Sustainability of US Organic Beef and Dairy Production Systems: Soil, Plant and Cattle Interactions
Abstract
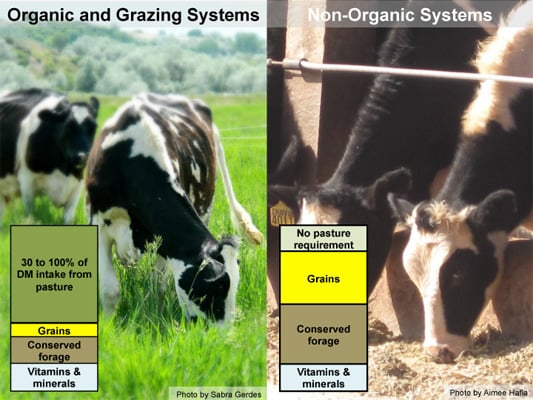
:1. Introduction: Organic vs. Non-Organic Cattle Feeding Practices
1.1. The Pasture Rule
1.2. Dairy Feeding Practices
1.3. Beef Feeding Practices
2. Organic Pasture Management
2.1. Soil Fertility in Organic Agricultural Systems
2.2. Soil Organic Matter
2.3. The Impact of Grazing Management on Forage Plants
3. The Effect of Grazing on Ruminants
- (1).
- Variation in the extent of absorption from the digestive tract.
- (2).
- Variation in the extent of excretion via the feces.
- (3).
- Variation in the extent of excretion via the urine.
- (4).
- Deposition of an excess in the tissues in harmless and/or reserve forms.
- (5).
- Variation in the extent of secretion into milk.
3.1. Forage Plant Nutrition in Pastures
3.2. Vitamins and Minerals in Organic Cattle Systems
3.3. Treatment of Parasites in Organic Cattle Systems
4. Grazing Impacts on the Nutritional Quality of Milk and Meat
4.1. Quality Differences of Dairy Products
4.2. Quality Differences of Beef Products
5. Greenhouse Gas Implications of Organic and Non-Organic Production
5.1. Greenhouse Gas Emissions in Dairy Systems
5.2. Greenhouse Gas Emissions in Beef Systems
6. Economics of Grazing-Based Cattle Systems
6.1. Economics of Dairy Systems
6.2. Economics of Beef Systems
7. Social Implications of Organic vs. Non-organic Cattle Production Systems
- The Principle of Health—Organic agriculture should sustain and enhance the health of soil, plant, animal, human and planet as one and indivisible.
- The Principle of Ecology—Organic agriculture should be based on living ecological systems and cycles, work with them, emulate them and help sustain them.
- The Principle of Fairness—Organic agriculture should build on relationships that ensure fairness with regard to the common environment and life opportunities.
- The Principle of Care—Organic agriculture should be managed in a precautionary and responsible manner to protect the health and well being of current and future generations and the environment.
- Rural Communities: Organic agriculture strives to be sustainable and therefore to protect the whole of the environment [127], including workers, owners, and the rural communities to which they belong. The greater use of pasture in organic dairy and beef production systems due to the pasture rule has the potential to influence farm size, which may be more important than farm type in predicting community participation and involvement. The premium that consumers are willing to pay for organically produced food allows small, labor-intensive businesses to prosper. Additionally, the use of locally available inputs is encouraged in organic agriculture, which in turn increases demand for other local businesses. Sustaining smaller organic dairies and beef producers by enforcing the pasture rule and supporting a premium price for organic beef should result in more farm residents, which should benefit communities [128]. However, the total number of organic producers within dairy or ranching communities is small, making the impact of this factor difficult to quantify.
- Land Stewardship: Protecting and enhancing soil fertility by adding manure, compost or by growing and plowing under cover crops is important in organic agriculture, not only to improve the current land productivity, but also to ensure production for future generations. Where perennial pastures replace cropping systems, there will be a decrease in soil erosion, cultivation and harvesting, and an increase in organic matter and (where nitrogen-fixing legumes are used) an increase in the nitrogen content of the soil, supporting productivity and the cycling of mineral nutrients through forage use and the return of nutrients to the soil as urine and dung.
- Human Health: Organic dairy and beef products are not contaminated with pesticide, hormone or antibiotic residues, and have been shown to have improved nutritional content, including increased beneficial nutrients such as omega-3 fatty acids and conjugated linoleic acids [129]. A strongly positive public perception of the perceived benefits results in increased consumption of organic foods by consumers.
8. Conclusions
References and Notes
- Marston, S.P.; Clark, G.W.; Anderson, G.W.; Kersbergen, R.J.; Lunak, M.; Marcinkowski, D.P.; Murphy, M.R.; Schwab, C.G.; Erickson, P.S. Maximizing profit on New England organic dairy farms: An economic comparison of 4 total mixed rations for organic Holsteins and Jerseys. J. Dairy Sci. 2011, 94, 3184–3201. [Google Scholar] [CrossRef]
- United States Department of Agricultue, National Agricultural Statistics Service (USDA-NASS), 2011 Certified Organic Production Survey; United States Department of Agriculture-National Agricultural Statistics Service: Washington, DC, USA, 2012.
