Artisanal Biochar Application: Enhancing Sandy Soil Fertility and Rice (Oryza sativa L.) Productivity
Abstract
1. Introduction
2. Materials and Methods
2.1. Obtaining and Treatment of the Studied Biochars
2.2. Characterization of the Biochars
2.2.1. Structural Analysis of Biochar
- (1)
- 0–45. ppm (CAlkyl-H,R);
- (2)
- 45–60. ppm (CAlkyl-O,N);
- (3)
- 60–90. ppm (CAlkyl-O);
- (4)
- 90–110. ppm (CAlkyl-di-O);
- (5)
- 110–145. ppm (CAromatic-H,R);
- (6)
- 145–160. ppm (CAromatic-O,N);
- (7)
- 160–190. ppm (CCOOH);
- (8)
- 190–220. ppm (CC=O).
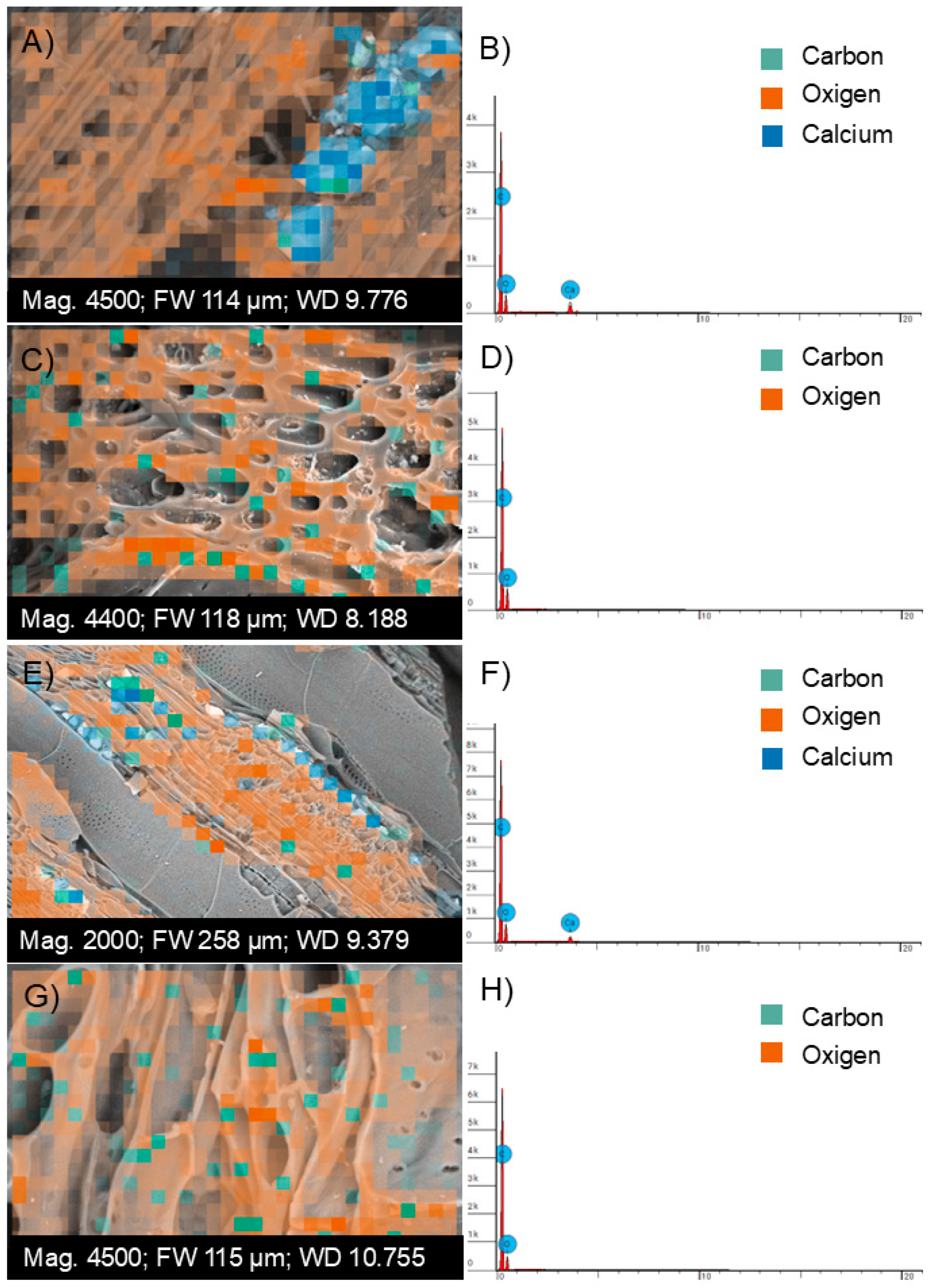
2.2.2. Characterization of Biochar by Energy Dispersive Spectroscopy (EDS) Microscopy
2.3. Bioactivity Experiment with Rice Plants
2.3.1. Quantification of Biomass Production and Growth
2.3.2. Quantification of Nutrients Present in the Aerial Biomass of Rice Plants
2.3.3. Analysis of the Photosynthetic Parameters of Rice Plants
2.3.4. Root Morphology
2.3.5. Statistical Analysis of Data
3. Results
3.1. Structural Analysis by CP MAS 13C NMR and Chemometric Analysis
3.2. Morphological Characterization of Biochar by Scanning Electron Microscopy (SEM) and Energy-Dispersive X-Ray Spectroscopy (EDS)
3.3. Effects of Biochar Application on Fresh and Dry Mass of the Root, Sheath, and Leaf of Rice Plants
3.4. Nutrient Contents in the Aerial Parts of Rice Plants
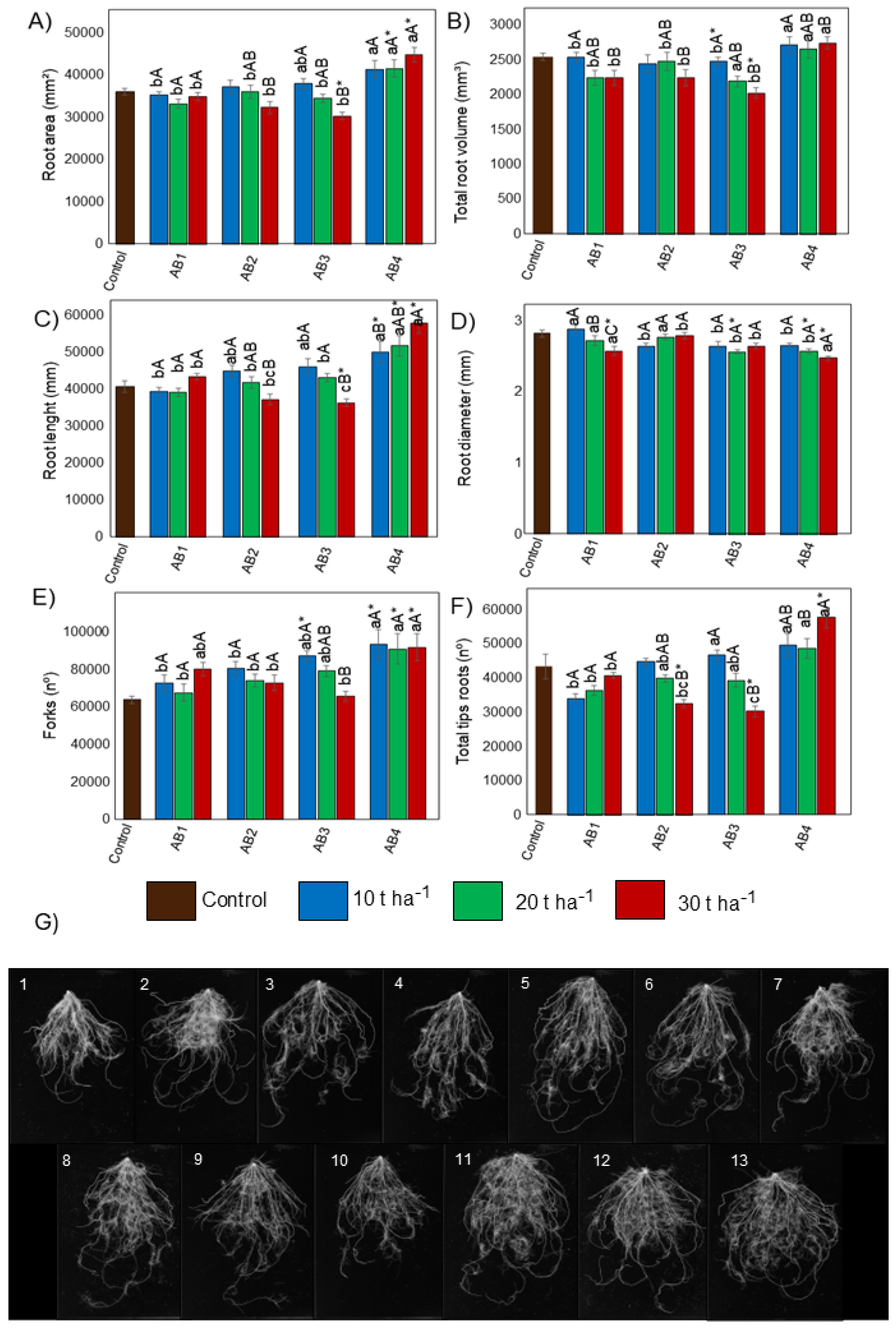
3.5. Effects of Biochar Application on Root Morphology
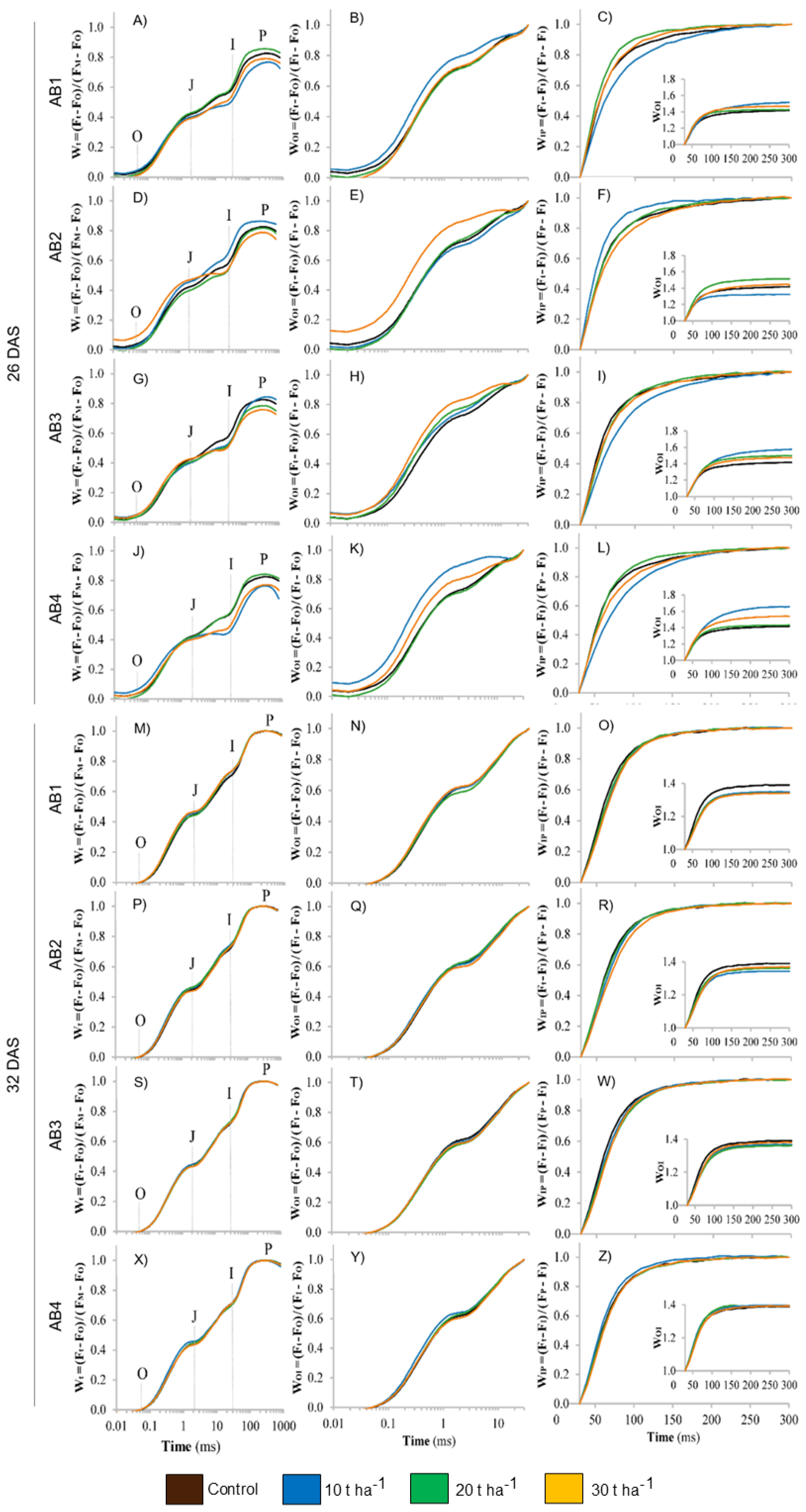
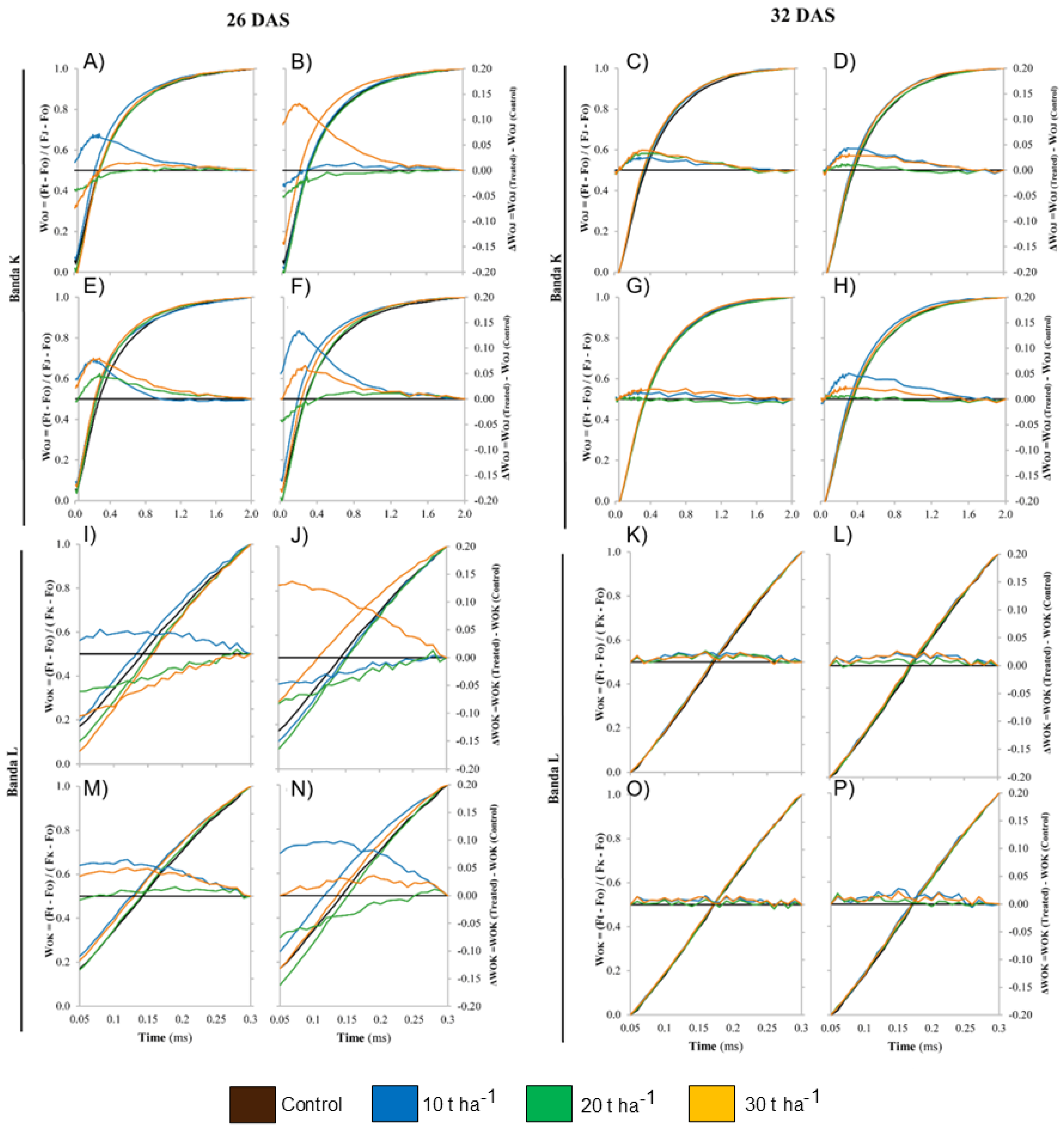
3.6. Effects of Biochar Application on Chlorophyll Fluorescence
4. Discussion
4.1. Structural and Morphological Characteristics of Artisanal Biochars
4.2. Influence of Artisanal Biochars on Nutrient Accumulation in the Aerial Part of Rice Plants
4.3. Effects of Artisanal Biochars on the Morphology of Rice Plant Roots
4.4. Effects of Artisanal Biochars on Photosynthetic Parameters
