Cassava: The Drought, War and Famine Crop in a Changing World
Abstract
:1. Introduction
2. Cassava: Origins, Distribution and Food Products



3. Cassava: Cyanide Production and Consequences for Consumers
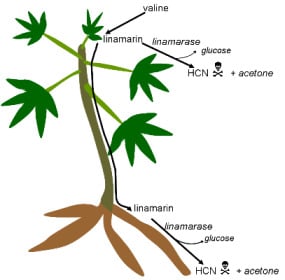
3.1. Cyanogenic Glycosides


3.2. Cyanogens in Cassava: Defence against Pests and Diseases
3.3. Cyanogens in Cassava: Variation within and Among Plants
3.4. Cyanogens in Cassava: Impacts on Human Health

| Body weight (kg) | Lethal dose range of HCN (mg) | Lethal amount of cassava product (kg) | |
|---|---|---|---|
| 10ppm HCN | 40ppm HCN | ||
| 10 | 5–35 | 0.5–3.5 | 0.13–0.88 |
| 20 | 10–70 | 1–7 | 0.25–1.75 |
| 40 | 20–140 | 2–14 | 0.50–3.50 |
| 60 | 30–210 | 3–21 | 0.75–5.25 |
| 80 | 40–280 | 4–28 | 1.00–7.00 |
| 100 | 50–350 | 5–35 | 1.25–8.75 |
3.5. Use of Genetic Engineering to Reduce Cyanogens in Cassava
4. Cassava: Sustainable Production in a Time of Environmental Change
4.1. Sustainable Production
4.2. Cassava and Sustainable Water Management
4.3. Cassava and Sustainable Nutrient Management
4.4. Cassava Production and Temperature
4.5. Cassava Production in a High CO2 World
| CO2 concentrations | Duration & Experiment type | Net CO2 assimilation rate | Root: shoot ratio | N supply | Reference |
|---|---|---|---|---|---|
| 350 ppm 700 ppm | 3 months, Glasshouse | Lower at elevated CO2 (measured after 2 months)a | Increased | Supplied as compound fertiliser twice during experiment | Imai et al. 1984 [191] |
| 360 ppm 550 ppm 710 ppm | 9 months, Glasshouse | Lower at 710 ppm than 550 and 360 ppm *, N.S. between 550 and 360 ppm (measured after 3 months)b | Decreased | 1 mM & 12 mM N in Hewitt’s solution, 3 times per week | Gleadow et al. 2009 [169] |
| 480 ppm 680 ppm | 8 months, Field | Higher at elevated CO2 but N.S (measured after 2.5 and 7 months)c | No change | N:P:K:Mg (18:18:18:3) 4.1 kg/ha, once at beginning of experiment | Fernandez et al. 2002 [192] |

5. Conclusions
Acknowledgements
References
- Baulcombe, D.; Crute, I.; Davies, B.; Dunwell, J.; Gale, M.; Jones, J.; Pretty, J.; Sutherland, W.; Toulmin, C.; Green, N.; et al. Reaping the Benefits: Science and the Sustainable Instensification of Global Agriculture; The Royal Society: London, UK, 2009. [Google Scholar]
- Gregory, P.J.; Ingram, J.S.I.; Brklacich, M. Climate Change and Food Security. Phil. Trans. R. Soc. B 2005, 306, 2139–2148. [Google Scholar] [CrossRef]
- Challinor, A.; Wheeler, T.; Garforth, C.; Craufurd, P.; Kassam, A. Assessing the vulnerability of food crop systems in Africa to climate change. Climatic Change 2007, 83, 831–399. [Google Scholar] [CrossRef]
- Cordell, D.; Drangert, J.O.; White, S. The story of Phosphorus: Global food security and food for thought. Global Environ. Change 2009, 19, 292–305. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Beier, C.; Calfapietra, C.; Ceulemans, R.; Durand-Tardif, M.; Farquhar, G.D.; Godbold, D.L.; Hendrey, G.R.; Hickler, T.; Kaduk, J.; et al. Next generation of elevated [CO2] experiments with crops: A critical investment for feeding the future world. Plant Cell Environ. 2008, 31, 1317–1324. [Google Scholar] [CrossRef] [PubMed]
- Funk, C.; Dettinger, M.D.; Michaelsen, J.C.; Verdin, J.P.; Brown, M.; Barlow, M.; Hoell, A. Warming of the Indian Ocean threatens eastern and southern African food security but could be mitigated by agricultural development. Proc. Nat. Acad. Sci. USA 2008, 105, 11081–11086. [Google Scholar] [CrossRef] [PubMed]
- Edgerton, M.D. Increasing crop productivity to meet global needs for feed, food, and fuel. Plant Physiol. 2009, 149, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.G.; Fritz, S.; van Wesenbeeck, C.F.A.; Fuchs, M.; You, L.Z.; Obersteiner, M.; Yang, H. A spatially explicit assessment of current and future hotspots of hunger in Sub-Saharan Africa in the context of global change. Global Planet. Change 2008, 64, 222–235. [Google Scholar] [CrossRef]
- Gleadow, R.M. Food Security in a Warming World. Australasian Science, Jan/Feb 2010; 31–33. [Google Scholar]
- Declaration on world food security. In Declaration on World Food Security; Food and Agriculture Organisation of the United Nations: Rome, Italy, 1996.
- Ziska, L.H.; Bunce, J.A. Predicting the impact of changing CO2 on crop yields: Some thoughts on food. New Phytol. 2007, 175, 607–618. [Google Scholar] [CrossRef] [PubMed]
- FAO. Why Cassava? Available online: http://www.fao.org/ag/AGP/agpc/gcds/index_en.html (accessed on 9 July 2010).
- FAOSTAT. Food and Agricultural Commodities Production. Available online: http://faostat.fao.org/ (accessed on 1 April 2010).
- Pearce, F. Cassava comeback. New Scientist, 21 April 2007; 38–39. [Google Scholar]
- Nassar, N.M.A.; Ortiz, R. Cassava improvement: Challenges and impacts. J. Agric. Sci. 2007, 145, 163–171. [Google Scholar] [CrossRef]
- Gallo, M.; Sayre, R. Removing allergens and reducing toxins from food crops. Curr. Opin. Biotechnol. 2009, 20, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Hillocks, R.J.; Thresh, J.M.; Bellotti, A.C. Cassava: Biology, Production and Utilization; CABI Publishing: Oxon, UK, 2002. [Google Scholar]
- El-Sharkawy, M.A. Cassava biology and physiology. Plant Mol. Biol. 2003, 53, 621–641. [Google Scholar] [CrossRef] [PubMed]
- De Tafur, S.M.; El-Sharkawy, M.A.; Cadavid, L.F. Response of cassava (Manihot esculenta Crantz) to water stress and fertilization. Photosynthetica 1997, 34, 233–239. [Google Scholar] [CrossRef]
- Dahniya, M.T. An overview of cassava in Africa. Afr. Crop Sci. J. 1994, 2, 337–343. [Google Scholar]
- Lebot, V. Tropical Root and Tuber Crops: Cassava, Sweet Potato, Yams and Aroids; CABI: Wallingford, UK, 2009. [Google Scholar]
- de Vries, S.C.; van de Ven, G.W.J.; van Ittersum, M.K.; Giller, K.E. Resource use efficiency and environmental performance of nine major biofuel crops, processed by first-generation conversion techniques. Biomass Bioenerg. 2010, 34, 588–601. [Google Scholar] [CrossRef]
- The World Cassava Economy: Facts, Trends and Outlooks; Food and Agriculture Organization of the United Nations and International Fund for Agricultural Development: Rome, Italy, 2000; pp. 1–59.
