Microplastics in the Typical Mulched Farmland of Sichuan Province: Characteristics That Vary Across Farming Scales and the Risk Significantly Contributed by Priority Polymers
Abstract
:1. Introduction
- (1)
- To select representative farmland soil sample and characterize their MP pollution from the abundance, the chemical composition, the morphological attributes (shapes and colors), and the size distribution of MPs.
- (2)
- To test the hypothesis that microplastic pollution characteristics vary with farming scales and to analyze potential sources.
- (3)
- To assess the ecological risks of MPs in agricultural soil via our optimized size-inclusive evaluation method and to analyze the major contributors to MP risk.
2. Materials and Methods
2.1. Study Area and Sampling
2.2. Soil Microplastic Extraction
2.3. Characterization of Extracted MPs
2.4. Risk Assessment
2.5. Analysis
3. Results
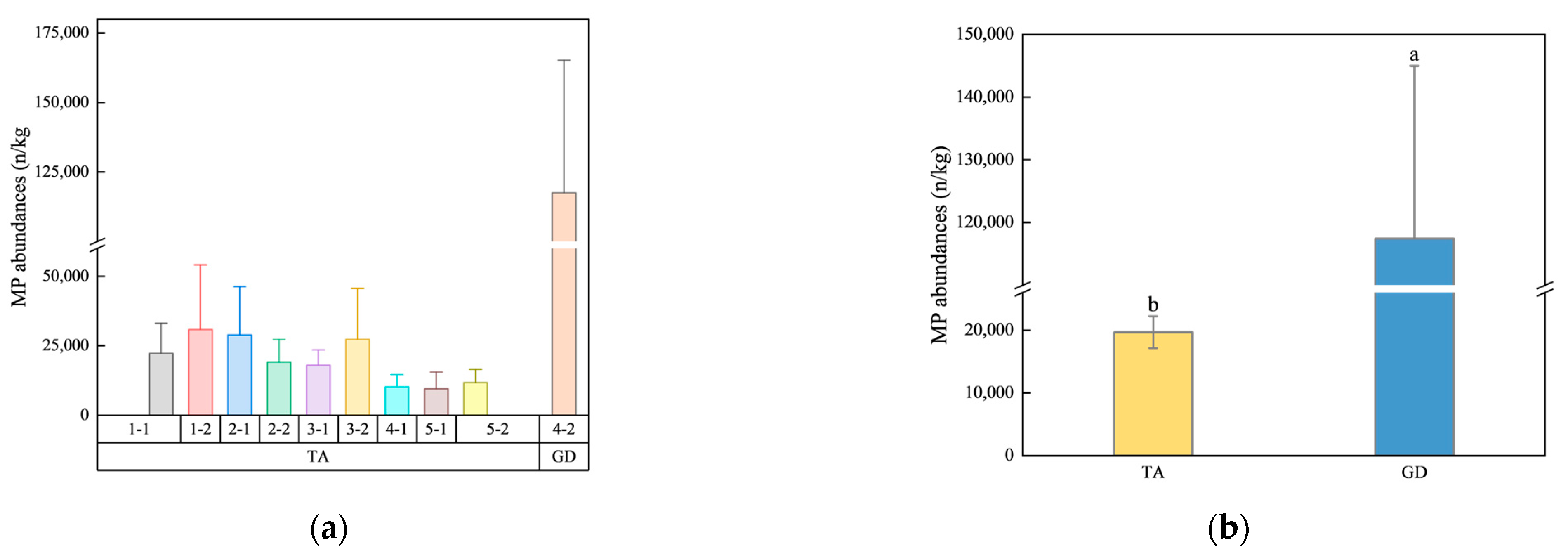
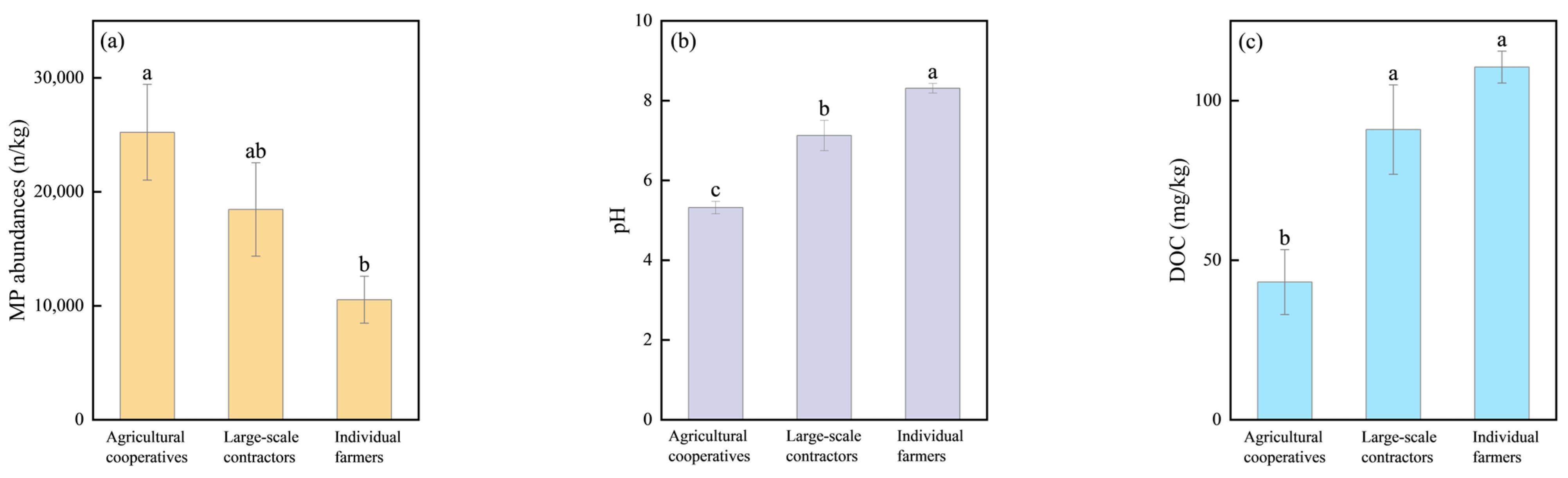
3.1. MP Abundances and Variation
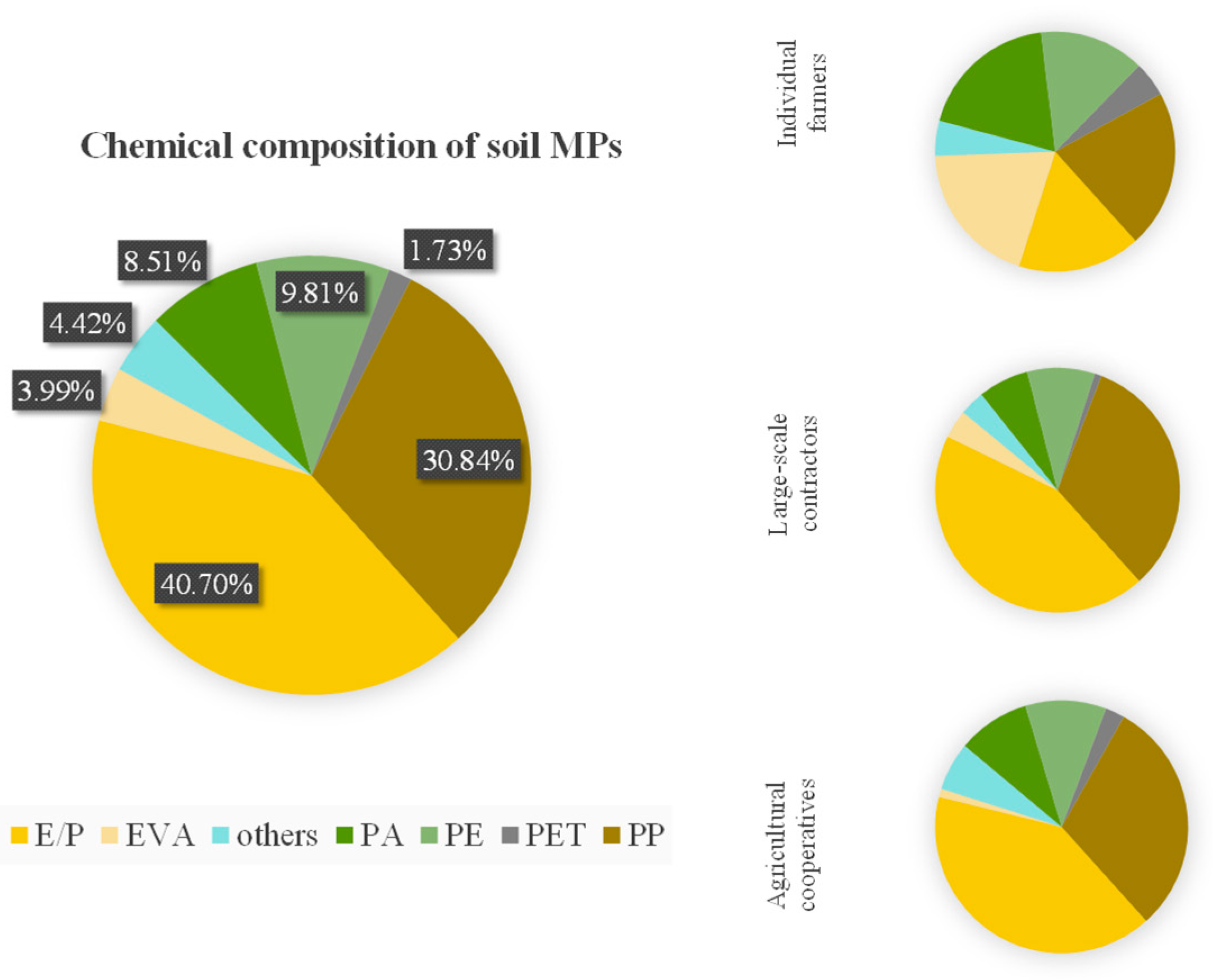
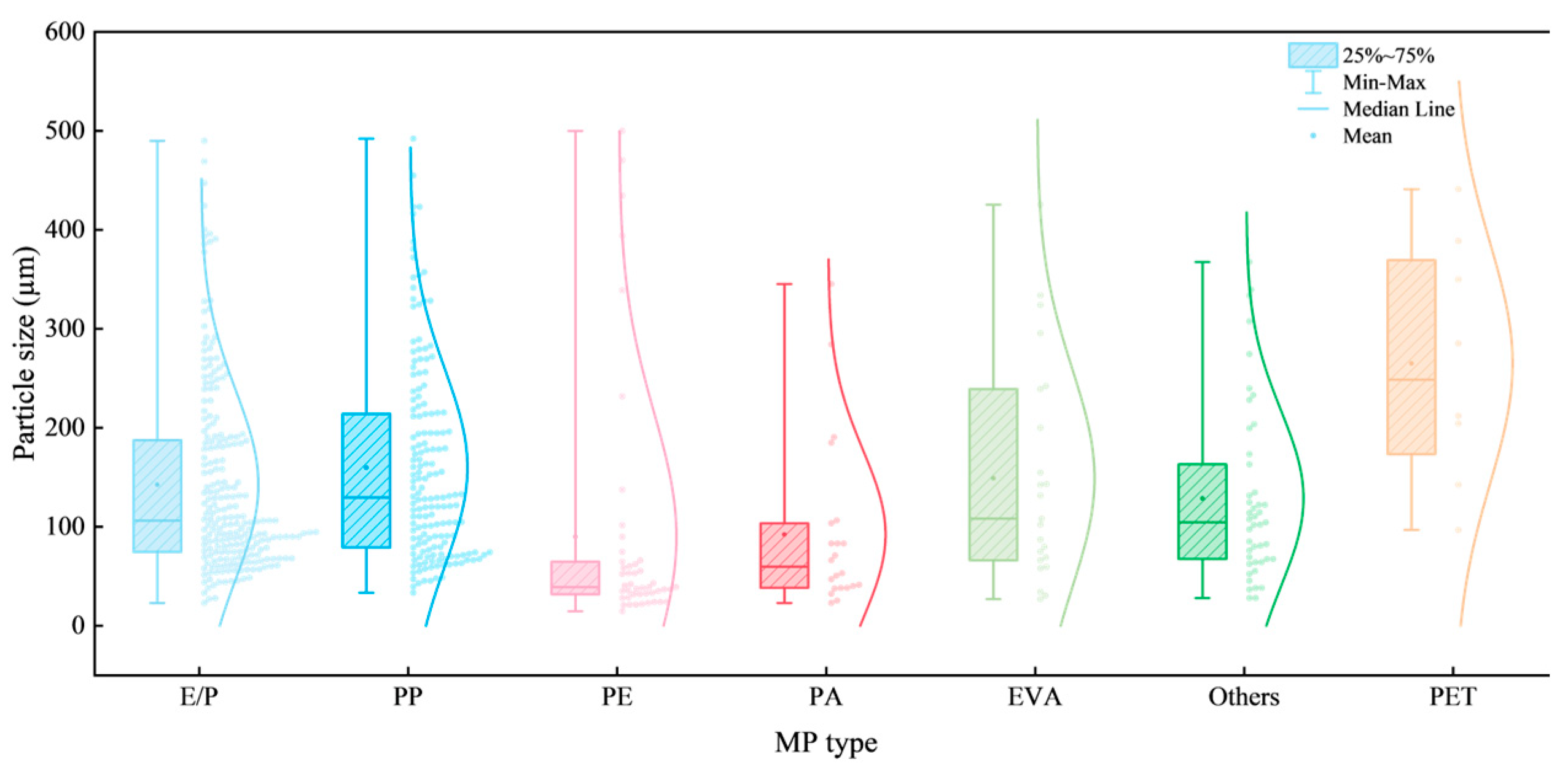
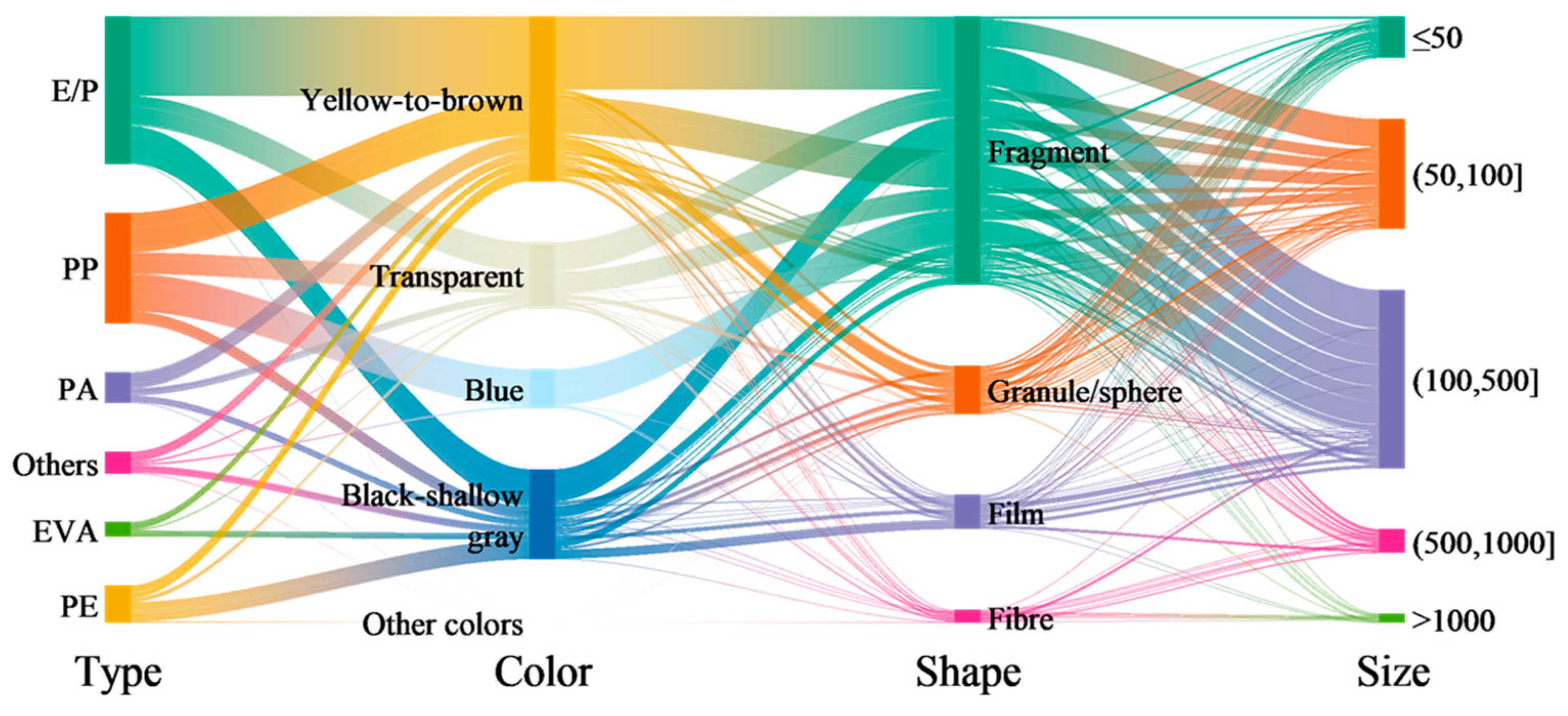
3.2. Chemical and Physical Characteristics
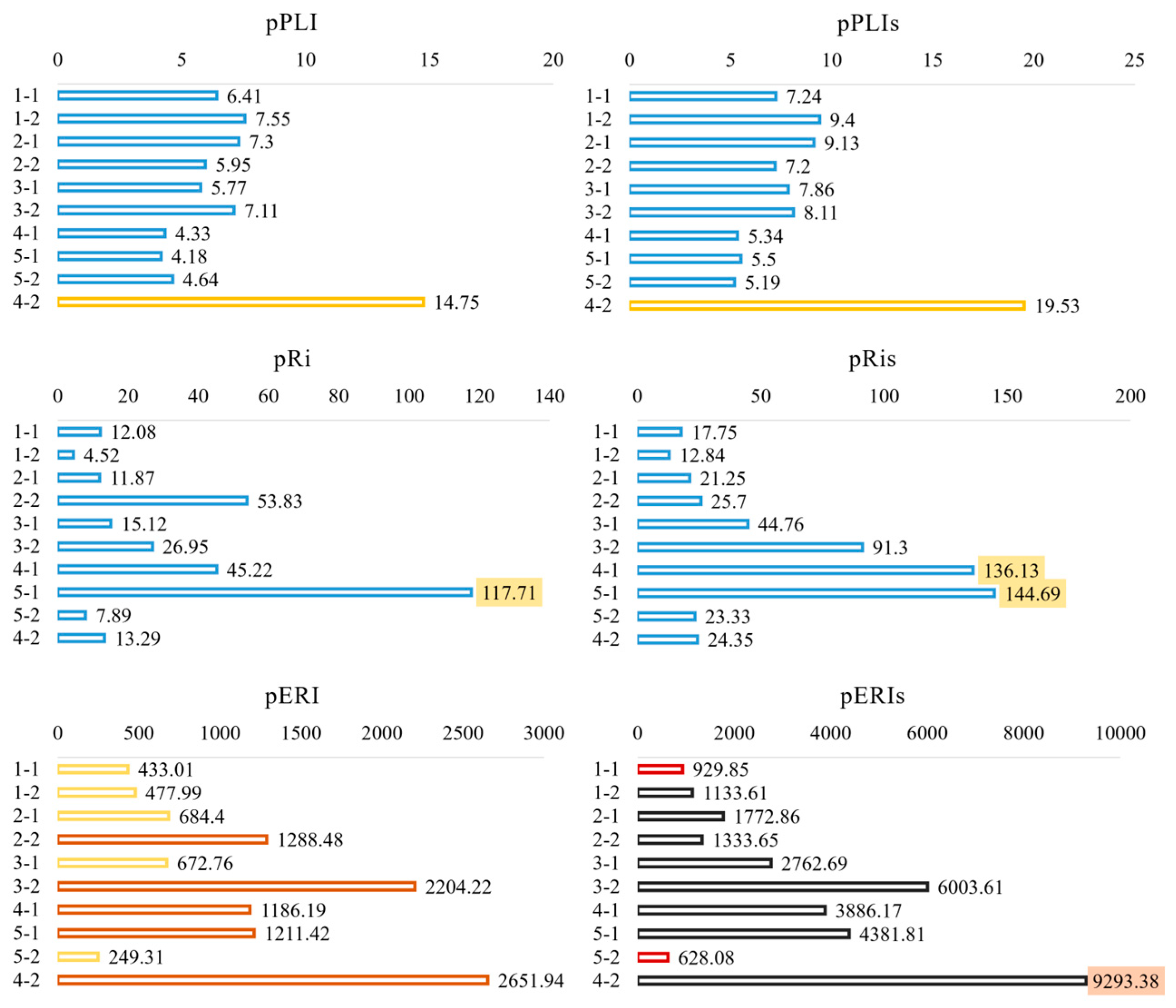
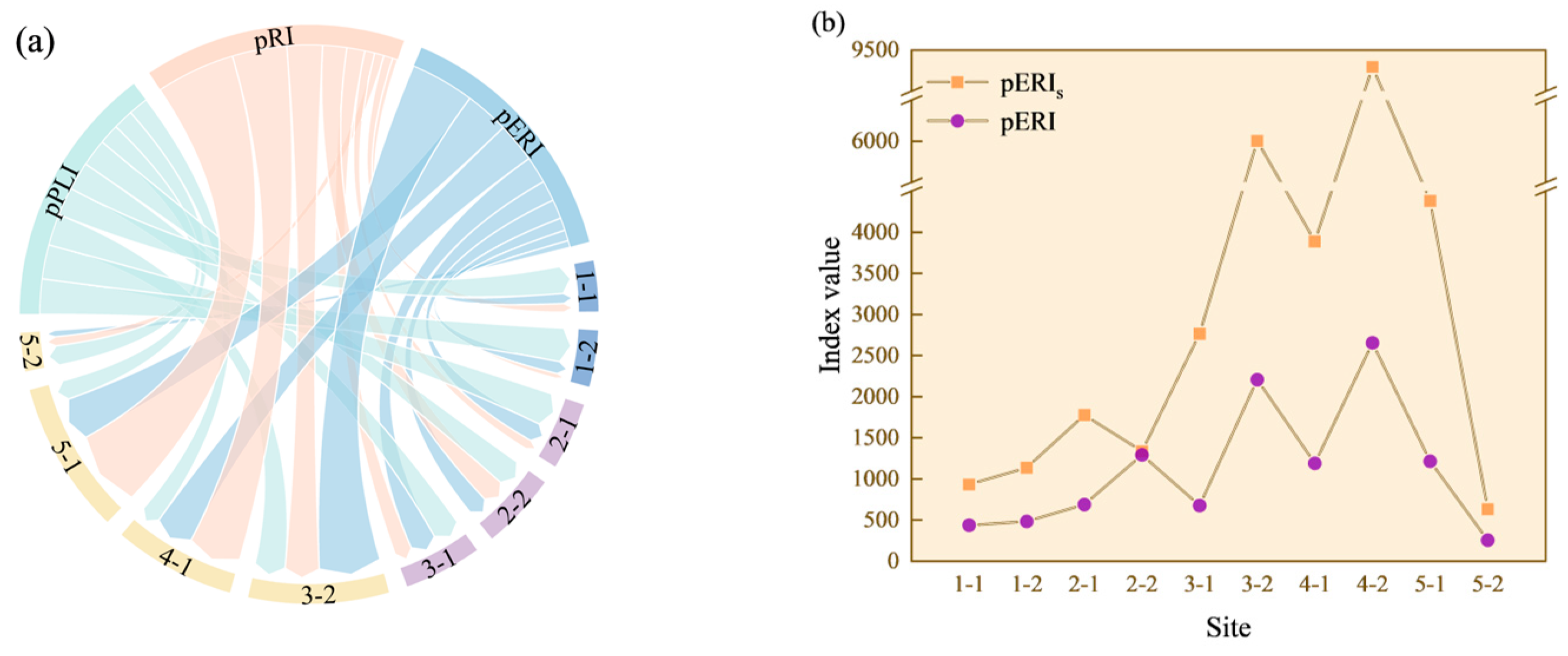
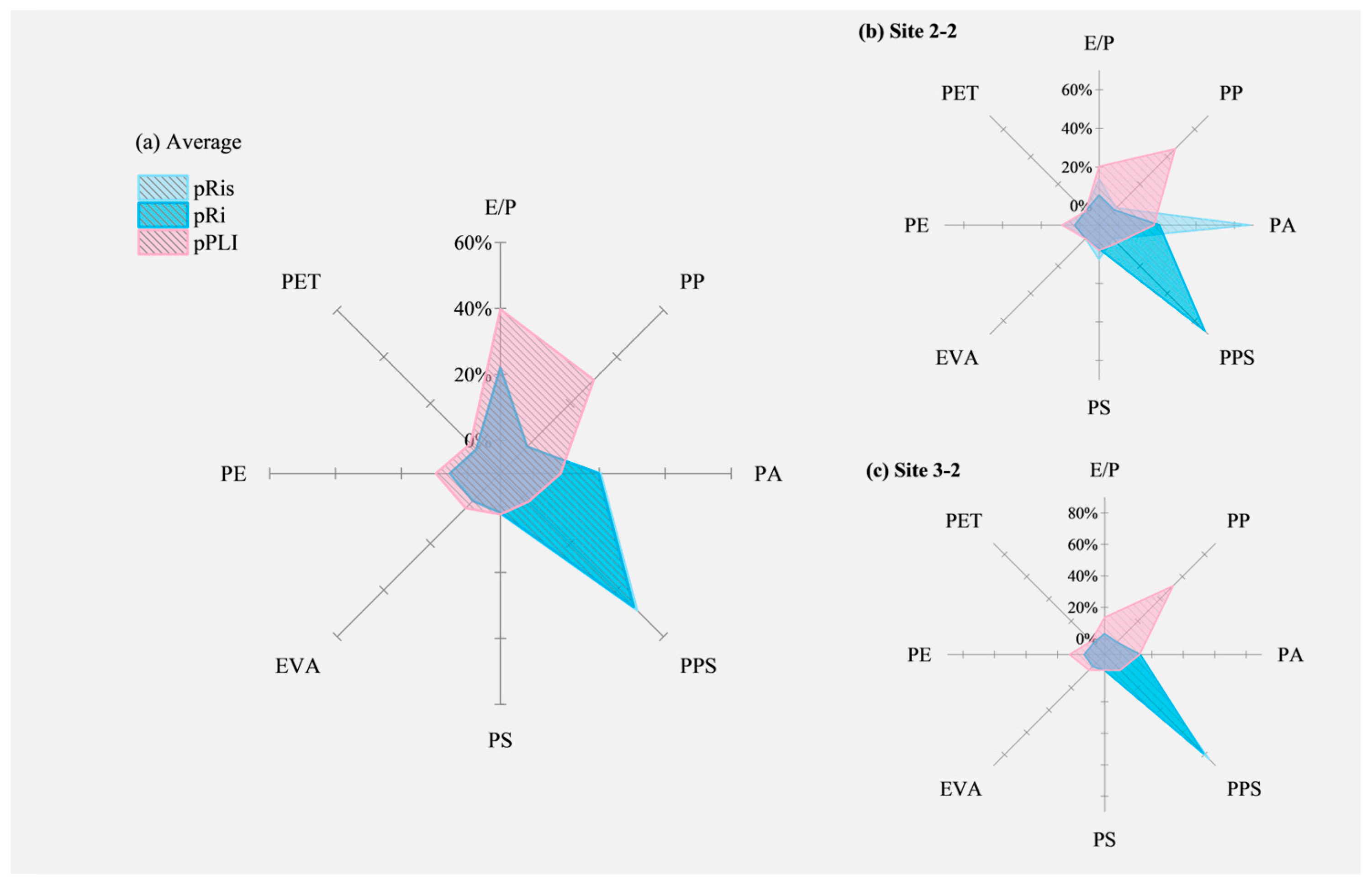
3.3. Risk Assessments and Comparisons
4. Discussion
4.1. MP Pollution Load and Distribution
4.2. Sources and Hazards of Farmland MPs
4.3. MP Occurrences Varying Across Farming Scales
4.4. Index-Dependent Ecological Risks and Major Contributors
4.5. MP Management Strategies
5. Conclusions
5.1. Environmental Implication
5.2. Limitations and Future Directions
- (1)
- Composition-Driven Uncertainties: variability in microplastic chemical composition (e.g., monomer toxicity and fragmentation rates) introduces uncertainties in soil risk assessments. Future work should prioritize critical polymers and integrate sensitivity factors (e.g., degradation rates) to refine risk models.