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lehmann, J.; Joseph, S. Biochar for Environmental Management; Routledge: London, UK, 2015; Volume 2, ISBN 9781844076581. [Google Scholar]
- Placido, J.; Capareda, S.; Karthikeyan, R. Production of Humic Substances from Cotton Stalks Biochar by Fungal Treatment with Ceriporiopsis Subvermispora. Sustain. Energy Technol. Assess. 2016, 13, 31–37. [Google Scholar] [CrossRef]
- Al-Wabel, M.I.; Al-Omran, A.; El-Naggar, A.H.; Nadeem, M.; Usman, A.R.A. Pyrolysis Temperature Induced Changes in Characteristics and Chemical Composition of Biochar Produced from Conocarpus Wastes. Bioresour. Technol. 2013, 131, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Canqui, H. Biochar and Soil Physical Properties. Soil Sci. Soc. Am. J. 2017, 81, 687–711. [Google Scholar] [CrossRef]
- Ibrahim, A.; Usman, A.R.A.; Al-Wabel, M.I.; Nadeem, M.; Ok, Y.S.; Al-Omran, A. Effects of Conocarpus Biochar on Hydraulic Properties of Calcareous Sandy Soil: Influence of Particle Size and Application Depth. Arch. Agron. Soil Sci. 2017, 63, 185–197. [Google Scholar] [CrossRef]
- Kameyama, K.; Miyamoto, T.; Iwata, Y.; Shiono, T. Influences of Feedstock and Pyrolysis Temperature on the Nitrate Adsorption of Biochar. Soil Sci. Plant Nutr. 2016, 62, 180–184. [Google Scholar] [CrossRef]
- Devereux, R.C.; Sturrock, C.J.; Mooney, S.J. The Effects of Biochar on Soil Physical Properties and Winter Wheat Growth. Earth Environ. Sci. Trans. R Soc. Edinb. 2013, 103, 13–18. [Google Scholar] [CrossRef]
- Torchia, D.F.d.O.; Zonta, E.; de Andrade, A.M.; García, A.C. Production and Characterization of Biochar Obtained from Different Biomass and Pyrolysis Temperature. Braz. J. Chem. Eng. 2021, 39, 415–427. [Google Scholar] [CrossRef]
- Lei, O.; Zhang, R. Effects of Biochars Derived from Different Feedstocks and Pyrolysis Temperatures on Soil Physical and Hydraulic Properties. J. Soils Sediments 2013, 13, 1561–1572. [Google Scholar] [CrossRef]