- Balagopalan, C. Cassava Utilization in Food, Feed and Industry. In Cassava: Biology, Production and Utilization; Hillocks, R.J., Thresh, J.M., Bellotti, A.C., Eds.; CABI Publishing: Oxon, UK, 2002; pp. 301–318. [Google Scholar]
- McKey, D.; Cavagnaro, T.R.; Cliff, J.; Gleadow, R.M. Chemical ecology in coupled human and natural systems: People, manioc, multitrophic interactions and global change. Chemoecology 2010, 20, 109–133. [Google Scholar] [CrossRef]
- Allem, A.C. The Origins and Taxonomy of Cassava. In Cassava: Biology, Production and Utilization; Hillocks, R.J., Thresh, J.M., Bellotti, A.C., Eds.; CABI Publishing: Oxon, UK, 2002; pp. 1–16. [Google Scholar]
- Jørgensen, K.; Bak, S.; Busk, P.K.; Sørensen, C.; Olsen, C.E.; Puonti-Kaerlas, J.; Møller, B.L. Cassava plants with a depleted cyanogenic glucoside content in leaves and tubers. Distribution of cyanogenic glucosides, their site of synthesis and transport, and blockage of the biosynthesis by RNA interference technology. Plant Physiol. 2005, 139, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Selmar, D.; Lieberei, R.; Biehl, B. Mobilization and utilization of cyanogenic glycosides: The linustatin pathway. Plant Physiol. 1988, 86, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Kongsawadworakul, P.; Viboonjun, U.; Romruensukharom, P.; Chantuma, P.; Ruderman, S.; Chrestin, H. The leaf, inner bark and latex cyanide potential of Hevea brasiliensis: Evidence for involvement of cyanogenic glucosides in rubber yield. Phytochemistry 2009, 70, 730–739. [Google Scholar] [CrossRef] [PubMed]
- Rival, L.; McKey, D. Domestication and diversity in manioc (Manihot esculenta Crantz ssp esculenta, Euphorbiaceae). Curr. Anthropol. 2008, 49, 1116–1125. [Google Scholar] [CrossRef]
- Leotard, G.; Duputie, A.; Kjellberg, F.; Douzery, E.J.P.; Debain, C.; de Granville, J.J.; McKey, D. Phylogeography and the origin of cassava: New insights from the northern rim of the Amazonian basin. Mol. Phylogenet. Evol. 2009, 53, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Duputie, A.; Massol, F.; David, P.; Haxaire, C.; McKey, D. Traditional Amerindian cultivators combine directional and ideotypic selection for sustainable management of cassava genetic diversity. J. Evol. Biol. 2009, 22, 1317–1325. [Google Scholar] [CrossRef] [PubMed]
- Montagnac, J.A.; Davis, C.R.; Tanumihardjo, S.A. Nutritional value of cassava for use as a staple food and recent advances for improvement. Compr. Rev. Food Sci. Food Saf. 2009, 8, 181–194. [Google Scholar] [CrossRef]
- Balagopalan, C.; Padmaja, G.; Nanda, S.K.; Moorthy, S.N. Cassava in Food, Feed and Industry; CRC Press: Boca Raton, FL, USA, 1988. [Google Scholar]
- Nhassico, D.; Muquingue, H.; Cliff, J.; Cumbana, A.; Bradbury, J.H. Rising African cassava production, diseases due to high cyanide intake and control measures. J. Sci. Food Agric. 2008, 88, 2043–2049. [Google Scholar] [CrossRef]
- Hillocks, R.J. Cassava in Africa. In Cassava: Biology, Production and Utilization; Hillocks, R.J., Thresh, J.M., Bellotti, A.C., Eds.; CABI Publishing: Oxon, UK, 2002; pp. 41–54. [Google Scholar]
- Achidi, A.U.; Ajayi, O.A.; Bokanga, M.; Maziya-Dixon, B. The use of cassava leaves as food in Africa. Ecol. Food Nutr. 2005, 44, 423–435. [Google Scholar] [CrossRef]
- van Oirschot, Q.E.A.; O’Brien, G.M.; Dufour, D.; El-Sharkawy, M.A.; Mesa, E. The effect of pre-harvest pruning of cassava upon root deterioration and quality characteristics. J. Sci. Food Agric. 2000, 80, 1866–1873. [Google Scholar] [CrossRef]
- Kolapo, A.L.; Sanni, M.O. A comparative evaluation of the macronutrient and micronutrient profiles of soybean-fortified gari and tapioca. Food Nutr. Bull. 2009, 30, 90–94. [Google Scholar] [PubMed]
- Shewry, P.R. Tuber storage proteins. Ann. Bot. 2003, 91, 755–769. [Google Scholar] [CrossRef] [PubMed]
- Cock, J.H. Cassava: A basic energy source in the tropics. Science 1982, 218, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Bouis, H.E.; Welch, R.M. Biofortification—A sustainable agricultural strategy for reducing micronutrient malnutrition in the global south. Crop Sci. 2010, 50, S20–S32. [Google Scholar] [CrossRef]
- Bayoumi, S.A.L.; Rowan, M.G.; Beeching, J.R.; Blagbrough, I.S. Constituents and secondary metabolite natural products in fresh and deteriorated cassava roots. Phytochemistry 2010, 71, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Cliff, J.; Martensson, J.; Lundqvist, P.; Rosling, H.; Sorbo, B. Association of high cyanide and low sulfur intake in cassava-induced spastic paraparesis. Lancet 1985, 2, 1211–1213. [Google Scholar] [CrossRef] [PubMed]
- Montagnac, J.A.; Davis, C.R.; Tanumihardjo, S.A. Nutritional value of cassava for use as a staple food and recent advances for improvement. Compr. Rev. Food Sci. Food Saf. 2009, 8, 181–194. [Google Scholar] [CrossRef]
- Poulton, J.E. Localization and Catabolism of Cyanogenic Glycosides. In Cyanide Compounds in Biology; Evered, D., Harnett, S., Eds.; John Wiley & Sons: Chichester, UK, 1988; pp. 67–91. [Google Scholar]
- Cliff, J.; Nicala, D. Long term follow-up of konzo patients. Trans. Roy. Soc. Trop. Med. Hyg. 1997, 91, 447–449. [Google Scholar] [CrossRef] [PubMed]
- Mlingi, N.; Poulter, N.H.; Rosling, H. An outbreak of acute intoxications from consumption of insufficiently processed cassava in Tanzania. Nutr. Res. 1992, 12, 677–687. [Google Scholar] [CrossRef]
