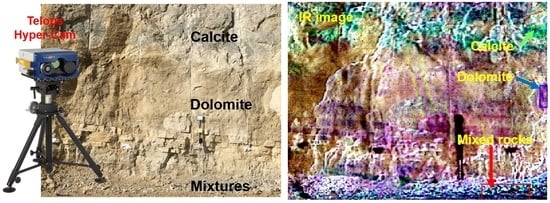
Thermal Infrared Hyperspectral Imaging for Mineralogy Mapping of a Mine Face
Abstract
:1. Introduction
2. Description of the Study Area
3. Background to LWIR Carbonate Spectroscopy
4. Data and Methodology
4.1. Instrumentation
4.2. Experimental Setup
4.3. Data Processing
4.4. Temperature and Emissivity Calculation.
5. Results
6. Discussion
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Axelsson, P. Processing of laser scanner data—Algorithms and applications. ISPRS J. Photogramm. Remote Sens. 1999, 54, 138–147. [Google Scholar] [CrossRef]
- Fröhlich, C.; Mettenleiter, M. Terrestrial laser scanning—New perspectives in 3D surveying. Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2004, 36, W2. [Google Scholar]
- Slob, S.; Hack, R. 3D terrestrial laser scanning as a new field measurement and monitoring technique. In Engineering Geology for Infrastructure Planning in Europe; Springer: New York, NY, USA, 2004; pp. 179–189. [Google Scholar]
- Buckley, S.J.; Howell, J.; Enge, H.; Kurz, T. Terrestrial laser scanning in geology: Data acquisition, processing and accuracy considerations. J. Geol. Soc. 2008, 165, 625–638. [Google Scholar] [CrossRef]
- Bellian, J.A.; Kerans, C.; Jennette, D.C. Digital outcrop models: Applications of terrestrial scanning LiDAR technology in stratigraphic modeling. J. Sediment. Res. 2005, 75, 166–176. [Google Scholar] [CrossRef]
- McCaffrey, K.; Jones, R.; Holdsworth, R.; Wilson, R.; Clegg, P.; Imber, J.; Holliman, N.; Trinks, I. Unlocking the spatial dimension: Digital technologies and the future of geoscience fieldwork. J. Geol. Soc. 2005, 162, 927–938. [Google Scholar] [CrossRef]
- Debba, P.; Carranza, E.J.; Stein, A.; van der Meer, F.D. Deriving optimal exploration target zones on mineral prospectivity maps. Math. Geosci. 2009, 41, 421. [Google Scholar] [CrossRef]
- Catani, F.; Farina, P.; Moretti, S.; Nico, G.; Strozzi, T. On the application of SAR interferometry to geomorphological studies: Estimation of landform attributes and mass movements. Geomorphology 2005, 66, 119–131. [Google Scholar] [CrossRef]
- Smith, M.; Rose, J.; Booth, S. Geomorphological mapping of glacial landforms from remotely sensed data: An evaluation of the principal data sources and an assessment of their quality. Geomorphology 2006, 76, 148–165. [Google Scholar] [CrossRef]
- Bishop, M.P.; Shroder, J.F., Jr.; Colby, J.D. Remote sensing and geomorphometry for studying relief production in high mountains. Geomorphology 2003, 55, 345–361. [Google Scholar] [CrossRef]
- Berger, Z. Satellite Hydrocarbon Exploration: Interpretation and Integration Techniques; Springer: New York, NY, USA, 2012. [Google Scholar]
- Fialko, Y.; Simons, M.; Agnew, D. The complete (3-D) surface displacement field in the epicentral area of the 1999 Mw7. 1 Hector Mine earthquake, California, from space geodetic observations. Geophys. Res. Lett. 2001, 28, 3063–3066. [Google Scholar] [CrossRef]
- Werner, C.; Wegmuller, U.; Strozzi, T.; Wiesmann, A. Interferometric point target analysis for deformation mapping. In Proceedings of the 2003 IEEE International Geoscience and Remote Sensing Symposium, IGARSS’03, Toulouse, France, 21–25 July 2003; Volume 7, pp. 4362–4364. [Google Scholar]
- Amelung, F.; Galloway, D.L.; Bell, J.W.; Zebker, H.A.; Laczniak, R.J. Sensing the ups and downs of Las Vegas: InSAR reveals structural control of land subsidence and aquifer-system deformation. Geology 1999, 27, 483–486. [Google Scholar] [CrossRef]