- Mirzaei-Aghsaghali, A.; Maheri-Sis, N. Nutritive value of some agro-industrial by-products for ruminants—A review. World J. Zool. 2008, 3, 40–46. [Google Scholar]
- Rinehart, L.; Baier, A. Pasture for Organic Ruminant Livestock: Understanding and Implementing the National Organic Program (NOP) Pasture Rule; United States Department of Agriculture, National Center for Appropriate Technology, National Sustainable Agriculture Information Service (ATTRA): Washington, DC, USA, 2011. [Google Scholar]
- Sato, K.; Bartlett, P.C.; Erskine, R.J.; Kaneene, J.B. A comparison of production and management between Wisconsin organic and conventional dairy herds. Livest. Prod. Sci. 2005, 93, 105–115. [Google Scholar]
- Gillespie, J.; Nehring, R.; Hallahan, C.; Sandretto, C. Pasture-based dairy systems: Who are the producers and are their operations more profitable than conventional dairies? J. Agr. Resour. Econ. 2009, 34, 412–427. [Google Scholar]
- Hoshide, A.K.; Halloran, J.M.; Kersbergen, R.J.; Griffin, T.S.; DeFauw, S.L.; LaGasse, B.J.; Jain, S. Effects of stored feed cropping systems and farm size on the profitability of Maine organic dairy farm simulations. J. Dairy Sci. 2011, 94, 5710–5723. [Google Scholar] [CrossRef]
- Zwald, A.G.; Ruegg, P.L.; Kaneene, J.B.; Warnick, L.D.; Wells, S.J.; Fossler, C.; Halbert, L.W. Management practices and reported antimicrobial usage on conventional and organic dairy farms. J. Dairy Sci. 2004, 87, 191–201. [Google Scholar] [CrossRef]
- Benbrook, C. Shades of Green: Quantifying the Benefits of Organic Dairy Production; The Organic Center: Washington, DC, USA, 2009. [Google Scholar]
- Matthews, K.H.; Johnson, R.J. Alternative Beef Production Systems: Issues and Implications. LDPM-218-01; United States Department of Agriculture, Economic Research Service, 2013. Available online: http://www.ers.usda.gov/media/1071057/ldpm-218-01.pdf (accessed on 2 July 2013).
- Cornucopia Institute, Position Paper on Organic Beef Finishing and Proposal for Three Tiered Labeling System for Organic Meat from Ruminants; Cornucopia Institute: Cornucopia, WI, USA, 2010.
- Coffey, L.; Baier, A.H. Guide for Organic Livestock Producers; United States Department of Agriculture, National Center for Appropriate Technology, National Sustainable Agriculture Information Service (ATTRA): Butte, MT, UDA, 2012. [Google Scholar]
- Kolok, A.S.; Sellin, M.K. The Environmental Impact of Growth-Promoting Compounds Employed by the United States Beef Cattle Industry: History, Current Knowledge, and Future Directions. In Reviews of Environmental Contamination and Toxicology; Whitacre, D.M., Ed.; Springer: New York, NY, USA, 2008; pp. 1–30. [Google Scholar]
- Rogers, S.; Haines, J. Detecting and Mitigating the Environmental Impact of Fecal Pathogens Originating from Confined Animal Feeding Operations: Review; EPA/600/R-06/021; United States Envrionmental Protection Agency, National Risk Management Research Laboratory, Office of Research and Development: Cincinnati, OH, USA, 2005. [Google Scholar]
- Allen, V.G.; Batello, C.; Berretta, E.J.; Hodgson, J.; Kothmann, M.; Li, X.; McIvor, J.; Milne, J.; Morris, C.; Peeters, A.; et al. An international terminology for grazing lands and grazing animals. Grass Forage Sci. 2011, 66, 2–28. [Google Scholar] [CrossRef]
- Walton, P.D.; Martinez, R.; Bailey, A.W. A comparison of continuous and rotational grazing. J. Range Manage. 1981, 34, 19–21. [Google Scholar] [CrossRef]
- Condron, L.M.; Cameron, K.C.; Di, H.J.; Clough, T.J.; Forbes, E.A.; McLaren, R.G.; Silva, R.G. A comparison of soil and environmental quality under organic and conventional farming systems in New Zealand. New Zeal. J. Agr. Res. 2000, 43, 443–466. [Google Scholar] [CrossRef]
- Reganold, J.P.; Palmer, A.S.; Lockhart, J.C.; Macgregor, A.N. Soil quality and financial performance of biodynamic and conventional farms in New Zealand. Science 1993, 260, 344–349. [Google Scholar] [CrossRef]
- Liebig, M.A.; Doran, J.W. Impact of organic production practices on soil quality indicators. J. Environ. Qual. 1999, 28, 1601–1609. [Google Scholar] [CrossRef]