- Cardoso, A.P.; Mirione, E.; Ernesto, M.; Massaza, F.; Cliff, J.; Haque, M.R.; Bradbury, J.H. Processing of cassava roots to remove cyanogens. J. Food Compos. Anal. 2005, 18, 451–460. [Google Scholar] [CrossRef]
- McKey, D.; Beckerman, S. Chemical Ecology, Plant Evolution and Traditional Manioc Cultivation Systems. In Tropical Forests, People and Food: Biocultural Interactions and Applications to Development; Hladick, A., Linares, O.F., Hladik, C.M., Pagezy, H., Semple, A., Hadley, M., Eds.; UNESCO: Paris, France, 1993; pp. 83–112. [Google Scholar]
- Jørgensen, K.; Moranta, A.V.; Kannangara, R.; Morant, M.; Jensen, N.B.; Olsen, C.E.; Motawia, M.S.; Møller, B.L.; Bak, S. CYP71E7: The oxime-metabolizing P450 in Manihot esculenta Crantz, cassava. Plant Physiol. 2010, in press. [Google Scholar]
- Solomonson, L.P. Cyanide as a Metabolic Inhibitor. In Cyanide in Biology; Vennesland, B., Conn, E.E., Knowles, C.J., Westley, J., Wissing, F., Eds.; Academic Press: London, UK, 1981; pp. 11–28. [Google Scholar]
- Møller, B.L. Functional diversifications of cyanogenic glucosides. Curr. Opin. Plant Biol. 2010, 13, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Conn, E.E. Biosynthesis of Cyanogenic Glycosides. In Cyanide in Biology; Vennesland, V., Conn, E.E., Knowles, C.J., Westly, J., Wissing, F., Eds.; Academic Press: New York, NY, USA, 1981; pp. 1–10. [Google Scholar]
- Bissett, F.H.; Clapp, R.C.; Coburn, R.A.; Ettlinger, M.G.; Long, L. Cyanogenesis in manioc: Concerning lotaustralin. Phytochemistry 1969, 8, 2235–2247. [Google Scholar] [CrossRef]
- Nartey, F. Studies on cassava, Manihot utilissima Pohl—I. Cyanogenesis: The biosynthesis of linamarin and lotaustralin in etiolated seedlings. Phytochemistry 1968, 7, 1307–1312. [Google Scholar] [CrossRef]
- Nambisan, B.; Sundaresan, S. Distribution of linamarin and its metabolizing enzymes in cassava tissues. J. Sci. Food Agric. 1994, 66, 503–507. [Google Scholar] [CrossRef]
- de Bruijn, G.H. The cyanogenic character of cassava (Manihot esculenta). In Chronic Cassava Toxicity, Proceedings of an Interdisciplinary Workshop, London, UK, 29–30 January 1973; Nestel, B., MacIntyre, R., Eds.; International Development Research Centre (Ottawa): Ottawa, Canada, 1973; pp. 43–48. [Google Scholar]
- Gleadow, R.M.; Woodrow, I.E. Constraints on effectiveness of cyanogenic glycosides in herbivore defense. J. Chem. Ecol. 2002, 28, 1301–1313. [Google Scholar] [CrossRef] [PubMed]
- Bellotti, A.C.; Riis, L. Cassava cyanogenic potential and resistance to pests and diseases. Acta Hortic. 1994, 375, 140–150. [Google Scholar]
- Kakes, P. The function of cyanogenesis in cassava. Acta Hortic. 1994, 375, 79–85. [Google Scholar]
- Jones, D.A. Cyanogenesis in Animal-Plant Interactions. In Cyanide Compounds in Biology; Evered, D., Harnett, S., Eds.; John Wiley & Sons: Chichester, UK, 1988; pp. 151–170. [Google Scholar]
- Morant, A.V.; Jorgensen, K.; Jorgensen, B.; Dam, W.; Olsen, C.E.; Moller, B.L.; Bak, S. Lessons learned from metabolic engineering of cyanogenic glucosides. Metabolomics 2007, 3, 383–398. [Google Scholar] [CrossRef]
- Tattersall, D.B.; Bak, S.; Jones, P.R.; Olsen, C.E.; Nielsen, J.K.; Hansen, M.L.; Høj, P.B.; Møller, B.L. Resistance to an herbivore through engineered cyanogenic glucoside synthesis. Science 2001, 293, 1826–1828. [Google Scholar] [CrossRef] [PubMed]
- Poulton, J.E. Cyanogenesis in plants. Plant Physiol. 1990, 94, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Vetter, J. Plant cyanogenic glycosides. Toxicon 2000, 38, 11–36. [Google Scholar] [CrossRef] [PubMed]
- Bokanga, M.; Ekanayake, I.J.; Dixon, A.G.O.; Porto, M.C.M. Genotype-environment interactions for cyanogenic potential in cassava. Acta Hortic. 1994, 375, 131–139. [Google Scholar]
- Koch, B.; Nielsen, V.S.; Halkier, B.A.; Olsen, C.E.; Moller, B.L. The biosynthesis of cyanogenic glucosides in seedlings of cassava (Manihot esculenta Crantz). Arch. Biochem. Biophys. 1992, 292, 141–150. [Google Scholar] [CrossRef] [PubMed]
- McMahon, J.M.; White, W.L.B.; Sayre, R.T. Cyanogenesis in cassava (Manihot esculenta Crantz). J. Exp. Bot. 1995, 46, 731–741. [Google Scholar] [CrossRef]
- Jones, D.A. Why are so many food plants cyanogenic? Phytochemistry 1998, 47, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Bellotti, A.C. Arthropod Pests. In Cassava: Biology, Production and Utilization; Hillocks, R.J., Thresh, J.M., Bellotti, A.C., Eds.; CABI Publishing: Oxon, UK, 2002; pp. 209–236. [Google Scholar]
- Gleadow, R.M.; Woodrow, I.E. Polymorphism in cyanogenic glycoside content and cyanogenic beta-glucosidase activity in natural populations of Eucalyptus cladocalyx. Aust. J. Plant Physiol. 2000, 27, 693–699. [Google Scholar]
- Hughes, M.A. The cyanogenic polymorphism in Trifolium repens L (white clover). Heredity 1991, 66, 105–115. [Google Scholar] [CrossRef]
- Mydans, S. Wasps to Fight Thai Cassava Plague. The New York Times. 18 July 2010. Available online: http://www.nytimes.com/2010/07/19/world/asia/19thai.html?_r=2 (accessed on 21 July 2010).
- Calvert, L.A.; Thresh, J.M. Viruses and Virus Diseases of Cassava. In Cassava: Biology, Production and Utilization; Hillocks, R.J., Thresh, J.M., Bellotti, A.C., Eds.; CABI Publishing: Oxon, UK, 2002; pp. 237–260. [Google Scholar]
- FAONewsroom. Combating Cassava Mosaic. Available online: http://www.fao.org/newsroom/en/field/2007/1000693/index.html (accessed on 22 July 2010).
