Metal-Doped Carbon Dots as Fenton-like Catalysts and Their Applications in Pollutant Degradation and Sensing
Abstract
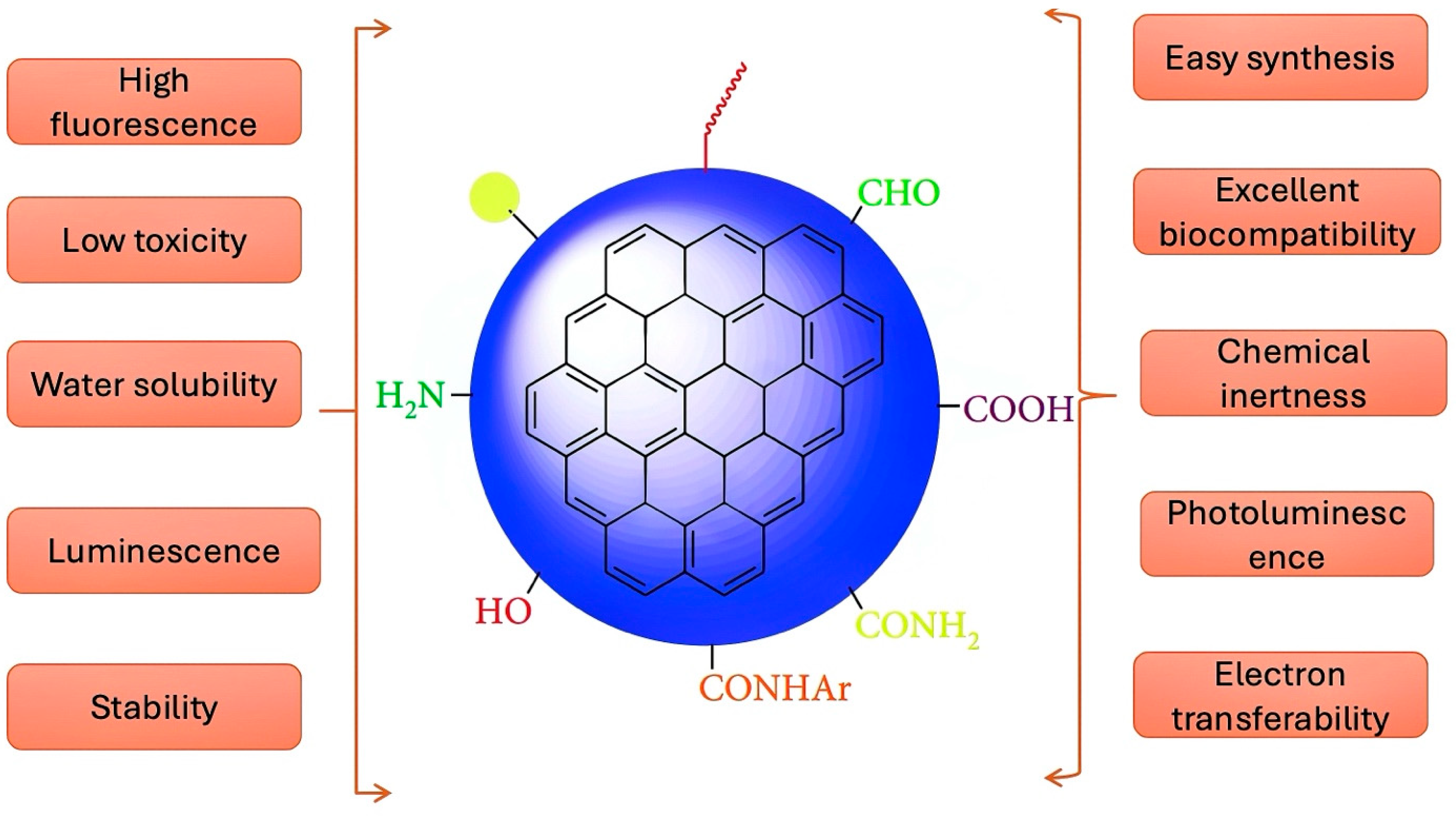
1. Introduction
2. Metal-Doped CDs vs. Non-Metal Doped CDs
| Advantages | Disadvantages | References | |
|---|---|---|---|
| Non-metal doped CDs |
|
| [8,9,42,48] |
| Metal-doped CDs |
|
| [34,49,50,51] |
3. Synthesis of Metal-Doped CDs
3.1. Raw Materials
3.2. Synthesis Methods
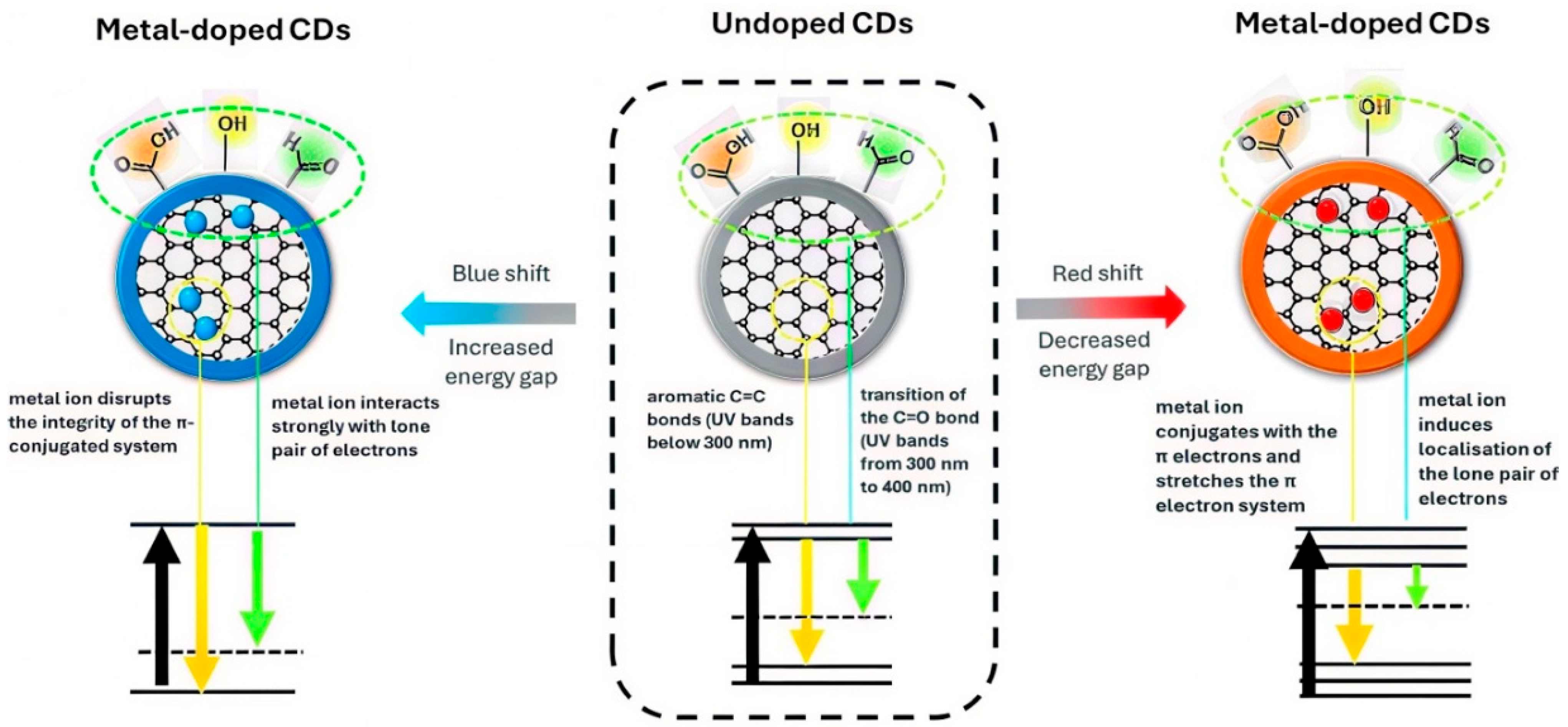
4. Effects of Metal Doping on the Catalytic Properties of CDs
4.1. Cu Dopants
4.2. Zn Dopants
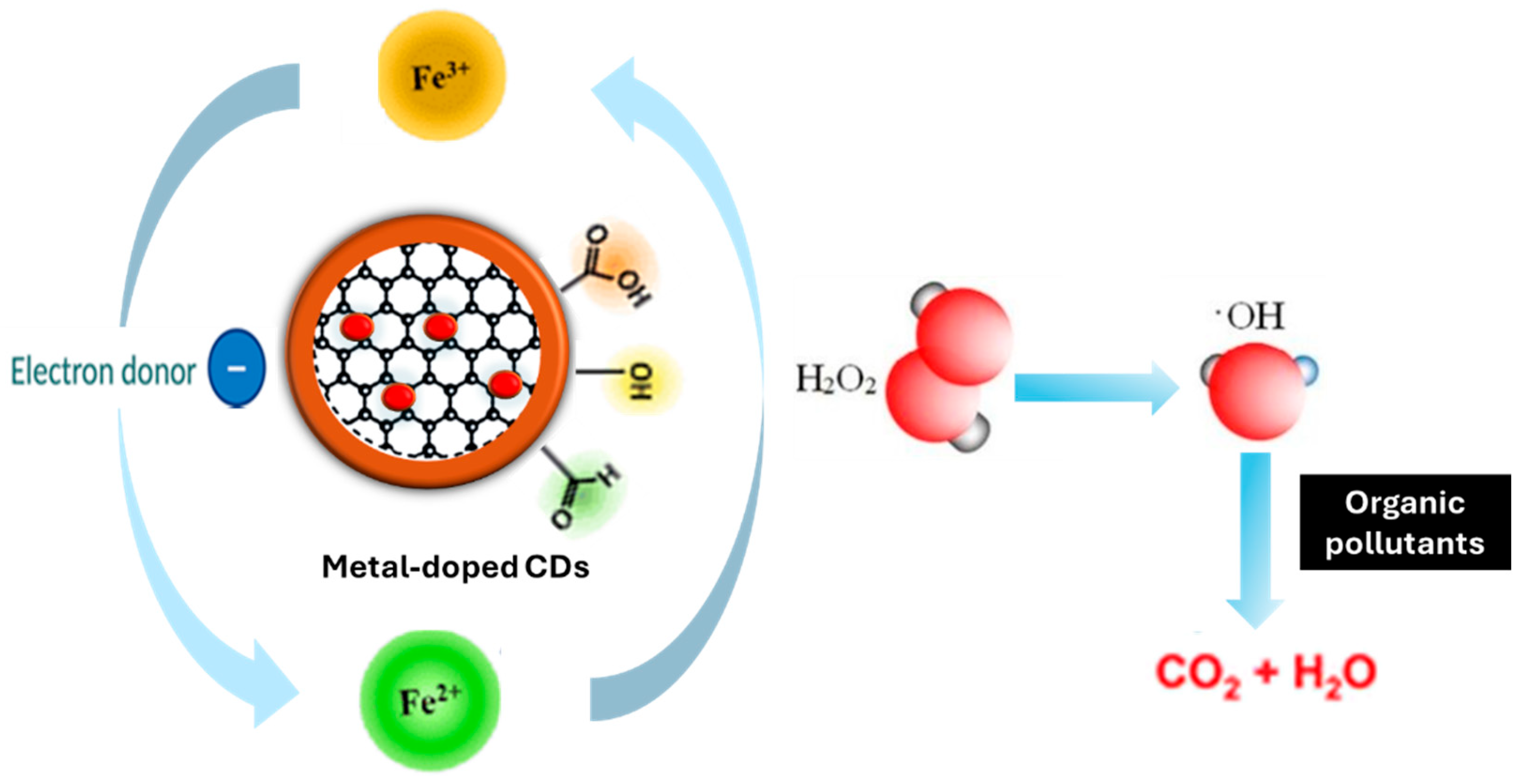
4.3. Fe Dopants
4.4. Mg Dopants
5. CDs as Fenton-like Catalysts for Pollutant Degradation
5.1. CDs Used in Combination with Traditional Fenton-like Catalysts
5.2. Metal-Doped CDs as Fenton-like Catalysts
5.3. Factors Affecting Metal-Doped CD Catalysed Fenton-like Reactions
5.3.1. Metal Elements and Their Valences
5.3.2. pH
5.3.3. Temperature
5.3.4. H2O2 Dosage and Fe2+ Dosage
6. Metal-Doped CDs for Sensing
6.1. Detection of H2O2 and Ferrous Ions
6.2. Detection of Glucose
6.3. Detection of Dopamine (DA)
6.4. Detection of Antioxidant Activity Index (AAI)
7. Summary and Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cui, L.; Ren, X.; Sun, M.; Liu, H.; Xia, L. Carbon dots: Synthesis, properties and applications. Nanomaterials 2021, 11, 3419. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-P.; Zhou, B.; Lin, Y.; Wang, W.; Fernando, K.S.; Pathak, P.; Meziani, M.J.; Harruff, B.A.; Wang, X.; Wang, H. Quantum-sized carbon dots for bright and colorful photoluminescence. J. Am. Chem. Soc. 2006, 128, 7756–7757. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Ngashangva, L.; Goswami, P. Carbon Dots: An Emerging Smart Material for Analytical Applications. Micromachines 2021, 12, 84. [Google Scholar] [CrossRef]
- Mansuriya, B.D.; Altintas, Z. Carbon dots: Classification, properties, synthesis, characterization, and applications in health care—An updated review (2018–2021). Nanomaterials 2021, 11, 2525. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gu, C.; Wu, L.; Tan, W.; Shang, Z.; Tian, Y.; Ma, J. Recent advances in carbon dots for electrochemical sensing and biosensing: A systematic review. Microchem. J. 2024, 207, 111687. [Google Scholar] [CrossRef]
- Dhiman, R.; Kumar, J.; Singh, M. Fluorescent carbon dots for sensing applications: A review. Anal. Sci. 2024, 40, 1387–1396. [Google Scholar] [CrossRef]
- Ge, G.; Li, L.; Wang, D.; Chen, M.; Zeng, Z.; Xiong, W.; Wu, X.; Guo, C. Carbon dots: Synthesis, properties and biomedical applications. J. Mater. Chem. B 2021, 9, 6553–6575. [Google Scholar] [CrossRef]
- Akram, Z.; Raza, A.; Mehdi, M.; Arshad, A.; Deng, X.; Sun, S. Recent advancements in metal and non-metal mixed-doped carbon quantum dots: Synthesis and emerging potential applications. Nanomaterials 2023, 13, 2336. [Google Scholar] [CrossRef]
- Xu, J.; Cui, K.; Gong, T.; Zhang, J.; Zhai, Z.; Hou, L.; Zaman, F.U.; Yuan, C. Ultrasonic-assisted synthesis of N-doped, multicolor carbon dots toward fluorescent inks, fluorescence sensors, and Logic Gate Operations. Nanomaterials 2022, 12, 312. [Google Scholar] [CrossRef]
- Umami, R.; Permatasari, F.A.; Sundari, C.D.D.; Muttaqien, F.; Iskandar, F. Surface functional groups effect on the absorption spectrum of carbon dots: Initial TD-DFT study. J. Phys. Conf. Ser. 2022, 2243, 012043. [Google Scholar] [CrossRef]
- Lin, L.; Luo, Y.; Tsai, P.; Wang, J.; Chen, X. Metal ions doped carbon quantum dots: Synthesis, physicochemical properties, and their applications. TrAC Trends Anal. Chem. 2018, 103, 87–101. [Google Scholar] [CrossRef]
- Akhter, F.; Ahmed, J.; Pinjaro, M.A.; Shaikh, F.A.; Arain, H.J.; Ahsan, M.J. Metal-doped carbon dots (MCDs) as efficient nano-adsorbents for detection, monitoring, and degradation of wastewater pollutants: Recent progress, challenges, and future prospects. Water Air Soil Pollut. 2023, 234, 672. [Google Scholar] [CrossRef]
- Liu, X.; Sang, Y.; Yin, H.; Liu, A.; Guo, Z.; Liu, Z. Progress in the mechanism and kinetics of Fenton reaction. MOJ Eco Env. Sci. 2018, 3, 10–14. [Google Scholar] [CrossRef]
- Krupińska, I. Application of Fenton’s Reaction for Removal of Organic Matter from Groundwater. Molecules 2024, 29, 5150. [Google Scholar] [CrossRef]