- (2)
- Sampling Representativeness: samples were targeted to areas with high microplastic contamination likelihood using literature and field data. Broader geographic sampling and larger sample sizes would strengthen generalizability.
- (3)
- Indirect Source Attribution: pollution sources were inferred from microplastic properties and site data. Future research could further verify the linkages by the direct comparative analysis (e.g., polymer fingerprinting) between soil microplastics and suspected sources.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adomat, Y.; Grischek, T. Sampling and processing methods of microplastics in river sediments—A review. Sci. Total Environ. 2021, 758, 143691. [Google Scholar] [CrossRef]
- Zhang, Y.; Kang, S.; Allen, S.; Allen, D.; Gao, T.; Sillanpää, M. Atmospheric microplastics: A review on current status and perspectives. Earth-Sci. Rev. 2020, 203, 103118. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Y.; Kang, S.; Wang, Z.; Wu, C. Microplastics in soil: A review on methods, occurrence, sources, and potential risk. Sci. Total Environ. 2021, 780, 146546. [Google Scholar] [CrossRef] [PubMed]
- Ya, H.; Jiang, B.; Xing, Y.; Zhang, T.; Lv, M.; Wang, X. Recent advances on ecological effects of microplastics on soil environment. Sci. Total Environ. 2021, 798, 149338. [Google Scholar] [CrossRef]
- An, Q.; Zhen, Z.; Zhong, N.; Qiu, D.; Xie, Y.; Yan, C. Effects of biodegradable microplastics on arsenic migration and transformation in paddy soils: A comparative analysis with conventional microplastics. J. Hazard. Mater. 2024, 469, 134053. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, T.; Jiang, M.; Xu, M.; Yu, Y.; Guo, B.; Chen, D.; Hu, S.; Jiang, J.; Zhang, Y.; et al. Assessment of Plastic Stocks and Flows in China: 1978–2017. Resour. Conserv. Recycl. 2020, 161, 104969. [Google Scholar] [CrossRef]