- Nguyen, M.L.; Haynes, R.J.; Goh, K.M. Nutrient budgets and status in three pairs of conventional and alternative mixed cropping farms in Canterbury, New Zealand. Agr. Ecosyst. Environ. 1995, 52, 149–162. [Google Scholar] [CrossRef]
- Løes, A.-K.; Øgaard, A.F. Long-term changes in extractable soil phosphorus (P) in organic dairy farming systems. Plant Soil 2001, 237, 321–332. [Google Scholar] [CrossRef]
- Cornforth, I.S.; Sinclair, A.G. Model for calculating maintenance phosphate requirements for grazed pastures. New Zeal. J. Exp. Agr. 1982, 10, 53–61. [Google Scholar] [CrossRef]
- Gosling, P.; Shepherd, M. Long-term changes in soil fertility in organic arable farming systems in England, with particular reference to phosphorus and potassium. Agr. Ecosyst. Environ. 2005, 105, 425–432. [Google Scholar] [CrossRef]
- Soder, K.J.; Holden, L.A. Use of anionic salts with grazing prepartum dairy cows. Prof. Anim. Scientist 1999, 15, 278–285. [Google Scholar]
- Bulluck, L.R.; Brosius, M.; Evanylo, G.K.; Ristaino, J.B. Organic and synthetic fertility amendments influence soil microbial, physical and chemical properties on organic and conventional farms. Appl. Soil Ecol. 2002, 19, 147–160. [Google Scholar] [CrossRef]
- National Academy of Sciences, Watershed Management for Potable Water Supply: Assessing the New York City Strategy; National Academy Press: Washington, DC, USA, 2000.
- Whitehead, D.C. Nutrient Elements in Grassland: Soil-Plant-Animal Relationships; CABI: Wallingford, Oxon, UK, 2000. [Google Scholar]
- Peterson, P.R.; Gerrish, J.R. Grazing management affects manure distribution by beef cattle. Available online: http://aes.missouri.edu/fsrc/research/afgc95pp.stm (accessed on 19 May 2013).
- Cederberg, C.; Mattsson, B. Life cycle assessment of milk production–a comparison of conventional and organic farming. J. Clean. Prod. 2000, 8, 49–60. [Google Scholar] [CrossRef]
- Ryschawy, J.; Choisis, N.; Choisis, J.P.; Joannon, A.; Gibon, A. Mixed crop-livestock systems: An economic and environmental-friendly way of farming? Animal 2012, 6, 1722–1730. [Google Scholar] [CrossRef]
- Drinkwater, L.E.; Wagoner, P.; Sarrantonio, M. Legume-based cropping systems have reduced carbon and nitrogen losses. Nature 1998, 396, 262–265. [Google Scholar] [CrossRef]
- Peoples, M.B.; Baldock, J.A. Nitrogen dynamics of pastures: Nitrogen fixation inputs, the impact of legumes on soil nitrogen, fertility, and the contributions of fixed nitrogen to Australian farming systems. Aust. J. Exp. Agr. 2001, 41, 324–346. [Google Scholar]
- Khan, S.A.; Mulvaney, R.L.; Ellsworth, T.R.; Boast, C.W. The myth of nitrogen fertilization for soil carbon sequestration. J. Environ. Qual. 2007, 36, 1821–1832. [Google Scholar] [CrossRef]
- Blanco-Canqui, H.; Schlegel, A.J. Implications of inorganic fertilization of irrigated corn on soil properties: Lessons learned after 50 years. J. Environ. Qual. 2013, 42, 861–871. [Google Scholar] [CrossRef]
- Brady, N. The Nature and Property of Soils, 10th ed.; Pearson: Upper Saddle River, NJ, USA, 1990. [Google Scholar]
- James, D.W.; Hanks, R.J.; Jurinak, J.J. Modern Irrigated Soils; Wiley: New York, NY, USA, 1982. [Google Scholar]
- Soder, K.J.; Stout, W.L. Effect of soil type and fertilization level on mineral concentration of pasture: Potential relationships to ruminant performance and health. J. Anim. Sci. 2003, 81, 1603–1610. [Google Scholar]
- Ball, D.M.; Hoveland, C.S.; Lacefield, G.D. Southern Forages: Modern Concepts for Forage Crop Management, 4th ed.; Graphic Communications Corporation: Lawrenceville, GA, USA, 2007. [Google Scholar]
- Church, D.C. The Ruminant Animal: Digestive Physiology and Nutrition; Waveland Press, Inc.: Prospect Heights, IL, USA, 1988. [Google Scholar]
- Van Soest, P.J. Nutritional Ecology of the Ruminant, 2nd ed.; Cornell University Press: Ithaca, New York, NY, USA, 1994. [Google Scholar]
- Goff, J.P.; Horst, R.L. Effects of the addition of potassium or sodium, but not calcium, to prepartum rations on milk fever in dairy cows. J. Dairy Sci. 1997, 80, 176–186. [Google Scholar] [CrossRef]
- Hardeng, F.; Edge, V.L. Mastitis, ketosis, and milk fever in 31 organic and 93 conventional Norwegian dairy herds. J. Dairy Sci. 2001, 84, 2673–2679. [Google Scholar] [CrossRef]