- Fischbacher, A.; von Sonntag, C.; Schmidt, T.C. Hydroxyl radical yields in the Fenton process under various pH, ligand concentrations and hydrogen peroxide/Fe(II) ratios. Chemosphere 2017, 182, 738–744. [Google Scholar] [CrossRef]
- Chen, T.; Zhu, Z.; Wang, Y.; Zhang, H.; Qiu, Y.; Yin, D. Efficient organics heterogeneous degradation by spinel CUFE2O4 supported porous carbon nitride catalyst: Multiple electron transfer pathways for reactive oxygen species generation. Chemosphere 2022, 300, 134511. [Google Scholar] [CrossRef]
- Meng, S.; Zhou, P.; Sun, Y.; Zhang, P.; Zhou, C.; Xiong, Z.; Zhang, H.; Liang, J.; Lai, B. Reducing agents enhanced Fenton-like oxidation (Fe(III)/Peroxydisulfate): Substrate specific reactivity of reactive oxygen species. Water 2022, 218, 118412. [Google Scholar] [CrossRef]
- Wang, J.; Li, S.; Qin, Q.; Peng, C. Sustainable and feasible reagent-free electro-fenton via sequential dual-cathode electrocatalysis. Proc. Natl. Acad. Sci. USA 2021, 118, e2108573118. [Google Scholar] [CrossRef]
- Audino, F.; Pérez-Moya, M.; Graells, M. A novel modeling approach for a generalizable photo-Fenton-based degradation of organic compounds. Environ. Sci. Pollut. Res. 2020, 27, 22913–22934. [Google Scholar] [CrossRef]
- Shen, Y.; Wang, Y.; Shan, P.; Xu, R.; Sun, X.; Hou, J.; Guo, F.; Li, C.; Shi, W. Boosted photo-self-fenton degradation activity by Fe-doped carbon dots as dual-function active sites for in-situ H2O2 generation and activation. Sep. Purif. Technol. 2025, 353, 128529. [Google Scholar] [CrossRef]
- Beker, S.A.; Cole, I.; Ball, A.S. A review on the catalytic remediation of dyes by tailored carbon dots. Water 2022, 14, 1456. [Google Scholar] [CrossRef]
- Han, W.; Zhang, H.; Li, D.; Qin, W.; Zhang, X.; Wang, S.; Duan, X. Surface engineered carbon quantum dots for efficient photocatalytic hydrogen peroxide production. Appl. Catal. B Environ. Energy 2024, 350, 123918. [Google Scholar] [CrossRef]
- Gao, F.; Liu, J.; Tang, Q.; Jiang, Y. The guidelines for the design and synthesis of transition metal atom doped carbon dots. ChemBioChem 2024, 25, e202300485. [Google Scholar] [CrossRef] [PubMed]
- John, V.L.; Nair, Y.; Vinod, T.P. Doping and surface modification of carbon quantum dots for enhanced functionalities and related applications. Part. Part. Syst. Charact. 2021, 38, 2100170. [Google Scholar] [CrossRef]
- Nguyen, K.G.; Baragau, I.-A.; Gromicova, R.; Nicolaev, A.; Thomson, S.A.J.; Rennie, A.; Power, N.P.; Sajjad, M.T.; Kellici, S. Investigating the effect of N-doping on carbon quantum dots structure, optical properties and metal ion screening. Sci. Rep. 2022, 12, 13806. [Google Scholar] [CrossRef]
- Wu, H.; Tong, C. Nitrogen- and sulfur-codoped carbon dots for highly selective and sensitive fluorescent detection of Hg2+ ions and sulfide in environmental water samples. J. Agric. Food Chem. 2019, 67, 2794–2800. [Google Scholar] [CrossRef]
- Li, C.; Huang, J.; Yuan, L.; Xie, W.; Ying, Y.; Li, C.; Yu, Y.; Pan, Y.; Qu, W.; Hao, H.; et al. Recent progress of emitting long-wavelength carbon dots and their merits for visualization tracking, target delivery and Theranostics. Theranostics 2023, 13, 3064–3102. [Google Scholar] [CrossRef]
- Li, Y.; Lin, H.; Luo, C.; Wang, Y.; Jiang, C.; Qi, R.; Huang, R.; Travas-Sejdic, J.; Peng, H. Aggregation induced red shift emission of phosphorus doped carbon dots. RSC Adv. 2017, 7, 32225–32228. [Google Scholar] [CrossRef]
- Li, F.; Li, T.; Sun, C.; Xia, J.; Jiao, Y.; Xu, H. Selenium-doped carbon quantum dots for free-radical scavenging. Angew. Chem. 2017, 129, 10042–10046. [Google Scholar] [CrossRef]
- Qian, Z.; Shan, X.; Chai, L.; Ma, J.; Chen, J.; Feng, H. Si-doped carbon quantum dots: A facile and general preparation strategy, bioimaging application, and Multifunctional Sensor. ACS Appl. Mater. Amp Interfaces 2014, 6, 6797–6805. [Google Scholar] [CrossRef]
- Zuo, G.; Xie, A.; Pan, X.; Su, T.; Li, J.; Dong, W. Fluorine-doped cationic carbon dots for efficient gene delivery. ACS Appl. Nano Mater. 2018, 1, 2376–2385. [Google Scholar] [CrossRef]
