Characterization and Evaluation of the Efficiency of Organic Amendments and Native Macrophytes for the Treatment of Acid Mine Drainage in Hualgayoc—A Case Study
Abstract
1. Introduction
2. Materials and Methods
2.1. A Description of the Case Study
2.2. Sampling and Characterization of AMD and Macrophytes
2.3. Production and Characterization of Amendments
2.4. AMD Preparation for Heavy Metal Determination
2.5. Determination of Heavy Metals Form Organic Ammendments
2.6. Preparation of Macrophytes for Heavy Metal Determination
2.7. Digestion and Determination of Metals and Metalloids in AMD, Organic Ammendments, and Macrophytes
2.8. Installation and Operating Conditions of the System and Sorption Capacity
2.9. Experimental Design and Data Analysis
3. Results and Discussion
3.1. Heavy Metals and Physicochemical Parameters of AMD
3.2. Physicochemical Properties and Heavy Metals of Amendments, Biochar, and Macrophytes
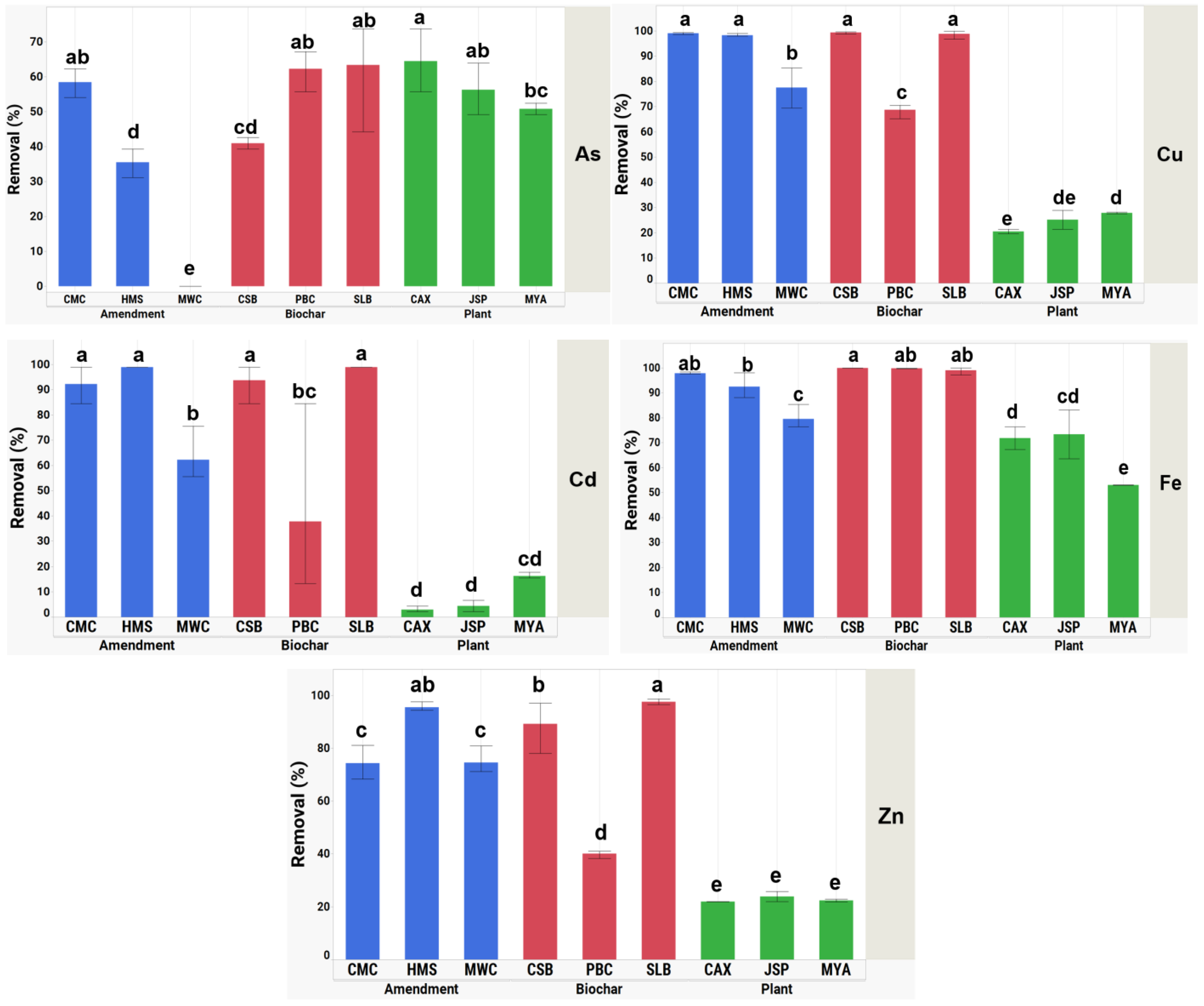
3.3. Sorption Capacity and Removal of Heavy Metals
| Description of Application | Concentration of Application | Concentration of Pollutant | Time | Pollutant | % Removal (%) | BCF | Reference |
|---|---|---|---|---|---|---|---|
| Carex riparia 1 Cyperus longus 2 Cyperus rotundu 3 | As: 0.450–0.500 mg/L | 90 days | As | - | 74.24 1, 40.29 2, 29.37 3 | [55] | |
| Cd: 2.000–2.500 mg/L | Cd | - | 12.64 1, 15.63 2, 24.45 3 | ||||
| - | Cu: 160–1000 mg/L | Cu | - | 4.42 1, 39.80 2, 34.94 3 | |||
| Fe: 250–400 mg/L | Fe | - | 108.58 1, 399.79 2, 413.11 3 | ||||
| Zn: 70–320 mg/L | Zn | - | 3.95 1, 14.24 2, 18.91 3 | ||||
| Myriophyllum aquaticum | - | Cu: 1000 ug/L TC: 3000 ug/L | 12 weeks | Cu + tetracycline (TC) | >80 | - | [19] |
| Myriophyllum aquaticum | - | Cu: 0.5 mg/L | 7 days | Cu | 95.20 | 200–300 | [62] |
| Myriophyllum aquaticum | - | Zn: 0.25 mg/L | Zn | 91.91 | 180–200 | ||
| Juncus effusus | - | Sulfate (SO42⁻): 4000 mg/L, Mn: 18 mg/L, Zn: 10 mg/L, Cd: 0.5 mg/L | 60 days | Sulfate (SO42⁻) | <50 | - | [1] |
| Juncus effusus | - | Sulfate (SO42⁻): 4000 mg/L, Mn: 18 mg/L, Zn: 10 mg/L, Cd: 0.5 mg/L | Mn | >99 | - | ||
| Carex flacca + Condensed Molasses Soluble (CMS) 1 Phragmites australis 2 | 1% CSM | 206 ± 45 | As | - | 2227 1, 295 2 | [8] | |
| 265 ± 100 | Cu | - | 371 1, 502 2 | ||||
| 885 ± 50 | 6 weeks | Pb | - | 1685 1, 1046 2 | |||
| 2780 ± 110 | Zn | - | 329 1, 3882 | ||||
| - | Cd | - | 401 1, 463 2 | ||||
| Fe-impregnated biochar (FBC) | 20% FBC | 10 mg/L As(V) | 48 h | As | 87.53 | - | [53] |
| Wood biochar (WB4, WB7) | - | 10 mg/L As (III) | 24 h | As | 58.1(WB7) | - | [54] |
| Rice husk biochar (RB4, RB7) | - | 10 mg/L As (III) | As | 54.0 (RB7) | - | ||
| Pine cone biochar (PBC) 1 Zn-loaded PCB 2 | - | 100 µg/L As (III) | - | As | 66.08 ± 3.94 1 87.62 ± 3.88 2 | - | [63] |
| Biochar inoculated bacteria (BIC) | BIC: 0.6 g/L | 5 mg/L (Cd2+) | Up to 120 h | Cd | 67.90 | - | [64] |