- Zollitsch, W.; Kristensen, T.; Krutzinna, C.; MacNaeihde, F.; Younie, D. Feeding for Health and Welfare: The Challenge of Formulating Well-balanced Rations in Organic Livestock Production. In Animal Health and Welfare in Organic Agriculture; Vaarst, M., Roderick, S., Lund, V., Lockeretz, W., Eds.; CABI Publishing: Wallingford, Oxon, UK, 2004; pp. 329–356. [Google Scholar]
- Beal, S. Free Choice Smorgasbord Vitamin and Mineral Supplementation for Livestock. NODPA News 2010, 10, 8–9, 30–31. [Google Scholar] [CrossRef]
- Weller, R.F.; Bowling, P.J. The yield and quality of plant species grown in mixed organic swards. In Organic Meat and Milk from Ruminants; Kyriazakis, I., Zervas, G., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2002; pp. 177–183. [Google Scholar]
- Soder, K.J.; Sanderson, M.A.; Stack, J.L.; Muller, L.D. Intake and performance of lactating cows grazing diverse forage mixtures. J. Dairy Sci. 2006, 89, 2158–2167. [Google Scholar] [CrossRef]
- Allen, V.G.; Pond, K.R.; Saker, K.E.; Fontenot, J.P.; Bagley, C.P.; Ivy, R.L.; Evans, R.R.; Schmidt, R.E.; Fike, J.H.; Zhang, X.; et al. Tasco: Influence of a brown seaweed on antioxidants in forages and livestock–A review. J. Anim. Sci. 2001, 79, E21–E31. [Google Scholar]
- Berry, M.H.; Turk, K.L. The value of kelp meal in rations for dairy cattle. J. Dairy Sci. 1944, 27, 861–866. [Google Scholar] [CrossRef]
- Soule, G.M.; Brito, A.F.; Miranda, A.; Chase, L.; Whitehouse, N.L.; Fletcher, E.S.; Antaya, N.T. Effects of kelp meal on performance and structural growth of conventional and organic dairy calves. J. Dairy Sci. 2012, 95 (Suppl. 2), 109. [Google Scholar] [CrossRef]
- Younie, D.; Thamsborg, S.M.; Ambrosini, F.; Roderick, S. Grassland Management and Parasite Control. In Animal Health and Welfare in Organic Agriculture; Vaarst, M., Roderick, S., Lund, V., Lockeretz, W., Eds.; CABI Publishing: Wallingford, Oxon, UK, 2004; pp. 308–328. [Google Scholar]
- Hoste, H.; Jackson, F.; Athanasiadou, S.; Thamsborg, S.M.; Hoskin, S.O. The effects of tannin-rich plants on parasitic nematodes in ruminants. Trends Parasitol. 2006, 22, 253–261. [Google Scholar] [CrossRef]
- Bernard, G.; Worku, M.; Ahmedna, M. The effects of diatomaceous earth on parasite-infected goats. Bull. Georgian Natl. Acad. Sci. 2009, 3, 129–135. [Google Scholar]
- Burke, J.M.; Wells, A.; Casey, P.; Miller, J.E. Garlic and papaya lack control over gastrointestinal nematodes in goats and lambs. Vet. Parasitol. 2009, 159, 171–174. [Google Scholar] [CrossRef]
- Kunkel, J.R.; Murphy, W.M.; Rogers, D.; Dugdale, D.T. Seasonal control of gastrointestinal parasites among dairy heifers. Bovine Pr. 1983, 18, 54–57. [Google Scholar]
- Myers, G.H. Strategies to control internal parasites in cattle and swine. J. Anim. Sci. 1988, 66, 1555–1564. [Google Scholar]
- Worthington, V. Nutritional quality of organic versus conventional fruits, vegetables, and grains. J. Altern. Complem. Med. 2001, 7, 161–173. [Google Scholar] [CrossRef]
- Heaton, S. Assessing organic food quality: Is it better for you? In UK Organic Research 2002, Proceedings of the COR Conference, 26–28 March 2002; Powell, J., Ed.; University of Wales: Aberystwyth, UK, 2002; pp. 55–60. [Google Scholar]
- Bourn, D.; Prescott, J. A comparison of the nutritional value, sensory qualities, and food safety of organically and conventionally produced foods. CRC Cr. Rev. Food Sci. 2002, 42, 1–34. [Google Scholar] [CrossRef]
- Magkos, F.; Arvaniti, F.; Zampelas, A. Organic food: Nutritious food or food for thought? A review of the evidence. Int. J. Food Sci. Nutr. 2003, 54, 357–371. [Google Scholar] [CrossRef]
- Givens, D.I. Milk and meat in our diet: Good or bad for health? Animal 2010, 4, 1941–1952. [Google Scholar] [CrossRef]
- Shingfield, K.J.; Bonnet, M.; Scollan, N.D. Recent developments in altering the fatty acid composition of ruminant-derived foods. Animal 2013, 7, 132–162. [Google Scholar] [CrossRef]
- Lock, A.L.; Garnsworthy, P.C. Seasonal variation in milk conjugated linoleic acid and delta9-desaturase activity in dairy cows. Livest. Prod. Sci. 2003, 79, 47–59. [Google Scholar] [CrossRef]
- Palmquist, D.L.; Beaulieu, D.; Barbano, D.M. Feed and animal factors influencing milk fat and composition. J. Dairy Sci. 1993, 76, 1753–1771. [Google Scholar] [CrossRef]