- Ding, L.; Zhang, S.; Wang, X.; Yang, X.; Zhang, C.; Qi, Y.; Guo, X. The occurrence and distribution characteristics of microplastics in the agricultural soils of Shaanxi Province, in north-western China. Sci. Total Environ. 2020, 720, 137525. [Google Scholar] [CrossRef] [PubMed]
- Miao, H.; Zhang, S.; Gao, W.; Zhou, J.; Cai, H.; Wu, L.; Liu, J.; Wang, Z.; Liu, T. Microplastics occurrence and distribution characteristics in mulched agricultural soils of Guizhou province. Sci. Rep. 2024, 14, 21505. [Google Scholar] [CrossRef]
- Zhang, J.; Zou, G.; Wang, X.; Ding, W.; Xu, L.; Liu, B.; Mu, Y.; Zhu, X.; Song, L.; Chen, Y. Exploring the Occurrence Characteristics of Microplastics in Typical Maize Farmland Soils With Long-Term Plastic Film Mulching in Northern China. Front. Mar. Sci. 2021, 8, 800087. [Google Scholar] [CrossRef]
- Ye, C.; Lin, J.; Li, Z.G.; Wang, G.H.; Li, Z.L. Characteristics of Microplastic Pollution in Agricultural Soils in Xiangtan, China. Sustainability 2024, 16, 7254. [Google Scholar] [CrossRef]
- Jia, Z.F.; Wei, W.; Wang, Y.H.; Chang, Y.J.; Lei, R.; Che, Y.H. Occurrence characteristics and risk assessment of microplastics in agricultural soils in the loess hilly gully area of Yan’ an, China. Sci. Total Environ. 2024, 912, 169627. [Google Scholar] [CrossRef]
- Chen, Y.; Leng, Y.; Liu, X.; Wang, J. Microplastic pollution in vegetable farmlands of suburb Wuhan, central China. Environ. Pollut. 2020, 257, 113449. [Google Scholar] [CrossRef]
- Medyńska-Juraszek, A.; Szczepańska, A. Microplastic Pollution in EU Farmland Soils: Preliminary Findings from Agricultural Soils (Southwestern Poland). Agriculture 2023, 13, 1733. [Google Scholar] [CrossRef]
- Harms, I.K.; Diekötter, T.; Troegel, S.; Lenz, M. Amount, distribution and composition of large microplastics in typical agricultural soils in Northern Germany. Sci. Total Environ. 2021, 758, 143615. [Google Scholar] [CrossRef]