| Food waste compost | 11,787.6 ppm | Fe | ≈100 | - | [61] | ||
| 20 g/L compost | 4.5 ppm | Cu | >90 | - | |||
| 20 g/L compost | 0.2 ppm | 2 h | Pb | ≈100 | - | ||
| 40 g/L compost | 61.2 ppm | Zn | ≈90 | - | |||
| 40 g/L compost | 5.9 ppm | Ni | <90 | - |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, A.; Zhang, Y.; Zhao, X.; Li, J.; Zhang, G.; Shi, H.; Guo, L.; Xu, S. Experimental Study on the Hydroponics of Wetland Plants for the Treatment of Acid Mine Drainage. Sustainability 2022, 14, 2148. [Google Scholar] [CrossRef]
- Luís, A.T.; Córdoba, F.; Antunes, C.; Loayza-Muro, R.; Grande, J.A.; Silva, B.; Diaz-Curiel, J.; Da Silva, E.F. Extremely Acidic Eukaryotic (Micro) Organisms: Life in Acid Mine Drainage Polluted Environments—Mini-Review. Int. J. Environ. Res. Public Health 2022, 19, 376. [Google Scholar]
- Jiao, Y.; Zhang, C.; Su, P.; Tang, Y.; Huang, Z.; Ma, T. A review of acid mine drainage: Formation mechanism, treatment technology, typical engineering cases and resource utilization. Process Saf. Environ. Prot. 2023, 170, 1240–1260. [Google Scholar]
- Singh, S.; Chakraborty, S. Performance of organic substrate amended constructed wetland treating acid mine drainage (AMD) of North-Eastern India. J. Hazard Mater. 2020, 397, 122719. [Google Scholar]
- Wibowo, Y.G.; Safitri, H.; Malik, I.B.I.; Sudibyo; Priyanto, S. Alternative Low-Cost Treatment for Real Acid Mine Drainage: Performance, Bioaccumulation, Translocation, Economic, Post-Harvest, and Bibliometric Analyses. Sustainability 2022, 14, 15404. [Google Scholar] [CrossRef]
- Anawar, H.M. Sustainable rehabilitation of mining waste and acid mine drainage using geochemistry, mine type, mineralogy, texture, ore extraction and climate knowledge. J. Environ. Manag. 2015, 158, 111–121. [Google Scholar]
- Zheng, Q.; Zhang, Y.; Zhang, Z.; Li, H.; Wu, A.; Shi, H. Experimental research on various slags as a potential adsorbent for the removal of sulfate from acid mine drainage. J. Environ. Manag. 2020, 270, 110880. [Google Scholar] [CrossRef] [PubMed]
- Nagy, A.; Magyar, T.; Juhász, C.; Tamás, J. Phytoremediation of acid mine drainage using by-product of lysine fermentation. Water Sci. Technol. 2020, 81, 1507–1517. [Google Scholar] [CrossRef]
- Kurkjian, R.; Dunlap, C.; Flegal, A.R. Long-range downstream effects of urban runoff and acid mine drainage in the Debed River, Armenia: Insights from lead isotope modeling. Appl. Geochem. 2004, 19, 1567–1580. [Google Scholar] [CrossRef]
- Mitra, S.; Chakraborty, A.J.; Tareq, A.M.; Emran, T.B.; Nainu, F.; Khusro, A.; Idris, A.M.; Khandaker, M.U.; Osman, H.; Alhumaydhi, F.A.; et al. Impact of heavy metals on the environment and human health: Novel therapeutic insights to counter the toxicity. J. King Saud. Univ. Sci. 2022, 34, 101865. [Google Scholar]