- Chilliard, Y.; Ferlay, A. Dietary lipids and forages interactions on cow and goat milk fatty acid composition and sensory properties. Reprod. Nutr. Dev. 2004, 44, 467–492. [Google Scholar] [CrossRef]
- Chilliard, Y.; Glasser, F.; Ferlay, A.; Bernard, L.; Rouel, J.; Doreau, M. Diet, rumen biohydrogenation and nutritional quality of cow and goat milk fat. Eur. J. Lipid Sci. Tech. 2007, 109, 828–855. [Google Scholar] [CrossRef]
- Elgersma, A.; Tamminga, S.; Dijkstra, J. Lipids in herbage. In Fresh Herbage for Dairy Cattle; Elgersma, A., Dijikstra, J., Tamminga, S., Eds.; Springer: Wageningen, The Netherlands, 2006; pp. 175–194. [Google Scholar]
- Ferlay, A.; Agabriel, C.; Sibra, C.; Journal, C.; Martin, B.; Chilliard, Y. Tanker milk variability in fatty acids according to farm feeding and husbandry practices in a French semi-mountain area. Dairy Sci. Technol. 2008, 88, 193–215. [Google Scholar] [CrossRef]
- Chilliard, Y.; Ferlay, A.; Doreau, M. Effect of different types of forages, animal fat or marine oils in cow’s diet on milk fat secretion and composition, especially conjugated linoleic acid (CLA) and polyunsaturated fatty acids. Livest. Prod. Sci. 2001, 70, 31–48. [Google Scholar] [CrossRef]
- Dewhurst, R.J.; Shingfield, K.J.; Lee, M.R.F.; Scollan, N.D. Increasing the concentrations of beneficial polyunsaturated fatty acids in milk produced by dairy cows in high-forage systems. Anim. Feed Sci. Tech. 2006, 131, 168–206. [Google Scholar] [CrossRef]
- Elgersma, A.; Tamminga, S.; Ellen, G. Modifying milk composition through forage. Anim. Feed Sci. Tech. 2006, 131, 207–225. [Google Scholar] [CrossRef]
- Palupi, E.; Jayanegara, A.; Ploeger, A.; Kahl, J. Comparison of nutritional quality between conventional and organic dairy products: A meta-analysis. J. Sci. Food Agric. 2012, 92, 2774–2781. [Google Scholar] [CrossRef]
- Ip, C.; Banni, S.; Angioni, E.; Carta, G.; McGinley, J.; Thompson, H.J.; Barbano, D.; Bauman, D. Conjugated linoleic acid–enriched butter fat alters mammary gland morphogenesis and reduces cancer risk in rats. J. Nutr. 1999, 129, 2135–2142. [Google Scholar]
- Butler, G.; Nielsen, J.H.; Slots, T.; Seal, C.; Eyre, M.D.; Sanderson, R.; Leifert, C. Fatty acid and fat-soluble antioxidant concentrations in milk from high- and low-input conventional and organic systems: Seasonal variation. J. Sci. Food Agric. 2008, 88, 1431–1441. [Google Scholar] [CrossRef]
- Butler, G.; Stergiadis, S.; Seal, C.; Eyre, M.; Leifert, C. Fat composition of organic and conventional retail milk in northeast England. J. Dairy Sci. 2011, 94, 24–36. [Google Scholar] [CrossRef]
- Wyss, U.; Miinger, A.; Collomb, M. Variation of fatty acid content in grass and milk during the grazing season. Grassland Sci. Eur. 2010, 15, 422–424. [Google Scholar]
- Duckett, S.K.; Neel, J.P.; Sonon, R.N.; Fontenot, J.P.; Clapham, W.M.; Scaglia, G. Effects of winter stocker growth rate and finishing system on: II. Ninth-tenth-eleventh-rib composition, muscle color, and palatability. J. Anim. Sci. 2007, 85, 2691–2698. [Google Scholar] [CrossRef]
- Duckett, S.K.; Neel, J.P.S.; Lewis, R.M.; Fontenot, J.P.; Clapham, W.M. Effects of forage species or concentrate finishing on animal performance, carcass and meat quality. J. Anim. Sci. 2013, 91, 1454–1467. [Google Scholar] [CrossRef]
- Neel, J.P.; Fontenot, J.P.; Clapham, W.M.; Duckett, S.K.; Felton, E.E.; Scaglia, G.; Bryan, W.B. Effects of winter stocker growth rate and finishing system on: I. Animal performance and carcass characteristics. J. Anim. Sci. 2007, 85, 2012–2018. [Google Scholar] [CrossRef]
- Scaglia, G.; Fontenot, J.P.; Swecker, W.S.; Corl, B.A.; Duckett, S.K.; Boland, H.T.; Smith, R.; Abaye, A.O. Performance, carcass, and meat characteristics of beef steers finished on 2 different forages or on a high-concentrate diet. Prof. Anim. Sci. 2012, 28, 194–203. [Google Scholar]
- Bennett, L.L.; Hammond, A.C.; Williams, M.J.; Kunkle, W.E.; Johnson, D.D.; Preston, R.L.; Miller, M.F. Performance, carcass yield, and carcass quality characteristics of steers finished on rhizoma peanut (Arachis glabrata)-tropical grass pasture or concentrate. J. Anim. Sci. 1995, 73, 1881–1887. [Google Scholar]