- Hu, S.; Ding, Y.; Chang, Q.; Yang, J.; Lin, K. Chlorine-functionalized carbon dots for highly efficient photodegradation of pollutants under visible-light irradiation. Appl. Surf. Sci. 2015, 355, 774–777. [Google Scholar] [CrossRef]
- Najaflu, M.; Shahgolzari, M.; Bani, F.; Khosroushahi, A.Y. Green synthesis of near-infrared copper-doped carbon dots from Alcea for cancer photothermal therapy. ACS Omega 2022, 7, 34573–34582. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, R.M.; Cardoso, I.M.F.; da Silva, L.P.; da Silva, J.G.E. Copper(ii)-doped carbon dots as catalyst for ozone degradation of textile dyes. Nanomaterials 2022, 12, 1211. [Google Scholar] [CrossRef]
- Huang, C.; Duan, M.; Shi, Y.; Liu, H.; Zhang, P.; Zuo, Y.; Yan, L.; Xu, Y.; Niu, Y. Insights into the antibacterial mechanism of iron doped carbon dots. J. Colloid Interface Sci. 2023, 645, 933–942. [Google Scholar] [CrossRef]
- Hu, F.; Fu, Q.; Li, Y.; Yan, C. Zinc-doped carbon quantum dots-based ratiometric fluorescence probe for rapid, specific, and visual determination of tetracycline hydrochloride. Food Chem. 2023, 431, 137097. [Google Scholar] [CrossRef]
- Hasanzadeh, A.; Radmanesh, F.; Hosseini, E.S.; Hashemzadeh, I.; Kiani, J.; Nourizadeh, H.; Naseri, M.; Fatahi, Y.; Chegini, F.; Madjd, Z.; et al. Highly Photoluminescent Nitrogen- and Zinc-Doped Carbon Dots for Efficient Delivery of CRISPR/Cas9 and mRNA. Bioconjugate Chem. 2021, 32, 1875–1887. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, D.; Han, X.; Chen, Z.; Li, M.; Jiang, L.; Zeng, J. Magnesium-doped carbon quantum dot nanomaterials alleviate salt stress in rice by scavenging reactive oxygen species to increase photosynthesis. ACS Nano 2024, 18, 31188–31203. [Google Scholar] [CrossRef]
- Zhang, Z.; Fan, Z. Application of magnesium ion doped carbon dots obtained via hydrothermal synthesis for arginine detection. New J. Chem. 2020, 44, 4842–4849. [Google Scholar] [CrossRef]
- Da Rocha, J.D.; Prado Cechinel, M.; Rocha, L.; Riella, H.; Padoin, N.; Soares, C. Exploring the potential of rare earth doped carbon dots: Concepts and applications. Chem. Eng. J. Adv. 2024, 17, 100583. [Google Scholar] [CrossRef]
- Kou, X.; Cong, Y.; Dong, W.-F.; Li, L. Rare earth-modified yellow carbon dots for long-term imaging in cells and zebrafish. Mater. Des. 2024, 240, 112855. [Google Scholar] [CrossRef]
- Yu, R.; Ou, M.; Hou, Q.; Li, C.; Qu, S.; Tan, Z. Metal and non-metal doped carbon dots: Properties and applications. Light Adv. Manuf. 2024, 5, 1. [Google Scholar] [CrossRef]
- Han, Y.; Kong, X.; Bao, R.; Yi, L.; Gu, Y.; Liu, L.; Lan, L.; Gan, Z.; Yi, J.; Zhang, X. Excitation-dependent photostability and high fluorescence quantum yield of Cr-doped carbon polymer dots for sensors, anticounterfeiting inks, and light-emitting devices. ACS Appl. Nano Mater. 2024, 7, 1674–1683. [Google Scholar] [CrossRef]
- Khare, P.; Bhati, A.; Anand, S.; Sonkar, S. Brightly fluorescent zinc-doped red-emitting carbon dots for the sunlight-induced photoreduction of Cr(VI) to Cr(III). ACS Omega 2018, 3, 5187–5194. [Google Scholar] [CrossRef]
- Mokoloko, L.L.; Forbes, R.P.; Coville, N.J. The behavior of carbon dots in catalytic reactions. Catalysts 2023, 13, 1201. [Google Scholar] [CrossRef]
- Kang, W.; Lee, A.; Tae, Y.; Lee, B.; Choi, J. Enhancing catalytic efficiency of carbon dots by modulating their Mn doping and chemical structure with metal salts. RSC Adv. 2023, 13, 8996–9002. [Google Scholar] [CrossRef]
- Fiszka Borzyszkowska, A.; Silowska, A.; Czaja, P.; Bielicka-Gieldon, A.; Zekker, I.; Zielinska-Jurek, A. ZnO-decorated green-synthesized multi-doped carbon dots from chlorella pyrenoidosa for sustainable photocatalytic carbamazepine degradation. RSC Adv. 2023, 13, 25529–25551. [Google Scholar] [CrossRef]
- Zhang, S.; Fan, X.; Guan, R.; Hu, Y.; Jiang, S.; Shao, X.; Wang, S.; Yue, Q. Carbon dots as metal-free photocatalyst for dye degradation with high efficiency within nine minutes in dark. Opt. Mater. 2022, 123, 111914. [Google Scholar] [CrossRef]
- Yang, W.; Huang, T.; Zhao, M.; Luo, F.; Weng, W.; Wei, Q.; Lin, Z.; Chen, G. High peroxidase-like activity of iron and nitrogen co-doped carbon dots and its application in Immunosorbent Assay. Talanta 2017, 164, 1–6. [Google Scholar] [CrossRef]
