Use of Black Soldier Fly Larvae for Bioconversion of Tomato Crop Residues
Abstract
1. Introduction
2. Materials and Methods
2.1. Black Soldier Fly Larvae Production
2.2. Substrate Preparation
2.3. Diets and Experimental Design
2.4. Larval Performance
2.5. Waste Reduction and Conversion Efficiency
2.6. Bromatologial Analysis
2.7. Statistical Analysis
3. Results
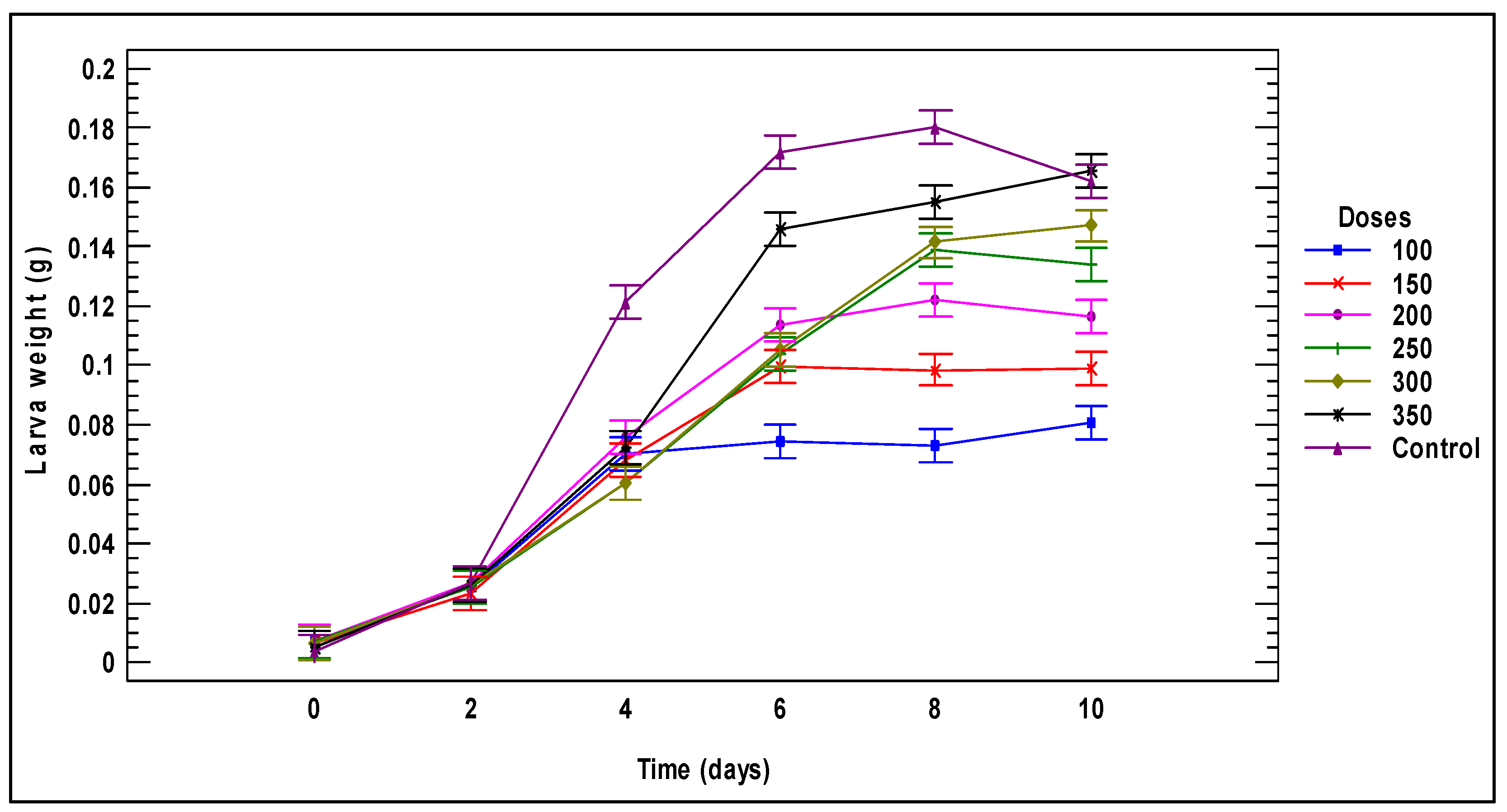
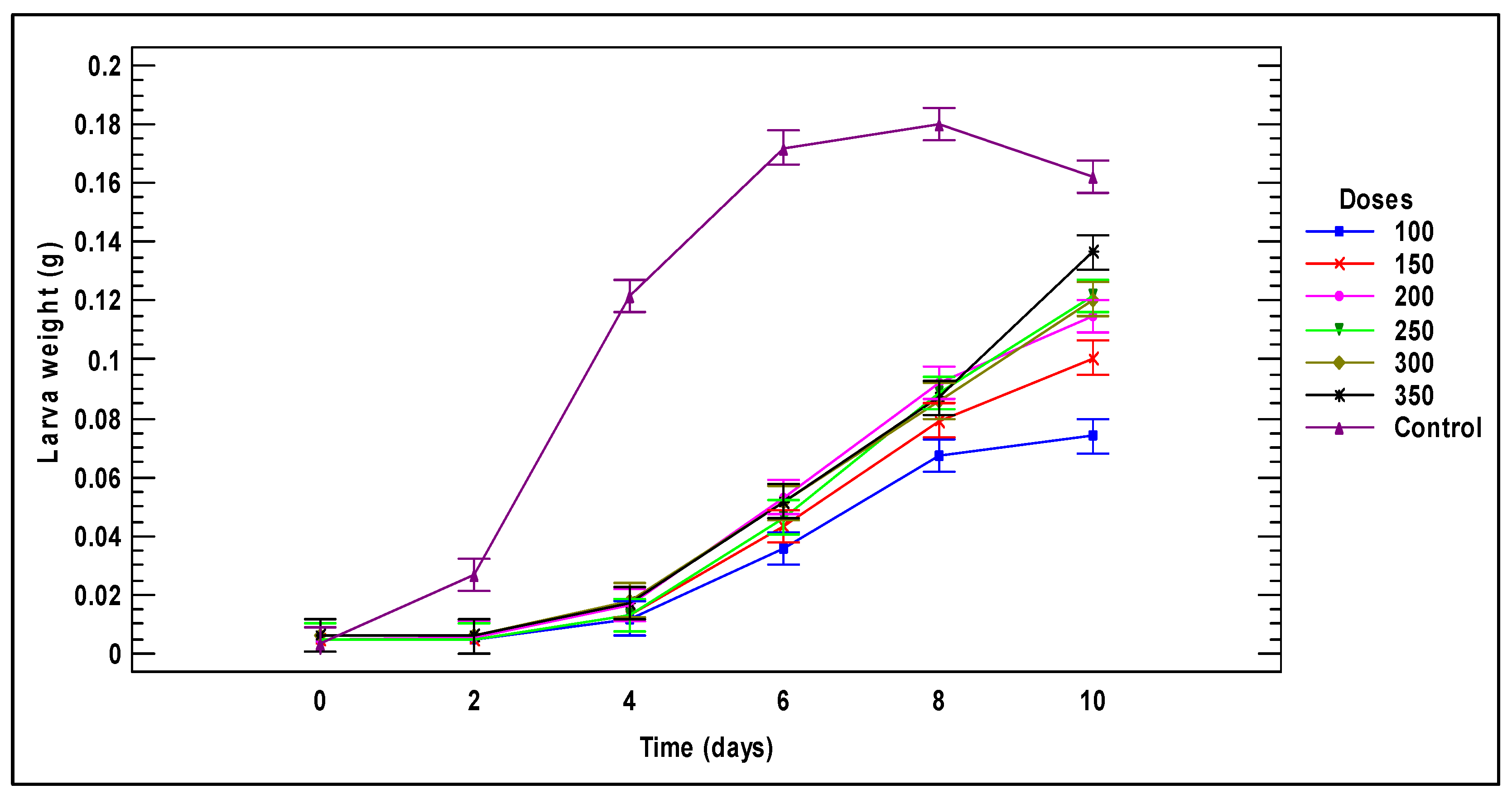
3.1. Larval Performance
3.2. Biomass Generated by Larvae Fed on Tomato Plant Substrates
3.2.1. Tomato Fruit
3.2.2. Tomato Leaves
3.2.3. Tomato Stems
3.3. Performance of Black Soldier Fly Larvae in Plant Tomato Waste
3.4. Proximal Substrates and Diet Composition
3.5. Proximal Larval Composition
4. Discussion
4.1. Larval Performance
4.2. Generation of Biomass by Larvae Fed on Tomato Plant Substrates
4.3. Bioconversion of Organic Waste of Tomato Plant
4.4. Proximal Substrates and Larval Composition
5. Conclusions
6. Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations (FAO). Agricultural Production Statistics. 2000–2020. FAOSTAT Analytical Brief Series No. 41. Rome, Italy. 2022. Available online: https://openknowledge.fao.org/server/api/core/bitstreams/b75223dd-4e30-43aa-85a9-4c587753b027/content (accessed on 2 January 2025).
- The Observatory of Economic Complexity (OCE). Tomates Frescos o Refrigerados. 2023. Available online: https://oec.world/es/profile/hs/tomatoes?yearSelector1=2023 (accessed on 4 January 2025).
- Servicio de Informacion Agroalimentaria y Pesquera (SIAP). Anuario Estadístico de la Producción Agrícola. Available online: https://nube.agricultura.gob.mx/cierre_agricola/ (accessed on 4 January 2025).
