Biochars as Potential Adsorbers of CH4, CO2 and H2S
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biochar Production and Characterization
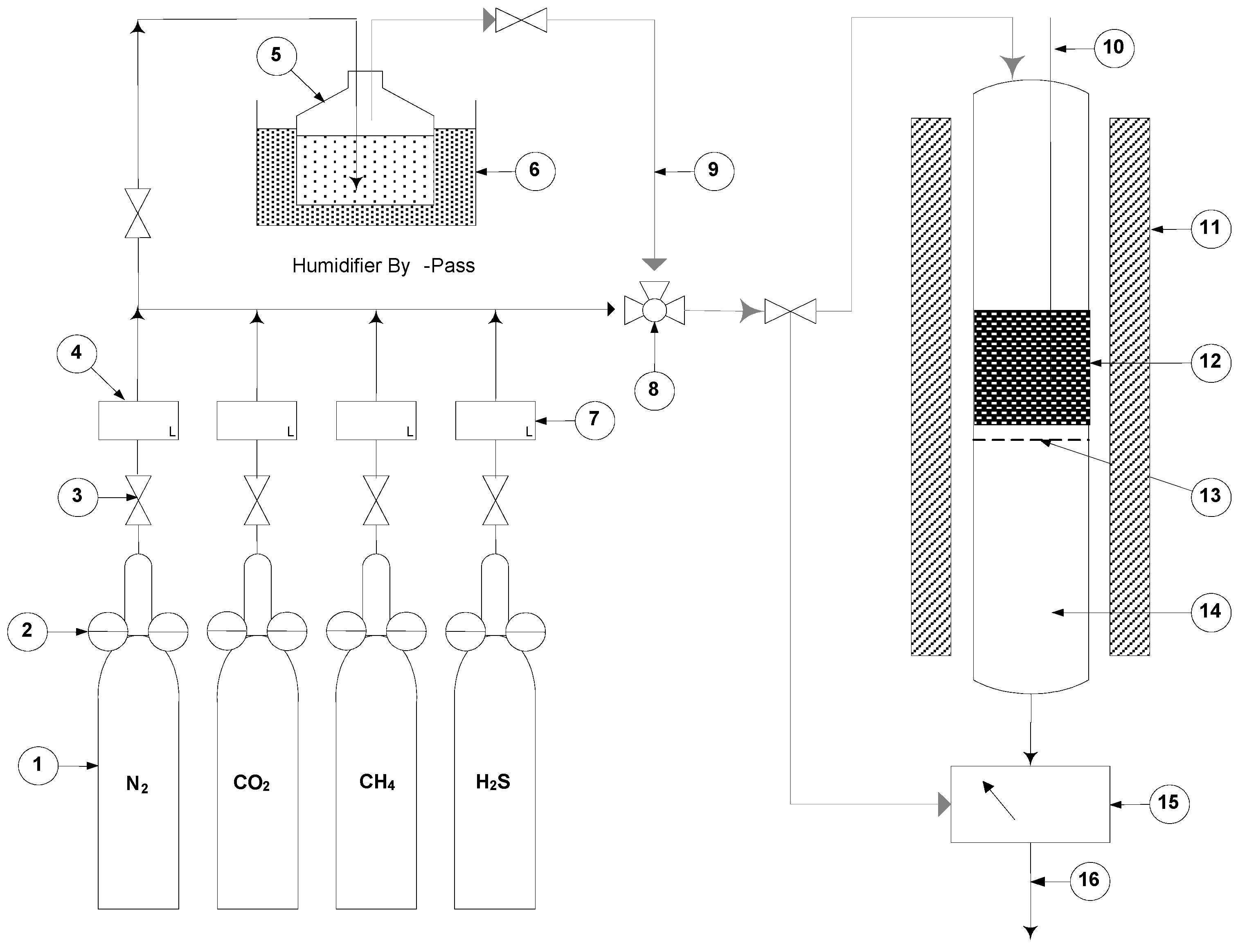
2.2. Biogas Adsorption
3. Results and Discussion
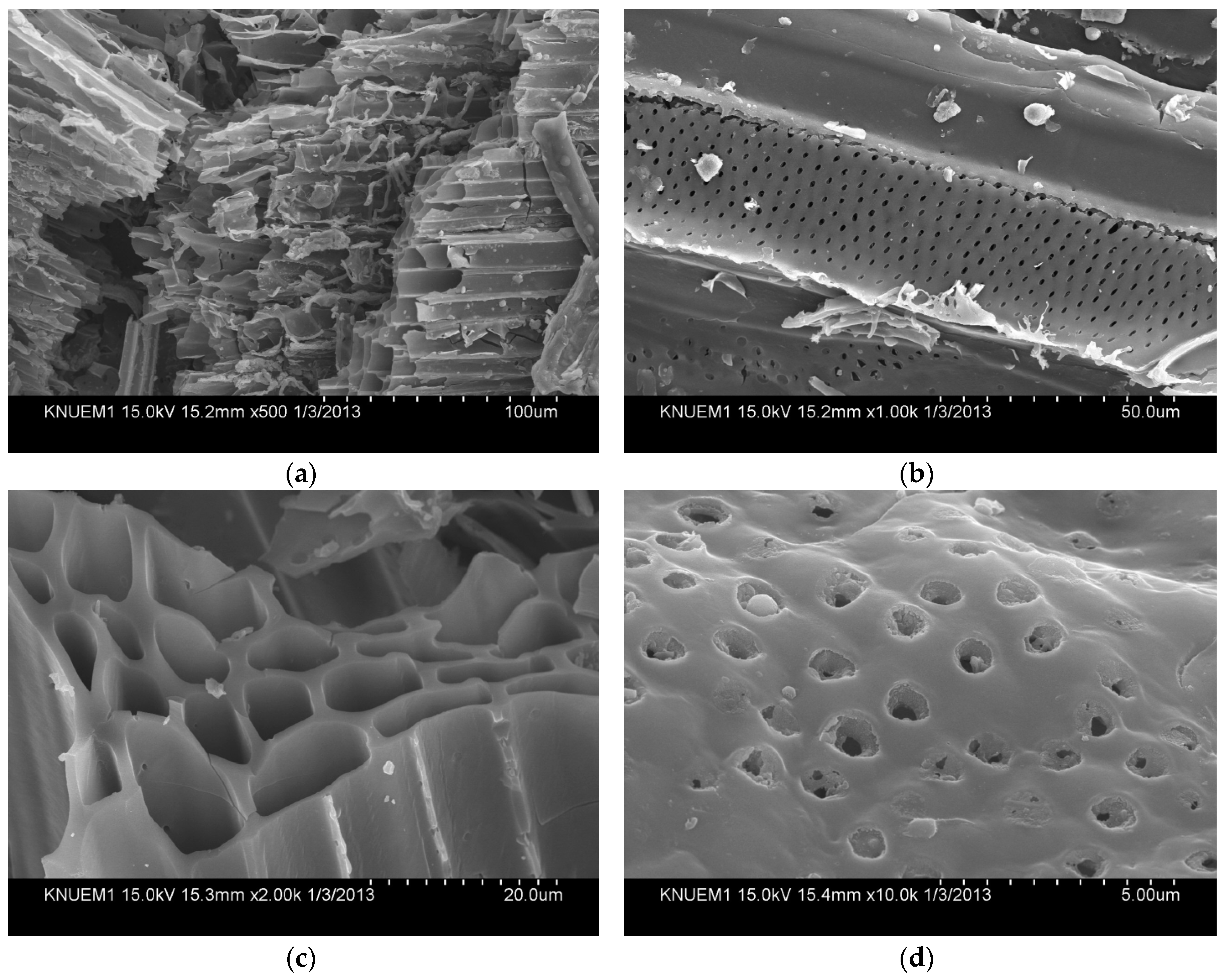
3.1. Characterization of Biochars
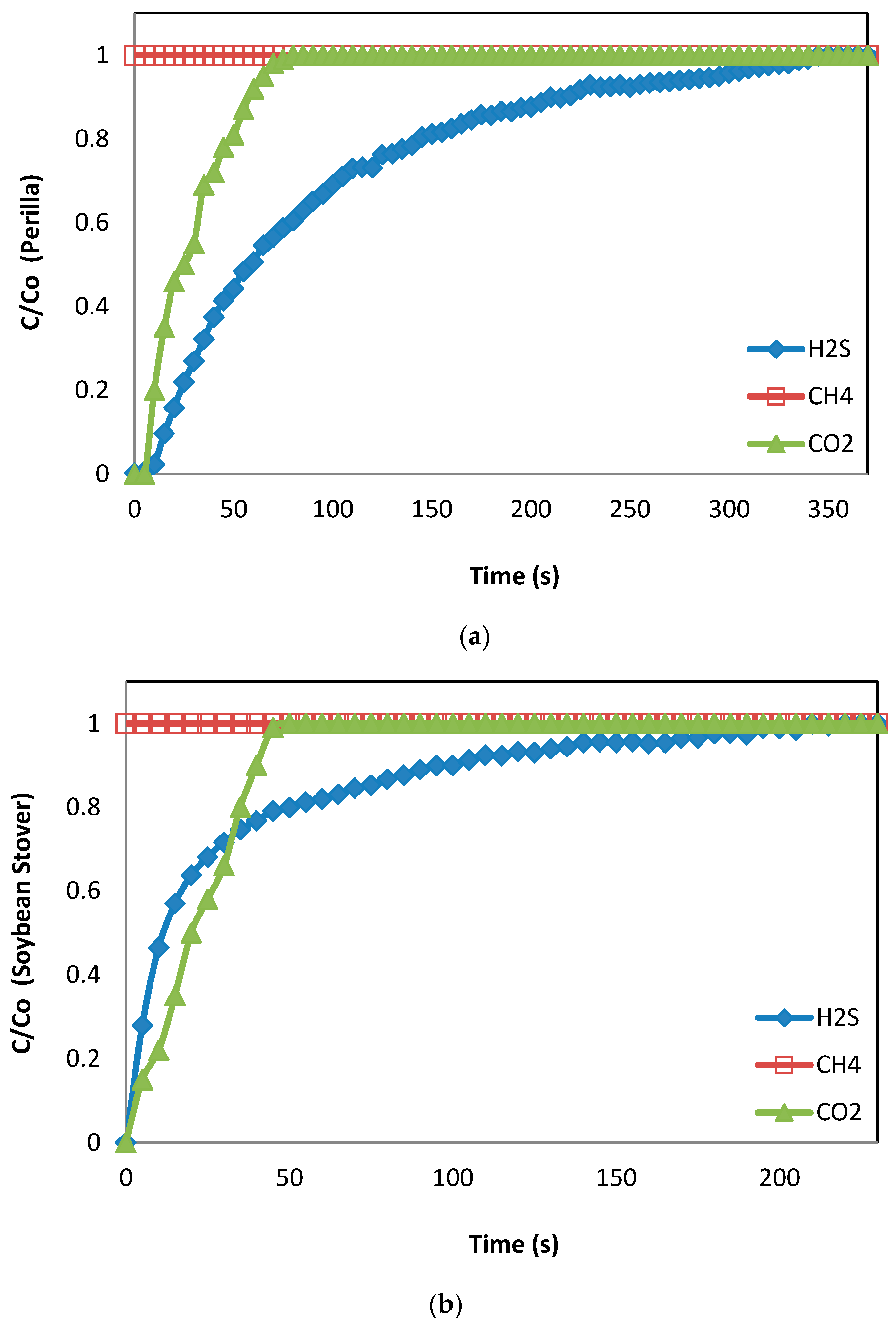
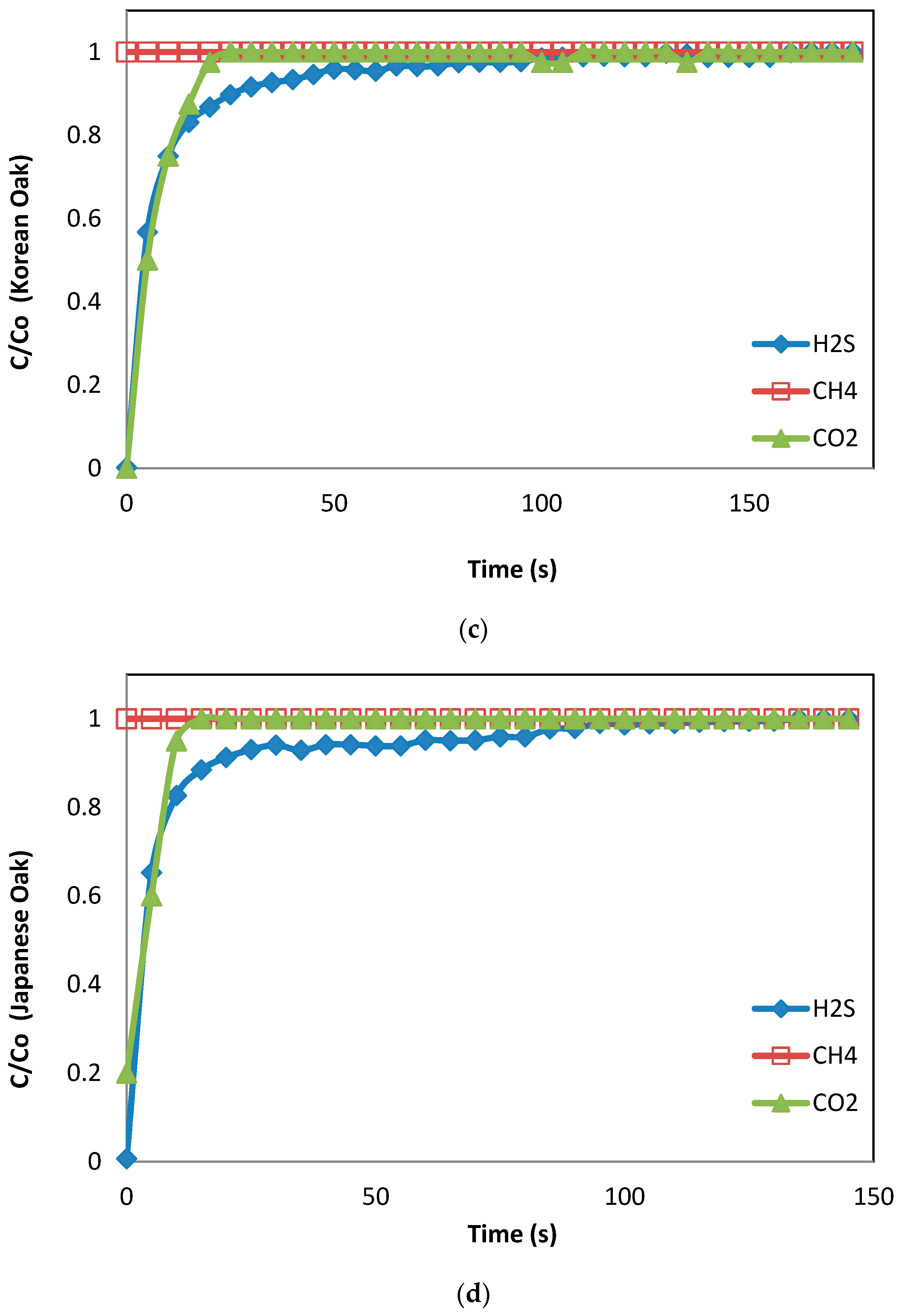
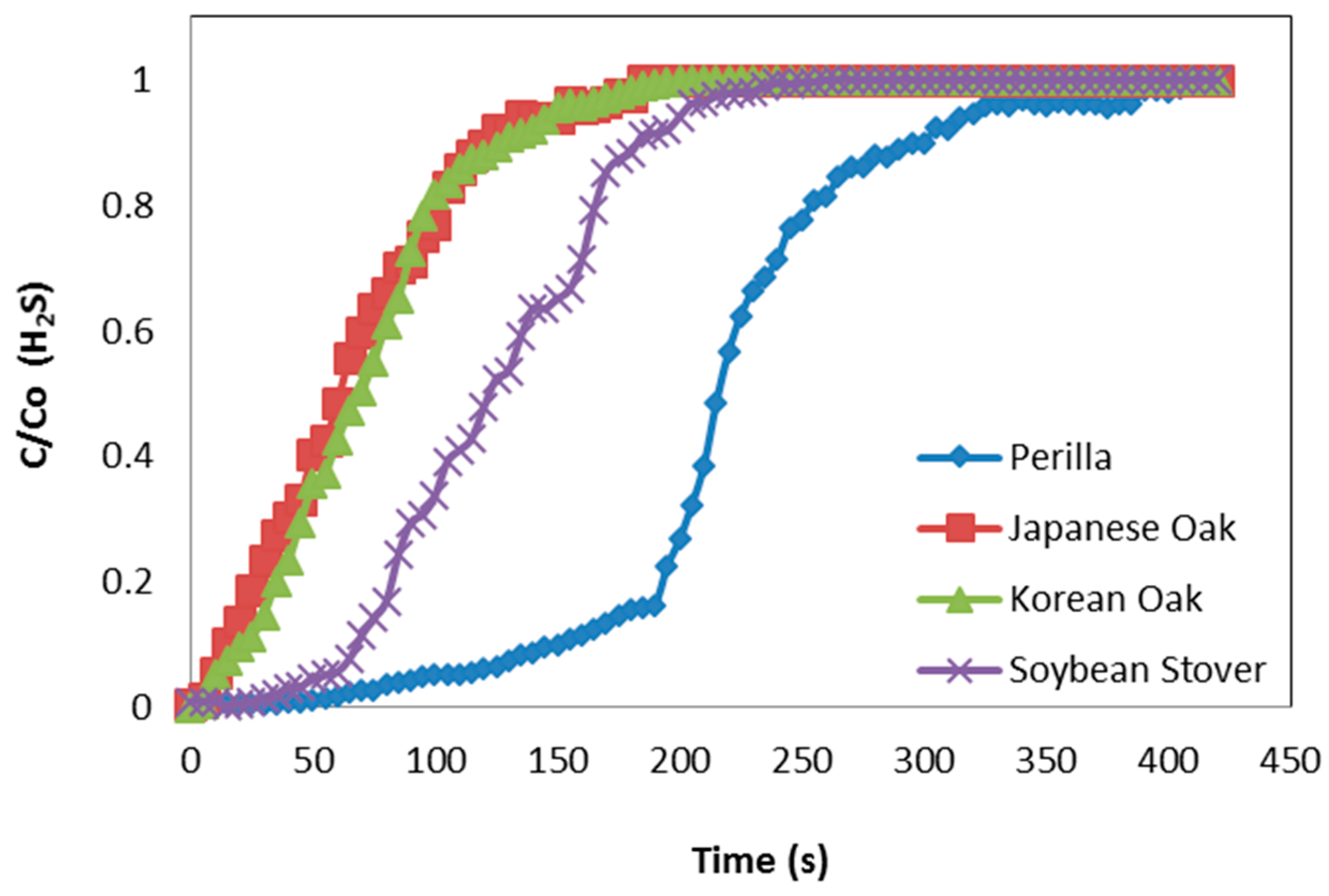
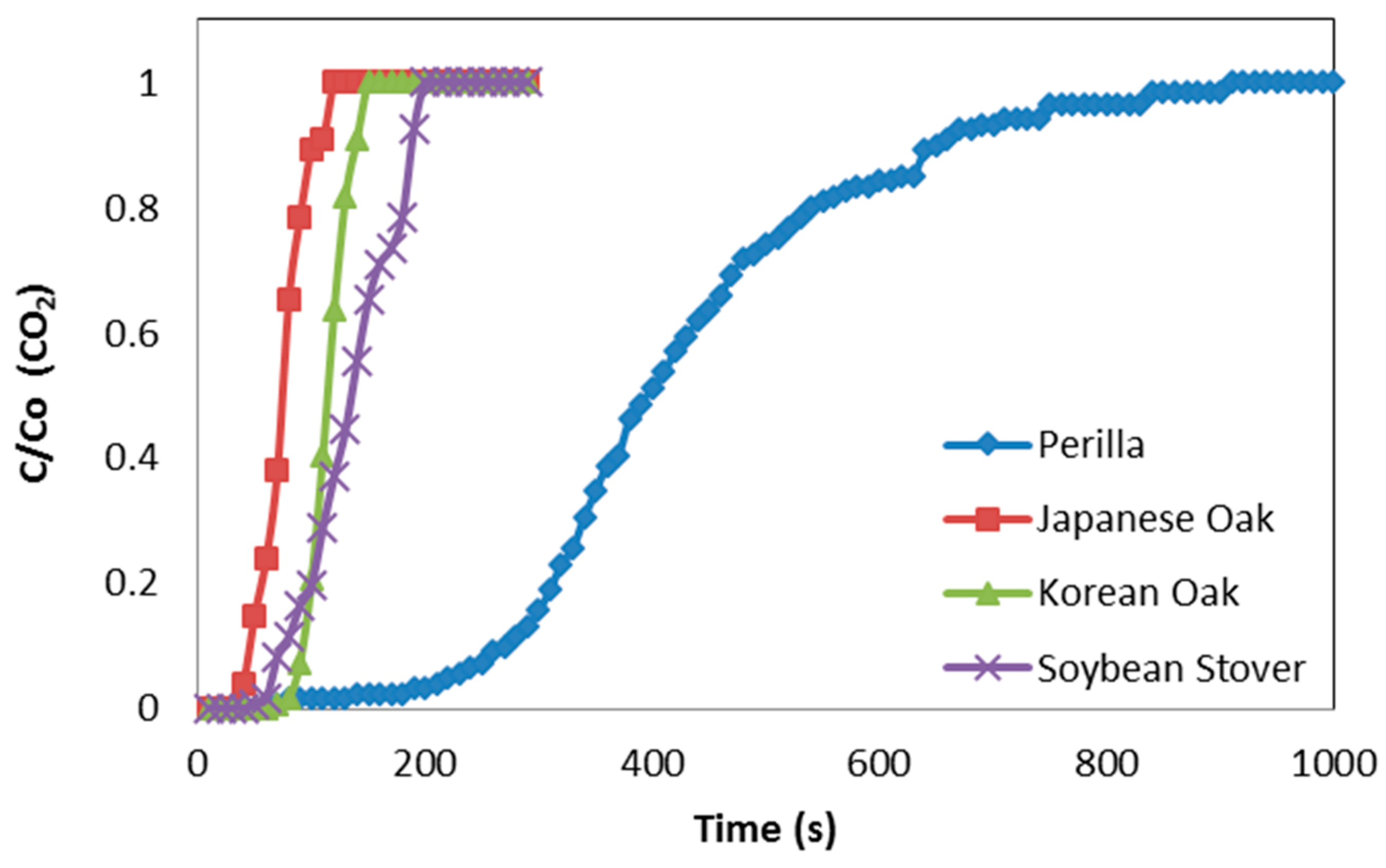
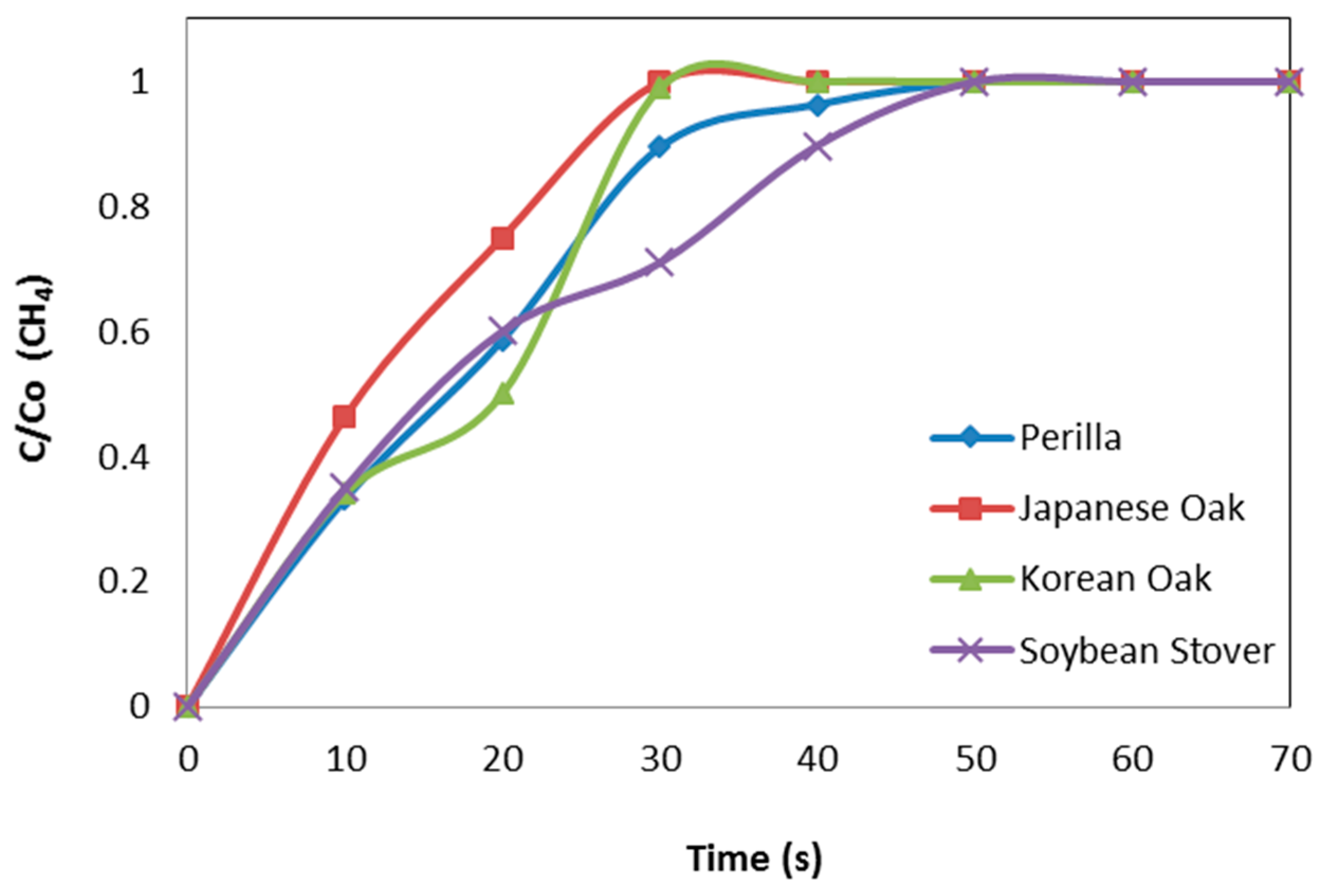
3.2. Breakthrough Capacity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ohimain, E.I.; Izah, S.C. A review of biogas production from palm oil mill effluents using different configurations of bioreactors. Renew. Sustain. Energy Rev. 2017, 70, 242–253. [Google Scholar] [CrossRef]
- Ji, C.M.; Eong, P.P.; Tia, T.B.; Seng, C.E.; Ling, C.K. Biogas from palm oil mill effluent (POME): Opportunities and challenges from Malaysia’s perspective. Renew. Sustain. Energy Rev. 2013, 26, 717–726. [Google Scholar]