- Jin, Z.; Li, Q.; Tang, P.; Li, G.; Liu, L.; Chen, D.; Wu, J.; Chai, Z.; Huang, G.; Chen, X. Copper-doped carbon dots with enhanced Fenton reaction activity for rhodamine B degradation. Nanoscale Adv. 2022, 4, 3073–3082. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, P.; Wu, X.; Ma, C.; Luo, S.; Xu, M.; Li, W.; Liu, S. Nitrogen and copper (II) co-doped carbon dots for applications in ascorbic acid determination by non-oxidation reduction strategy and cellular imaging. Talanta 2020, 210, 120649. [Google Scholar] [CrossRef] [PubMed]
- Etefa, H.F.; Tessema, A.A.; Dejene, F.B. Carbon dots for future prospects: Synthesis, characterizations and recent applications: A review (2019–2023). C 2024, 10, 60. [Google Scholar] [CrossRef]
- Humaera, N.A.; Fahri, A.N.; Armynah, B.; Tahir, D. Natural source of carbon dots from part of a plant and its applications: A review. Luminescence 2021, 36, 1354–1364. [Google Scholar] [CrossRef] [PubMed]
- Kurian, M.; Paul, A. Recent trends in the use of green sources for carbon dot synthesis—A short review. Carbon Trends 2021, 3, 100032. [Google Scholar] [CrossRef]
- Dhandapani, E.; Duraisamy, N.; Mohan Raj, R. Green synthesis of carbon quantum dots from food waste. Mater. Today Proc. 2022, 51, 1696–1700. [Google Scholar] [CrossRef]
- Kaur, P.; Verma, G. Converting fruit waste into carbon dots for bioimaging applications. Mater. Today Sustain. 2022, 18, 100137. [Google Scholar] [CrossRef]
- Chen, W.; Yin, H.; Cole, Y.; Houshyar, S.; Wang, L. Carbon dots derived from non-biomass waste: Methods, applications, and future perspectives. Molecules 2024, 29, 2441. [Google Scholar] [CrossRef]
- Dursun, S.; Yavuz, E.; Çetinkaya, Z. In situ reduction of chloroauric acid (HAuCl4) for generation of catalytic au nanoparticle embedded triazine based covalent organic polymer networks. RSC Adv. 2019, 9, 38538–38546. [Google Scholar] [CrossRef]
- Chen, H.; Wang, G.D.; Tang, W.; Todd, T.; Zhen, Z.; Tsang, C.; Hekmatyar, K.; Cowger, T.; Hubbard, R.B.; Zhang, W.; et al. Gd-encapsulated carbonaceous dots with efficient renal clearance for magnetic resonance imaging. Adv. Mater. 2014, 26, 6761–6766. [Google Scholar] [CrossRef]
- Yang, X.; Hou, S.; Chu, T.; Han, J.; Li, R.; Guo, Y.; Gong, Y.; Li, H.; Wan, Z. Preparation of magnesium, nitrogen-codoped carbon quantum dots from lignin with bright green fluorescence and sensitive pH response. Ind. Crops Prod. 2021, 167, 113507. [Google Scholar] [CrossRef]
- Liang, M.; Wang, Y.; Ma, K.; Yu, S.; Chen, Y.; Deng, Z.; Liu, Y.; Wang, F. Engineering inorganic nanoflares with elaborate enzymatic specificity and efficiency for versatile biofilm eradication. Small 2020, 16, 2002348. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yan, Y.; Zhou, J.; Huang, C.; Zhen, S.; Zhan, L. Fe-doped carbon dots: A novel fluorescent nanoprobe for cellular hypochlorous acid imaging. Anal. Sci. 2024, 40, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zhang, R.; Wang, Y. One-pot hydrothermal synthesis of metal-doped carbon dot nanozymes using protein cages as precursors. RSC Adv. 2023, 13, 6760–6767. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Guo, Y.; Zhang, J.; Yue, Y.; Fan, L.; Li, F.; Dong, C.; Shuang, S. A high catalytic activity nanozyme based on cobalt-doped carbon dots for biosensor and anticancer cell effect. ACS Appl. Mater. Interfaces 2022, 14, 57206–57214. [Google Scholar] [CrossRef]
- Qi, J.; Zhang, P.; Zhang, T.; Zhang, R.; Zhang, Q.; Wang, J.; Zong, M.; Gong, Y.; Liu, X.; Wu, X.; et al. Metal-doped carbon dots for biomedical applications: From design to implementation. Heliyon 2024, 10, e32133. [Google Scholar] [CrossRef]
- Egorova, M.; Tomskaya, A.; Smagulova, S.A. Optical properties of carbon dots synthesized by the hydrothermal method. Materials 2023, 16, 4018. [Google Scholar] [CrossRef]
- Byrappa, K.; Adschiri, T. Hydrothermal technology for Nanotechnology. Prog. Cryst. Growth Charact. Mater. 2007, 53, 117–166. [Google Scholar] [CrossRef]
- Liu, M.L.; Bin Chen, B.; Yang, T.; Wang, J.; Liu, X.D.; Huang, C.Z. One-pot carbonization synthesis of europium-doped carbon quantum dots for highly selective detection of tetracycline. Methods Appl. Fluoresc. 2017, 5, 015003. [Google Scholar] [CrossRef]