- Guerrero-Ibañez, A.; Reyes-Muñoz, A. Monitoring Tomato Leaf Disease through Convolutional Neural Networks. Electronics 2023, 12, 229. [Google Scholar] [CrossRef]
- Przybylska, S.; Tokarczyk, G. Lycopene in the Prevention of Cardiovascular Diseases. Int. J. Mol. Sci. 2022, 23, 1957. [Google Scholar] [CrossRef]
- Perveen, R.; Suleria, H.A.; Anjum, F.M.; Butt, M.S.; Pasha, I.; Ahmad, S. Tomato (Solanum lycopersicum) Carotenoids and Lycopenes Chemistry; Metabolism, Absorption, Nutrition, and Allied Health Claims—A Comprehensive Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 919–929. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Jin, N.; Jin, L.; Xiao, X.; Hu, L.; Liu, Z.; Wu, Y.; Xie, Y.; Zhu, W.; Lyu, J.; et al. Response of Tomato Fruit Quality Depends on Period of LED Supplementary Light. Front. Nutr. 2022, 9, 833723. [Google Scholar] [CrossRef]
- Morote, L.; Lobato-Gómez, M.; Ahrazem, O.; Argandoña, J.; Olmedilla-Alonso, B.; López-Jiménez, A.J.; Diretto, G.; Cuciniello, R.; Bergamo, P.; Frusciante, S.; et al. Crocins-rich tomato extracts showed enhanced protective effects in vitro. J. Funct. Foods 2023, 101, 105432. [Google Scholar] [CrossRef]
- Salehi, B.; Sharifi-Rad, R.; Sharopov, F.; Namiesnik, J.; Roointan, A.; Kamle, M.; Kumar, P.; Martins, N.; Sharifi-Rad, J. Beneficial effects and potential risks of tomato consumption for human health: An overview. Nutrition 2019, 62, 201–208. [Google Scholar] [CrossRef]
- Arfin, N.; Podder, M.K.; Kabir, S.R.; Asaduzzaman, A.K.M.; Hasan, I. Antibacterial, antifungal and in vivo anticancer activities of chitin-binding lectins from Tomato (Solanum lycopersicum) fruits. Arab. J. Chem. 2022, 15, 104001. [Google Scholar] [CrossRef]
- Banihani, S.A. Tomato (Solanum lycopersicum L.) and type 2 diabetes. Int. J. Food Prop. 2018, 21, 99–105. [Google Scholar] [CrossRef]
- Sharma, B.; Vaish, B.; Monika; Singh, U.K.; Singh, P.; Singh, R.P. Recycling of Organic Wastes in Agriculture: An Environmental Perspective. Int. J. Environ. Res. 2019, 13, 409–429. [Google Scholar] [CrossRef]
- Sharma, K.; Garg, V.K. Chapter 10—Vermicomposting of Waste: A Zero-Waste Approach for Waste Management. In Sustainable Resource Recovery and Zero Waste Approaches; Taherzadeh, M.J., Bolton, K., Wong, J., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 133–164. [Google Scholar]
- Badgujar, K.C.; Bhanage, B.M. Chapter 1—Dedicated and Waste Feedstocks for Biorefinery: An Approach to Develop a Sustainable Society. In Waste Biorefinery; Bhaskar, T., Pandey, A., Mohan, S.V., Lee, D.-J., Khanal, S.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 3–38. [Google Scholar]
- Dupuis, I. Estimación de los residuos agrícolas generados en la isla de Tenerife, Servicio Técnico de Agricultura y Desarrollo Rural. 2006. Available online: https://www.agrocabildo.org/publica/Publicaciones/sost_28_L_estima_residu_agricola.pdf (accessed on 4 January 2025).
- Mizael, W.C.F.; Costa, R.G.; Rodrigo Beltrão Cruz, G.; Ramos de Carvalho, F.F.; Ribeiro, N.L.; Lima, A.; Domínguez, R.; Lorenzo, J.M. Effect of the Use of Tomato Pomace on Feeding and Performance of Lactating Goats. Animals 2020, 10, 1574. [Google Scholar] [CrossRef]
- Durmuş, M.; Kızılkaya, R. The Effect of Tomato Waste Compost on Yield of Tomato and Some Biological Properties of Soil. Agronomy 2022, 12, 1253. [Google Scholar] [CrossRef]
- Rashwan, M.A.; Naser Alkoaik, F.; Abdel-Razzak Saleh, H.; Blanqueza Fulleros, R.; Nagy Ibrahim, M. Maturity and stability assessment of composted tomato residues and chicken manure using a rotary drum bioreactor. J. Air Waste Manag. Assoc. 2021, 71, 529–539. [Google Scholar] [CrossRef]
- Özbay, N.; Yargıç, A.Ş.; Yarbay Şahin, R.Z.; Yaman, E. Chapter 3.6—Research on the Pyrolysis Characteristics of Tomato Waste With Fe–Al2O3 Catalyst. In Exergetic, Energetic and Environmental Dimensions; Dincer, I., Colpan, C.O., Kizilkan, O., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 815–828. [Google Scholar]