- Zong, Y.; Chen, D.; Lu, S. Impact of Biochars on Swell-Shrinkage Behavior, Mechanical Strength, and Surface Cracking of Clayey Soil. J. Plant Nutr. Soil Sci. 2014, 177, 920–926. [Google Scholar] [CrossRef]
- Zheng, R.; Li, C.; Sun, G.; Xie, Z.; Chen, J.; Wu, J.; Wang, Q. The Influence of Particle Size and Feedstock of Biochar on the Accumulation of Cd, Zn, Pb, and As by Brassica chinensis L. Environ. Sci. Pollut. Res. 2017, 24, 22340–22352. [Google Scholar] [CrossRef]
- Alvarez-Campos, O.; Lang, T.A.; Bhadha, J.H.; McCray, J.M.; Glaz, B.; Daroub, S.H. Biochar and Mill Ash Improve Yields of Sugarcane on a Sand Soil in Florida. Agric. Ecosyst. Environ. 2018, 253, 122–130. [Google Scholar] [CrossRef]
- Gonzaga, M.I.S.; Mackowiak, C.; de Almeida, A.Q.; de Carvalho Junior, J.I.T.; Andrade, K.R. Positive and Negative Effects of Biochar from Coconut Husks, Orange Bagasse and Pine Wood Chips on Maize (Zea mays L.) Growth and Nutrition. Catena 2018, 162, 414–420. [Google Scholar] [CrossRef]
- Jien, S.H.; Wang, C.S. Effects of Biochar on Soil Properties and Erosion Potential in a Highly Weathered Soil. Catena 2013, 110, 225–233. [Google Scholar] [CrossRef]
- He, Y.; Yao, Y.; Ji, Y.; Deng, J.; Zhou, G.; Liu, R.; Shao, J.; Zhou, L.; Li, N.; Zhou, X.; et al. Biochar Amendment Boosts Photosynthesis and Biomass in C3 but Not C4 Plants: A Global Synthesis. GCB Bioenergy 2020, 12, 605–617. [Google Scholar] [CrossRef]
- Corbeels, M.; Marchão, R.L.; Neto, M.S.; Ferreira, E.G.; Madari, B.E.; Scopel, E.; Brito, O.R. Evidence of limited carbon sequestration in soils under no-tillage systems in the Cerrado of Brazil. Sci. Rep. 2016, 6, 21450. [Google Scholar] [CrossRef]
- Santos, R.S.; Santana, A.S. agricultura e agroindústria rural Na região sudeste segundo dados do censo agropecuário de 2017. Bol. Reg. Urbano E Ambient. Ipea 2020, 23, 123–133. [Google Scholar] [CrossRef]