- Pakkath, S.A.R.; Chetty, S.; Selvarasu, P.; Murugan, A.V.; Kumar, Y.; Periyasamy, L.; Santhakumar, M.; Sadras, S.R.; Santhakumar, K. Transition metal ion (Mn2+, Fe2+, Co2+, and Ni2+)-doped carbon dots synthesized via microwave-assisted pyrolysis: A potential nanoprobe for magneto-fluorescent dual-modality bioimaging. ACS Biomater. Sci. Eng. 2018, 4, 2582–2596. [Google Scholar] [CrossRef]
- Romero, M.P.; Alves, F.; Stringasci, M.D.; Buzza, H.H.; Ciol, H.; Inada, N.M.; Bagnato, V.S. One-pot microwave-assisted synthesis of carbon dots and in vivo and in vitro antimicrobial photodynamic applications. Front. Microbiol. 2021, 12, 662149. [Google Scholar] [CrossRef]
- Kumar, V.B.; Kumar, R.; Gedanken, A.; Shefi, O. Fluorescent metal-doped carbon dots for neuronal manipulations. Ultrason. Sonochemistry 2019, 52, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Rong, M.; Deng, X.; Chi, S.; Huang, L.; Zhou, Y.; Shen, Y.; Chen, X. Ratiometric fluorometric determination of the anthrax biomarker 2, 6-dipicolinic acid by using europium(III)-doped carbon dots in a test stripe. Microchim. Acta 2018, 185, 201. [Google Scholar] [CrossRef] [PubMed]
- Jiao, M.; Wang, Y.; Wang, W.; Zhou, X.; Xu, J.; Xing, Y.; Chen, L.; Zhang, Y.; Chen, M.; Xu, K.; et al. Gadolinium-doped red-emissive carbon dots as targeted theranostic agents for fluorescence and MR imaging guided cancer phototherapy. Chem. Eng. J. 2022, 440, 135965. [Google Scholar] [CrossRef]
- Muradov, M.B.; Mammadyarove, S.J.; Eynazova, G.M.; Balayeva, O.O.; Hasanova, I.; Aliyeva, G.; Melikova, S.Z.; Mahammadali, A. The effect of Cu doping on structural, optical properties and photocatalytic activity of Co3O4 nanoparticles synthesized by sonochemical method. Opt. Mater. 2023, 142, 114001. [Google Scholar] [CrossRef]
- Wu, W.; Zhan, L.; Fan, W.; Song, J.; Li, X.; Li, Z.; Wang, R.; Zhang, J.; Zheng, J.; Wu, M.; et al. Cu-N dopants boost electron transfer and photooxidation reactions of carbon dots. Angew. Chem. Int. Ed. 2015, 54, 6540–6544. [Google Scholar] [CrossRef]
- Duan, Y.; Huang, Y.; Chen, S.; Zuo, W.; Shi, B. Cu-doped carbon dots as catalysts for the chemiluminescence detection of glucose. ACS Omega 2019, 4, 9911–9917. [Google Scholar] [CrossRef]
- Xing, D.; Koubaa, A.; Tao, Y.; Magdouli, S.; Li, P.; Bouafif, H.; Zhang, J. Copper-doped carbon Nanodots with superior photocatalysis, directly obtained from chromium-copper-arsenic-treated wood waste. Polymers 2022, 15, 136. [Google Scholar] [CrossRef]
- Gao, F.; Huang, J.; Ruan, Y.; Li, H.; Gong, P.; Wang, F.; Tang, Q.; Jiang, Y. Unraveling the structure transition and peroxidase mimic activity of copper sites over atomically dispersed copper-doped carbonized polymer dots. Angew. Chem. Int. Ed. 2022, 135, e202214042. [Google Scholar] [CrossRef]
- Das, P.; Ganguly, S.; Bose, M.; Mondal, S.; Choudhary, S.; Gangopadhyay, S.; Das, A.K.; Banerjee, S.; Das, N.C. Zinc and nitrogen ornamented bluish white luminescent carbon dots for engrossing bacteriostatic activity and Fenton based bio-sensor. Mater. Sci. Eng. C 2018, 88, 115–129. [Google Scholar] [CrossRef]
- He, H.; Yang, Y.; Li, J.; Lai, X.; Chen, X.; Wang, L.; Zhang, W.; Huang, Y.; Zhang, P. Enhanced fluorescence of Zn-doped carbon quantum dots using zinc citrate chelate as precursor for fluorescent sensor applications. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2021, 264, 114955. [Google Scholar] [CrossRef]
- Tammina, S.K.; Yang, D.; Li, X.; Koppala, S.; Yang, Y. High photoluminescent nitrogen and zinc doped carbon dots for sensing Fe3+ ions and temperature. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 222, 117141. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Mondal, U.S.; Paul, S. Highly fluorescent metal-doped carbon quantum dots prepared from hen feather demonstrating pH-dependent dual sensing of 4-nitrophenol and Hg2+ ion. Appl. Surf. Sci. 2023, 638, 157998. [Google Scholar] [CrossRef]
- Xu, Q.; Liu, Y.; Su, R.; Cai, L.; Li, B.; Zhang, Y.; Zhang, L.; Wang, Y.; Wang, Y.; Li, N.; et al. Highly fluorescent Zn-doped carbon dots as Fenton reaction-based bio-sensors: An integrative experimental–theoretical consideration. Nanoscale 2016, 8, 17919–17927. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.M.; Lai, C.W.; Ngai, K.S.; Juan, J.C. Recent developments of zinc oxide based photocatalyst in water treatment technology: A review. Water Res. 2016, 88, 428–448. [Google Scholar] [CrossRef]