- Rena; Mohammed Bin Zacharia, K.; Yadav, S.; Machhirake, N.P.; Kim, S.-H.; Lee, B.-D.; Jeong, H.; Singh, L.; Kumar, S.; Kumar, R. Bio-hydrogen and bio-methane potential analysis for production of bio-hythane using various agricultural residues. Bioresour. Technol. 2020, 309, 123297. [Google Scholar] [CrossRef] [PubMed]
- Tabrika, I.; Azim, K.; Mayad, E.H.; Zaafrani, M. Composting of tomato plant residues: Improvement of composting process and compost quality by integration of sheep manure. Org. Agric. 2020, 10, 229–242. [Google Scholar] [CrossRef]
- Travieso, M.D.C.; de Evan, T.; Marcos, C.N.; Molina-Alcaide, E. CHAPTER TWO—Tomato by-products as animal feed. In Tomato Processing by-Products; Jeguirim, M., Zorpas, A., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 33–76. [Google Scholar]
- Ventura, M.R.; Pieltain, M.C.; Castanon, J.I.R. Evaluation of tomato crop by-products as feed for goats. Anim. Feed Sci. Technol. 2009, 154, 271–275. [Google Scholar] [CrossRef]
- Yasmin, N.; Jamuda, M.; Panda, A.K.; Samal, K.; Nayak, J.K. Emission of greenhouse gases (GHGs) during composting and vermicomposting: Measurement, mitigation, and perspectives. Energy Nexus 2022, 7, 100092. [Google Scholar] [CrossRef]
- Madia, V.N.; De Vita, D.; Ialongo, D.; Tudino, V.; De Leo, A.; Scipione, L.; Di Santo, R.; Costi, R.; Messore, A. Recent Advances in Recovery of Lycopene from Tomato Waste: A Potent Antioxidant with Endless Benefits. Molecules 2021, 26, 130–136. [Google Scholar] [CrossRef]
- Grossule, V.; Fang, D.; Yue, D.; Lavagnolo, M.C. Treatment of wastewater using black soldier fly larvae, under different degrees of biodegradability and oxidation of organic content. J. Environ. Manag. 2022, 319, 115734. [Google Scholar] [CrossRef]
- Liu, T.; Klammsteiner, T.; Dregulo, A.M.; Kumar, V.; Zhou, Y.; Zhang, Z.; Awasthi, M.K. Black soldier fly larvae for organic manure recycling and its potential for a circular bioeconomy: A review. Sci. Total Environ. 2022, 833, 155122. [Google Scholar] [CrossRef]
- Ribeiro, N.; Costa, R.; Ameixa, O.M.C.C. The Influence of Non-Optimal Rearing Conditions and Substrates on the Performance of the Black Soldier Fly (Hermetia illucens). Insects 2022, 13, 639. [Google Scholar] [CrossRef]
- Gold, M.; Cassar, C.M.; Zurbrügg, C.; Kreuzer, M.; Boulos, S.; Diener, S.; Mathys, A. Biowaste treatment with black soldier fly larvae: Increasing performance through the formulation of biowastes based on protein and carbohydrates. Waste Manag. 2020, 102, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Lalander, C.; Diener, S.; Zurbrügg, C.; Vinnerås, B. Effects of feedstock on larval development and process efficiency in waste treatment with black soldier fly (Hermetia illucens). J. Clean. Prod. 2019, 208, 211–219. [Google Scholar] [CrossRef]
- Raksasat, R.; Abdelfattah, E.A.; Liew, C.S.; Rawindran, H.; Kiatkittipong, K.; Mohamad, M.; Mohd Zaid, H.F.; Jumbri, K.; Lam, M.K.; Lim, J.W. Enriched sewage sludge from anaerobic pre-treatment in spurring valorization potential of black soldier fly larvae. Environ. Res. 2022, 212, 113447. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Ristow, B.; Rahayu, T.; Putra, N.S.; Widya Yuwono, N.; Nisa, K.; Mategeko, B.; Smetana, S.; Saki, M.; Nawaz, A.; et al. Black soldier fly larvae (BSFL) and their affinity for organic waste processing. Waste Manag. 2022, 140, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Xie, P.; Ding, Z.; Niu, G.; Wen, T.; Gu, W.; Lu, Y.; Wang, F.; Li, W.; Zeng, J.; et al. Inhibition of pathogenic microorganisms in solid organic waste via black soldier fly larvae-mediated management. Sci. Total Environ. 2024, 913, 169767. [Google Scholar] [CrossRef] [PubMed]
- Broeckx, L.; Frooninckx, L.; Slegers, L.; Berrens, S.; Noyens, I.; Goossens, S.; Verheyen, G.; Wuyts, A.; Van Miert, S. Growth of Black Soldier Fly Larvae Reared on Organic Side-Streams. Sustainability 2021, 13, 12953. [Google Scholar] [CrossRef]