- Torchia, D.F.d.O.; van Tol de Castro, T.A.; Tavares, O.C.H.; Souza, C.C.B.; da Silva, H.F.O.; de Moura, O.V.T.; da Silva, K.C.M.; de Souza Rocha, F.; Zonta, E.; García, A.C. Effects of Artisanal Biochar Applied to Rice Cultivation in Fragile Sandy Planosol at Rio de Janeiro (Brazil). J. Soil Sci. Plant Nutr. 2024, 24, 172–189. [Google Scholar] [CrossRef]
- Mazzei, P.; Cangemi, S.; Malakshahi Kurdestani, A.; Mueller, T.; Piccolo, A. Quantitative Evaluation of Noncovalent Interactions between 3,4-Dimethyl-1 H-Pyrazole and Dissolved Humic Substances by NMR Spectroscopy. Environ. Sci. Technol. 2022, 56, 11771–11779. [Google Scholar] [CrossRef] [PubMed]
- Portz, A.; Resende, A.S.; Teixeira, A.J.; Abboud, A.C.S.; Martins, C.A.C.; Carvalho, C.A.B.; Lima, E.; Zonta, E.; Pereira, J.B.A.; Balieiro, F.C.; et al. Recomendações de Adubos, Corretivos e de Manejo Da Matéria Para as Principais Culturas Do Estado Do Rio de Janeiro. In Manual de Calagem e Adubação do Estado do Rio de Janeiro; Luiz Rodrigues Freire, Ed.; Embrapa: Brasília, Brazil, 2013; Volume 1, pp. 1–434. [Google Scholar]
- Glaser, B.; Lehmann, J.; Zech, W. Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal—A review. Biol. Fertil. Soils 2002, 35, 219–230. [Google Scholar] [CrossRef]
- Uzoma, K.C.; Inoue, M.; Andry, H.; Fujimaki, H.; Zahoor, A.; Nishihara, E. Effect of cow manure biochar on maize productivity under sandy soil condition. Soil Use Manag. 2011, 27, 205–211. [Google Scholar] [CrossRef]
- Malavolta, E.; Vitti, G.C.; Oliveira, S.A. Evaluation of the Nutritional Status of Plants: Principles and Applications, 2nd ed.; Potafos: Piracicaba, Brazil, 1997. [Google Scholar]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the Chlorophyll a Fluorescence Transient. In Chlorophyll a Fluorescence; Papageorgiou, G.C., Govindjee, Eds.; Springer: Berlin/Heidelberg, Germany, 2004; Volume 19, pp. 321–362. [Google Scholar]