- Palazot, M.; Soccalingame, L.; Froger, C.; Jolivet, C.; Bispo, A.; Kedzierski, M.; Bruzaud, S. First national reference of microplastic contamination of French soils. Sci. Total Environ. 2024, 918, 170564. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Tang, J.; Lyu, H.; Wang, L.; Gillmore, A.B.; Schaeffer, S.M. Activities of Microplastics (MPs) in Agricultural Soil: A Review of MPs Pollution from the Perspective of Agricultural Ecosystems. J. Agric. Food Chem. 2022, 70, 4182–4201. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Ouyang, D.; Lei, J.; Tan, Q.; Xie, L.; Li, Z.; Liu, T.; Xiao, Y.; Farooq, T.H.; et al. Systematical review of interactions between microplastics and microorganisms in the soil environment. J. Hazard. Mater. 2021, 418, 126288. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Jinjin, C.; Ji, R.; Ma, Y.; Yu, X. Microplastics in agricultural soils: Sources, effects, and their fate. Curr. Opin. Environ. Sci. Health 2022, 25, 100311. [Google Scholar] [CrossRef]
- Wang, X.; Feng, Q.; Yang, L.; Li, M.; Zhao, F.; Tang, J.; Bu, Q.; Zhang, Y.; Chen, L. Effect of land use on occurrence and spatial variability of soil microplastics in agricultural watershed. Ecol. Front. 2025, 45, 497–506. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, Y.; Xu, Y.; Guo, X.; Zhu, L. Distribution characteristics and mechanism of microplastics mediated by soil physicochemical properties. Sci. Total Environ. 2020, 726, 138389. [Google Scholar] [CrossRef]
- Chen, X.; Lu, Z.; Heng, L.; Chappell, A.; Oshunsanya, S.O.; Adu-Gyamfi, J.; Liu, W.; Yu, H. The spatio-temporal variability of soil microplastic distribution and erosion-induced microplastic export under extreme rainfall event using sediment fingerprinting and 7Be in intensive agricultural catchment. J. Hazard. Mater. 2025, 488, 137378. [Google Scholar] [CrossRef] [PubMed]