- Lalander, C.; Ermolaev, E.; Wiklicky, V.; Vinnerås, B. Process efficiency and ventilation requirement in black soldier fly larvae composting of substrates with high water content. Sci. Total Environ. 2020, 729, 138968. [Google Scholar] [CrossRef]
- Barbi, S.; Macavei, L.I.; Fuso, A.; Luparelli, A.V.; Caligiani, A.; Ferrari, A.M.; Maistrello, L.; Montorsi, M. Valorization of seasonal agri-food leftovers through insects. Sci. Total Environ. 2020, 709, 136209. [Google Scholar] [CrossRef]
- Addeo, N.F.; Vozzo, S.; Secci, G.; Mastellone, V.; Piccolo, G.; Lombardi, P.; Parisi, G.; Asiry, K.A.; Attia, Y.A.; Bovera, F. Different Combinations of Butchery and Vegetable Wastes on Growth Performance, Chemical-Nutritional Characteristics and Oxidative Status of Black Soldier Fly Growing Larvae. Animals 2021, 11, 3515. [Google Scholar] [CrossRef]
- Nyakeri, E.M.; Ayieko, M.A.; Amimo, F.A.; Salum, H.; Ogola, H.J.O. An optimal feeding strategy for black soldier fly larvae biomass production and faecal sludge reduction. J. Insects Food Feed 2019, 5, 201–214. [Google Scholar] [CrossRef]
- Vodounnou, J.V.; Iko, R.; Sintondji, W.; Tossavi, E.; Kpogue, D.; Micha, J.-C. Rearing of black soldier fly (Hermetia illucens) larvae as a tool for managing agricultural byproducts. Discov. Agric. 2024, 2, 91. [Google Scholar] [CrossRef]
- Shumo, M.; Khamis, F.M.; Tanga, C.M.; Fiaboe, K.K.M.; Subramanian, S.; Ekesi, S.; van Huis, A.; Borgemeister, C. Influence of Temperature on Selected Life-History Traits of Black Soldier Fly (Hermetia illucens) Reared on Two Common Urban Organic Waste Streams in Kenya. Animals 2019, 9, 79. [Google Scholar] [CrossRef]
- Liland, N.S.; Biancarosa, I.; Araujo, P.; Biemans, D.; Bruckner, C.G.; Waagbø, R.; Torstensen, B.E.; Lock, E.-J. Modulation of nutrient composition of black soldier fly (Hermetia illucens) larvae by feeding seaweed-enriched media. PLoS ONE 2017, 12, e0183188. [Google Scholar] [CrossRef]
- Loiotine, Z.; Gasco, L.; Biasato, I.; Resconi, A.; Bellezza Oddon, S. Effect of larval handling on black soldier fly life history traits and bioconversion efficiency. Front. Vet. Sci. 2024, 11, 1330342. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency (EPA). Method 3546, Microwave Extraction. 2007. Available online: https://www.epa.gov/hw-sw846/sw-846-test-method-3546-microwave-extraction (accessed on 9 January 2025).
- Janssen, R.H.; Vincken, J.-P.; van den Broek, L.A.M.; Fogliano, V.; Lakemond, C.M.M. Nitrogen-to-Protein Conversion Factors for Three Edible Insects: Tenebrio molitor, Alphitobius diaperinus, and Hermetia illucens. J. Agric. Food Chem. 2017, 65, 2275–2278. [Google Scholar] [CrossRef] [PubMed]
- Simões, S.; Santos, R.; Bento-Silva, A.; Santos, M.V.; Mota, M.; Duarte, N.; Sousa, I.; Raymundo, A.; Prista, C. Improving nutritional quality of unripe tomato through fermentation by a consortium of yeast and lactic acid bacteria. J. Sci. Food Agric. 2022, 102, 1422–1429. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhao, G.; Zhang, Y.; Han, L.; Xiao, W. Nitrogen-to-Protein Conversion Factors for Crop Residues and Animal Manure Common in China. J. Agric. Food Chem. 2017, 65, 9186–9190. [Google Scholar] [CrossRef]
- Deruytter, D.; Gasco, L.; Yakti, W.; Katz, H.; Coudron, C.L.; Gligorescu, A.; Frooninckx, L.; Noyens, I.; Meneguz, M.; Grosso, F.; et al. Standardising black soldier fly larvae feeding experiments: An initial protocol and variability estimates. J. Insects Food Feed 2023, 10, 1685–1696. [Google Scholar] [CrossRef]
- Hewitt, B.R. Spectrophotometric Determination of Total Carbohydrate. Nature 1958, 182, 246–247. [Google Scholar] [CrossRef]
- PROY-NOM-211-SSA1-2002; Norma Oficial Mexicana. Productos y Servicios-Método de Prueba Fisicoquímicos. Deter-minación de Humedad y Sólidos Totales en Alimentos por Secado en Estufa. Diario Oficial de la Federación: Mexico City, Mexico, 2003.
- NMX-F-607-NORMEX-2020; Norma Oficial Mexicana. Alimentos Determinación de Cenizas en Alimentos Método de Prueba. Diario Oficial de la Federación: Mexico City, Mexico, 2022.