- Legg, J.P.; Owor, B.; Sseruwagi, P.; Ndunguru, J. Cassava Mosaic Virus Disease in East and Central Africa: Epidemiology and Management of a Regional Pandemic. In Plant Virus Epidemiology; Elsevier Academic Press: San Diego, CA, USA, 2006; Volume 67, pp. 355–418. [Google Scholar]
- Alicai, T.; Omongo, C.A.; Maruthi, M.N.; Hillocks, R.J.; Baguma, Y.; Kawuki, R.; Bua, A.; Otim-Nape, G.W.; Colvin, J. Re-emergence of cassava brown streak disease in Uganda. Plant Dis. 2007, 91, 24–29. [Google Scholar] [CrossRef]
- Mtunda, K.J. Breeding, Evaluation and Selection of Cassava for High Starch Content and Yield in Tanzania; University of KwaZulu-Natal: Pietermaritzburg, South Africa, 2009. [Google Scholar]
- Dixon, A.G.O.; Ssemakula, G. Prospects for cassava breeding in Sub-Saharan Africa in the next decade. J. Food Agric. Environ. 2008, 6, 256–262. [Google Scholar]
- Hillocks, R.J.; Wydra, K. Bacterial, Fungal and Nematode Diseases. In Cassava: Biology, Production and Utilization; Hillocks, R.J., Thresh, J.M., Bellotti, A.C., Eds.; CABI Publishing: Oxon, UK, 2002; pp. 261–280. [Google Scholar]
- Okolie, P.N.; Obasi, B.N. Diurnal variation of cyanogenic glucosides, thiocyanate and rhodanese in cassava. Phytochemistry 1993, 33, 775–778. [Google Scholar] [CrossRef]
- Oluwole, O.S.A. Cyanogenicity of cassava varieties and risk of exposure to cyanide from cassava food in Nigerian communities. J. Sci. Food Agric. 2008, 88, 962–969. [Google Scholar] [CrossRef]
- King, N.L.R.; Bradbury, J.H. Bitterness of cassava: Identification of a new apiosyl glucoside and other compounds that affect its bitter taste. J. Sci. Food Agric. 1995, 68, 223–230. [Google Scholar] [CrossRef]
- Mkumbira, J.; Chiwona-Karltun, L.; Lagercrantz, U.; Mahungu, N.M.; Saka, J.; Mhone, A.; Bokanga, M.; Brimer, L.; Gullberg, U.; Rosling, H. Classification of cassava into ‘bitter ’ and ‘cool’ in Malawi: From farmers’ perception to characterisation by molecular markers. Euphytica 2003, 132, 7–22. [Google Scholar] [CrossRef]
- Alves, A.A.C. Cassava Botany and Physiology. In Cassava: Biology, Production and Utilization; Hillocks, R.J., Thresh, J.M., Bellotti, A.C., Eds.; CABI Publishing: Oxon, UK, 2002; pp. 67–89. [Google Scholar]
- Bourdoux, P.; Seghers, P.; Mafuta, M.; Vanderpas, J.; Vanderpas-Rivera, M.; Delange, F.; Ermans, A.M. Cassava products: HCN content and detoxification processes. In Nutrional Factors Involved in the Goitrogenic Action of Cassava; Delange, F., Iteke, F.B., Ermans, A.M., Eds.; International Development Research Centre (IDRC): Ottawa, Canada, 1982; pp. 51–58. [Google Scholar]
- Montagnac, J.A.; Davis, C.R.; Tanumihardjo, S.A. Processing techniques to reduce toxicity and antinutrients of cassava for use as a staple food. Compr. Rev. Food Sci. Food Saf. 2009, 8, 17–27. [Google Scholar] [CrossRef]
- Kobawila, S.C.; Louembe, D.; Keleke, S.; Hounhouigan, J.; Gamba, C. Reduction of the cyanide content during fermentation of cassava roots and leaves to produce bikedi and ntoba mbodi, two food products from Congo. Afr. J. Biotechnol. 2005, 4, 689–696. [Google Scholar] [CrossRef]
- Chiwona-Karltun, L.; Tylleskar, T.; Mkumbira, J.; Gebre-Medhin, M.; Rosling, H. Low dietary cyanogen exposure from frequent consumption of potentially toxic cassava in Malawi. Int. J. Food Sci. Nutr. 2000, 51, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Wilson, W.M.; Dufour, D.L. Why “bitter” cassava? Productivity of “bitter” and “sweet” cassava in a Tukanoan Indian settlement in the northwest Amazon. Econ. Bot. 2002, 56, 49–57. [Google Scholar] [CrossRef]
- Kayode, G.O. Effects of various planting and harvesting times on the yield, HCN, dry-matter accumulation and starch content of four cassava varieties in a tropical rainforest region. J. Agric. Sci. 1983, 101, 633–636. [Google Scholar] [CrossRef]
- Gershenzon, J. The Cost of Plant Chemical Defense Against Herbivory: A Biochemical Perspective. In Insect-plant Interactions; Bernays, E.A., Ed.; CRC Press: New York, NY, USA, 1989; Volume 5, pp. 105–173. [Google Scholar]
- Goodger, J.Q.D.; Choo, T.Y.S.; Woodrow, I.E. Ontogenetic and temporal trajectories of chemical defence in a cyanogenic eucalypt. Oecologia 2007, 153, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Kakes, P. An analysis of the costs and benefits of the cyanogenic system in Trifolium repens L. Theor. Appl. Genet. 1989, 77, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Jenrich, R.; Trompetter, I.; Bak, S.; Olsen, C.E.; Moller, B.L.; Piotrowski, M. Evolution of heteromeric nitrilase complexes in Poaceae with new functions in nitrile metabolism. Proc. Nat. Acad. Sci. USA 2007, 104, 18848–18853. [Google Scholar] [CrossRef] [PubMed]
- Tylleskar, T.; Banea, M.; Bikangi, N.; Fresco, L.; Persson, L.A.; Rosling, H. Epidemiologic evidence from Zaire for a dietrary etiology of konzo, an upper motor neuron disease. Bull. World Health Organ. 1991, 69, 581–589. [Google Scholar] [PubMed]
- Cliff, J.; Coutinho, J. Acute intoxication from newly-introduced cassava during drought in Mozambique. Trop. Doct. 1995, 25, 193. [Google Scholar] [PubMed]
- Cardoso, A.P.; Ernesto, M.; Nicala, D.; Mirione, E.; Chavane, L.; N’Zwalo, H.; Chikumba, S.; Cliff, J.; Mabota, A.P.; Haque, M.R.; et al. Combination of cassava flour cyanide and urinary thiocyanate measurements of school children in Mozambique. Int. J. Food Sci. Nutr. 2004, 55, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Cliff, J.; Nicala, D.; Saute, F.; Givragy, R.; Azambuja, G.; Taela, A.; Chavane, L.; Gani, A. Ankle clonus and thiocyanate, linamarin, and inorganic sulphate excretion in school children in communities with Konzo, Mozambique. J. Trop. Pediatr. 1999, 45, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Yeoh, H.H.; Chew, M.Y. Protein content and amino-acid composition of cassava leaf. Phytochemistry 1976, 15, 1597–1599. [Google Scholar] [CrossRef]
- Stephenson, K.; Amthor, R.; Mallowa, S.; Nungo, R.; Maziya-Dixon, B.; Gichuki, S.; Mbanaso, A.; Manary, M. Consuming cassava as a staple food places children 2–5 years old at risk for inadequate protein intake, an observational study in Kenya and Nigeria. Nutr. J. 2010, 9. [Google Scholar] [CrossRef]