- George, S.; Yin, H.; Liu, Z.; Shen, S.; Cole, I.; Khiong, C.W. Hazard profiling of a combinatorial library of zinc oxide nanoparticles: Ameliorating light and dark toxicity through surface passivation. J. Hazard. Mater. 2022, 434, 128825. [Google Scholar] [CrossRef]
- Zinc in Freshwater. 2021. Available online: https://www.waterquality.gov.au/anz-guidelines/guideline-values/default/water-quality-toxicants/toxicants/zinc-2000 (accessed on 10 March 2025).
- Jung, H.; Sapner, V.S.; Adhikari, A.; Sathe, B.R.; Patel, R. Recent progress on carbon quantum dots based photocatalysis. Front. Chem. 2022, 10, 881495. [Google Scholar] [CrossRef]
- Dang, Y.; Li, B.; Feng, X.; Jia, J.; Li, K.; Zhang, Y. Preparation of iron-doped carbon dots and their application in photocatalytic reduction of carbon dioxide. ChemPhotoChem 2022, 7, e202200156. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, B.; Lu, M.; Li, S.; Guo, J.; Chen, F.; Xiong, X.; Yin, Z.; Liu, H.; Zhou, D. Ultrasmall Fe-doped carbon dots nanozymes for photoenhanced antibacterial therapy and wound healing. Bioact. Mater. 2022, 12, 246–256. [Google Scholar] [CrossRef]
- Zhang, I.; Cao, X.; Gu, L.; Yu, H.; Wu, F.; Liu, Y.; Liu, X.; Gao, Y.; Zhang, H. Embedded iron and nitrogen co-doped carbon quantum dots within g-C3N4 as an exceptional PMS photocatalytic activator for sulfamethoxazole degradation: The key role of FeN bridge. Sep. Purif. Technol. 2024, 342, 126975. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J. Multivalent metal catalysts in Fenton/Fenton-like oxidation system: A critical review. Chem. Eng. J. 2023, 466, 143147. [Google Scholar] [CrossRef]
- Luo, H.; Liu, H.; Sun, C. Removal of sulfide ions from Kraft washing effluents by photocatalysis with n and fe codoped carbon dots. Polymers 2023, 15, 679. [Google Scholar] [CrossRef] [PubMed]
- Soares, O.S.; Rodrigues, C.S.; Maderia, L.M.; Pereira, M.F. Heterogeneous fenton-like degradation of P-nitrophenol over tailored carbon-based materials. Catalysts 2019, 9, 258. [Google Scholar] [CrossRef]
- Gatou, M.-A.; Skylla, E.; Dourou, P.; Pippa, N.; Gazouli, M.; Lagopati, N.; Pavlatou, E.A. Magnesium oxide (MgO) nanoparticles: Synthetic Strategies and Biomedical Applications. Crystals 2024, 14, 215. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, G.; Zhang, L.; Li, N.; Hou, Y.; Zhang, R.; Wang, W.; Du, X.; Chen, F.; Li, B. A versatile synthetic strategy for Mg-doped carbon dots derived from the traditional Chinese herb mulberry and its application in osteoblastic differentiation. J. Photochem. Photobiol. A Chem. 2024, 446, 115135. [Google Scholar] [CrossRef]
- Verma, R.; Naik, K.K.; Gangwar, J.; Srivastava, A.K. Morphology, mechanism and optical properties of nanometer-sized MgO synthesized via facile wet chemical method. Mater. Chem. Phys. 2014, 148, 1064–1070. [Google Scholar] [CrossRef]
- Gopi, P.; Ponnusamy, K. Influence of magnesium doping on carbon dots for enhancing photocurrent generation in photodetectors. Phys. Scr. 2025, 100, 035944. [Google Scholar] [CrossRef]
- Bhati, A.; Anand, S.R.; Kumar, G.; Garg, A.K.; Khare, P.; Sonkar, S. Sunlight-induced photocatalytic degradation of pollutant dye by highly fluorescent red-emitting MG-n-embedded carbon dots. ACS Sustain. Chem. Amp; Eng. 2018, 6, 9246–9256. [Google Scholar] [CrossRef]
- Liu, J.; Li, R.; Yang, B. Carbon dots: A new type of carbon-based nanomaterial with wide applications. ACS Cent. Sci. 2020, 6, 2179–2195. [Google Scholar] [CrossRef]
- Abu-Melha, S. Metal-doped carbon quantum dots for catalytic discoloration of methylene blue in day light. J. Photochem. Photobiol. A Chem. 2024, 447, 115233. [Google Scholar] [CrossRef]
- Shen, S.; Li, R.; Wang, H.; Fu, J. Carbon dot–doped titanium dioxide sheets for the efficient photocatalytic performance of refractory pollutants. Front. Chem. 2021, 9, 706343. [Google Scholar] [CrossRef]
- Camilli, E.; Pighin, A.F.; Copello, G.J.; Villanueva, E. Cobalt Nanoparticles Decorated with Carbon Quantum Dots as an Improved Catalyst for Fenton-Like Reaction. 2023. Available online: https://ssrn.com/abstract=4336523 (accessed on 10 March 2025).