- Meneguz, M.; Schiavone, A.; Gai, F.; Dama, A.; Lussiana, C.; Renna, M.; Gasco, L. Effect of rearing substrate on growth performance, waste reduction efficiency and chemical composition of black soldier fly (Hermetia illucens) larvae. J. Sci. Food Agric. 2018, 98, 5776–5784. [Google Scholar] [CrossRef]
- Pérez-Pacheco, R.; Hinojosa-Garro, D.; Ruíz-Ortíz, F.; Camacho-Chab, J.C.; Ortega-Morales, B.O.; Alonso-Hernández, N.; Fonseca-Muñoz, A.; Landero-Valenzuela, N.; Loeza-Concha, H.J.; Diego-Nava, F.; et al. Growth of the Black Soldier Fly Hermetia illucens (Diptera: Stratiomyidae) on Organic-Waste Residues and Its Application as Supplementary Diet for Nile Tilapia Oreochromis niloticus (Perciformes: Cichlidae). Insects 2022, 13, 326. [Google Scholar] [CrossRef] [PubMed]
- Yakti, W.; Förster, N.; Müller, M.; Mewis, I.; Ulrichs, C. Hemp Waste as a Substrate for Hermetia illucens (L.) (Diptera: Stratiomyidae) and Tenebrio molitor L. (Coleoptera: Tenebrionidae) Rearing. Insects 2023, 14, 183. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kumari, K. An inclusive approach for organic waste treatment and valorisation using Black Soldier Fly larvae: A review. J. Environ. Manag. 2019, 251, 109569. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tian, X.; Liu, Z.; Liu, Z.; Shang, S.; Li, H.; Qu, J.; Chen, P. Rearing of Black Soldier Fly Larvae with Corn Straw and the Assistance of Gut Microorganisms in Digesting Corn Straw. Insects 2024, 15, 734. [Google Scholar] [CrossRef]
- Jucker, C.; Lupi, D.; Moore, C.; Leonardi, M.; Savoldelli, S. Nutrient Recapture from Insect Farm Waste: Bioconversion with Hermetia illucens (L.) (Diptera: Stratiomyidae). Sustainability 2020, 12, 362. [Google Scholar] [CrossRef]
- Bekker, N.S.; Heidelbach, S.; Vestergaard, S.Z.; Nielsen, M.E.; Riisgaard-Jensen, M.; Zeuner, E.J.; Bahrndorff, S.; Eriksen, N.T. Impact of substrate moisture content on growth and metabolic performance of black soldier fly larvae. Waste Manag. 2021, 127, 73–79. [Google Scholar] [CrossRef]
- Cattaneo, A.; Belperio, S.; Sardi, L.; Martelli, G.; Nannoni, E.; Dabbou, S.; Meneguz, M. Black Soldier Fly Larvae’s Optimal Feed Intake and Rearing Density: A Welfare Perspective (Part II). Insects 2025, 16, 5. [Google Scholar] [CrossRef]
- Cheng, J.Y.K.; Chiu, S.L.H.; Lo, I.M.C. Effects of moisture content of food waste on residue separation, larval growth and larval survival in black soldier fly bioconversion. Waste Manag. 2017, 67, 315–323. [Google Scholar] [CrossRef]
- Xiang, F.; Zhang, Q.; Xu, X.; Zhang, Z. Black soldier fly larvae recruit functional microbiota into the intestines and residues to promote lignocellulosic degradation in domestic biodegradable waste. Environ. Pollut. 2024, 340, 122676. [Google Scholar] [CrossRef]
- Kuppusamy, G.; Kong, C.K.; Segaran, G.C.; Tarmalingam, E.; Herriman, M.; Ismail, M.F.; Mehmood Khan, T.; Low, L.E.; Goh, B.-H. Hummingbird-Leaves-Reared Black Soldier Fly Prepupae: Assessment of Nutritional and Heavy Metal Compositions. Biology 2020, 9, 274. [Google Scholar] [CrossRef]
- Hopkins, I.; Newman, L.P.; Gill, H.; Danaher, J. The Influence of Food Waste Rearing Substrates on Black Soldier Fly Larvae Protein Composition: A Systematic Review. Insects 2021, 12, 608. [Google Scholar] [CrossRef] [PubMed]
- Naser El Deen, S.; van Rozen, K.; Elissen, H.; van Wikselaar, P.; Fodor, I.; van der Weide, R.; Hoek-van den Hil, E.F.; Rezaei Far, A.; Veldkamp, T. Bioconversion of Different Waste Streams of Animal and Vegetal Origin and Manure by Black Soldier Fly Larvae Hermetia illucens L. (Diptera: Stratiomyidae). Insects 2023, 14, 204. [Google Scholar] [CrossRef] [PubMed]