- Banea-Mayambu, J.P.; Tylleskar, T.; Tylleskar, K.; Gebre-Medhin, M.; Rosling, H. Dietary cyanide from insufficiently processed cassava and growth retardation in children in the Democratic Republic of Congo (formerly Zaire). Ann. Trop. Paediatr. 2000, 20, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Zagrobelny, M.; Bak, S.; Møller, B.L. Cyanogenesis in plants and arthropods. Phytochemistry 2008, 69, 1457–1468. [Google Scholar] [CrossRef] [PubMed]
- Westley, J. Mammalian Cyanide Detoxification with Sulphane Sulphur. In Cyanide Compounds in Biology; Evered, D., Harnett, S., Eds.; John Wiley & Sons: Chichester, UK, 1988; pp. 212–218. [Google Scholar]
- Tylleskar, T.; Banea, M.; Bikangi, N.; Cooke, R.D.; Poulter, N.H.; Rosling, H. Cassava cyanogens and konzo, an upper motoneuron disease found in Africa. Lancet 1992, 339, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Howlett, W.P.; Brubaker, G.R.; Mlingi, N.; Rosling, H. Konzo, an epidemic upper motor neurone disease studied in Tanzania. Brain 1990, 113, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Dufour, D.L. Biocultural approaches in human biology. Amer. J. Hum. Biol. 2006, 18, 1–9. [Google Scholar] [CrossRef]
- Cliff, J. The Burden of Cassava Cyanide-induced Disease: Estimates for the World Health Organization; CCDNN News: Canberrra, Australia, 2010; p. 4. [Google Scholar]
- Cliff, J. Cassava safety in times of war and drought in Mozambique. Acta Hortic. 1994, 375, 372–378. [Google Scholar]
- Ernesto, M.; Cardoso, A.P.; Nicala, D.; Mirione, E.; Massaza, F.; Cliff, J.; Haque, M.R.; Bradbury, J.H. Persistent konzo and cyanogen toxicity from cassava in northern Mozambique. Acta Trop. 2002, 82, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Banea-Mayambu, J.P.; Gitebo, N.D.; Nkuadi, M.A. Bitter cassava consumption and konzo in Kahemba territory, Bandudu Province, DRC. In Proceedings of the Workshop on Toxico-nutritional Neurodegenerations Konzo and Lathyrism, Ghent, Belgium, 21–22 September 2009; Bradbury, J.H., Ed.; CCDNN News: Canberrra, Australia, 2009; Volume 14, pp. 5–6. [Google Scholar]
- Howlett, W.P. Konzo: A new human disease entity. Acta Hortic. 1994, 375, 323–329. [Google Scholar]
- Osuntokun, B.O. Chronic cyanide intoxication of dietary origin and a degenerative neuropathy in Nigerians. Acta Hortic. 1994, 375, 311–321. [Google Scholar]
- Oluwole, O.S.A.; Onabolu, A.O.; Cotgreave, I.A.; Rosling, H.; Persson, A.; Link, H. Incidence of endemic ataxic polyneuropathy and its relation to exposure to cyanide in a Nigerian community. J. Neurol. Neurosurg. Psychiat. 2003, 74, 1417–1422. [Google Scholar] [CrossRef] [PubMed]
- Oluwole, O.S.A.; Onabolu, A.O.; Cotgreave, I.A.; Rosling, H.; Persson, A.; Link, H. Low prevalence of ataxic polyneuropathy in a community with high exposure to cyanide from cassava foods. J. Neurol. 2002, 249, 1034–1040. [Google Scholar] [CrossRef] [PubMed]
- Onabolu, A.O.; Oluwole, O.S.A.; Bokanga, M.; Rosling, H. Ecological variation of intake of cassava food and dietary cyanide load in Nigerian communities. Public Health Nutr. 2001, 4, 871–876. [Google Scholar] [PubMed]
- Halstrøm, F.; Møller, K.D. The content of cyanide in human organs from cases of poisoning with cyanide taken by mouth. With a contribution to the toxicology of cyanides. Acta Pharmacol. Toxicol. 1945, 1, 18–28. [Google Scholar] [CrossRef]
- Bradbury, J.H. Monitoring of Cyanide Content in Cassava Roots and Flour Using Picrate Kits; CCDNN News: Canberrra, Australia, 2010. Available online: http://online.anu.edu.au/BoZo/CCDN/three.html (accessed on 7 October 2010).
- Bradbury, M.G.; Egan, S.V.; Bradbury, J.H. Picrate paper kits for determination of total cyanogens in cassava roots and all forms of cyanogens in cassava products. J. Sci. Food Agric. 1999, 79, 593–601. [Google Scholar] [CrossRef]
- Egan, S.V.; Yeoh, H.H.; Bradbury, J.H. Simple picrate paper kit for determination of the cyanogenic potential of cassava flour. J. Sci. Food Agric. 1998, 76, 39–48. [Google Scholar] [CrossRef]
- Bradbury, J.H.; Denton, I.C. Rapid wetting method to reduce cyanogen content of cassava flour. Food Chem. 2010, 121, 591–594. [Google Scholar] [CrossRef]
- Nambisan, B.; Sundaresan, S. Effect of processing on the cyanoglucoside content of cassava. J. Sci. Food Agric. 1985, 36, 1197–1203. [Google Scholar] [CrossRef]
- Adewoye, S.O.; Fawole, O.O.; Owolabi, O.D.; Omotosho, J.S. Toxicity of cassava wastewater effluents to African catfish: Clarias gariepinus (Burchell, 1822). Ethiopian J. Sci. 2005, 28, 189–194. [Google Scholar]
- Ehiagbonare, J.E.; Adjarhore, R.Y.; Enabulele, S.A. Effect of cassava effluent on Okada natural water. Afr. J. Biotechnol. 2009, 8, 2816–2818. [Google Scholar]
- Dufour, D.L. Effectiveness of cassava detoxification techniques used by indigenous peoples in northwest Amazonia. Interciencia 1989, 14, 86–91. [Google Scholar]
- Dorea, J.G. Fish are central in the diet of Amazonian riparians: Should we worry about their mercury concentrations? Environ. Res. 2003, 92, 232–244. [Google Scholar] [CrossRef] [PubMed]
- Dorea, J.G. Cassava cyanogens and fish mercury are high but safely consumed in the diet of native Amazonians. Ecotoxicol. Environ. Safty 2004, 57, 248–256. [Google Scholar] [CrossRef]
- Aalbersberg, W.G.L.; Limalevu, L. Cyanide content in fresh and processed Fijian cassava (Manihot esculenta) cultivars. Trop. Sci. 1991, 31, 249–256. [Google Scholar]
- Bradbury, J.H. Simple wetting method to reduce cyanogen content of cassava flour. J. Food Compos. Anal. 2006, 19, 388–393. [Google Scholar] [CrossRef]
- Cumbana, A.; Mirione, E.; Cliff, J.; Bradbury, J.H. Reduction of cyanide content of cassava flour in Mozambique by the wetting method. Food Chem. 2007, 101, 894–897. [Google Scholar] [CrossRef]