- Tavares, O.C.H.; Santos, L.A.; Filho, D.F.; Ferreira, L.M.; García, A.C.; Castro, T.A.V.T.; Zonta, E.; Pereira, M.G.; Fernandes, M.S. Response Surface Modeling of Humic Acid Stimulation of the Rice (Oryza sativa L.) Root System. Arch. Agron. Soil Sci. 2021, 67, 1046–1059. [Google Scholar] [CrossRef]
- Demirbas, A. Effects of Temperature and Particle Size on Bio-Char Yield from Pyrolysis of Agricultural Residues. J. Anal. Appl. Pyrolysis 2004, 72, 243–248. [Google Scholar] [CrossRef]
- Rutherford, D.W.; Wershaw, R.L.; Cox, L.G. Changes in Composition and Porosity Occurring During the Thermal Degradation of Wood and Wood Components; US Department of the interior, US Geological Survey: Reston, WA, USA, 2005. [Google Scholar]
- Antal, M.J.; Grønli, M. The Art, Science, and Technology of Charcoal Production. Ind. Eng. Chem. Res. 2003, 42, 1619–1640. [Google Scholar] [CrossRef]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar Effects on Soil Biota—A Review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Spokas, K.A.; Novak, J.M.; Venterea, R.T. Biochar’s role as an alternative N-fertilizer: Ammonia capture. Plant Soil 2012, 350, 35–42. [Google Scholar] [CrossRef]
- Keiluweit, M.; Nico, P.S.; Johnson, M.G.; Kleber, M. Dynamic molecular structure of plant biomass-derived black carbon. Environ. Sci. Technol. 2010, 44, 1247–1253. [Google Scholar] [CrossRef]
- Singh, B.P.; Cowie, A.L.; Smernik, R.J. Biochar stability in a clayey soil as a function of feedstock and pyrolysis temperature. Environ. Sci. Technol. 2010, 46, 11770–11778. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, B.T.; Lehmann, J. Black carbon decomposition under varying water regimes. Org. Geochem. 2009, 40, 846–853. [Google Scholar] [CrossRef]