- Kedzierski, M.; Cirederf-Boulant, D.; Palazot, M.; Yvin, M.; Bruzaud, S. Continents of plastics: An estimate of the stock of microplastics in agricultural soils. Sci. Total Environ. 2023, 880, 163294. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, J.; Liu, Y.; Chen, L.; Tao, S.; Liu, W. Distribution characteristics of microplastics in agricultural soils from the largest vegetable production base in China. Sci. Total Environ. 2021, 756, 143860. [Google Scholar] [CrossRef]
- Deng, L.; Chen, L.; Zhao, J.J.; Wang, R.M. Comparative analysis on environmental and economic performance of agricultural cooperatives and smallholder farmers: The case of grape production in Hebei, China. PLoS ONE 2021, 16, e0245981. [Google Scholar] [CrossRef]
- Wang, Y.; Xv, X.; Shao, T.; He, Q.; Guo, Z.; Wang, Y.; Guo, Q.; Xing, B. A case on source to soil to solutions: Distribution characteristics of microplastics in farmland soil of the largest vegetable base in Northwest China. Sci. Total Environ. 2024, 907, 167910. [Google Scholar] [CrossRef] [PubMed]
- Yakub, A.S.; Bassey, B.O.; Bello, A.I.; Bello, B.O.; Olapoju, O.A.; Agwu, O.A.; Balogun, K.J.; Igbo, J.K.; Ajani, G.; Odedere, A.O.; et al. Eco-toxic Risk Assessment of microplastics in water and sediment across Nigeria Offshore, Gulf of Guinea. Environ. Monit. Assess. 2024, 196, 906. [Google Scholar] [CrossRef]
- Ren, S.; Kai, W.; Jinrui, Z.; Jingjing, L.; Hanyue, Z.; Ruimin, Q.; Wen, X.; Changrong, Y.; Xuejun, L.; Fusuo, Z.; et al. Potential sources and occurrence of macro-plastics and microplastics pollution in farmland soils: A typical case of China. Crit. Rev. Environ. Sci. Technol. 2024, 54, 533–556. [Google Scholar] [CrossRef]
- Ministry of Ecology and Environment, The People’s Republic of China. Letter of Reply to Proposal No. 01680 (Resources and Environment Category No. 122) of the Second Meeting of the 14th National Committee of the CPPCC. 2024. Available online: https://www.mee.gov.cn/xxgk2018/xxgk/xxgk13/202411/t20241125_1096649_wh.html (accessed on 12 April 2025).