- Siritunga, D.; Sayre, R. Engineering cyanogen synthesis and turnover in cassava (Manihot esculenta). Plant Mol. Biol. 2004, 56, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Siritunga, D.; Arias-Garzon, D.; White, W.; Sayre, R.T. Over-expression of hydroxynitrile lyase in transgenic cassava roots accelerates cyanogenesis and food detoxification. Plant Biotechnol. J. 2004, 2, 37–43. [Google Scholar] [CrossRef] [PubMed]
- El-Sharkawy, M.A. Drought-tolerant cassava for Africa, Asia and Latin-America. Bioscience 1993, 43, 441–451. [Google Scholar] [CrossRef]
- Leihner, D.E. Agronomy and Cropping Systems. In Cassava: Biology, Production and Utilization; Hillocks, R.J., Thresh, J.M., Bellotti, A.C., Eds.; CABI Publishing: Oxon, UK, 2002; pp. 91–113. [Google Scholar]
- Olasantan, F.O.; Ezumah, H.C.; Lucas, E.O. Effects of intercropping with maize on the micro-environment, growth and yield of cassava. Agr. Ecosyst. Environ. 1996, 57, 149–158. [Google Scholar] [CrossRef]
- Saidou, A.; Kuyper, T.W.; Kossou, D.K.; Tossou, R.; Richards, P. Sustainable soil fertility management in Benin: Learning from farmers. NJAS-Wagen. J. Life Sci. 2004, 52, 349–369. [Google Scholar] [CrossRef]
- Cardoso, I.M.; Kuyper, T.W. Mycorrhizas and tropical soil fertility. Agric. Ecosyst. Environ. 2006, 116, 72–84. [Google Scholar] [CrossRef]
- Fermont, A.M.; van Asten, P.J.A.; Giller, K.E. Increasing land pressure in East Africa: The changing role of cassava and consequences for sustainability of farming systems. Agric. Ecosyst. Environ. 2008, 128, 239–250. [Google Scholar] [CrossRef]
- Onwueme, I.C. Cassava in Asia and the Pacific. In Cassava: Biology, Production and Utilization; Hillocks, R.J., Thresh, J.M., Bellotti, A.C., Eds.; CABI Publishing: Oxon, UK, 2002; pp. 55–65. [Google Scholar]
- Refsgaard, K.; Bjarnholt, N.; Møller, B.L.; Saddik, M.M.; Hansen, H.C.B. Dissipation of cyanogenic glucosides and cyanide in soil amended with white clover (Trifolium repens L.). Soil Biol. Biochem. 2010, 42, 1108–1113. [Google Scholar] [CrossRef]
- Bjarnholt, N.; Laegdsmand, M.; Hansen, H.C.B.; Jacobsen, O.H.; Møller, B.L. Leaching of cyanogenic glucosides and cyanide from white clover green manure. Chemosphere 2008, 72, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.H.; Fu, R.; Liang, C.X.; Dong, D.F.; Luo, X.L. Allelopathic effects of cassava (Manihot esculenta Crantz) on radish (Raphanus sativus L.) and ryegrass (Lolium perenne L.). Allelopathy J. 2010, 25, 155–162. [Google Scholar]
- Fermont, A.M.; Tittonell, P.A.; Baguma, Y.; Ntawuruhunga, P.; Giller, K.E. Towards understanding factors that govern fertilizer response in cassava: Lessons from East Africa. Nutr. Cycl. Agroecosyst. 2010, 86, 133–151. [Google Scholar] [CrossRef] [Green Version]
- Forster, P.; Ramaswamy, V.; Artaxo, P.; Berntsen, T.; Betts, R.; Fahey, D.W.; Haywood, J.; Lean, J.; Lowe, D.C.; Myhre, G.; et al. Changes in Atmospheric Constituents and in Radiative Forcing. In Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Solomon, S., Qin, D., Manning, M., Chen, Z., Marquis, M., Averyt, K.B., Tignor, M., Miller, H.L., Eds.; Cambridge University Press: Cambridge, UK/New York, NY, USA, 2007. [Google Scholar]
- Ainsworth, E.A.; Long, S.P. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol. 2005, 165, 351–371. [Google Scholar] [CrossRef] [PubMed]
- Agricultural Productivity and Climate Change. In Proceedings of the Theo Murphy High Flyers Think Tank, Melbourne, Australia, 22–23 October 2009; Australian Academy of Science: Canberra, Australia, 2010.
- Meehl, G.A.; Stocker, T.F.; Collins, W.D.; Friedlingstein, P.; Gaye, A.T.; Gregory, J.M.; Kitoh, A.; Knutti, R.; Murphy, J.M.; Noda, A.; et al. Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Solomon, S., Qin, D., Manning, M., Chen, Z., Marquis, M., Averyt, K.B., Tignor, M., Miller, H.L., Eds.; Cambridge University Press: Cambridge, UK/ New York, NY, USA, 2007. [Google Scholar]
- Okogbenin, E.; Ekanayake, I.J.; Porto, M.C.M. Genotypic variability in adaptation responses of selected clones of cassava to drought stress in the Sudan savanna zone of Nigeria. J. Agron. Crop Sci. 2003, 189, 376–389. [Google Scholar] [CrossRef]
- Bakayoko, S.; Tschannen, A.; Nindjin, C.; Dao, D.; Girardin, O.; Assa, A. Impact of water stress on fresh tuber yield and dry matter content of cassava (Manihot esculenta Crantz) in Cote d’Ivoire. Afr. J. Agric. Res. 2009, 4, 21–27. [Google Scholar]
- Baker, G.R.; Fukai, S.; Wilson, G.L. The response of cassava to water deficits at various stages of growth in the subtropics. Aust. J. Agric. Res. 1989, 40, 517–528. [Google Scholar] [CrossRef]
- Hahn, S.K.; Reynolds, L.; Egbunike, G.N. Cassava as Livestock Feed in Africa. In Proceedings of the IITA/ILCA/University of Ibadan Workshop on the Potential Utilization of Cassava as Livestock Feed in Africa, Ibadan, Nigeria, 14–18 November 1988; Hahn, S.K., Reynolds, L., Egbunike, G.N., Eds.; International Institute of Tropical Agriculture: Ibadan, Nigeria, 1992; pp. 7–15. [Google Scholar]
- Chauynarong, N.; Elangovan, A.V.; Iji, P.A. The potential of cassava products in diets for poultry. World Poultry Sci. J. 2009, 65, 23–35. [Google Scholar] [CrossRef]
- Oso, A.O.; Oso, O.; Bamgbose, A.M.; Eruvbetine, D. Utilization of unpeeled cassava (Manihot esculenta) root meal in diets of weaner rabbits. Livest. Sci. 2010, 127, 192–196. [Google Scholar] [CrossRef]
- Thang, C.M.; Ledin, I.; Bertilsson, J. Effect of using cassava products to vary the level of energy and protein in the diet on growth and digestibility in cattle. Livest. Sci. 2010, 128, 166–172. [Google Scholar] [CrossRef]
- Alves, A.A.C.; Setter, T.L. Abscisic acid accumulation and osmotic adjustment in cassava under water deficit. Environ. Exp. Bot. 2004, 51, 259–271. [Google Scholar] [CrossRef]
- Santisopasri, V.; Kurotjanawong, K.; Chotineeranat, S.; Piyachomkwan, K.; Sriroth, K.; Oates, C.G. Impact of water stress on yield and quality of cassava starch. Ind. Crop. Prod. 2001, 13, 115–129. [Google Scholar] [CrossRef]
- Nambisan, B. Impact of Environmental Factors on Cyanide Content in Cassava; CCDNN News: Canberrra, Australia, 2003; pp. 2–3. [Google Scholar]