- Berek, A.K.; Hue, N.V. Characterization of Biochars and Their Use as an Amendment to Acid Soils. Soil Sci. 2016, 181, 412–426. [Google Scholar] [CrossRef]
- Chia, C.H.; Munroe, P.; Joseph, S.; Lin, Y. Microscopic Characterisation of Synthetic Terra Preta. Aust. J. Soil Res. 2010, 48, 593–605. [Google Scholar] [CrossRef]
- Torres, G.N.; Amorin, R.S.S.; DiRaimo, L.A.D.; Faria, O.C.O. Water retention in sandy soils of different origins with the addition of biochar. Caatinga 2023, 37, e11792. [Google Scholar]
- Kamarudin, N.S.; Dahalan, F.A.; Hasan, M.; An, O.S.; Parmin, N.A.; Ibrahim, N.; Hamdzah, M.; Zain, N.A.M.; Muda, K.; Wikurendra, E.A. Biochar: A Review of Its History, Characteristics, Factors That Influence Its Yield, Methods of Production, Application in Wastewater Treatment and Recent Development. Biointerface Res. Appl. Chem. 2022, 12, 7914–7926. [Google Scholar] [CrossRef]
- Uchimiya, M.; Wartelle, L.H.; Lima, I.M.; Klasson, K.T. Sorption of Deisopropylatrazine on Broiler Litter Biochars. J. Agric. Food Chem. 2010, 58, 12350–12356. [Google Scholar] [CrossRef]
- Nguyen, B.T.; Lehmann, J.; Kinyangi, J.; Smernik, R.; Riha, S.J.; Engelhard, M.H. Long-Term Black Carbon Dynamics in Cultivated Soil. Biogeochemistry 2008, 89, 295–308. [Google Scholar] [CrossRef]
- Dietrich, C.C.; Rahaman, M.A.; Robles-Aguilar, A.A.; Latif, S.; Intani, K.; Müller, J.; Jablonowski, N.D. Nutrient Loaded Biochar Doubled Biomass Production in Juvenile Maize Plants (Zea mays L.). Agronomy 2020, 10, 567. [Google Scholar] [CrossRef]
- Silva Gonzaga, M.I.; Oliveira da Silva, P.S.; Carlos de Jesus Santos, J.; Ganassali de Oliveira Junior, L.F. Biochar Increases Plant Water Use Efficiency and Biomass Production While Reducing Cu Concentration in Brassica juncea L. in a Cu-Contaminated Soil. Ecotoxicol. Environ. Saf. 2019, 183, 109557. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, C.; Gray, E.M.; Boyd, S.E.; Yang, H.; Zhang, D. Roles of Biochar in Improving Phosphorus Availability in Soils: A Phosphate Adsorbent and a Source of Available Phosphorus. Geoderma 2016, 276, 1–6. [Google Scholar] [CrossRef]
