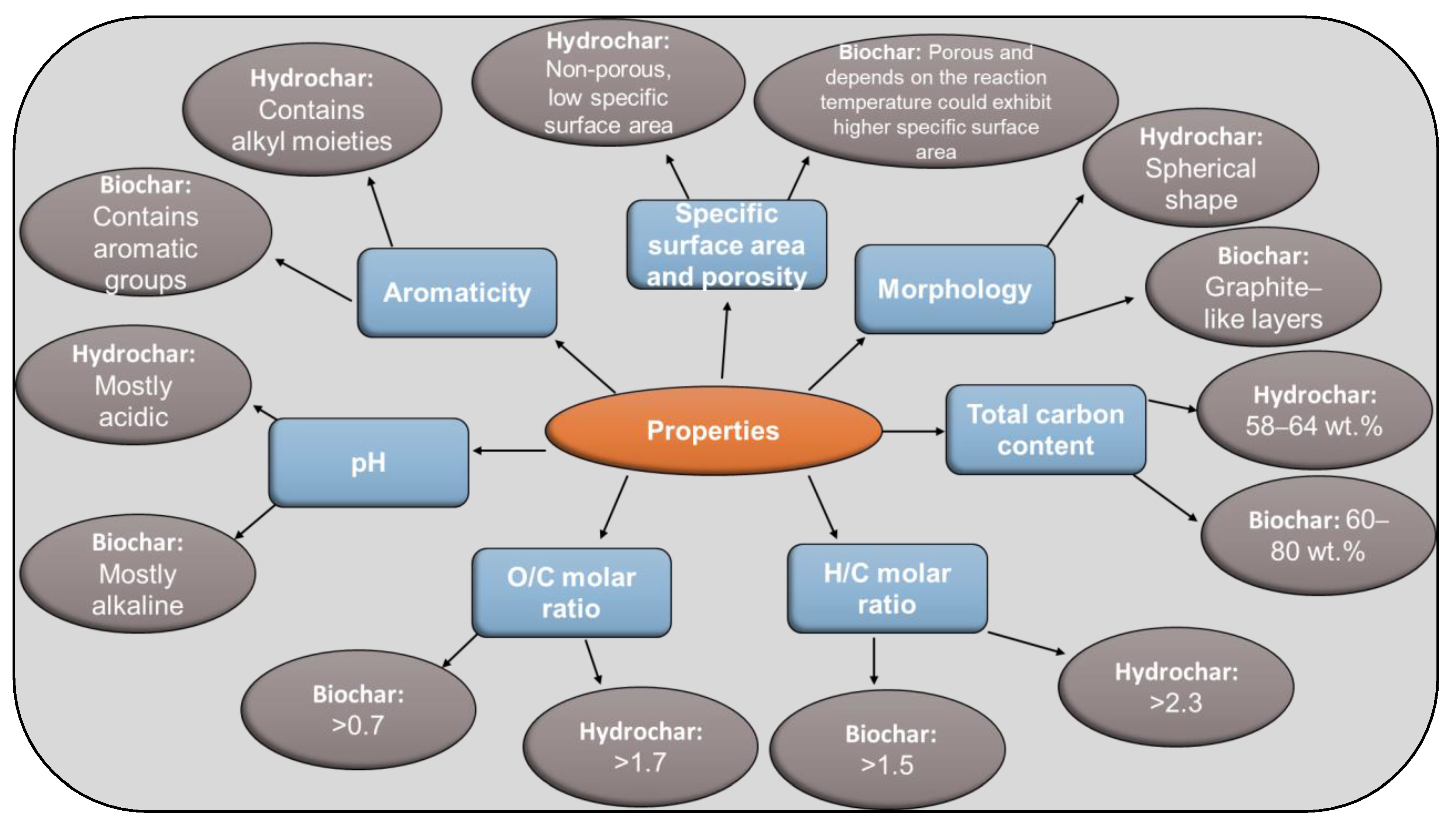
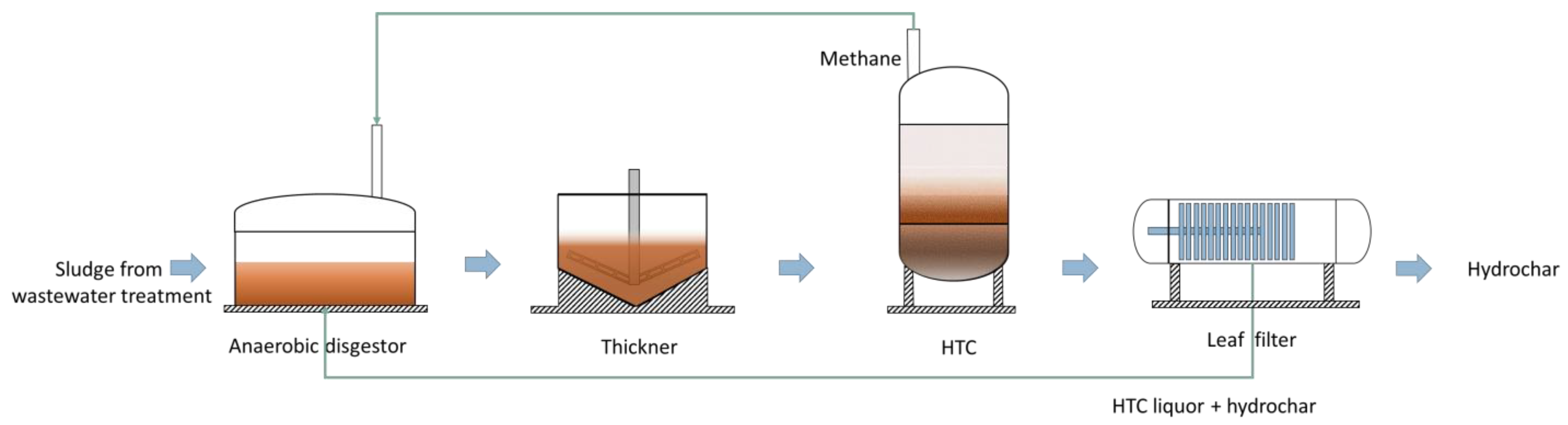
| Properties | Biochar | Hydrochar |
|---|---|---|
| Aromaticity | Contains aromatic groups | Contains alkyl moieties |
| Specific surface area and porosity | Porous and, depending on the reaction temperature, could exhibit higher specific surface area (>400 m2 g−1) | Non-porous, low specific surface area |
| pH | Mostly alkaline | Mostly acidic |
| Morphology | Graphite-like layers | Spherical shape |
| O/C molar ratio | >0.7 | >1.7 |
| Total carbon content (wt%) | 60–80 | 58–64 |
| H/C molar ratio | >1.5 | >2.3 |
| Raw Material | Temperature (°C) | Time (h) | Hydrochar Yield (wt%) | Carbon Content (wt%) | H/C Atomic Ratio | Higher Calorific Value (MJ/kg) | Reference |
|---|---|---|---|---|---|---|---|
| Rice husk | 200 | 6 | 66 | 4.8 | 1.27 | 15.7 | [77] |
| Pinewood chips | 250 | 6 | 74 | 53.3 | - | - | [78] |
| Pinewood meal | 265 | 20 | 60 | 74.2 | 5.6 | - | [79] |
| Wood meal | 260 | 0.5 | 68 | 67.4 | 5.8 | - | [80] |
| Rubberwood fiber | 260 | 9 | 91 | 66.9 | 6.4 | - | [81] |
| Eucalyptus wood | 240 | 3 | 72 | - | - | - | [82] |
| Pinewood | 240 | 1 | 79 | 65.4 | 6.6 | - | [83] |
| Sweet potato peels | 300 | 2 | 64 | 46.9 | 6.2 | - | [84] |
| Sugarcane bagasse | 300 | 0.5 | 88 | 70 | 0.6 | - | [85] |
| Microalgae | 222 | 0.58 | 15.4 | 55.6 | 0.12 | - | [86] |
| Sewage sludge | 220 | 0.5 | 56 | 7.9 | 0.16 | 3.6 | [87] |
| Microalgae | 272 | 0.16 | 12.1 | 54.3 | 0.12 | - | [88] |
| Wastewater-grown algae | 350 | 0.5 | - | 27 | 0.06 | 20.3 | [89] |
| Microalgae | 250 | 1 | 22.7 | 27.33 | 0.12 | - | [90] |
| Sugarcane bagasse and vinasse | 230 | 13 | 27 | 62.7 | 5.4 | - | [91] |