- Hosseini, S.E.; Wahid, M.A. Utilization of biogas released from palm oil mill effluent for power generation using self-preheated reactor. Energy Convers. Manag. 2015, 105, 957–966. [Google Scholar] [CrossRef]
- Taliwa, Y.; Matsumoto, T.; Oshita, K.; Takaoka, M.; Morisawa, S.; Takeda, N. Characterization of trace constituents in landfill gas and a comparison of sites in Asia. J. Mater. Cycles Waste Manag. 2009, 11, 305–311. [Google Scholar]
- Ho, K.L.; Chung, Y.C.; Lin, Y.H.; Tseng, C.P. Microbial populations analysis and field application of biofilter for the removal of volatile-sulfur compounds from swine wastewater treatment system. J. Hazard. Mater. 2008, 152, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez Ortiz, F.J.; Aguilera, P.G.; Ollero, P. Biogas desulfurization by adsorption on thermally treated sewage-sludge. Sep. Purif. Technol. 2014, 123, 200–213. [Google Scholar] [CrossRef]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Lee, S.; Oh, S.E.; Mohan, D.; Moon, D.; Lee, Y.; Ok, Y.S. Modeling adsorption kinetics of trichloroethylene onto biochars derived from soybean stover and peanut shell wastes. Environ. Sci. Pollut. Res. 2013, 20, 8364–8373. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Lee, S.S.; Rajapaksha, A.U.; Vithanage, M.; Zhang, M.; Cho, J.S.; Lee, S.E.; Ok, Y.S. Trichloroethylene adsorption by pine needle biochars produced at various pyrolysis temperatures. Bioresour. Technol. 2013, 143, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Awad, Y.M.; Blagodatskaya, E.; Ok, Y.S.; Kuzyakov, Y. Effects of polyacrylamide, biopolymer and biochar on the decomposition of 14C-labeled maize residues and on their stabilization in soil aggregates. Eur. J. Soil Sci. 2013, 64, 488–499. [Google Scholar] [CrossRef]