- Chen, W.; Chen, Y.; Yang, H.; Li, K.; Chen, X.; Chen, H. Investigation on Biomass Nitrogen-Enriched Pyrolysis: Influence of Temperature. Bioresour. Technol. 2018, 249, 247–253. [Google Scholar] [CrossRef]
- Li, S.; Chen, G. Thermogravimetric, Thermochemical, and Infrared Spectral Characterization of Feedstocks and Biochar Derived at Different Pyrolysis Temperatures. Waste Manag. 2018, 78, 198–207. [Google Scholar] [CrossRef]
- Zhang, L.; Chang, L.; Liu, H.; de Jesús Puy Alquiza, M.; Li, Y. Biochar application to soils can regulate soil phosphorus availability: A review. Biochar 2025, 7, 13. [Google Scholar] [CrossRef]
- Tian, J.; Kuang, X.; Tang, M.; Chen, X.; Huang, F.; Cai, Y.; Cai, K. Biochar Application under Low Phosphorus Input Promotes Soil Organic Phosphorus Mineralization by Shifting Bacterial PhoD Gene Community Composition. Sci. Total Environ. 2021, 779, 146556. [Google Scholar] [CrossRef] [PubMed]
- Laird, D.A.; Fleming, P.; Davis, D.D.; Horton, R.; Wang, B.; Karlen, D.L. Impact of biochar amendments on the quality of a typical Midwestern agricultural soil. Geoderma 2010, 158, 443–449. [Google Scholar] [CrossRef]
- Meng, Z.; Huang, S.; Xu, T.; Lin, X.; Wu, J. Competitive adsorption, immobilization, and desorption risks of Cd, Ni, and Cu in saturated-unsaturated soils by biochar under combined aging. Hazard. Mater. 2022, 34, 128903. [Google Scholar] [CrossRef] [PubMed]
- Bhadha, J.H.; Daroub, S.H.; Lang, T.A. Effect of Kinetic Control, Soil: Solution Ratio, Electrolyte Cation, and Others, on Equilibrium Phosphorus Concentration. Geoderma 2012, 173–174, 209–214. [Google Scholar] [CrossRef]
- Prendergast-Miller, M.T.; Duvall, M.; Sohi, S.P. Biochar-Root Interactions Are Mediated by Biochar Nutrient Content and Impacts on Soil Nutrient Availability. Eur. J. Soil Sci. 2014, 65, 173–185. [Google Scholar] [CrossRef]