- Zhang, Y.; Guo, P.; Jin, M.; Gao, G.; Xi, Q.; Zhou, H.; Xu, G.; Xu, J. Promoting the Photo-Fenton catalytic activity with carbon dots: Broadening light absorption, higher applicable pH and better reuse performance. Mol. Catal. 2020, 481, 110254. [Google Scholar] [CrossRef]
- Beker, S.A.; Khudur, L.S.; Cole, I.; Ball, A.S. Catalytic degradation of methylene blue using iron and nitrogen-containing carbon dots as Fenton-like catalysts. New J. Chem. 2022, 46, 263–275. [Google Scholar] [CrossRef]
- Kremer, M.L. Initial steps in the reaction of H2O2 with Fe2+ and Fe3+ ions: Inconsistency in the free radical theory. Reactions 2023, 4, 171–175. [Google Scholar] [CrossRef]
- Walkey, C.; Das, D.; Seal, S.; Erlicham, J.; Heckman, K.; Ghibeli, L.; Traversa, E.; McGinnis, J.F.; Self, W.F. Catalytic properties and biomedical applications of cerium oxide nanoparticles. Environ. Sci. Nano 2015, 2, 33–53. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, W.; Yuan, P.; Chi, C.; Zhang, J.; Zhou, N. Synthesis of lanthanum-doped carbon dots for detection of mercury ion, multicolor imaging of cells and tissue, and bacteriostasis. Chem. Eng. J. 2017, 330, 1137–1147. [Google Scholar] [CrossRef]
- Zhu, P.; Zhao, X.; Zhang, Y.; Liu, Y.; Zhao, Z.; Yang, Z.; Liu, X.; Zhang, W.; Guo, Z.; Wang, X.; et al. Mn3+/Mn4+ ion-doped carbon dots as Fenton-like catalysts for fluorescence dual-signal detection of dopamine. Front. Bioeng. Biotechnol. 2022, 10, 964814. [Google Scholar] [CrossRef]
- Tejwan, N.; Saini, A.K.; Sharma, A.; Singh, T.A.; Kumar, N.; Das, J. Metal-doped and hybrid carbon dots: A comprehensive review on their synthesis and biomedical applications. J. Control Release 2021, 330, 132–150. [Google Scholar] [CrossRef]
- Yang, K.; Chen, M.; Liu, C.; Lin, X.; Hou, D.; Zheng, Y.; Sun, H.; Yang, F.; Sun, J. Tunable catalytic performance of Pd-doped carbon dots for catalytic polymerization of phenylacetylenes via modification of surface functional groups. ACS Appl. Nano Mater. 2025, 8, 3064–3072. [Google Scholar] [CrossRef]
- Gallareta-Olivares, G.; Rivas-Sanchez, A.; Curz-Cruz, A.; Hussain, S.M.; Gonzalez-Gonzaliez, R.B.; Cardenas-Alcaide, M.F.; Lqbal, H.M.; Parra-Saldivar, R.P. Metal-doped carbon dots as robust nanomaterials for the monitoring and degradation of water pollutants. Chemosphere 2023, 312, 137190. [Google Scholar] [CrossRef]
- Hussain, S.; Aneggi, E.; Goi, D. Catalytic activity of metals in heterogeneous Fenton-like oxidation of wastewater contaminants: A Review. Environ. Chem. Lett. 2021, 19, 2405–2424. [Google Scholar] [CrossRef]
- Jung, Y.S.; Lim, W.T.; Park, J.; Kim, Y. Effect of pH on Fenton and Fenton-like oxidation. Environ. Technol. 2009, 30, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Delnavaz, E.; Amjadi, M. Platinum, palladium, and nitrogen co-doped carbon dots as efficient enhancers for acetonitrile-hydrogen peroxide chemiluminescence reaction and its application for sensitive determination of Fe(II) ions. Synth. Met. 2024, 305, 117604. [Google Scholar] [CrossRef]
- Zhuo, S.; Guan, Y.; Li, H.; Fang, J.; Zhang, P.; Du, J.; Zhu, C.Q. Facile fabrication of fluorescent Fe-doped carbon quantum dots for dopamine sensing and bioimaging application. Analyst 2019, 144, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, S.; Xie, B.; Li, B.; Huang, H. Zn-doped carbon dots-based versatile bioanalytical probe for precise estimation of antioxidant activity index of multiple samples via Fenton chemistry. Sens. Actuators B Chem. 2022, 363, 131558. [Google Scholar] [CrossRef]
- Yuan, C.; Qin, X.; Xu, Y.; Jing, Q.; Shi, R.; Wang, Y. High sensitivity detection of H2O2 and glucose based on carbon quantum dots-catalyzed 3, 3′, 5, 5′-tetramethylbenzidine oxidation. Microchem. J. 2020, 159, 105365. [Google Scholar] [CrossRef]
- Liu, X.; Liu, J. Biosensors and sensors for dopamine detection. View 2021, 2, 20200102. [Google Scholar] [CrossRef]
- He, W.; Liu, R.; Zhou, P.; Liu, Q.; Cui, T. Flexible micro-sensors with self-assembled graphene on a polyolefin substrate for dopamine detection. Biosens. Bioelectron. 2020, 167, 112473. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Shi, H.; Yang, M.; Yao, Z.; Zhang, B.; Liu, E.; Hu, X.; Xue, W.; Fan, J. Biocompatible sulfur nitrogen co-doped carbon quantum dots for highly sensitive and selective detection of dopamine. Colloids Surf. B Biointerfaces 2021, 205, 111874. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, C.; Zhou, T.; Ma, H. Gold nanoparticle based colorimetric probe for dopamine detection based on the interaction between dopamine and melamine. Microchim. Acta 2014, 182, 1003–1008. [Google Scholar] [CrossRef]
- Zhang, R.; Jin, G.D.; Chen, D.; Hu, X.Y. Simultaneous electrochemical determination of dopamine, ascorbic acid and uric acid using poly(acid Chrome Blue K) modified glassy carbon electrode. Sens. Actuators B Chem. 2009, 138, 174–181. [Google Scholar] [CrossRef]