- Barreto-Pio, C.; Bravo-Toledo, L.; Virú-Vásquez, P.; Borda-Contreras, A.; Zarate-Sarapura, E.; Pilco, A. Optimization Applying Response Surface Methodology in the Co-treatment of Urban and Acid Wastewater from the Quiulacocha Lagoon, Pasco (Peru). Environ. Res. Eng. Manag. 2023, 79, 90–109. [Google Scholar] [CrossRef]
- Vriens, B.; Peterson, H.; Laurenzi, L.; Smith, L.; Aranda, C.; Mayer, K.U.; Beckie, R.D. Long-term monitoring of waste-rock weathering at the Antamina mine, Peru. Chemosphere 2019, 215, 858–869. [Google Scholar] [CrossRef] [PubMed]
- Cruzado-Tafur, E.; Torró, L.; Bierla, K.; Szpunar, J.; Tauler, E. Heavy metal contents in soils and native flora inventory at mining environmental liabilities in the Peruvian Andes. J. S. Am. Earth Sci. 2021, 106, 103107. [Google Scholar] [CrossRef]
- Cruz, D.; Pimentel, M.; Russo, A.; Cabral, W. Charge Neutralization Mechanism Efficiency in Water with High Color Turbidity Ratio Using Aluminium Sulfate and Flocculation Index. Water 2020, 12, 572. [Google Scholar] [CrossRef]
- Blanco, I.; Sapsford, D.J.; Trumm, D.; Pope, J.; Kruse, N.; Cheong, Y.-w.; McLauchlan, H.; Sinclair, E.; Weber, P.; Olds, W. Internationale Erprobungen vertikaler Durchflussfilter für die Aufbereitung von Grubenwasser aus dem Kohlebergbau. Mine Water Environ. 2018, 37, 4–17. [Google Scholar] [CrossRef]
- Pat-Espadas, A.M.; Portales, R.L.; Amabilis-Sosa, L.E.; Gómez, G.; Vidal, G. Review of Constructed Wetlands for Acid Mine Drainage Treatment. Water 2018, 10, 1685. [Google Scholar] [CrossRef]
- Roé-Sosa, A.; Rangel-Peraza, J.G.; Rodríguez-Mata, A.E.; Pat-Espadas, A.; Bustos-Terrones, Y.; Diaz-Peña, I.; Vu, C.M.; Amabilis-Sosa, L.E. Emulating natural wetlands oxygen conditions for the removal of N and P in agricultural wastewaters. J. Environ. Manag. 2019, 236, 351–357. [Google Scholar] [CrossRef]
- Ebrahimbabaie, P.; Smith, A.; Zahran, E.M.; Pichtel, J. Phytoremediation of Engineered Nanoparticles Using Typha latifolia and Carex rostrata. Appl. Environ. Soil Sci. 2023, 2023, 3417525. [Google Scholar] [CrossRef]
- Guo, X.; Liu, M.; Zhong, H.; Li, P.; Zhang, C.; Wei, D.; Zhao, T. Potential of Myriophyllum aquaticum for phytoremediation of water contaminated with tetracycline antibiotics and copper. J. Environ. Manag. 2020, 270, 110867. [Google Scholar] [CrossRef]
- Syranidou, E.; Christofilopoulos, S.; Kalogerakis, N. Juncus spp.—The helophyte for all (phyto)remediation purposes? N. Biotechnol. 2017, 38, 43–55. [Google Scholar] [CrossRef]
- Wibowo, Y.G.; Ramadan, B.S.; Sudibyo, S.; Safitri, H.; Rohman, A.; Syarifuddin, H. Efficient remediation of acid mine drainage through sustainable and economical biochar-CaO composite derived from solid waste. Environ. Dev. Sustain. 2024, 26, 16803–16826. [Google Scholar] [CrossRef]
- Gibert, O.; De Pablo, J.; Cortina, J.L.; Ayora, C. Evaluation of municipal compost/limestone/iron mixtures as filling material for permeable reactive barriers for in-situ acid mine drainage treatment. J. Chem. Technol. Biotechnol. 2003, 78, 489–496. [Google Scholar] [CrossRef]