| Moso bamboo | 220 | 1 | 51.8 | - | - | 19.8 | [92] |
| Green waste | 190 | 1 | 80 | 48.8 | 1.2 | 19.2 | [93] |
| Cotton stalk | 240 | 2 | 57 | 69 | 0.91 | - | [94] |
| Cotton stalk | 180 | 4 | 60 | 51.2 | 1.2 | - | [94] |
| Corn cob residue | 250 | 0.55 | 46.6 | 61.7 | 0.08 | 24.3 | [95] |
| Bamboo shoot bark | 210 | 0.5 | 56.4 | 51.3 | - | - | [96] |
| Grape pomace | 190 | - | - | 55.7 | 0.1 | - | [97] |
| Apple pulp with chips | 190 | - | - | 55.9 | 0.13 | - | [97] |
| rotten apple | 190 | - | - | 62.5 | 0.09 | - | [97] |
| Apple juice pulp | 190 | - | - | 53.9 | 0.11 | - | [97] |
| Chinese fan palm | 210 | 0.5 | 60 | 57.3 | 1.62 | 24.9 | [98] |
| Chinese fan palm | 180 | 1 | 61 | 55.9 | 1.64 | 24.2 | [98] |
| Biomass | Hydrothermal Carbonization | Adsorbate | Kinetic Model | Isotherm/Qmax (mg g−1) | Reuse (Cycles) | Ref. |
|---|---|---|---|---|---|---|
| Sawdust | 190 °C for 12 h (50 mL of 0.1 M N-cyclohexyl sulfamic acid + 10 g) | Benzotriazole | Pseudo-second order | Langmuir/160 | 7 | [172] |
| Cu (II) | - | Temkin/299 | - | |||
| Orange peels | 190 °C for 24 h, (110 mL of distilled water + 15 g), (30 mL 70% of HNO3 with 1 g) | Methylene Blue | Pseudo-second order | Langmuir/107 | - | [173] |
| Corn cobs | 300 °C for 30 min (240 mL of ultrapure water + 40 g) | Cr (IV) | - | Langmuir/34 | - | [174] |
| Ni (II) | - | Freundlich/29 | - | |||
| Bamboo | 200 °C for 24 H (160 mL of HCl + 40 g) | Methylene Blue | Pseudo-second order | Langmuir/141 | - | [175] |
| Cu (II) | - | 1239 | - | |||
| The mixture of walnut and peanut tree residues | 200 °C total of 6 h, with 2 h being under CO2 flux of 150 mL/min) | Acetone | Pseudo-second order | 39.4 | 5 | [176] |