- Hedrick, H.B.; Paterson, J.A.; Matches, A.G.; Thomas, J.D.; Morrow, R.E.; Stringer, W.C.; Lipsey, R.J. Carcass and palatability characteristics of beef produced on pasture, corn silage and corn grain. J. Anim. Sci. 1983, 57, 791–801. [Google Scholar]
- Muir, P.D.; Deaker, J.M.; Bown, M.D. Effects of forage- and grain-based feeding systems on beef quality: A review. New Zeal. J Agr. Sci. 1998, 41, 623–635. [Google Scholar] [CrossRef]
- Scollan, N.; Hocquette, J.F.; Nuernberg, K.; Dannenberger, D.; Richardson, I.; Moloney, A. Innovations in beef production systems that enhance the nutritional and health value of beef lipids and their relationship with meat quality. Meat Sci. 2006, 74, 17–33. [Google Scholar] [CrossRef]
- Duckett, S.K.; Neel, J.P.; Fontenot, J.P.; Clapham, W.M. Effects of winter stocker growth rate and finishing system on: III. Tissue proximate, fatty acid, vitamin, and cholesterol content. J. Anim. Sci. 2009, 87, 2961–2970. [Google Scholar] [CrossRef]
- Noci, F.; Monahan, F.J.; French, P.; Moloney, A.P. The fatty acid composition of muscle fat and subcutaneous adipose tissue of pasture-fed beef heifers: Influence of the duration of grazing. J. Anim. Sci. 2005, 83, 1167–1178. [Google Scholar]
- Gilmore, L.A.; Walzem, R.L.; Crouse, S.F.; Smith, D.R.; Adams, T.H.; Vaidyanathan, V.; Cao, X.; Smith, S.B. Consumption of high-oleic acid ground beef increases HDL-cholesterol concentration but both high- and low-oleic acid ground beef decrease HDL particle diameter in normocholesterolemic men. J. Nutr. 2011, 141, 1188–1194. [Google Scholar] [CrossRef]
- Kris-Etherton, P.M.; Pearson, T.A.; Wan, Y.; Hargrove, R.L.; Moriarty, K.; Fishell, V.; Etherton, T.D. High–monounsaturated fatty acid diets lower both plasma cholesterol and triacylglycerol concentrations. Am. J. Clin. Nutr. 1999, 70, 1009–1015. [Google Scholar]
- Chait, A.; Brazg, R.L.; Tribble, D.L.; Krauss, R.M. Susceptibility of small, dense, low-density lipoproteins to oxidative modification in subjects with the atherogenic lipoprotein phenotype, pattern B. Am. J. Med. 1993, 94, 350–356. [Google Scholar] [CrossRef]
- Kouba, M. Quality of organic animal products. Livest. Prod. Sci. 2003, 80, 33–40. [Google Scholar] [CrossRef]
- Caroprese, M.; Marzano, A.; Marino, R.; Gliatta, G.; Muscio, A.; Sevi, A. Flaxseed supplementation improves fatty acid profile of cow milk. J. Dairy Sci. 2010, 93, 2580–2588. [Google Scholar] [CrossRef]
- Kronberg, S.L.; Scholljegerdes, E.J.; Leper, A.N.; Berg, E.P. The effect of flaxseed supplementation on growth, carcass characteristics, fatty acid profile, retail shelf life, and sensory characteristics of beef from steers finished on grasslands of the northern Great Plains. J. Anim. Sci. 2011, 89, 2892–2903. [Google Scholar] [CrossRef]
- Donovan, D.C.; Schingoethe, D.J.; Baer, R.J.; Ryali, J.; Hippen, A.R.; Franklin, S.T. Influence of dietary fish oil on conjugated linoleic acid and other fatty adids in milk fat from lactating dairy cows. J. Dairy Sci. 2000, 83, 2620–2628. [Google Scholar] [CrossRef]
- Olesen, J.E.; Schelde, K.; Weiske, A.; Weisbjerg, M.R.; Asman, W.A.H.; Djurhuus, J. Modelling greenhouse gas emissions from European conventional and organic dairy farms. Agr. Ecosyst. Environ. 2006, 112, 207–220. [Google Scholar] [CrossRef]
- Thomassen, M.A.; van Calker, K.J.; Smits, M.C.J.; Iepema, G.L.; de Boer, I.J.M. Life cycle assessment of conventional and organic milk production in the Netherlands. Agr. Syst. 2008, 96, 95–107. [Google Scholar] [CrossRef]
- Lynch, D.; MacRae, R.; Martin, R. The carbon and global warming potential impacts of organic farming: Does it have a significant role in an energy constrained world? Sustainability 2011, 3, 322–362. [Google Scholar] [CrossRef]
- Capper, J.L.; Castaneda-Gutierrez, E.; Cady, R.A.; Bauman, D.E. The environmental impact of recombinant bovine somatotropin (rbST) use in dairy production. Proc. Natl. Acad. Sci. USA 2008, 105, 9668–9673. [Google Scholar]
- FAO, Greenhouse Gas Emissions from the Dairy Sector: A Life Cycle Assessment; Food and Agiculture Organization of the United Nations, Animal Production and Health Division: Rome, Italy, 2010.