- Virú-Vásquez, P.; Huapaya Pardavé, R.; Bravo-Toledo, L.; Flor, M.; Coral, C.; Curaqueo, G. Biochar and Compost in the Soil: A Bibliometric Analysis of Scientific Research. Environ. Res. Eng. Manag. 2022, 78, 73–95. [Google Scholar] [CrossRef]
- Palacios-Hugo, R.; Calle-Maravi, J.; Césare-Coral, M.F.; Iparraguirre, J.; Virú-Vásquez, P. Physicochemical Characterization and Stability of Biochar Obtained from 5 Species of Forest Biomass in Peru. Environ. Res. Eng. Manag. 2023, 79, 35–51. [Google Scholar] [CrossRef]
- Gibert, O.; De Pablo, J.; Cortina, J.L.; Ayora, C. Municipal compost-based mixture for acid mine drainage bioremediation: Metal retention mechanisms. Appl. Geochem. 2005, 20, 1648–1657. [Google Scholar] [CrossRef]
- Guo, H.-n.; Liu, H.-t.; Wu, S. Immobilization pathways of heavy metals in composting: Interactions of microbial community and functional gene under varying C/N ratios and bulking agents. J. Hazard Mater. 2022, 426, 128103. [Google Scholar] [CrossRef]
- Wijesekara, H.; Bolan, N.S.; Vithanage, M.; Xu, Y.; Mandal, S.; Brown, S.L.; Hettiarachchi, G.M.; Pierzynski, G.M.; Huang, L.; Ok, Y.S.; et al. Utilization of Biowaste for Mine Spoil Rehabilitation. Adv. Agron. 2016, 138, 97–173. [Google Scholar]
- Wang, C.; Jia, Y.; Li, J.; Li, P.; Wang, Y.; Yan, F.; Wu, M.; Fang, W.; Xu, F.; Qiu, Z. Influence of microbial augmentation on contaminated manure composting: Metal immobilization, matter transformation, and bacterial response. J. Hazard Mater. 2023, 441, 129762. [Google Scholar] [CrossRef]
- Moreno-Barriga, F.; Díaz, V.; Acosta, J.A.; Muñoz, M.Á.; Faz, Á.; Zornoza, R. Organic matter dynamics, soil aggregation and microbial biomass and activity in Technosols created with metalliferous mine residues, biochar and marble waste. Geoderma 2017, 301, 19–29. [Google Scholar] [CrossRef]
- Matos, G.D.; Arruda, M.A.Z. Vermicompost as natural adsorbent for removing metal ions from laboratory effluents. Process Biochem. 2003, 39, 81–88. [Google Scholar] [CrossRef]
- Das, D.; Abbhishek, K.; Banik, P.; Bhattacharya, P. A valorisation approach in recycling of organic wastes using low-grade rock minerals and microbial culture through vermicomposting. Environ. Chall. 2021, 5, 100225. [Google Scholar] [CrossRef]
- Poornima, S.; Dadi, M.; Subash, S.; Manikandan, S.; Karthik, V.; Deena, S.R.; Balachandar, R.; Kumaran, S.K.N.; Subbaiya, R. Review on advances in toxic pollutants remediation by solid waste composting and vermicomposting. Sci. Afr. 2024, 23, e02100. [Google Scholar] [CrossRef]
- Sánchez-Tello, S. Zonas de Vida de Cajamarca. Cajamarca-Perú. 2011. Available online: https://zeeot.regioncajamarca.gob.pe/sites/default/files/ZonasVidasZEESegunMapaNacional.pdf (accessed on 8 November 2022).