- Vithanage, M.; Rajapaksha, A.U.; Tang, X.; Thiele-Bruhn, S.; Kim, K.H.; Lee, S.E.; Ok, Y.S. Sorption and transport of sulfamethazine in agricultural soils amended with invasive-plant-derived biochar. J. Environ. Manag. 2014, 141, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Kong, Z.; Liaw, S.B.; Gao, X.; Yu, Y.; Wu, H. Leaching characteristics of inherent inorganic nutrients in biochars from the slow and fast pyrolysis of mallee biomass. Fuel 2014, 128, 433–441. [Google Scholar] [CrossRef]
- Sun, Y.; Gao, B.; Yao, Y.; Fang, J.; Zhang, M.; Zhou, Y.; Chen, H.; Yang, L. Effects of feedstock type, production method, and pyrolysis temperature on biochar and hydrochar properties. Chem. Eng. J. 2014, 240, 574–578. [Google Scholar] [CrossRef]
- Creamer, A.E.; Gao, B.; Zhang, M. Carbon dioxide capture using biochar produced from sugarcane bagasse and hickory wood. Chem. Eng. J. 2014, 249, 174–179. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. Biochar for Environmental Management: Science and Technology; Earthscan: London, UK; Sterling, VA, USA, 2009. [Google Scholar]
- Park, J.H.; Ok, Y.S.; Kim, S.H.; Kang, S.W.; Cho, J.S.; Heo, J.S.; Dalaune, R.D.; Seo, D.C. Characteristics of biochars derived from fruit tree pruning wastes and their effects on lead adsorption. J. Korean Soc. Appl. Biol. Chem. 2015, 58, 751–760. [Google Scholar] [CrossRef]
- Adinata, D.; Daud, W.M.A.W.; Aroua, M.K. Production of carbon molecular sieves from palm shell based activated carbon by pore sizes modification with benzene for methane selective separation. Fuel Process. Technol. 2007, 88, 599–605. [Google Scholar] [CrossRef]
- Shang, G.; Shen, G.; Liu, L.; Chen, Q.; Xu, Z. Kinetics and mechanisms of hydrogen sulfide adsorption by biochars. Bioresour. Technol. 