- United States Department of Agriculture, Economic Research Service (USDA-ERS). Farm Milk Production. Available online: http://www.ers.usda.gov/topics/animal-products/dairy/background.aspx (accessed on 24 March 2013).
- Schultz, M. Organic Dairy Profile; Agricultural Marketing Resource Center: Washington, DC, USA, 2013. [Google Scholar]
- Peters, G.M.; Rowley, H.V.; Wiedemann, S.; Tucker, R.; Short, M.D.; Schulz, M. Red meat production in Australia: Life cycle assessment and comparison with overseas studies. Envir. Sci. Tech. 2010, 44, 1327–1332. [Google Scholar]
- Crosson, P.; Shalloo, L.; O’Brien, D.; Lanigan, G.J.; Foley, P.A.; Boland, T.M.; Kenny, D.A. A review of whole farm systems models of greenhouse gas emissions from beef and dairy cattle production systems. Anim. Feed Sci. Tech. 2011, 166–167, 29–45. [Google Scholar] [CrossRef]
- Kim, S.; Dale, B.E. Cumulative energy and global warming impact from the production of biomass for biobased products. J. Ind. Ecol. 2004, 7, 147–162. [Google Scholar]
- CAST, Carbon Sequestration and Greenhouse Gas Fluxes in Agriculture: Challenges and Opportunities; Task Force Report No. 142; Council for Agricultural Science and Technology: Ames, IA, USA, 2011.
- Paustian, K.; Antle, J.M.; Sheehan, J.; Paul, E.A. Agriculture’s Role in Greenhouse Gas Mitigation; Pew Center on Global Climate Change: Arlington, VA, USA, 2006. [Google Scholar]
- Follett, R.F.; Reed, D.A. Soil carbon sequestration in grazing lands: Societal benefits and policy implications. Rangel Ecol. Manag. 2010, 63, 4–15. [Google Scholar] [CrossRef]
- Sayre, N.F.; Carlisle, L.; Huntsinger, L.; Fisher, G.; Shattuck, A. The role of rangelands in diversified farming systems: Innovations, obstacles, and opportunities in the USA. Ecol. Soc. 2012, 17, 43. [Google Scholar]
- Greene, C. Data Track the Expansion of International and U.S. Organic Farming. Amber Waves 2007, 5, 36–37. [Google Scholar]
- White, S.L.; Benson, G.A.; Washburn, S.P.; Green, J.T. Milk production and economic measures in confinement or pasture systems using seasonally calved Holstein and Jersey cows. J. Dairy Sci. 2002, 85, 95–104. [Google Scholar] [CrossRef]
- Dartt, B.A.; Lloyd, J.W.; Radke, B.R.; Black, J.R.; Baneene, J.B. A comparison of profitability and economic efficiencies between management-intensive grazing and conventionally managed dairies in Michigan. J. Dairy Sci. 1999, 82, 2412–2420. [Google Scholar] [CrossRef]
- Hanson, G.D.; Cunningham, L.C.; Morehart, M.J.; Parsons, R.L. Profitability of moderate intensive grazing of dairy cows in the Northeast. J. Dairy Sci. 1998, 81, 821–829. [Google Scholar] [CrossRef]
- Hanson, J.C.; Johnson, D.M.; Lichtenberg, E.; Minegishi, K. Competitiveness of management-intensive grazing dairies in the mid-Atlantic region from 1995 to 2009. J. Dairy Sci. 2013, 96, 1894–1904. [Google Scholar] [CrossRef]
- Parker, W.J.; Muller, L.D.; Buckmaster, D.R. Management and economic implications of intensive grazing on dairy farms in the northeastern states. J. Dairy Sci. 1992, 75, 2587–2597. [Google Scholar] [CrossRef]
- McBride, W.D.; Greene, C. Characteristics, Costs, and Issues for Organic Dairy Farming; Research Report Number 82; United States Department of Agriculture, Economic: Washington, DC, USA, 2009. [Google Scholar]
- Berentsen, P.B.; Kovacs, K.; van Asseldonk, M.A. Comparing risk in conventional and organic dairy farming in the Netherlands: an empirical analysis. J. Dairy Sci. 2012, 95, 3803–3811. [Google Scholar] [CrossRef]
- Lund, V. Natural living—a precondition for animal welfare in organic farming. Livest. Sci. 2006, 100, 71–83. [Google Scholar] [CrossRef]