- Van derWerff, H.; Van der Meijde, M.; Jansma, F.; Van der Meer, F.; Groothuis, G.J. A spatial-spectral approach for visualization of vegetation stress resulting from pipeline leakage. Sensors 2008, 8, 3733–3743. [Google Scholar] [CrossRef] [PubMed]
- Sabins, F.F. Remote sensing for mineral exploration. Ore Geol. Rev. 1999, 14, 157–183. [Google Scholar] [CrossRef]
- Roonwal, G. Remote Sensing in Mineral Exploration. In Mineral Exploration: Practical Application; Springer: New York, NY, USA, 2018; pp. 119–153. [Google Scholar]
- Hunt, G.R. Electromagnetic radiation: The communication link in remote sensing. In Remote Sensing in Geololgy, 3rd ed.; Wiely: New York, NY, USA, 1980; pp. 5–45. [Google Scholar]
- Al Fasatwi, Y.; Van Dijk, P. Lineament and geomorphic analysis of remote sensing data as an aid to hydrocarbon exploration, Sirt Basin, Libya. ITC J. 1990, 2, 137–144. [Google Scholar]
- Ellingsrud, S.; Eidesmo, T.; Johansen, S.; Sinha, M.; MacGregor, L.; Constable, S. Remote sensing of hydrocarbon layers by seabed logging (SBL): Results from a cruise offshore Angola. Lead. Edge 2002, 21, 972–982. [Google Scholar] [CrossRef]
- Khan, S.D.; Jacobson, S. Remote sensing and geochemistry for detecting hydrocarbon microseepages. Geol. Soc. Am. Bull. 2008, 120, 96–105. [Google Scholar] [CrossRef]
- Clark, R.N.; King, T.V.; Klejwa, M.; Swayze, G.A.; Vergo, N. High spectral resolution reflectance spectroscopy of minerals. J. Geophys. Res. Solid Earth 1990, 95, 12653–12680. [Google Scholar] [CrossRef]
- Weng, Q. Thermal infrared remote sensing for urban climate and environmental studies: Methods, applications, and trends. ISPRS J. Photogramm. Remote Sens. 2009, 64, 335–344. [Google Scholar] [CrossRef]
- Lefsky, M.A.; Cohen, W.B.; Parker, G.G.; Harding, D.J. Lidar remote sensing for ecosystem studies: Lidar, an emerging remote sensing technology that directly measures the three-dimensional distribution of plant canopies, can accurately estimate vegetation structural attributes and should be of particular interest to forest, landscape, and global ecologists. AIBS Bull. 2002, 52, 19–30. [Google Scholar]
- Goetz, A.F.; Vane, G.; Solomon, J.E.; Rock, B.N. Imaging spectrometry for earth remote sensing. Science 1985, 228, 1147–1153. [Google Scholar] [CrossRef] [PubMed]
- Bowen, B.B.; Martini, B.A.; Chan, M.A.; Parry, W.T. Reflectance spectroscopic mapping of diagenetic heterogeneities and fluid-flow pathways in the Jurassic Navajo Sandstone. AAPG Bull. 2007, 91, 173–190. [Google Scholar] [CrossRef]
- Harris, J.; Rogge, D.; Hitchcock, R.; Ijewliw, O.; Wright, D. Mapping lithology in Canada’s Arctic: Application of hyperspectral data using the minimum noise fraction transformation and matched filtering. Can. J. Earth Sci. 2005, 42, 2173–2193. [Google Scholar] [CrossRef]
- Gagnon, M.A.; Tremblay, P.; Savary, S.; Duval, M.; Farley, V.; Lagueux, P.; Guyot, É.; Chamberland, M. Airborne thermal infrared hyperspectral imaging for mineral mapping. In Proceedings of the International Workshop on Advanced Infrared Technology & Applications, Pisa, Italy, 29 September–2 October 2015; Volume 29, pp. 83–86. [Google Scholar]
- Hapke, B. Theory of Reflectance and Emittance Spectroscopy; Cambridge University Press: Cambridge, UK, 2012. [Google Scholar]
- Gillespie, A.R.; Rokugawa, S.; Hook, S.J.; Matsunaga, T.; Kahle, A.B. Temperature/Emissivity Separation Algorithm Theoretical Basis Document, Version 2.4; ATBD Contract NAS5-31372; NASA: Greenbelt, Maryland, 1999. [Google Scholar]