- Pinto Herrera, H. Los pasivos mineros ambientales y los conflictos sociales en Hualgayoc. Investig. Soc. 2013, 17, 265–277. [Google Scholar] [CrossRef]
- Nguegang, B.; Masindi, V.; Msagati Makudali, T.A.; Tekere, M. Effective treatment of acid mine drainage using a combination of MgO-nanoparticles and a series of constructed wetlands planted with Vetiveria zizanioides: A hybrid and stepwise approach. J. Environ. Manag. 2022, 310, 114751. [Google Scholar] [CrossRef]
- Césare, M.; Zamora, G. Manual Técnico de Obtención de Biochar de Bambú. 2020. Available online: https://www.researchgate.net/publication/346961977_Contrato_N174-2015_FONDECYT-UNALM_OBTENCION_DE_BIOCHAR_DE_BAMBU_Procesos_de_produccion_del_biochar_y_usos_Elaborado_por_el_Circulo_de_Investigacion_para_el_Desarrollo_de_la_Cadena_de_Valor_del_Bambu_p (accessed on 8 November 2022).
- Rajkovich, S.; Enders, A.; Hanley, K.; Hyland, C.; Zimmerman, A.R.; Lehmann, J. Corn growth and nitrogen nutrition after additions of biochars with varying properties to a temperate soil. Biol. Fertil. Soils 2011, 48, 271–284. [Google Scholar] [CrossRef]
- Iglesias Jiménez, E.; Pérez García, V. Relationships between organic carbon and total organic matter in municipal solid wastes and city refuse composts. Bioresour. Technol. 1992, 41, 265–272. [Google Scholar] [CrossRef]
- LECO Corporation. Carbon, Hydrogen, and Nitrogen in Coal 2013. Available online: https://knowledge.leco.com/index.php/component/edocman/ (accessed on 8 November 2022).
- Schinner, F.; Ohlinger, R.; Kandeler, E.; Margesin, R. Methods in Soil Biology; Springer: Berlin/Heidelberg, Germany, 1996. [Google Scholar]
- APHA; AWWA; WEF. Standard Methods for the Examination of Water and Wastewater, 24th ed.; APHA Press: Washington, DC, USA, 2023. [Google Scholar]
- EPA (Environmental Protection Agency). Method 3050B-Acid Digestion of Sediments, Sludges, and Soils; EPA: Sydney, Australia, 1996.
- Soon, Y.K. Dertermination of Cadmium, Chromium, Cobalt, Lead, and Nickel in Plant Tissue. In Handbook on Reference Methods for Plants Analysis; Kalra, Y.P., Ed.; CRC Press: Boca Raton, FL, USA, 1998; pp. 193–198. [Google Scholar]
- Tokalioglu, Ş.; Kartal, Ş.; Güneş, A.A. Determination of Heavy Metals in Soil Extracts and Plant Tissues at Around of a Zinc Smelter. Int. J. Environ. Anal. Chem. 2001, 80, 201–217. [Google Scholar] [CrossRef]
- Mirosławski, J.; Paukszto, A. Determination of the cadmium, chromium, nickel, and lead ions relays in selected polish medicinal plants and their infusion. Biol. Trace Elem. Res. 2018, 182, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.; He, J.; Lü, C.; Ren, L.; Fan, Q.; Wang, J.; Xie, Z. Distribution characteristics and potential ecological risk assessment of heavy metals (Cu, Pb, Zn, Cd) in water and sediments from Lake Dalinouer, China. Ecotoxicol. Environ. Saf. 2013, 93, 135–144. [Google Scholar] [CrossRef]