- Bocquier, F.; Gonzalez-Garcia, E. Sustainability of ruminant agriculture in the new context: Feeding strategies and features of animal adaptability into the necessary holistic approach. Animal 2010, 4, 1258–1273. [Google Scholar] [CrossRef]
- Hodgson, J. Grazing Management: Science into Practice; Longman Scientific and Technical: Harlow, Essex, UK, 1990. [Google Scholar]
- Dillon, P.; Hennessy, T.; Shalloo, L.; Thorne, F.; Horan, B. Future outlook for the Irish dairy industry: A study of international competitiveness, influence of international trade reform and requirement for change. Int. J. Dairy Technol. 2008, 61, 16–29. [Google Scholar] [CrossRef]
- Peyraud, J.L.; Delagarde, R. Managing variations in dairy cow nutrient supply under grazing. Animal 2013, 7, 57–67. [Google Scholar] [CrossRef]
- Butler, L.J. The economics of organic milk production in California: A comparison with conventional costs. Am. J. Alternative Agr. 2002, 17, 83–91. [Google Scholar] [CrossRef]
- Rotz, C.A.; Kamphuis, G.H.; Karsten, H.D.; Weaver, R.D. Organic dairy production systems in Pennsylvania: A case study evaluation. J. Dairy Sci. 2007, 90, 3961–3979. [Google Scholar] [CrossRef]
- Rouquette, F.M.; Redmon, L.A.; Aiken, G.E.; Hill, G.M.; Sollenberger, L.E.; Andrae, J. ASAS Centennial Paper: Future needs of research and extension in forage utilization. J. Anim. Sci. 2009, 87, 438–446. [Google Scholar]
- Younie, D. An organic approach to meat production. Food Sci. Technol. Today 1992, 6, 163–166. [Google Scholar]
- Fernández, M.I.; Woodward, B.W. Comparison of conventional and organic beef production systems I. Feedlot performance and production costs. Livest. Prod. Sci. 1999, 61, 213–223. [Google Scholar] [CrossRef]
- Lewis, J.M.; Klopfenstein, T.J.; Pfeiffer, G.A.; Stock, R.A. An economic evaluation of the differences between intensive and extensive beef production systems. J. Anim. Sci. 1990, 68, 2506–2516. [Google Scholar]
- Reichl, R. Michael Pollan and Ruth Reichl hash out the food revolution. Smithsonian 2013, 44, 74–83. [Google Scholar]
- Papendick, R.I.; Elliot, L.F.; Dalgren, R.B. Environmental consequences of modern production agriculture: How can alternative agriculture address these concerns? Am. J. Alternative Agr. 1986, 1, 3–10. [Google Scholar] [CrossRef]
- Lasley, P.; Hoiberg, E.; Bultena, G. Is sustainable agriculture an elixir for rural communities? Am. J. Alternative Agr. 1993, 8, 133–139. [Google Scholar] [CrossRef]
- Realini, C.E.; Duckett, S.K.; Brito, G.W.; Dalla Rizza, M.; de Mattos, D. Effect of pasture vs. concentrate feeding with or without antioxidants on carcass characteristics, fatty acid composition, and quality of Uruguayan beef. Meat Sci. 2004, 66, 567–577. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hafla, A.N.; MacAdam, J.W.; Soder, K.J. Sustainability of US Organic Beef and Dairy Production Systems: Soil, Plant and Cattle Interactions. Sustainability 2013, 5, 3009-3034. https://doi.org/10.3390/su5073009
Hafla AN, MacAdam JW, Soder KJ. Sustainability of US Organic Beef and Dairy Production Systems: Soil, Plant and Cattle Interactions. Sustainability. 2013; 5(7):3009-3034. https://doi.org/10.3390/su5073009
Chicago/Turabian StyleHafla, Aimee N., Jennifer W. MacAdam, and Kathy J. Soder. 2013. "Sustainability of US Organic Beef and Dairy Production Systems: Soil, Plant and Cattle Interactions" Sustainability 5, no. 7: 3009-3034. https://doi.org/10.3390/su5073009
APA StyleHafla, A. N., MacAdam, J. W., & Soder, K. J. (2013). Sustainability of US Organic Beef and Dairy Production Systems: Soil, Plant and Cattle Interactions. Sustainability, 5(7), 3009-3034. https://doi.org/10.3390/su5073009