- Bruno, D.; Orlando, M.; Testa, E.; Carnevale Miino, M.; Pesaro, G.; Miceli, M.; Pollegioni, L.; Barbera, V.; Fasoli, E.; Draghi, L.; et al. Valorization of organic waste through black soldier fly: On the way of a real circular bioeconomy process. Waste Manag. 2025, 191, 123–134. [Google Scholar] [CrossRef]
- Permana, A.D.; Ramadhani Eka Putra, J.E.N. Growth of Black Soldier Fly (Hermetia illucens) Larvae Fed on Spent Coffee Ground. IOP Conf. Ser. Earth Environ. Sci. 2018, 187, 012070. [Google Scholar] [CrossRef]
- Peguero, D.A.; Gold, M.; Vandeweyer, D.; Zurbrügg, C.; Mathys, A. A Review of Pretreatment Methods to Improve Agri-Food Waste Bioconversion by Black Soldier Fly Larvae. Front. Sustain. Food Syst. 2022, 5, 745894. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; He, J.; Lu, H.; Sun, S.; Ji, F.; Dong, X.; Bao, Y.; Xu, J.; He, G.; et al. Chronological and Carbohydrate-Dependent Transformation of Fatty Acids in the Larvae of Black Soldier Fly Following Food Waste Treatment. Molecules 2023, 28, 1903. [Google Scholar] [CrossRef]
- Fuso, A.; Barbi, S.; Macavei, L.I.; Luparelli, A.V.; Maistrello, L.; Montorsi, M.; Sforza, S.; Caligiani, A. Effect of the Rearing Substrate on Total Protein and Amino Acid Composition in Black Soldier Fly. Foods 2021, 10, 1773. [Google Scholar] [CrossRef]
- Zandi-Sohani, N.; Tomberlin, J.K. Comparison of Growth and Composition of Black Soldier Fly (Hermetia illucens L.) Larvae Reared on Sugarcane By-Products and Other Substrates. Insects 2024, 15, 771. [Google Scholar] [CrossRef]


| Substrate | Doses (mg/Larva/Day) | Survival Rate (%) | Growth Rate (mg/Day) | Wet Total Biomass (mg) |
|---|---|---|---|---|
| Fruit | 100 | 100.0 ± 0.0 a | 7.3 ± 0.7 a | 6609.4 ± 217.0 a |
| 150 | 100.0 ± 0.0 a | 9.2 ± 0.5 b | 8317.6 ± 153.5 b | |
| 200 | 100.0 ± 0.0 a | 10.9 ± 0.0 c | 9866.7 ± 21.9 c | |
| 250 | 100.0 ± 0.0 a | 12.4 ± 0.5 d | 11,245.4 ± 165.4 d | |
| 300 | 99.0 ± 1.7 a | 13.9 ± 0.3 e | 12,562.3 ± 115.9 e | |
| 350 | 96.6 ± 3.6 a | 15.2 ± 0.2 f | 13,716.8 ± 88.9 f | |
| Control | 97.0 ± 3.8 a | 15.6 ± 0.2 f | 14,112.9 ± 72.7 g | |
| Stem | 100 | 100.0 ± 0.0 a | 3.6 ± 0.6 a | 2191.5 ± 186.1 a |
| 150 | 100.0 ± 0.0 a | 6.2 ± 1.0 b | 5626.7 ± 321.6 b | |
| 200 | 100.0 ± 0.0 a | 9.2 ± 1.6 c | 8363.4 ± 483.0 c | |
| 250 | 100.0 ± 0.0 a | 9.3 ± 0.1 c | 8382.9 ± 55.7 c | |
| 300 | 98.6 ± 4.0 a | 10.1 ± 0.8 c | 9110.7 ± 248.4 c | |
| 350 | 100.0 ± 0.0 a | 12.7 ± 0.1 d | 11,475.8 ± 36.7 d | |
| Control | 97.0 ± 3.8 a | 15.6 ± 0.2 e | 14,112.9 ± 72.7 e | |
| Leaf | 100 | 98.0 ± 1.7 ab | 6.7 ± 0.3 a | 6067.2 ± 110.3 a |
| 150 | 94.6 ± 4.0 ab | 9.6 ± 2.8 b | 8696.1 ± 841.9 b | |
| 200 | 100.0 ± 0.0 b | 10.9 ± 0.3 bc | 9890.7 ± 97.7 bc | |
| 250 | 98.0 ± 1.7 ab | 11.4 ± 0.9 bc | 10,299.7 ± 285.9 bc | |
| 300 | 88.0 ± 1.7 a | 9.9 ± 1.3 bc | 8992.2 ± 399.0 bc | |
| 350 | 94.3 ± 5.1 b | 12.2 ± 1.5 c | 11,033.8 ± 453.1 c | |
| Control | 200 | 97.0 ± 3.8 ab | 15.6 ± 0.2 d | 14,112.9 ± 72.7 d |
| Substrate | Doses | WRI | SR (%) | FCR | ECD (%) | BR (%) |
|---|---|---|---|---|---|---|
| Fruit | 100 | 7.5 ± 0.4 b | 75.2 ± 4.8 b | 3.2 ± 0.4 a | 27.4 ± 4.1 b | 23.0 ± 3.0 d |
| 150 | 7.6 ± 0.4 b | 76.8 ± 4.0 b | 3.3 ± 0.1 ab | 24.7 ± 1.0 b | 20.4 ± 0.3 c | |
| 200 | 7.3 ± 0.5 b | 73.8 ± 5.0 b | 3.9 ± 0.4 ab | 23.9 ± 3.0 b | 18.8 ± 1.0 bc | |
| 250 | 7.3 ± 0.4 b | 73.9 ± 1.7 b | 4.0 ± 0.2 ab | 23.4 ± 1.5 b | 18.2 ± 0.8 bc | |