- Lutgens, F.K.; Tarbuck, E.J.; Tasa, D.G. Essentials of Geology; Pearson Higher Education: San Francisco, CA, USA, 2014. [Google Scholar]
- Hatch, F.H.; Rastall, R.H.; Greensmith, J.T. Textbook of Petrology V2: The Petrology of the Sedimentary Rocks; Allen & Unwin: Boston, MA, USA, 1971. [Google Scholar]
- Bissell, H.J.; Chilingar, G.V. Classification of sedimentary carbonate rocks. In Developments in Sedimentology; Elsevier: Amsterdam, The Netherlands, 1967; Volume 9, pp. 87–168. [Google Scholar]
- Hunt, G.R.; Salisbury, J.W. Visible and near infrared spectra of minerals and rocks. II. Carbonates. Mod. Geol. 1971, 2, 23–30. [Google Scholar]
- Dietrich, R.V.; Skinner, B.J. Rocks and Rock Minerals; Technical Report; Wiley: New York, NY, USA, 1979. [Google Scholar]
- Hamilton, W.; Bishop, A.; Woolley, A. Minerals, Rocks and Fossils; Hamlyn Publishing Group: London, UK, 1987. [Google Scholar]
- Lane, M.D.; Christensen, P.R. Thermal infrared emission spectroscopy of anhydrous carbonates. J. Geophys. Res. 1997, 102, 25581–25592. [Google Scholar] [CrossRef] [Green Version]
- Baldridge, A.; Hook, S.; Grove, C.; Rivera, G. The ASTER spectral library version 2.0. Remote Sens. Environ. 2009, 113, 711–715. [Google Scholar] [CrossRef]
- Kahle, A.B.; Alley, R.E. Separation of temperature and emittance in remotely sensed radiance measurements. Remote Sens. Environ. 1992, 42, 107–111. [Google Scholar] [CrossRef]
- Salvaggio, C.; Miller, C.J. Comparison of field-and laboratory-collected midwave and longwave infrared emissivity spectra/data reduction techniques. In Algorithms for Multispectral, Hyperspectral, and Ultraspectral Imagery VII International Society for Optics and Photonics; SPIE: Bellingham, WA, USA, 2001; Volume 4381, pp. 549–559. [Google Scholar]
- Horton, K.A.; Johnson, J.R.; Lucey, P.G. Infrared measurements of pristine and disturbed soils 2. Environmental effects and field data reduction. Remote Sens. Environ. 1998, 64, 47–52. [Google Scholar] [CrossRef]
- Bower, N.; Knuteson, R.; Revercomb, H. High Spectral Resolution Land Surface Temperature and Emissivity Measurements in the Thermal Infrared; Co-operative Institute for Meteorological and Satellite Studies at the University of Wisconsin: Madison, WI, USA, 1999. [Google Scholar]
- Berk, A.; Conforti, P.; Kennett, R.; Perkins, T.; Hawes, F.; van den Bosch, J. MODTRAN® 6: A major upgrade of the MODTRAN® radiative transfer code. In Proceedings of the 2014 6th Workshop on IEEE Hyperspectral Image and Signal Processing: Evolution in Remote Sensing (WHISPERS), Zurich, Switzerland, 24–27 June 2014; pp. 1–4. [Google Scholar]
- Green, D.; Schodlok, M. Characterisation of carbonate minerals from hyperspectral TIR scanning using features at 14,000 and 11,300 nm. Aust. J. Earth Sci. 2016, 63, 951–957. [Google Scholar]
- Clark, R.; Swayze, G.; Gallagher, A.; Gorelick, N.; Kruse, F. Mapping with imaging spectrometer data using the complete band shape least-squares algorithm simultaneously fit to multiple spectral features from multiple materials. In Proceedings of the Third Airborne Visible/Infrared Imaging Spectrometer (AVIRIS) Workshop; JPL Publication 91-28; Jet Propulsion Laboratory: Pasadena, CA, USA, 1991; Volume 42, pp. 2–3. [Google Scholar]
- Clark, R.N.; Swayze, G.A.; Livo, K.E.; Kokaly, R.F.; Sutley, S.J.; Dalton, J.B.; McDougal, R.R.; Gent, C.A. Imaging spectroscopy: Earth and planetary remote sensing with the USGS Tetracorder and expert systems. J. Geophys. Res. 2003, 108, 44. [Google Scholar] [CrossRef]