- Lehmann, J.; Pereira da Silva, J., Jr.; Steiner, C.; Nehls, T.; Zech, W.; Glaser, B. Nutrient Availability and Leaching in an Archaeological Anthrosol and a Ferralsol of the Central Amazon Basin: Fertilizer, Manure and Charcoal Amendments. Plant Soil 2003, 249, 343–357. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torchia, D.F.d.O.; Castro, T.A.v.T.d.; Rodrigues, N.F.; da Silva, H.F.d.O.; Spaccini, R.; Cangemi, S.; Tavares, O.C.H.; Berbara, R.L.L.; Zonta, E.; García, A.C. Artisanal Biochar Application: Enhancing Sandy Soil Fertility and Rice (Oryza sativa L.) Productivity. Sustainability 2025, 17, 3306. https://doi.org/10.3390/su17083306
Torchia DFdO, Castro TAvTd, Rodrigues NF, da Silva HFdO, Spaccini R, Cangemi S, Tavares OCH, Berbara RLL, Zonta E, García AC. Artisanal Biochar Application: Enhancing Sandy Soil Fertility and Rice (Oryza sativa L.) Productivity. Sustainability. 2025; 17(8):3306. https://doi.org/10.3390/su17083306
Chicago/Turabian StyleTorchia, Danielle França de Oliveira, Tadeu Augusto van Tol de Castro, Natália Fernandes Rodrigues, Hellen Fernanda de Oliveira da Silva, Riccardo Spaccini, Silvana Cangemi, Orlando Carlos Huertas Tavares, Ricardo Luiz Louro Berbara, Everaldo Zonta, and Andrés Calderín García. 2025. "Artisanal Biochar Application: Enhancing Sandy Soil Fertility and Rice (Oryza sativa L.) Productivity" Sustainability 17, no. 8: 3306. https://doi.org/10.3390/su17083306
APA StyleTorchia, D. F. d. O., Castro, T. A. v. T. d., Rodrigues, N. F., da Silva, H. F. d. O., Spaccini, R., Cangemi, S., Tavares, O. C. H., Berbara, R. L. L., Zonta, E., & García, A. C. (2025). Artisanal Biochar Application: Enhancing Sandy Soil Fertility and Rice (Oryza sativa L.) Productivity. Sustainability, 17(8), 3306. https://doi.org/10.3390/su17083306