2013, 133, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Song, W.; Sun, G.; Xie, L.; Wang, J.; Li, K.; Sun, C.; Liu, H.; Snape, C.E.; Drage, T. CO2 capture with activated carbon grafted by nitrogenous functional groups. Energy Fuel 2013, 27, 4818–4823. [Google Scholar] [CrossRef]
- Sitthikhankaew, R.; Chadwick, D.; Assabumrungrat, S.; Laosiripojana, N. Effects of humidity, O2, and CO2 on H2S adsorption onto upgraded and KOH impregnated activated carbons. Fuel Process. Technol. 2014, 124, 249–257. [Google Scholar] [CrossRef]
- Arami-Niya, A.; Daud, W.M.A.W.; Mjalli, F.S. Comparative study of the textural characteristics of oil palm shell activated carbon produced by chemical and physical activation for methane adsorption. Chem. Eng. Res. Des. 2010, 89, 657–664. [Google Scholar] [CrossRef]
| Biochar | Moisture | Mobile Matter | Fixed Matter | Ash | pH † | C * | H * | O * | N * | S * | BET Surface Area | Pore Volume | Pore Size |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | m2/g | cm3/g | nm | ||||||||||
| Perilla | 0.1 | 6.5 | 51.6 | 41.9 | 10.6 | 71.8 | 0.9 | 15.3 | 1.5 | 0.1 | 473.4 | 0.1 | 3.4 |
| Korean oak | 6.8 | 31.4 | 56.1 | 5.1 | 10.2 | 88.7 | 1.2 | 9.7 | 0.4 | 0.0 | 270.8 | 0.1 | 1.1 |
| Japanese oak | 1.5 | 31.3 | 63.9 | 3.3 | 9.9 | 89.9 | 2.4 | 7.5 | 0.2 | 0.0 | 475.6 | 0.2 | 1.1 |
| Soybean stover a | 0.4 | 14.7 | 67.8 | 17.2 | 11.3 | 81.9 | 1.4 | 15.5 | 1.3 | 0.0 | 420.3 | 0.2 | 1.1 |
| Biochar | Adsorption Capacity (mmol/g) | |||||
|---|---|---|---|---|---|---|
| Simultaneous | Single | |||||
| H2S | CO2 | CH4 | ||||
| Perilla | 0.208 | 0.126 | 0.000 | 0.537 | 2.312 | 0.099 |
| Korean oak | 0.022 | 0.027 | 0.000 | 0.178 | 0.597 | 0.092 |
| Japanese oak | 0.018 | 0.012 | 0.000 | 0.167 | 0.379 | 0.064 |
| Soybean stover | 0.072 | 0.082 | 0.000 | 0.308 | 0.707 | 0.094 |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sethupathi, S.; Zhang, M.; Rajapaksha, A.U.; Lee, S.R.; Mohamad Nor, N.; Mohamed, A.R.; Al-Wabel, M.; Lee, S.S.; Ok, Y.S. Biochars as Potential Adsorbers of CH4, CO2 and H2S. Sustainability 2017, 9, 121. https://doi.org/10.3390/su9010121
Sethupathi S, Zhang M, Rajapaksha AU, Lee SR, Mohamad Nor N, Mohamed AR, Al-Wabel M, Lee SS, Ok YS. Biochars as Potential Adsorbers of CH4, CO2 and H2S. Sustainability. 2017; 9(1):121. https://doi.org/10.3390/su9010121
Chicago/Turabian StyleSethupathi, Sumathi, Ming Zhang, Anushka Upamali Rajapaksha, Sang Ryong Lee, Norhusna Mohamad Nor, Abdul Rahman Mohamed, Mohammad Al-Wabel, Sang Soo Lee, and Yong Sik Ok. 2017. "Biochars as Potential Adsorbers of CH4, CO2 and H2S" Sustainability 9, no. 1: 121. https://doi.org/10.3390/su9010121
APA StyleSethupathi, S., Zhang, M., Rajapaksha, A. U., Lee, S. R., Mohamad Nor, N., Mohamed, A. R., Al-Wabel, M., Lee, S. S., & Ok, Y. S. (2017). Biochars as Potential Adsorbers of CH4, CO2 and H2S. Sustainability, 9(1), 121. https://doi.org/10.3390/su9010121