| Synthesis Methods | Method Description | Advantages | Disadvantages | References |
|---|---|---|---|---|
| Hydrothermal synthesis | Reactions in high-temperature aqueous solutions under a high vapour pressure. |
|
| [66,67] |
| Chemical oxidation | Carbonisation of small organic molecules with strong oxidising acids. |
|
| [68] |
| Microwave assisted synthesis | Rapid heating of carbon precursors using microwaves. |
|
| [69,70] |
| Ultrasound assisted synthesis | Using energy generated by ultrasound to dehydrate, polymerise, and carbonise the precursors. |
|
| [71] |
| Pyrolysis | High-temperature reaction involving carbonisation. |
|
| [72] |
| Solvent thermal synthesis | Synthesis at elevated temperature and high pressure in organic solvents. |
|
| [73] |
| CD Type | Dye Target | pH | Degradation Efficiency (%) | Half-Life (t1/2, min) | k (min−1) | Normalised k (per mg CD/mM H2O2) | Experimental Conditions | References |
|---|---|---|---|---|---|---|---|---|
| Undoped CDs | Methylene Blue | 6.8 | 42.0% (300 min) | 68.5 | 0.0101 | 0.002 min−1·mg−1 (10 mg catalyst, 1 mM H2O2) | Daylight, 50 °C, 1 mM H2O2, 10 mg CD, MB = 0.25 mg/mL | [100] |
| Non-metal-doped CDs (N-CDs) | Rhodamine B | 7.0 | ~55% (180 min) | — | ~0.0078 | ~0.0015 min−1·mg−1 (estimated) | Visible light, room temp, 1 mM H2O2, ~10 mg CD, RhB = 0.25 mg/mL | [101] |
| Metal-doped CDs (Ag, Pd, Cu, Fe) | Methylene Blue | 6.8 | 78–100% (≤300 min) | 22.6–30.9 | 0.022–0.0316 | ~0.005–0.007 min−1·mg−1·mM−1 | Daylight, 50 °C, 1 mM H2O2, 10 mg CD, MB = 0.25 mg/mL | [100,101] |
| Metal-doped CDs (Cu, Fe) | Rhodamine B/Orange II | 7.0 | 90–98% (≤120 min) | 2.3–21.9 | 0.0316–1.002 | Up to 0.10 min−1·mg−1·mM−1 | No light (ozonation) or visible light; room temp; 1–3 mM H2O2/O3; 5–10 mg catalyst; RhB/O-II = 0.1–0.2 mg/mL | [34] |
| Metal Ion | Electronic Configuration | Common Oxidation States | Redox Potential<br> (V vs. SHE) | d-Orbital Occupancy | ROS Generation Potential | Charge Transfer Efficiency | Pollutant Degradation Behaviour | References |
|---|---|---|---|---|---|---|---|---|
| Fe3+/Fe2+ | [Ar] 3d5/3d6 | +2, +3 | +0.77 (Fe3+/Fe2+) | Half-filled | High (•OH via H2O2) | High (Fenton-active centre) | Fast MB degradation | [15,90] |
| Cu2+/Cu+ | [Ar] 3d9/3d10 | +1, +2 | +0.17 (Cu2+/Cu+) | Nearly full | Moderate (•OH, O2•−) | High (visible-light active) | Strong for RhB, moderate for MB | [34,74] |
| Zn2+ | [Ar] 3d10 | +2 | −0.76 (Zn2+/Zn) | Full (d10) | Low (non-redox active) | Indirect (bandgap tuning) | High for RhB via adsorption | [47,103] |
| Mg2+ | [Ne] | +2 | −2.37 (Mg2+/Mg) | No d-electrons | Minimal | Weak (structural tuning) | Mild, stable light-activated CD | [95,98] |
| Doped CDs | Precursors | Analytes | Signals | Linear Range | LOD | Ref |
|---|---|---|---|---|---|---|
| Zn-doped CDs | sodium citrate and zinc chloride | hydrogen peroxide | fluorescence | 10–80 μM | 10 nM | [83] |
| N, Zn-doped CDs | citric acid, tris(hydroxymethyl)aminomethane, and zinc acetate | hydrogen peroxide | fluorescence | 10–70 μM | 8 nM | [79] |
| Pt, Pd, N-doped CDs | citric acid monohydrate, diethylenetriamine, chloroplatinic acid, and palladium chloride | Fe(II) | chemiluminescence | 0.002 to 8.0 µM | 1.0 nM | [114] |
| Zn-doped CDs | sodium citrate and zinc chloride | glucose | fluorescence | 5–100 μM | 5 nM | [83] |
| Cu-doped CDs | citric acid and Cu(NO3)2·3H2O | glucose | chemiluminescence | 1–48 μM | 0.32 μM | [76] |
| Co-doped CDs | vitamin B12 and citric acid | glucose | colourimetric | 0.500 to 200 μM | 0.145 μM | [64] |
| Mn3+/Mn4+-doped CDs | O-phenylenediamine and manganese acetate | dopamine | dual fluorescence | 100–275 nM and 325–525 nM | 3 and 12 nM | [108] |
| Fe-doped CDs | ethylenediamine tetraacetic acid salts and ferric nitrate | dopamine | fluorescence | 0.01–50 μM | 5 nM | [115] |
| Zn-doped CDs | ZnCl2 and trisodium citrate dihydrate | antioxidant activity index (AAI) | fluorescence | 2–10 µg/mL | 0.210 µg/mL | [116] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.; Ball, A.S.; Cole, I.; Yin, H. Metal-Doped Carbon Dots as Fenton-like Catalysts and Their Applications in Pollutant Degradation and Sensing. Sustainability 2025, 17, 3642. https://doi.org/10.3390/su17083642
Chen W, Ball AS, Cole I, Yin H. Metal-Doped Carbon Dots as Fenton-like Catalysts and Their Applications in Pollutant Degradation and Sensing. Sustainability. 2025; 17(8):3642. https://doi.org/10.3390/su17083642
Chicago/Turabian StyleChen, Weiyun, Andrew S. Ball, Ivan Cole, and Hong Yin. 2025. "Metal-Doped Carbon Dots as Fenton-like Catalysts and Their Applications in Pollutant Degradation and Sensing" Sustainability 17, no. 8: 3642. https://doi.org/10.3390/su17083642
APA StyleChen, W., Ball, A. S., Cole, I., & Yin, H. (2025). Metal-Doped Carbon Dots as Fenton-like Catalysts and Their Applications in Pollutant Degradation and Sensing. Sustainability, 17(8), 3642. https://doi.org/10.3390/su17083642