- Cadavid, L.F.; El-Sharkawy, M.A.; Acosta, A.; Sanchez, T. Long-term effects of mulch, fertilization and tillage on cassava grown in sandy soils in northern Colombia. Field Crop. Res. 1998, 57, 45–56. [Google Scholar] [CrossRef]
- Molina, J.L.; El-Sharkawy, M.A. Increasing crop productivity in cassava by fertilizing production of planting material. Field Crop. Res. 1995, 44, 151–157. [Google Scholar] [CrossRef]
- Howeler, R.H. Long-term effect of cassava cultivation on soil productivity. Field Crop. Res. 1991, 26, 1–18. [Google Scholar] [CrossRef]
- Reuter, D.J.; Robinson, J.B.; Dutkiewicz, C. Plant Analysis: An Interpretation Manual, 2nd ed.; CSIRO Publishing: Melbourne, Australia, 1997. [Google Scholar]
- Busk, P.K.; Moller, B.L. Dhurrin synthesis in sorghum is regulated at the transcriptional level and induced by nitrogen fertilization in older plants. Plant Physiol. 2002, 129, 1222–1231. [Google Scholar] [CrossRef] [PubMed]
- Endris, S. Modification of Nutritional Quality of Cassava Through Plant Nutrition; CCDNN News: Canberrra, Australia, 2007; pp. 2–3. [Google Scholar]
- Gleadow, R.M.; Edwards, E.J.; Evans, J.R. Changes in nutritional value of cyanogenic Trifolium repens grown at elevated atmospheric CO2. J. Chem. Ecol. 2009, 35, 476–478. [Google Scholar] [CrossRef] [PubMed]
- Simon, J.; Gleadow, R.M.; Woodrow, I.E. Allocation of nitrogen to chemical defence and plant functional traits is constrained by soil N. Tree Physiol. 2010, 30, 1111–1117. [Google Scholar] [CrossRef]
- Webber, B.L.; Woodrow, I.E. Intra-plant variation in cyanogenesis and the continuum of foliar plant defense traits in the rainforest tree Ryparosa kurrangii (Achariaceae). Tree Physiol. 2008, 28, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.E.; Gleadow, R.M.; Woodrow, I.E. Cyanogenesis in tropical Prunus turneriana: Characterisation, variation and response to low light. Funct. Plant Biol. 2004, 31, 491–503. [Google Scholar] [CrossRef]
- Gleadow, R.M.; Evans, J.R.; McCaffery, S.; Cavagnaro, T.R. Growth and nutritive value of cassava (Manihot esculenta Cranz.) are reduced when grown in elevated CO2. Plant Biol. 2009, 11, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Susan John, K.; Ravindran, C.S.; George, J. Long Term Fertilizer Experiments: Three Decades Experience in Cassava; John, S.K., Ravindran, C.S., George, J., Eds.; Central Tuber Crops Research Institute: Kerala, India, 2005; p. 83. [Google Scholar]
- Cavagnaro, T.R. The role of arbuscular mycorrhzas in improving plant zinc nutrition under low soil zinc concentrations: A review. Plant Soil 2008, 304, 315–325. [Google Scholar] [CrossRef]
- Cavagnaro, T.R.; Jackson, L.E.; Six, J.; Ferris, H.; Goyal, S.; Asami, D.; Scow, K.M. Arbuscular mycorrhizas, microbial communities, nutrient availability, and soil aggregates in organic tomato production. Plant Soil 2006, 282, 209–225. [Google Scholar] [CrossRef]
- Brown, K.H.; Wuehler, S.E. Zinc and Human Health: Results of Recent Trials and Implications for Program Interventions and Research; International Development Research Centre: Ottawa, Canada, 2000. [Google Scholar]
- Azcón-Aguilar, C.; Cantos, M.; Troncoso, A.; Barea, J.M. Beneficial effect of arbuscular mycorrhizas on acclimatization of micropropagated cassava plantlets. Sci. Hortic. 1997, 72, 63–71. [Google Scholar] [CrossRef]
- Khasa, P.; Furlan, V.; Fortin, J.A. Response of some tropical plant-species to endomychorrhizal fungi under field conditions. Trop. Agric. 1992, 69, 279–283. [Google Scholar]
- Sieverding, E. Should VAM inocula contain single or several fungal species? Agric. Ecosyst. Environ. 1990, 29, 391–396. [Google Scholar] [CrossRef]
- Carretero, C.L.; Cantos, M.; Garcia, J.L.; Azcon, R.; Troncoso, A. Arbuscular-mycorrhizal contributes to alleviation of salt damage in cassava clones. J. Plant Nutr. 2008, 31, 959–971. [Google Scholar] [CrossRef]
- Liasu, M.O.; Atayese, M.O.; Osonubi, O. Effect of mycorrhiza and pruning regimes on seasonality of hedgerow tree mulch contribution to alley-cropped cassava in Ibadan, Nigeria. Afr. J. Biotechnol. 2006, 5, 1341–1349. [Google Scholar]
- Sieverding, E.; Howeler, R.H. Influence of species of VA mycorrhizal fungi on cassava yield response to phosphorus fertilization. Plant Soil 1985, 88, 213–221. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: Cambridge, UK, 2008. [Google Scholar]
- Straker, C.J.; Hilditch, A.J.; Rey, M.E.C. Arbuscular mycorrhizal fungi associated with cassava (Manihot esculenta Crantz) in South Africa. S. Afr. J. Bot. 2010, 76, 102–111. [Google Scholar] [CrossRef]
- El-Sharkawy, M.A.; Tafur, S.M.D.; Cadavid, L.F. Potential photosynthesis of cassava as affected by growth conditions. Crop Sci. 1992, 32, 1336–1342. [Google Scholar] [CrossRef]
- Irikura, Y.; Cock, J.H.; Kawano, K. The physiological basis of genotype—Temperature interactions in cassava. Field Crop. Res. 1979, 2, 227–239. [Google Scholar] [CrossRef]
- Stockmal, A.; Oleszek, W. Changes of cyanogenic glucosides in white clover (Trifolium repens L.) during the growing season. J. Agric. Food Chem. 1997, 45, 4333–4336. [Google Scholar] [CrossRef]
- Collinge, D.B.; Hughes, M.A. Developmental and physiological studies on the cyanogenic glucosides of white clover, Trifolium repens L. J. Exp. Bot. 1982, 33, 154–161. [Google Scholar] [CrossRef]
- El-Sharkawy, M.A. International research on cassava photosynthesis, productivity, eco-physiology, and responses to environmental stresses in the tropics. Photosynthetica 2006, 44, 481–512. [Google Scholar] [CrossRef]
- Drake, B.G.; Gonzalez-Meler, M.A.; Long, S.P. More efficient plants: A consequence of rising atmospheric CO2? 1997, 48, 609–639. [Google Scholar]
- Jackson, L.E.; Burger, M.; Cavagnaro, T.R. Roots, nitrogen transformations, and ecosystem services. Annu. Rev. Plant Biol. 2008, 59, 341–363. [Google Scholar] [CrossRef] [PubMed]
- Craigon, J.; Fangmeier, A.; Jones, M.; Donnelly, A.; Bindi, M.; De Temmerman, L.; Persson, K.; Ojanpera, K. Growth and marketable-yield responses of potato to increased CO2 and ozone. Eur. J. Agron. 2002, 17, 273–289. [Google Scholar] [CrossRef]