| Bamboo | 200 °C, HCL (1 M) with 40 g (1:2 w/w C4H2O3, maleic anhydride, +200 mL of NaHCO3, sodium bicarbonate at 140 °C for 20 min) | Methylene Blue | Pseudo-second order | Langmuir/621 | 4 | [177] |
| Cd (II) | - | 49 | - | |||
| The mixture of walnut and peanut tree residues | 200 °C for 6 h of 1:1 (w/w) with activation with KOH (50%) or H3PO4 (85%) for 1 h at 600 °C | Acetone | Pseudo-second order | 50.5 | 5 | [178] |
| Cyclohexane | - | 159.66 | - | |||
| Corn stover | 240 °C for 12 (H3PO4) | Pb (II) | Pseudo-second order | Langmuir/354 | - | [172] |
| Polyethyleneimine (240 °C for 12 h) | Langmuir/214 | - | ||||
| Sugarcane bagasse | 200 °C 3 h (100 mL, 10% of H2O2 + 5 g) | Cd (II) | Pseudo-second order | Langmuir/323 | - | [49] |
| Pb (II) | - | 357 | - | |||
| Sucrose | 800 °C process for 12 h with a mixture of 0.75 mol/L of sucrose 1 to 3 (w/w) with KOH with N2 flux of 100 mL/min | Acetone | - | 226 | 4 | [179] |
| Toluene | - | 251 | - | |||
| Acetic ether | - | 241 | - | |||
| Rice straw | 200 °C for 70 with 0.1 g per mL using a microwave reactor | Dye | - | Langmuir/221 | - | [180] |
| Berberine | - | Freundlich/174 | - | |||
| Coffee residue (grounds) | 160 °C for a period of 2 to 12 h with a mixture of 1:10 | Sulfadiazine | Pseudo-second order | Langmuir/0.08 | - | [181] |
| Sawdust | 220 °C for 60 days using a 150 L anaerobic fermenter. | Cd (II) | Pseudo-second order | Langmuir/20 | - | [182] |
| Biomass | Magnetization Technique Used | Fe Source | Adsorbate | Isotherm Model/Qmax (mg/g) | Kinetic Model | Ref. |
|---|---|---|---|---|---|---|
| Vinasse and red mud | Co-hydrothermal treatment | Fe2O3 | Pb(11) | Freundlich/223.144 | Pseudo-second order | [212] |