| 300 | 7.4 ± 0.3 b | 74.0 ± 3.0 b | 4.1 ± 0.4 b | 23.3 ± 2.4 b | 18.0 ± 1.2 b | |
| 350 | 7.0 ± 0.8 b | 70.9 ± 8.2 b | 3.9 ± 0.5 ab | 24.7 ± 3.3 b | 17.7 ± 0.4 b | |
| Control | 200 | 5.4 ± 0.1 a | 54.5 ± 1.2 a | 6.3 ± 0.5 c | 15.6 ± 1.3 a | 8.6 ± 0.6 a |
| Stem | 100 | 2.7 ± 0.2 a | 27.7 ± 2.1 a | 6.8 ± 1.2 ab | 12.7 ± 2.6 ab | 4.0 ± 0.3 a |
| 150 | 3.3 ± 0.6 abc | 33.0 ± 6.6 abc | 7.5 ± 2.5 ab | 12.8 ± 3.8 ab | 4.5 ± 0.7 a | |
| 200 | 3.3 ± 0.3 abc | 33.5 ± 3.3 abc | 5.9 ± 1.0 a | 16.2 ± 2.7 b | 5.7 ± 0.7 ab | |
| 250 | 3.0 ± 0.2 ab | 30.3 ± 2.5 ab | 6.7 ± 0.5 ab | 14.1 ± 1.2 ab | 4.5 ± 0.0 a | |
| 300 | 3.5 ± 0.0 bc | 35.0 ± 0.2 bc | 8.2 ± 0.4 b | 11.6 ± 0.5 a | 4.2 ± 0.2 a | |
| 350 | 3.6 ± 0.3 c | 36.7 ± 3.0 c | 7.6 ± 0.3 ab | 12.6 ± 0.5 ab | 4.8 ± 0.3 a | |
| Control | 200 | 5.4 ± 0.1 d | 54.5 ± 1.2 d | 6.3 ± 0.5 ab | 15.6 ± 1.3 b | 8.6 ± 0.6 c |
| Leaf | 100 | 3.7 ± 0.4 abc | 37.3 ± 4.4 abc | 4.0 ± 0.9 a | 24.2 ± 6.8 c | 9.4 ± 1.3 c |
| 150 | 4.0 ± 0.3 de | 40.4 ± 3.8 de | 4.6 ± 1.4 a | 21.8 ± 7.1 bc | 9.0 ± 2.0 bc | |
| 200 | 4.6 ± 0.2 d | 46.3 ± 2.4 d | 5.6 ± 0.8 ab | 17.4 ± 2.9 abc | 8.3 ± 0.9 bc | |
| 250 | 4.0 ± 0.1 bcd | 40.2 ± 1.0 bcd | 5.6 ± 0.6 ab | 17.3 ± 1.7 abc | 7.2 ± 0.8 ab | |
| 300 | 3.4 ± 0.5 ab | 34.4 ± 5.5 ab | 6.6 ± 0.7 b | 14.4 ± 1.8 a | 5.2 ± 1.2 a | |
| 350 | 3.3 ± 0.3 a | 33.9 ± 3.0 a | 6.3 ± 0.8 b | 15.3 ± 2.0 ab | 5.4 ± 0.5 a | |
| Control | 200 | 5.4 ± 0.1 e | 54.5 ± 1.2 e | 6.3 ± 0.5 b | 15.6 ± 1.3 ab | 8.6 ± 0.6 bc |
| Component | Substrate | Initial | Final |
|---|---|---|---|
| Carbohydrates (%) | Gainesville | 55.85 ± 5.34 c | 9.43 ± 2.89 b |
| Leaf | 4.18 ± 1.93 a | 2.06 ± 0.00 a | |
| Stem | 14.39 ± 5.75 ab | 13.02 ± 5.55 b | |
| Fruit | 15.97 ± 2.53 b | 2.33 ± 0.20 a | |
| Protein (mg/g) | Gainesville | 16.26 ± 1.22 a | 19.78 ± 2.84 c |
| Leaf | 10.09 ± 0.08 b | 12.45 ± 0.55 b | |
| Stem | 4.61 ± 0.84 c | 2.35 ± 0.26 a | |
| Fruit | 12.18 ± 0.97 d | 10.85 ± 0.01 b | |
| Lipids (%) | Gainesville | 4.10 ± 0.20 c | 1.37 ± 0.30 b |
| Leaf | 2.49 ± 0.16 b | 3.13 ± 0.24 c | |
| Stem | 1.48 ± 0.25 a | 0.48 ± 0.06 a | |
| Fruit | 4.71 ± 0.24 d | 6.98 ± 0.29 d | |
| Ashes (%) | Gainesville | 10.48 ± 0.07 a | 30.56 ± 4.81 c |
| Leaf | 37.21 ± 2.86 c | 40.71 ± 1.64 d | |
| Stem | 12.20 ± 1.23 ab | 11.37 ± 1.52 a | |
| Fruit | 13.92 ± 3.73 b | 19.17 ± 2.47 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parra-Pacheco, B.; Aguirre-Becerra, H.; Feregrino-Pérez, A.A.; Chandrakasan, G.; González-Lara, H.; García-Trejo, J.F. Use of Black Soldier Fly Larvae for Bioconversion of Tomato Crop Residues. Sustainability 2025, 17, 3578. https://doi.org/10.3390/su17083578
Parra-Pacheco B, Aguirre-Becerra H, Feregrino-Pérez AA, Chandrakasan G, González-Lara H, García-Trejo JF. Use of Black Soldier Fly Larvae for Bioconversion of Tomato Crop Residues. Sustainability. 2025; 17(8):3578. https://doi.org/10.3390/su17083578
Chicago/Turabian StyleParra-Pacheco, Benito, Humberto Aguirre-Becerra, Ana Angelica Feregrino-Pérez, Gobinath Chandrakasan, Hugo González-Lara, and Juan Fernando García-Trejo. 2025. "Use of Black Soldier Fly Larvae for Bioconversion of Tomato Crop Residues" Sustainability 17, no. 8: 3578. https://doi.org/10.3390/su17083578
APA StyleParra-Pacheco, B., Aguirre-Becerra, H., Feregrino-Pérez, A. A., Chandrakasan, G., González-Lara, H., & García-Trejo, J. F. (2025). Use of Black Soldier Fly Larvae for Bioconversion of Tomato Crop Residues. Sustainability, 17(8), 3578. https://doi.org/10.3390/su17083578