- Edwards, G.E.; Sheta, E.; Moore, B.D.; Dai, Z.; Franceschi, V.R.; Cheng, S.H.; Lin, C.H.; Ku, M.S.B. Photosynthetic characteristics of cassava (Manihot esculenta Crantz), a C3 species with chlorenchymatous bundle sheath cells. Plant Cell Physiol. 1990, 31, 1199–1206. [Google Scholar]
- Imai, K.; Coleman, D.F.; Yanagisawa, T. Elevated atmospheric partial pressue of carbon dioxide and dry matter production of cassava (Manihot esculenta Crantz). Jpn. J. Crop Sci. 1984, 53, 479–485. [Google Scholar] [CrossRef]
- Fernandez, M.D.; Tezara, W.; Rengifo, E.; Herrera, A. Lack of downregulation of photosynthesis in a tropical root crop, cassava, grown under an elevated CO2 concentration. Funct. Plant Biol. 2002, 29, 805–814. [Google Scholar] [CrossRef]
- Heagle, A.S.; Miller, J.E.; Pursley, W.A. Growth and yield responses of potato to mixtures of carbon dioxide and ozone. J. Environ. Qual. 2003, 32, 1603–1610. [Google Scholar] [CrossRef] [PubMed]
- Lawson, T.; Craigon, J.; Black, C.R.; Colls, J.J.; Tulloch, A.M.; Landon, G. Effects of elevated carbon dioxide and ozone on the growth and yield of potatoes (Solanum tuberosum) grown in open-top chambers. Environ. Poll. 2001, 111, 479–491. [Google Scholar] [CrossRef]
- Miglietta, F.; Magliulo, V.; Bindi, M.; Cerio, L.; Vaccari, F.P.; Loduca, V.; Peressotti, A. Free air CO2 enrichment of potato (Solanum tuberosum L.): Development, growth and yield. Global Change Biol. 1998, 4, 163–172. [Google Scholar] [CrossRef]
- Lobell, D.B.; Burke, M.B.; Tebaldi, C.; Mastrandrea, M.D.; Falcon, W.P.; Naylor, R.L. Prioritizing climate change adaptation needs for food security in 2030. Science 2008, 319, 607–610. [Google Scholar] [CrossRef] [PubMed]
- Idso, S.B. The aerial fertilization effect of CO2 and its implications for global carbon cycling and maximum greenhouse warming. Bull. Amer. Meteorol. Soc. 1991, 72, 962–965. [Google Scholar] [CrossRef]
- Hogy, P.; Fangmeier, A. Atmospheric CO2 enrichment affects potatoes: 2. Tuber quality traits. Eur. J. Agron. 2009, 30, 85–94. [Google Scholar] [CrossRef]
- Taub, D.R.; Miller, B.; Allen, H. Effects of elevated CO2 on the protein concentration of food crops: a meta-analysis. Global Change Biol. 2008, 14, 565–575. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Ineson, P.; Scott, A. Elevated CO2 reduces the nitrogen concentration of plant tissues. Global Change Biol. 1998, 4, 43–54. [Google Scholar] [CrossRef]
- de Graaff, M.A.; van Groenigen, K.J.; Six, J.; Hungate, B.; van Kessel, C. Interactions between plant growth and soil nutrient cycling under elevated CO2: A meta-analysis. Global Change Biol. 2006, 12, 2077–2091. [Google Scholar] [CrossRef]
- Hu, S.; Chapin, F.S.; Firestone, M.K.; Field, C.B.; Chiariello, N.R. Nitrogen limitation of microbial decomposition in a grassland under elevated CO2. Nature 2001, 409, 188–191. [Google Scholar] [CrossRef] [PubMed]
- Reich, P.B.; Hungate, B.A.; Luo, Y.Q. Carbon-nitrogen interactions in terrestrial ecosystems in response to rising atmospheric carbon dioxide. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 611–636. [Google Scholar] [CrossRef]
- Lieffering, M.; Kim, H.Y.; Kobayashic, K.; Okadad, M. The impact of elevated CO2 on the elemental concentrations of field-grown rice grains. Field Crop. Res. 2004, 88, 279–286. [Google Scholar]
- Seneweera, S.P.; Conroy, J.P. Growth, grain yield and quality of rice (Oryza sativa L.) in response to elevated CO2 and phosphorous nutrition. Soil Sci. Plant Nutr. 1997, 43, 1131–1136. [Google Scholar]
- Manderscheid, R.; Bender, J.; Jaiger, H.J.; Weigel, H.J. Effects of season long CO2 enrichment on cereals. II. Nutrient concentrations and grain quality. Agric. Ecosyst. Environ. 1995, 54, 175–185. [Google Scholar] [CrossRef]
- Fangmeier, A.; DeTemmerman, L.; Mortensen, L.; Kemp, K.; Burke, J.; Mitchell, R.; van Oijen, M.; Weigel, H.J. Effects on nutrients and on grain quality in spring wheat crops grown under elevated CO2 concentrations and stress conditions in the European, multiple-site experiment ‘ESPACE-wheat’. Eur. J. Agron. 1999, 10, 215–229. [Google Scholar] [CrossRef]
- Frehner, M.; Luscher, A.; Hebeisen, T.; Zanetti, S.; Schubiger, F.; Scalet, M. Effects of elevated partial pressure of carbon dioxide and season of the year on forage quality and cyanide concentration of Trifolium repens L. from a FACE experiment. Acta Oecol. 1997, 18, 297–304. [Google Scholar] [CrossRef]
- Gleadow, R.M.; Foley, W.J.; Woodrow, I.E. Enhanced CO2 alters the relationship between photosynthesis and defence in cyanogenic Eucalyptus cladocalyx F. Muell. Plant Cell Environ. 1998, 21, 12–22. [Google Scholar] [CrossRef]
- Brouder, S.M.; Volenec, J.J. Impact of climate change on crop nutrient and water use efficiencies. Physiol. Plant. 2008, 133, 705–724. [Google Scholar] [CrossRef] [PubMed]
- Wall, G.W.; Garcia, R.L.; Kimball, B.A.; Hunsaker, D.J.; Pinter, P.J.; Long, S.P.; Osborne, C.P.; Hendrix, D.L.; Wechsung, F.; Wechsung, G.; et al. Interactive effects of elevated carbon dioxide and drought on wheat. Agron. J. 2006, 98, 354–381. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Burns, A.; Gleadow, R.; Cliff, J.; Zacarias, A.; Cavagnaro, T. Cassava: The Drought, War and Famine Crop in a Changing World. Sustainability 2010, 2, 3572-3607. https://doi.org/10.3390/su2113572
Burns A, Gleadow R, Cliff J, Zacarias A, Cavagnaro T. Cassava: The Drought, War and Famine Crop in a Changing World. Sustainability. 2010; 2(11):3572-3607. https://doi.org/10.3390/su2113572
Chicago/Turabian StyleBurns, Anna, Roslyn Gleadow, Julie Cliff, Anabela Zacarias, and Timothy Cavagnaro. 2010. "Cassava: The Drought, War and Famine Crop in a Changing World" Sustainability 2, no. 11: 3572-3607. https://doi.org/10.3390/su2113572
APA StyleBurns, A., Gleadow, R., Cliff, J., Zacarias, A., & Cavagnaro, T. (2010). Cassava: The Drought, War and Famine Crop in a Changing World. Sustainability, 2(11), 3572-3607. https://doi.org/10.3390/su2113572