| Watermelon | Co-precipitation | FeCl3.6H2O | Cadmium | Freundlich/347.2 | Pseudo-second order | [213] |
| Waste lignin | Hydrothermal treatment | FeCl3.6H2O | Thallium | Langmuir/278.9 | Pseudo-second order | [210] |
| Raffia fibers | Hydrothermal treatment | Fe3O4 | Cadmium | Temkin/16.34 | Elovich | [211] |
| Coffee husk | Co-precipitation | Fe3O4 | Methylene blue dye | Freundlich/78 | Pseudo-second order | [214] |
| Phytolacca acinose | Hydrothermal treatment | Fe3O4 | Cadmium | Langmuir/246.6 | Pseudo-second order | [215] |
| Wheat straw | Pyrolysis | Fe solution | Tetracycline and Mercury | Langmuir/268.3/127.4 | - | [205] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ighalo, J.O.; Akaeme, F.C.; Georgin, J.; de Oliveira, J.S.; Franco, D.S.P. Biomass Hydrochar: A Critical Review of Process Chemistry, Synthesis Methodology, and Applications. Sustainability 2025, 17, 1660. https://doi.org/10.3390/su17041660
Ighalo JO, Akaeme FC, Georgin J, de Oliveira JS, Franco DSP. Biomass Hydrochar: A Critical Review of Process Chemistry, Synthesis Methodology, and Applications. Sustainability. 2025; 17(4):1660. https://doi.org/10.3390/su17041660
Chicago/Turabian StyleIghalo, Joshua O., Florence C. Akaeme, Jordana Georgin, Jivago Schumacher de Oliveira, and Dison S. P. Franco. 2025. "Biomass Hydrochar: A Critical Review of Process Chemistry, Synthesis Methodology, and Applications" Sustainability 17, no. 4: 1660. https://doi.org/10.3390/su17041660
APA StyleIghalo, J. O., Akaeme, F. C., Georgin, J., de Oliveira, J. S., & Franco, D. S. P. (2025). Biomass Hydrochar: A Critical Review of Process Chemistry, Synthesis Methodology, and Applications. Sustainability, 17(4), 1660. https://doi.org/10.3390/su17041660