- Liu, Y.; Liao, Q.; Shao, Z.; Gao, W.; Cao, J.; Chen, C.; Liao, G.; He, P.; Lin, Z. Spatial Distribution Characteristics and Influencing Factors of Cultivated Land Productivity in a Large City: Case Study of Chengdu, Sichuan, China. Land 2025, 14, 239. [Google Scholar] [CrossRef]
- Qiu, Y.; Zhou, S.; Zhang, C.; Chen, L.; Qin, W.; Zhang, Q. Vertical distribution and weathering characteristic of microplastics in soil profile of different land use types. Sci. Total Environ. 2023, 905, 166902. [Google Scholar] [CrossRef]
- Du, A.; Hu, C.; Wang, X.; Zhao, Y.; Xia, W.; Dai, X.; Wang, L.; Zhang, S. Experimental Study on the Migration and Distribution of Microplastics in Desert Farmland Soil Under Drip Irrigation. Environ. Toxicol. Chem. 2024, 43, 1250–1259. [Google Scholar] [CrossRef]
- Chia, R.W.; Lee, J.-Y.; Cha, J.; Rodríguez-Seijo, A. Methods of soil sampling for microplastic analysis: A review. Environ. Chem. Lett. 2024, 22, 227–238. [Google Scholar] [CrossRef]
- Everaert, G.; Van Cauwenberghe, L.; De Rijcke, M.; Koelmans, A.A.; Mees, J.; Vandegehuchte, M.; Janssen, C.R. Risk assessment of microplastics in the ocean: Modelling approach and first conclusions. Environ. Pollut. 2018, 242, 1930–1938. [Google Scholar] [CrossRef] [PubMed]
- Lithner, D.; Larsson, Å.; Dave, G. Environmental and health hazard ranking and assessment of plastic polymers based on chemical composition. Sci. Total Environ. 2011, 409, 3309–3324. [Google Scholar] [CrossRef]
- Wu, P.; Lin, S.; Cao, G.; Wu, J.; Jin, H.; Wang, C.; Wong, M.H.; Yang, Z.; Cai, Z. Absorption, distribution, metabolism, excretion and toxicity of microplastics in the human body and health implications. J. Hazard. Mater. 2022, 437, 129361. [Google Scholar] [CrossRef]
- Yuan, Z.; Nag, R.; Cummins, E. Ranking of potential hazards from microplastics polymers in the marine environment. J. Hazard. Mater. 2022, 429, 128399. [Google Scholar] [CrossRef] [PubMed]
- Panqing, Y.; Abliz, A.; Xiaoli, S.; Aisaiduli, H. Human health-risk assessment of heavy metal–contaminated soil based on Monte Carlo simulation. Sci. Rep. 2023, 13, 7033. [Google Scholar] [CrossRef]
- Qi, R.; Tang, Y.; Jones, D.L.; He, W.; Yan, C. Occurrence and characteristics of microplastics in soils from greenhouse and open-field cultivation using plastic mulch film. Sci. Total Environ. 2023, 905, 166935. [Google Scholar] [CrossRef]
- Sun, H.; Shi, Y.; Li, C.; He, S.; Bai, Y.; Zhao, P.; Qiu, D.; Liu, J.; He, S. Factors controlling the heavy metal ion activity in soil contaminated by microplastics with different mulch durations: Partial least squares path model. J. Hazard. Mater. 2024, 464, 133027. [Google Scholar] [CrossRef]
- Pan, J.; Zhang, Q.; Zhang, K.; Zhang, Z.; Guo, X. Occurrence of microplastics in agricultural soils in ecologically fragile areas of China. Sci. Total Environ. 2023, 904, 166350. [Google Scholar] [CrossRef]
- Bai, R.; Liu, H.; Cui, J.; Wu, Y.; Guo, X.; Liu, Q.; Liu, Q.; Gao, H.; Yan, C.; He, W. The characteristics and influencing factors of farmland soil microplastic in Hetao Irrigation District, China. J. Hazard. Mater. 2024, 465, 133472. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Shi, Q.; Liu, Y.; Lei, H.; Chen, Y. Microplastics in arid soils: Impact of different cropping systems (Altay, Xinjiang). Environ. Pollut. 2022, 303, 119162. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.-l.; Tang, Q.-x.; Yang, J.-y. Microplastic characteristics and microplastic-heavy metal synergistic contamination in agricultural soil under different cultivation modes in Chengdu, China. J. Hazard. Mater. 2023, 459, 132270. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Wu, Y.; Liu, X.; Wu, J.; Wu, P.; Wu, W. Microplastics abundance associated with farmland use types and the impact on soil microbial communities: A case study in Southern China. J. Hazard. Mater. 2024, 480, 136477. [Google Scholar] [CrossRef]
- Cao, L.; Wu, D.; Liu, P.; Hu, W.; Xu, L.; Sun, Y.; Wu, Q.; Tian, K.; Huang, B.; Yoon, S.J.; et al. Occurrence, distribution and affecting factors of microplastics in agricultural soils along the lower reaches of Yangtze River, China. Sci. Total Environ. 2021, 794, 148694. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Y.; Li, X.; Zhang, Y.; Gao, W.; Jiang, J.; Mo, A.; He, D. Size/shape-dependent migration of microplastics in agricultural soil under simulative and natural rainfall. Sci. Total Environ. 2022, 815, 152507. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Chen, X.; Liu, Q.; Hu, H.; Wu, C.; Xue, Q. Informal landfill contributes to the pollution of microplastics in the surrounding environment. Environ. Pollut. 2022, 293, 118586. [Google Scholar] [CrossRef]
- Liu, X.; Lin, H.; Xu, S.; Yan, Y.; Yu, R.; Hu, G. Occurrence, distribution, and characteristics of microplastics in agricultural soil around a solid waste treatment center in southeast China. J. Soils Sediments 2023, 23, 936–946. [Google Scholar] [CrossRef]
- Corcoran, P.L. Degradation of microplastics in the environment. In Handbook of Microplastics in the Environment; Springer: Berlin/Heidelberg, Germany, 2022; pp. 531–542. [Google Scholar]
- Nakajima, R.; Lindsay, D.J.; Tsuchiya, M.; Matsui, R.; Kitahashi, T.; Fujikura, K.; Fukushima, T. A small, stainless-steel sieve optimized for laboratory beaker-based extraction of microplastics from environmental samples. MethodsX 2019, 6, 1677–1682. [Google Scholar] [CrossRef]
- Qadeer, A.; Ajmal, Z.; Usman, M.; Zhao, X.; Chang, S. Agricultural plastic mulching as a potential key source of microplastic pollution in the terrestrial ecosystem and consequences. Resour. Conserv. Recycl. 2021, 175, 105855. [Google Scholar] [CrossRef]
- Rillig, M.C. Microplastic Disguising As Soil Carbon Storage. Environ. Sci. Technol. 2018, 52, 6079–6080. [Google Scholar] [CrossRef]
- Wang, J.; Song, M.; Lu, M.; Wang, C.; Zhu, C.; Dou, X. Insights into effects of conventional and biodegradable microplastics on organic carbon decomposition in different soil aggregates. Environ. Pollut. 2024, 359, 124751. [Google Scholar] [CrossRef]
- Li, R.; Xi, B.; Tan, W.; Yuan, Y. Spatiotemporal heterogeneous effects of microplastics input on soil dissolved organic matter (DOM) under field conditions. Sci. Total Environ. 2022, 847, 157605. [Google Scholar] [CrossRef]