- Rodríguez-Vila, A.; Selwyn-Smith, H.; Enunwa, L.; Smail, I.; Covelo, E.F.; Sizmur, T. Predicting Cu and Zn sorption capacity of biochar from feedstock C/N ratio and pyrolysis temperature. Environ. Sci. Pollut. Res. 2018, 25, 7730–7739. [Google Scholar] [CrossRef]
- Khan, S.; Ullah, A.; Ayaz, T.; Aziz, A.; Aman, K.; Habib, M.; Yilmaz, S.; Farid, A.; Yasmin, H.; Ali, Q. Phycoremediation of industrial wastewater using Vaucheria debaryana and Cladophora glomerata. Environ. Monit. Assess 2023, 195, 825. [Google Scholar]
- Irfan, M.; Mudassir, M.; Khan, M.J.; Dawar, K.M.; Muhammad, D.; Mian, I.A.; Ali, W.; Fahad, S.; Saud, S.; Hayat, Z.; et al. Heavy metals immobilization and improvement in maize (Zea mays L.) growth amended with biochar and compost. Sci. Rep. 2021, 11, 18416. [Google Scholar]
- Wu, B.; Peng, H.; Sheng, M.; Luo, H.; Wang, X.; Zhang, R.; Xu, F.; Xu, H. Evaluation of phytoremediation potential of native dominant plants and spatial distribution of heavy metals in abandoned mining area in Southwest China. Ecotoxicol. Environ. Saf. 2021, 220, 112368. [Google Scholar] [PubMed]
- Barbafieri, M.; Dadea, C.; Tassi, E.; Bretzel, F.; Fanfani, L. Uptake of Heavy Metals by Native Species Growing in a Mining Area in Sardinia, Italy: Discovering Native Flora for Phytoremediation. Int. J. Phytoremediat. 2011, 13, 985–997. [Google Scholar]
- Alghamdi, S.A.; El-Zohri, M. Phytoremediation Characterization of Heavy Metals by Some Native Plants at Anthropogenic Polluted Sites in Jeddah, Saudi Arabia. Resources 2024, 13, 98. [Google Scholar] [CrossRef]
- He, R.; Peng, Z.; Lyu, H.; Huang, H.; Nan, Q.; Tang, J. Synthesis and characterization of an iron-impregnated biochar for aqueous arsenic removal. Sci. Total Environ. 2018, 612, 1177–1186. [Google Scholar]
- Zama, E.F.; Li, G.; Tang, Y.T.; Reid, B.J.; Ngwabie, N.M.; Sun, G.X. The removal of arsenic from solution through biochar-enhanced precipitation of calcium-arsenic derivatives. Environ. Pollut. 2022, 292, 118241. [Google Scholar] [PubMed]
- Velasco-Arroyo, B.; Curiel-Alegre, S.; Khan, A.H.A.; Rumbo, C.; Pérez-Alonso, D.; Rad, C.; De Wilde, H.; Pérez-de-Mora, A.; Barros, R. Phytostabilization of metal(loid)s by ten emergent macrophytes following a 90-day exposure to industrially contaminated groundwater. N. Biotechnol. 2024, 79, 50–59. [Google Scholar]
- Inyang, M.I.; Gao, B.; Yao, Y.; Xue, Y.; Zimmerman, A.; Mosa, A.; Pullammanappallil, P.; Ok, Y.S.; Cao, X. A review of biochar as a low-cost adsorbent for aqueous heavy metal removal. Crit. Rev. Environ. Sci. Technol. 2015, 46, 406–433. [Google Scholar]
- Qiu, B.; Tao, X.; Wang, H.; Li, W.; Ding, X.; Chu, H. Biochar as a low-cost adsorbent for aqueous heavy metal removal: A review. J. Anal. Appl. Pyrolysis 2021, 155, 105081. [Google Scholar]