- Clark, R.N. Spectroscopy of rocks and minerals, and principles of spectroscopy. Manu. Remote Sens. 1999, 3, 2. [Google Scholar]
- Clark, R.N.; Roush, T.L. Reflectance spectroscopy: Quantitative analysis techniques for remote sensing applications. J. Geophys. Res. Solid Earth 1984, 89, 6329–6340. [Google Scholar] [CrossRef]
- Adams, J.B. Imaging spectroscopy: Interpretation based on spectral mixture analysis. In Remote Geochemical Analysis: Elemental and Mineralogical Composition; Cambridge University Press: Cambridge, UK, 1993; pp. 145–166. [Google Scholar]
- Ichoku, C.; Karnieli, A. A review of mixture modeling techniques for sub-pixel land cover estimation. Remote Sens. Rev. 1996, 13, 161–186. [Google Scholar] [CrossRef]







 |  |  |  |
|---|---|---|---|
| = 9.25 m (1080 cm) | = 11.36 m (880 cm) | = 6.99 m (1430 cm) | = 13.98 m (715 cm) |
| Nondegenerate | Nondegenerate symmetric | Doubly degenerate | Doubly degenerate |
| symmetric stretch | out-of-plane bend | asymmetric stretch | asymmetric in-plane bend |
| Pixel | 1/ (cm) | FWHM (cm) | Depth | 1/ (cm) | FWHM (cm) | Depth |
|---|---|---|---|---|---|---|
| #1 | 885.62 ± 0.86 | 17.75 ± 4.01 | 0.092 | 1033.16 ± 1.41 | 140.82 ± 13.03 | 0.064 |
| #2 | 884.45 ± 0.78 | 14.56 ± 3.65 | 0.096 | 1033.44 ± 2.03 | 74.57 ± 9.27 | 0.059 |
| #3 | 895.35 ± 0.66 | 18.11 ± 3.09 | 0.082 | |||
| #4 | 887.47 ± 0.65 | 47.12 ± 6.09 | 0.136 | |||
| #5 | 889.57 ± 0.63 | 29.44 ± 4.83 | 0.109 | |||
| #6 | 892.24 ± 0.69 | 32.10 ± 5.22 | 0.141 | |||
| #7 | 892.48 ± 1.32 | 10.53 ± 5.37 | 0.033 |
| Mineral | 1/ (cm) | FWHM (cm) | 1/ (cm) | FWHM (cm) |
|---|---|---|---|---|
| Malachite | 819.99 ± 0.42 | 11.09 ± 1.57 | 1030.07 ± 0.88 | 82.33 ± 6.79 |
| Azurite | 817.86 ± 0.72 | 8.36 ± 3.04 | 1031.05 ± 0.73 | 74.74 ± 3.14 |
| Dolomite | 892.20 ± 0.304 | 22.15 ± 4.45 | ||
| Calcite | 883.58 ± 0.26 | 23.93 ± 8.69 | 1080.18 ± 1.28 | |
| Smithsonite | 876.17 ± 0.59 | 24.52 ± 5.72 | ||
| Siderite | 875.88 ± 0.46 | 18.19 ± 3.87 | 1144.61 ± 1.54 | |
| Rhodochrosite | 875.01 ± 0.48 | 6.86 ± 3.99 | ||
| Magnesite | 906.03 ± 0.398 | 30.07 ± 1.84 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boubanga-Tombet, S.; Huot, A.; Vitins, I.; Heuberger, S.; Veuve, C.; Eisele, A.; Hewson, R.; Guyot, E.; Marcotte, F.; Chamberland, M. Thermal Infrared Hyperspectral Imaging for Mineralogy Mapping of a Mine Face. Remote Sens. 2018, 10, 1518. https://doi.org/10.3390/rs10101518
Boubanga-Tombet S, Huot A, Vitins I, Heuberger S, Veuve C, Eisele A, Hewson R, Guyot E, Marcotte F, Chamberland M. Thermal Infrared Hyperspectral Imaging for Mineralogy Mapping of a Mine Face. Remote Sensing. 2018; 10(10):1518. https://doi.org/10.3390/rs10101518
Chicago/Turabian StyleBoubanga-Tombet, Stephane, Alexandrine Huot, Iwan Vitins, Stefan Heuberger, Christophe Veuve, Andreas Eisele, Rob Hewson, Eric Guyot, Frédérick Marcotte, and Martin Chamberland. 2018. "Thermal Infrared Hyperspectral Imaging for Mineralogy Mapping of a Mine Face" Remote Sensing 10, no. 10: 1518. https://doi.org/10.3390/rs10101518
APA StyleBoubanga-Tombet, S., Huot, A., Vitins, I., Heuberger, S., Veuve, C., Eisele, A., Hewson, R., Guyot, E., Marcotte, F., & Chamberland, M. (2018). Thermal Infrared Hyperspectral Imaging for Mineralogy Mapping of a Mine Face. Remote Sensing, 10(10), 1518. https://doi.org/10.3390/rs10101518