- Wu, J.; Cao, X.; Zhang, T.; Ouyang, L. Effect of polyethylene microplastics on soil organic carbon pool dynamics in the soil-rhizosphere-alfalfa system. J. Environ. Chem. Eng. 2024, 12, 114724. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, J.; Liu, X.; Qu, F.; Wang, X.; Wang, X.; Li, Y.; Sun, Y. Microplastics in the environment: A review of analytical methods, distribution, and biological effects. Trac-Trends Anal. Chem. 2019, 111, 62–72. [Google Scholar] [CrossRef]
- De Falco, F.; Di Pace, E.; Cocca, M.; Avella, M. The contribution of washing processes of synthetic clothes to microplastic pollution. Sci. Rep. 2019, 9, 6633. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Jin, X.; Wang, Y.; Jin, P. Advanced Treatment of Laundry Wastewater by Electro-Hybrid Ozonation–Coagulation Process: Surfactant and Microplastic Removal and Mechanism. Water 2022, 14, 4138. [Google Scholar] [CrossRef]
- Zhang, Y.; Lan, Z.; Pang, K.; Zhou, Y.; Li, T. Experimental transmittance of eva and po film mulches. Eng. Agrícola 2024, 44. Available online: https://www.scielo.br/j/eagri/a/LJPqzL38R9kb4PcrYVLpNrC/ (accessed on 7 April 2025). [CrossRef]
- Olguner, B.; Mulayim, A.; Gunduz, S.K. Microplastic concentration in the sediment of the Istanbul Strait (the Sea of Marmara, Turkiye). J. Soils Sediments 2023, 23, 2892–2904. [Google Scholar] [CrossRef]
- Amare, G.; Desta, B. Coloured plastic mulches: Impact on soil properties and crop productivity. Chem. Biol. Technol. Agric. 2021, 8, 4. [Google Scholar] [CrossRef]
- Huang, F.; Liu, Z.; Li, Z.; Wang, B.; Zhang, P.; Jia, Z. Is biodegradable film an alternative to polyethylene plastic film for improving maize productivity in rainfed agricultural areas?—Evidence from field experiments. Agric. Water Manag. 2022, 272, 107868. [Google Scholar] [CrossRef]
- Sun, Y.; Yuan, J.; Zhou, T.; Zhao, Y.; Yu, F.; Ma, J. Laboratory simulation of microplastics weathering and its adsorption behaviors in an aqueous environment: A systematic review. Environ. Pollut. 2020, 265, 114864. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Zhao, Y.; Li, Y.; Xiang, Y.; He, D.; Pan, X. Aging of microplastics affects their surface properties, thermal decomposition, additives leaching and interactions in simulated fluids. Sci. Total Environ. 2020, 714, 136862. [Google Scholar] [CrossRef]
- Hermabessiere, L.; Dehaut, A.; Paul-Pont, I.; Lacroix, C.; Jezequel, R.; Soudant, P.; Duflos, G. Occurrence and effects of plastic additives on marine environments and organisms: A review. Chemosphere 2017, 182, 781–793. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Liu, C.; Chen, Q.; Zhang, Z.; Han, J.; Liang, A.; Zhu, D.; Wang, H.; Lv, M.; Chen, L. Extractable additives in microplastics: A hidden threat to soil fauna. Environ. Pollut. 2022, 294, 118647. [Google Scholar] [CrossRef] [PubMed]
- Huerta Lwanga, E.; Mendoza Vega, J.; Ku Quej, V.; de los Angeles Chi, J.; Sanchez del Cid, L.; Chi, C.; Escalona Segura, G.; Gertsen, H.; Salanki, T.; van der Ploeg, M.; et al. Field evidence for transfer of plastic debris along a terrestrial food chain. Sci. Rep. 2017, 7, 14071. [Google Scholar] [CrossRef]
- Wang, W.; Ge, J.; Yu, X. Bioavailability and toxicity of microplastics to fish species: A review. Ecotoxicol. Environ. Saf. 2020, 189, 109913. [Google Scholar] [CrossRef]
- Taylor, S.E.; Pearce, C.I.; Sanguinet, K.A.; Hu, D.; Chrisler, W.B.; Kim, Y.-M.; Wang, Z.; Flury, M. Polystyrene nano- and microplastic accumulation at Arabidopsis and wheat root cap cells, but no evidence for uptake into roots. Environ. Sci.-Nano 2020, 7, 1942–1953. [Google Scholar] [CrossRef]
- Teuten, E.L.; Rowland, S.J.; Galloway, T.S.; Thompson, R.C. Potential for Plastics to Transport Hydrophobic Contaminants. Environ. Sci. Technol. 2007, 41, 7759–7764. [Google Scholar] [CrossRef]
- Zhang, X.; Zheng, M.; Yin, X.; Wang, L.; Lou, Y.; Qu, L.; Liu, X.; Zhu, H.; Qiu, Y. Sorption of 3,6-dibromocarbazole and 1,3,6,8-tetrabromocarbazole by microplastics. Mar. Pollut. Bull. 2019, 138, 458–463. [Google Scholar] [CrossRef]
- Ren, X.; Tang, J.; Liu, X.; Liu, Q. Effects of microplastics on greenhouse gas emissions and the microbial community in fertilized soil. Environ. Pollut. 2020, 256, 113347. [Google Scholar] [CrossRef]
- Hao, Y.; Sun, H.; Zeng, X.; Dong, G.; Kronzucker, H.J.; Min, J.; Xia, C.; Lam, S.S.; Shi, W. Smallholder vegetable farming produces more soil microplastics pollution than large-scale farming. Environ. Pollut. 2023, 317, 120805. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Waldman, W.R.; Kim, T.-Y.; Rillig, M.C. Effects of Different Microplastics on Nematodes in the Soil Environment: Tracking the Extractable Additives Using an Ecotoxicological Approach. Environ. Sci. Technol. 2020, 54, 13868–13878. [Google Scholar] [CrossRef]
- O’Connor, D.; Pan, S.; Shen, Z.; Song, Y.; Jin, Y.; Wu, W.-M.; Hou, D. Microplastics undergo accelerated vertical migration in sand soil due to small size and wet-dry cycles. Environ. Pollut. 2019, 249, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Schnepf, U. Realistic Risk Assessment of Soil Microplastics Is Hampered by a Lack of Eligible Data on Particle Characteristics: A Call for Higher Reporting Standards. Environ. Sci. Technol. 2023, 57, 3–4. [Google Scholar] [CrossRef]
- Monson, L.; Braunwarth, M.; Extrand, C.W. Moisture absorption by various Polyamides and their associated dimensional changes. J. Appl. Polym. Sci. 2008, 107, 355–363. [Google Scholar] [CrossRef]
- Aekjira Kuyyakanont, M.I.A. Study of different degradation effects in UV-sensitive polymers using xenon lamp and deuterium lamp to simulate UV irradiation in space environment. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2024, 549, 165267. [Google Scholar] [CrossRef]
- Moshood, T.D.; Nawanir, G.; Mahmud, F.; Mohamad, F.; Ahmad, M.H.; AbdulGhani, A. Biodegradable plastic applications towards sustainability: A recent innovations in the green product. Clean. Eng. Technol. 2022, 6, 100404. [Google Scholar] [CrossRef]