- Liu, Z.; Xu, Z.; Xu, L.; Buyong, F.; Chay, T.C.; Li, Z.; Cai, Y.; Hu, B.; Zhu, Y.; Wang, X. Modified biochar: Synthesis and mechanism for removal of environmental heavy metals. Carbon Res. 2022, 1, 8. [Google Scholar]
- Patra, J.M.; Panda, S.S.; Dhal, N.K. Biochar as a low-cost adsorbent for heavy metal removal: A review. Int. J. Res. BioSci. (IJRBS) 2017, 6, 1–7. [Google Scholar]
- Liu, J.; Xu, Z.; Zhang, W. Unraveling the role of Fe in As(III & V) removal by biochar via machine learning exploration. Sep. Purif. Technol. 2023, 311, 123245. [Google Scholar]
- Liu, W.; Rast, S.; Wang, X.; Lan, S.; Owusu-Fordjour, E.Y.; Yang, X. Enhanced removal of Fe, Cu, Ni, Pb, and Zn from acid mine drainage using food waste compost and its mechanisms. Green Smart Min. Eng. 2024, 1, 375–386. [Google Scholar]
- Romero-Hernández, J.A.; Amaya-Chávez, A.; Balderas-Hernández, P.; Roa-Morales, G.; González-Rivas, N.; Balderas-Plata, M.Á. Tolerance and hyperaccumulation of a mixture of heavy metals (Cu, Pb, Hg, and Zn) by four aquatic macrophytes. Int. J. Phytoremediat. 2017, 19, 239–245. [Google Scholar]
- Van Vinh, N.; Zafar, M.; Behera, S.K.; Park, H.S. Arsenic(III) removal from aqueous solution by raw and zinc-loaded pine cone biochar: Equilibrium, kinetics, and thermodynamics studies. Int. J. Environ. Sci. Technol. 2015, 12, 1283–1294. [Google Scholar]
- Wu, Z.; Firmin, K.A.; Cheng, M.; Wu, H.; Si, Y. Biochar enhanced Cd and Pb immobilization by sulfate-reducing bacterium isolated from acid mine drainage environment. J. Clean. Prod. 2022, 366, 132823. [Google Scholar]
- Kikis, C.; Thalassinos, G.; Antoniadis, V. Soil Phytomining: Recent Developments—A Review. Soil Syst. 2024, 8, 8. [Google Scholar] [CrossRef]

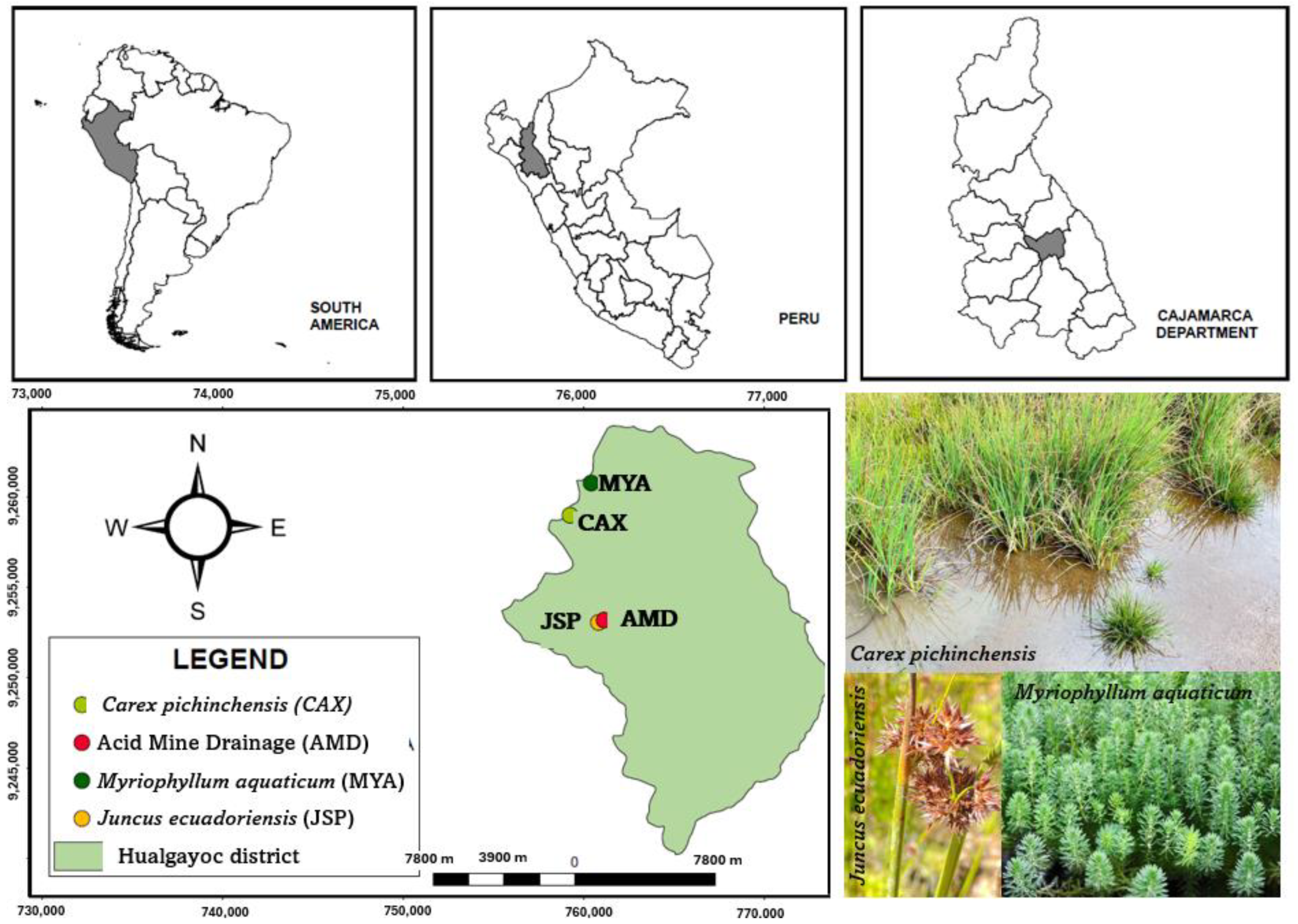
| EC (mS/m) | pH | Heavy Metals (mg/kg) | |||||
|---|---|---|---|---|---|---|---|
| As | Cd | Cu | Fe | Zn | |||
| AMD | 3.78 | 2.515 | 0.61 | 0.45 | 27.09 | 374.96 | 32.31 |
| Treatment | Physicochemical Parameters | Heavy Metals (mg/kg) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | EC (mS/m) | P (mg/kg) | K (mg/kg) | C (%) | OM (%) | N (meq/ 100 g) | CEC (meq/ 100 g) | Ca (meq/ 100 g) | Mg2+ (meq/ 100 g) | K+ (meq/ 100 g) | Na+ (meq/ 100 g) | As | Cu | Cd | Fe | Zn | |
| MWC | 10.03 | 12.78 | 125 | 28,656 | 22.52 | 38.83 | 1.74 | 154.3 | 33.55 | 19.32 | 72.2 | 29.2 | 20.19 | 0.74 | 0 | 12.6 | 0.46 |
| CMC | 6.33 | 1.8 | 77 | 1669 | 21.09 | 36.36 | 1.63 | 48 | 23.98 | 6.88 | 3.39 | 0.65 | 19.7 | 0.14 | 0 | 1.09 | 1.89 |
| HMS | 7.5 | 1.37 | 65 | 2261 | 14.22 | 24.52 | 1.1 | 37.81 | 26.2 | 5.99 | 4.74 | 0.88 | 18.87 | 0.16 | 0 | 0.4 | 0.07 |
| PBC | 9.81 | 0.63 | 18 | 1236 | 51.73 | 89.91 | 4.01 | 9.96 | 6.63 | 0.69 | 2.52 | 0.12 | 2.83 | 0.11 | 0 | 10.18 | 44.26 |
| CSB | 10.58 | 2.69 | 54 | 9328 | 49.77 | 85.82 | 3.96 | 28.9 | 3.59 | 2.03 | 23 | 0.28 | 11.63 | 0.4 | 0 | 4.79 | 4.72 |
| SLB | 8.59 | 2.52 | 49 | 233.2 | 7.85 | 13.54 | 0.6 | 21.49 | 19.9 | 0.78 | 0.55 | 0.25 | 63.5 | 0.07 | 0.9 | 0.08 | 0 |