- Han, X.; Lu, X.; Vogt, R.D. An optimized density-based approach for extracting microplastics from soil and sediment samples. Environ. Pollut. 2019, 254, 113009. [Google Scholar] [CrossRef]
- Kang, Y.; Hirota, Y.; Tsukayama, K.; Ikejima, K.; Sakurai, K. Separation and Measurement of Microplastics in Paddy Soil. J. Environ. Prot. 2024, 15, 1016–1021. [Google Scholar] [CrossRef]
- Li, W.X.; Wu, Y.M.; Wang, K.; Chen, W.; Tian, J.Y.; Xu, L. The Method for Determination of Microplastics in Typical Chinese Soils. J. Ecol. Rural. Environ. 2023, 39, 568–575. [Google Scholar]
- Liu, M.; Song, Y.; Lu, S.; Qiu, R.; Hu, J.; Li, X.; Bigalke, M.; Shi, H.; He, D. A method for extracting soil microplastics through circulation of sodium bromide solutions. Sci. Total Environ. 2019, 691, 341–347. [Google Scholar] [CrossRef]
- Goli, V.S.N.S.; Singh, D.N. Effect of ultrasonication conditions on polyethylene microplastics sourced from landfills: A precursor study to establish guidelines for their extraction from environmental matrices. J. Hazard. Mater. 2023, 459, 132230. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Pei, Z.; Li, Y.; Yang, R.; Wang, P.; Liang, Y.; Zhang, J.; Zhang, Q.; Jiang, G. A high-precision, effective method for extraction and identification of small-sized microplastics from soil. Talanta 2024, 272, 125802. [Google Scholar] [CrossRef]
- Maw, M.M.; Boontanon, N.; Fujii, S.; Boontanon, S.K. Rapid and efficient removal of organic matter from sewage sludge for extraction of microplastics. Sci. Total Environ. 2022, 853, 158642. [Google Scholar] [CrossRef] [PubMed]
- Prosenc, F.; Leban, P.; Šunta, U.; Bavcon Kralj, M. Extraction and Identification of a Wide Range of Microplastic Polymers in Soil and Compost. Polymers 2021, 13, 4069. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.; Hao, X.; Zhang, S. Extraction and decontamination of microplastics from high organic matter soils: A simple, cost-saving and high efficient method. J. Environ. Manag. 2023, 344, 118381. [Google Scholar] [CrossRef]
- Gupta, E.; Mishra, V.K.; Patel, A.; Srivastava, P.K. A modified methodology for extraction and quantification of microplastics in soil. NanoImpact 2024, 35, 100525. [Google Scholar] [CrossRef]
- Radford, F.; Zapata-Restrepo, L.M.; Horton, A.A.; Hudson, M.D.; Shaw, P.J.; Williams, I.D. Developing a systematic method for extraction of microplastics in soils. Anal. Methods 2021, 13, 1695–1705. [Google Scholar] [CrossRef]


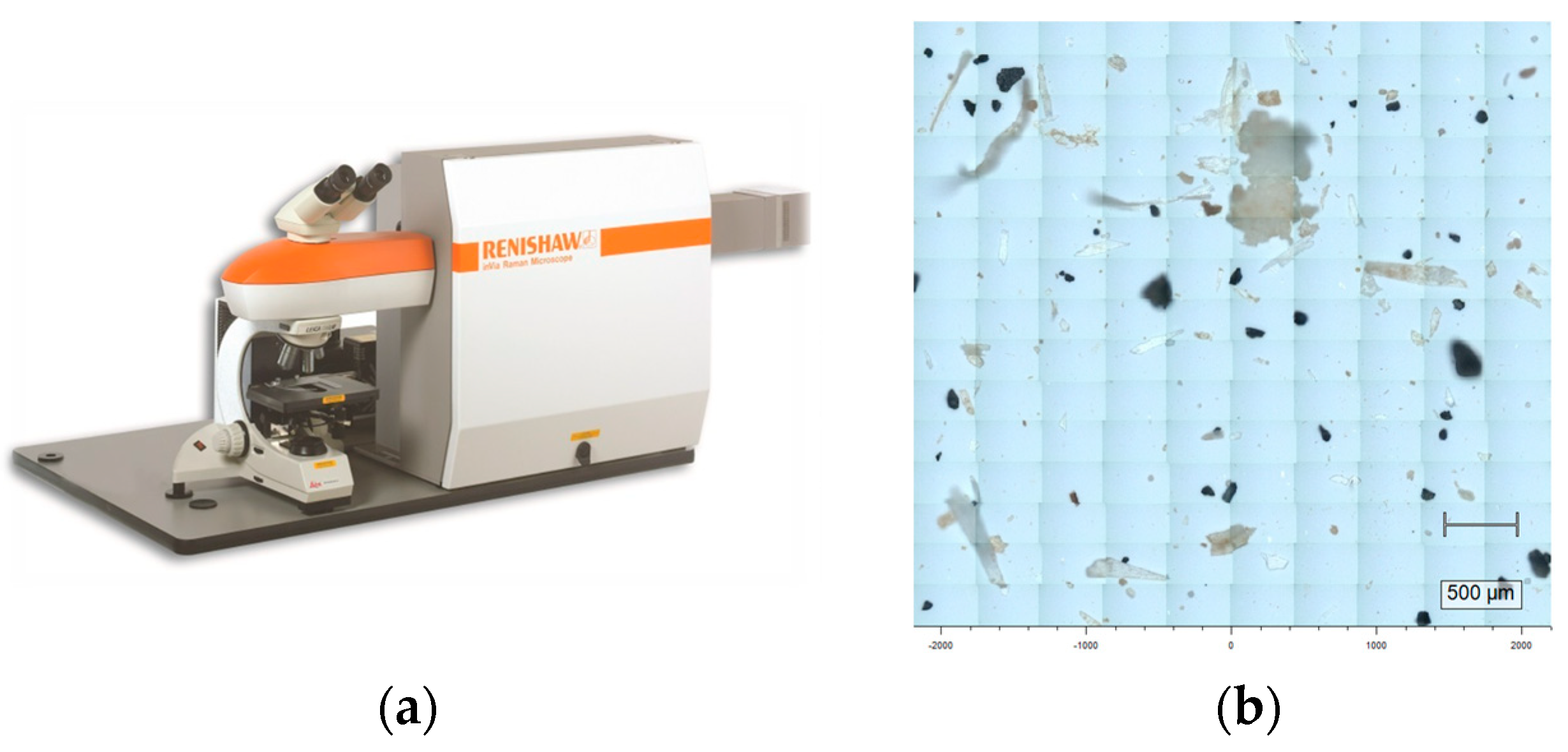








| No. | Location (Province) | Pretreatment | Abundance Average (n/kg) | Abundance Range (n/kg) | Major Size Range (μm), Percentage | Major MPs Types | Reference | ||
|---|---|---|---|---|---|---|---|---|---|
| Pore/Mesh Size in Separation (μm) | Separation for MPs and Solution | Density of Flotation Reagents (g/cm3) | |||||||
| 1 | Shaanxi | / | / | 1.5 | / | 1430 to 3410 | 0–490, 81% | PS, PE, PP, HDPE, PVC, PET | [7] |
| 2 | Guizhou | 0.45 | Vacuum filtration | 1.2 | 1150.60 ± 647.86 | 143.28 to 3283.46 | 10~100, 64.79% | / | [8] |
| 3 | Hebei | 0.45 | Vacuum filtration | 1.2 | 754 ± 477 | 240 to 2253 | <500, 50% | PE, PP, PET, rayon, PEA, cellophane, PAN | [9] |
| 4 | Hunan | 0.45 | Vacuum filtration | 1.5 | 4377.44 | 776.33 to 12,292.33 | <100, as high as 45.99% | PE, PP, PS, PA, PVC | [10] |
| 5 | Shandong | 48 | Sieve | 1.2 | 34,900 | / | 50–250, 80% | PE, PP, PS, PA, UF, POM | [38] |
| 6 | Beijing | 18,300 | |||||||
| 7 | Xinjiang | 33,200 | |||||||
| 8 | Qinghai | 0.45 | Vacuum filtration | 1.2 | / | 168.18 to 259.72 | <3000, 90.3% | PE, PP, PS, PA, PVC | [39] |
| 9 | Tibet | 183.33 to 611.11 | |||||||
| 10 | Shaanxi | 13 | Vacuum filtration | 1.7 | 4505 ± 435 | 1398 to 8568 | <100, 90% | PE, PET, EVA, PU, PVC | [11] |
| 11 | Shandong | 20 | Vacuum filtration | 1.2 | 1444 ± 986 | 310 to 5698 | <500, 58% | PP, E/P, PE | [23] |
| 12 | Hubei | 0.45 | Vacuum filtration | 1.5 | 2020 | 320 to 12,560 | <200, 70% | PA, PP | [12] |
| 13 | Xinjiang, Guizhou | 0.45 | Vacuum filtration | 1.5 | 2685 ± 938 | / | 0~490, 81.71% | PE, PP, PS, PVC | [40] |
| 14 | Inner Mongolia | 10 | Vacuum filtration | 1.46 | / | 1810 to 86,331 | <180, 90% | PE, PA, PP | [41] |
| 15 | Xinjiang | 0.22 | Vacuum filtration | 1.42 | 52,081.7 | 11,347 to 78,061 | <200, 70% | PE | [42] |
| 16 | Sichuan | 20 | Vacuum filtration | 1.19 | 6544 ± 2561 | 1333 ± 138 to 15,067 ± 2638 | 50–100, up to 29% | PP, PE | [43] |
| 17 | Guangdong | 20 | Vacuum filtration | 1.7 | 10,562 | 528 to 39,864 | 0–30, up to 51.7% | PET, PVC, FKM | [44] |
| 18 | Jiangsu, Shanghai | 0.03 | Vacuum filtration | 1.2 | 37.32 | 4.94 to 252.70 | 100~500, 49% | PP, PE, rayon | [45] |
| 19 | Shaanxi | 0.45 | Vacuum filtration | 1.2 | 1955 | 200.00 to 4733.33 | <100, 96.65% | PE, PET | [25] |
| 20 | Yunnan | 5 | Vacuum filtration | 1.6 | 595.00 ± 740.00 | 50.000 to 3450.0 | <1000, 75.7% | PP, EPR, PE | [46] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Liu, S.; Gao, C.; Huang, J.; Liang, H.; Zhang, L.; Xiao, G.; Wu, Y. Microplastics in the Typical Mulched Farmland of Sichuan Province: Characteristics That Vary Across Farming Scales and the Risk Significantly Contributed by Priority Polymers. Sustainability 2025, 17, 3516. https://doi.org/10.3390/su17083516
Zhang Y, Liu S, Gao C, Huang J, Liang H, Zhang L, Xiao G, Wu Y. Microplastics in the Typical Mulched Farmland of Sichuan Province: Characteristics That Vary Across Farming Scales and the Risk Significantly Contributed by Priority Polymers. Sustainability. 2025; 17(8):3516. https://doi.org/10.3390/su17083516
Chicago/Turabian StyleZhang, Yuqing, Shuyuan Liu, Cheng Gao, Jialiang Huang, Huan Liang, Li Zhang, Guangli Xiao, and Yi Wu. 2025. "Microplastics in the Typical Mulched Farmland of Sichuan Province: Characteristics That Vary Across Farming Scales and the Risk Significantly Contributed by Priority Polymers" Sustainability 17, no. 8: 3516. https://doi.org/10.3390/su17083516
APA StyleZhang, Y., Liu, S., Gao, C., Huang, J., Liang, H., Zhang, L., Xiao, G., & Wu, Y. (2025). Microplastics in the Typical Mulched Farmland of Sichuan Province: Characteristics That Vary Across Farming Scales and the Risk Significantly Contributed by Priority Polymers. Sustainability, 17(8), 3516. https://doi.org/10.3390/su17083516