| CAX | - | - | - | - | - | - | - | - | - | - | - | - | 10.93 | 14.16 | 0.62 | 1664.6 | 63.2 |
| JSP | - | - | - | - | - | - | - | - | - | - | - | - | 17.17 | 52.2 | 0.96 | 3809.73 | 99.55 |
| MYA | - | - | - | - | - | - | - | - | - | - | - | - | 7.17 | 3.72 | 0.005 | 1146.98 | 18.64 |
| Capacidad de Sorción (mg/g) | |||||
|---|---|---|---|---|---|
| Treatment | As | Cd | Cu | Fe | Zn |
| MWC | −0.004 | 0.001 | 0.039 | 0.546 | 0.044 |
| CMC | 0.001 | 0.001 | 0.049 | 0.672 | 0.044 |
| HMS | 0.000 | 0.001 | 0.049 | 0.636 | 0.057 |
| PBC | 0.001 | 0.000 | 0.044 | 0.879 | 0.030 |
| CSB | 0.001 | 0.002 | 0.139 | 1.942 | 0.149 |
| SLB | 0.000 | 0.001 | 0.030 | 0.419 | 0.036 |
| Heavy Metal | SS | F Ratio | p-Value |
|---|---|---|---|
| As | 10,223.762 | 23.692 | <0.0001 |
| Cd | 41,448.574 | 24.647 | <0.0001 |
| Cu | 28,804.231 | 353.221 | <0.0001 |
| Fe | 6662.845 | 43.949 | <0.0001 |
| Zn | 25,515.522 | 158.865 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nuñez-Bustamante, E.; Césare-Coral, M.F.; Cuba Torre, H.R.; Nuñez-Bustamante, N.; Sempértegui-Rafael, R.M.; Cornejo-La Torre, M.; Cueva, M.D.; Arribasplata-Vargas, M.A.; Castro-Pantoja, J.B.; Virú-Vásquez, P. Characterization and Evaluation of the Efficiency of Organic Amendments and Native Macrophytes for the Treatment of Acid Mine Drainage in Hualgayoc—A Case Study. Sustainability 2025, 17, 3570. https://doi.org/10.3390/su17083570
Nuñez-Bustamante E, Césare-Coral MF, Cuba Torre HR, Nuñez-Bustamante N, Sempértegui-Rafael RM, Cornejo-La Torre M, Cueva MD, Arribasplata-Vargas MA, Castro-Pantoja JB, Virú-Vásquez P. Characterization and Evaluation of the Efficiency of Organic Amendments and Native Macrophytes for the Treatment of Acid Mine Drainage in Hualgayoc—A Case Study. Sustainability. 2025; 17(8):3570. https://doi.org/10.3390/su17083570
Chicago/Turabian StyleNuñez-Bustamante, Ever, Mary Flor Césare-Coral, Hector Ricardo Cuba Torre, Nelve Nuñez-Bustamante, Roxana Mabel Sempértegui-Rafael, Melitza Cornejo-La Torre, Mario D. Cueva, Marco Antonio Arribasplata-Vargas, Jhimy Brayam Castro-Pantoja, and Paul Virú-Vásquez. 2025. "Characterization and Evaluation of the Efficiency of Organic Amendments and Native Macrophytes for the Treatment of Acid Mine Drainage in Hualgayoc—A Case Study" Sustainability 17, no. 8: 3570. https://doi.org/10.3390/su17083570
APA StyleNuñez-Bustamante, E., Césare-Coral, M. F., Cuba Torre, H. R., Nuñez-Bustamante, N., Sempértegui-Rafael, R. M., Cornejo-La Torre, M., Cueva, M. D., Arribasplata-Vargas, M. A., Castro-Pantoja, J. B., & Virú-Vásquez, P. (2025). Characterization and Evaluation of the Efficiency of Organic Amendments and Native Macrophytes for the Treatment of Acid Mine Drainage in Hualgayoc—A Case Study. Sustainability, 17(8), 3570. https://doi.org/10.3390/su17083570









