Spectral Properties of Coniferous Forests: A Review of In Situ and Laboratory Measurements
Abstract
:1. Introduction
2. Spectral Properties of Coniferous Needles
2.1. Measurements of Needle Optical Properties
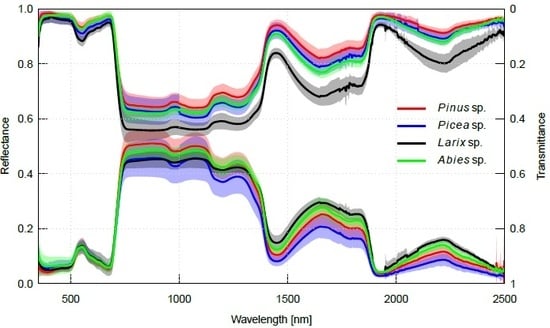
2.2. Interspecific Variation in Needle Spectra
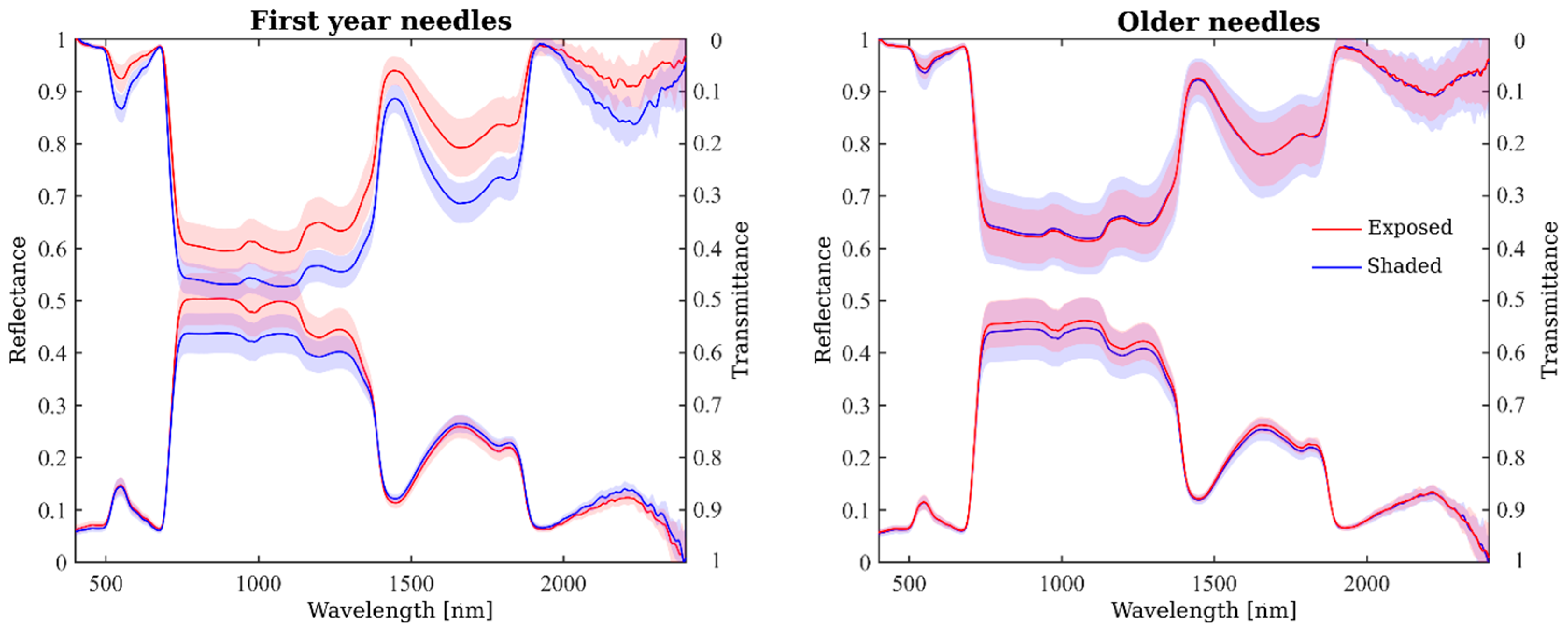
2.3. Spatial Variation in Needle Spectra
2.4. Seasonal Variation in Needle Spectra
2.5. Directional Variation in Needle Spectra
2.6. Drivers of Spectral Variability in Needle Spectra
3. Spectral and Directional Optical Properties of Coniferous Shoots
4. Spectral Properties of Woody Parts
5. Spectral Properties of Understory in Coniferous Forests
5.1. Spectral Differences between Understory Types
5.2. Seasonal Variation in Understory Spectra
5.3. Spatial and Directional Variation in Understory Spectra
5.4. Drivers of Spectral Variability in Understory Spectra
6. Case Studies: Spectra of Coniferous Canopies
6.1. Introduction to Case Studies
6.2. Nested Scales of Spectra of Coniferous Canopies
6.3. Angular Variation in Spectra of Coniferous Canopies
6.4. Seasonal Variation in Spectra of Coniferous Canopies
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
| Dataset | Region | Biome | nm | Geom. 1 | R or T 2 | Species |
|---|---|---|---|---|---|---|
| [14] | - | - | VIS/NIR 3 | IS | R | Pinus strobus, Thuja occidentalis |
| [15] | - | - | 400–1100 | IS | R | Pinus resinosa |
| [50] | ON, Canada | Temperate | VIS/NIR 3 | BF | R | Abies balsamea, Pinus sylvestris, Thuja occidentalis |
| [24] | VT, USA | Temperate | 400–1100 | IS | R, T | Picea abies, Pinus resinosa, Pinus strobus |
| [28] | ME, USA | Temperate | 400–1100 | IS | R, T | Picea rubens, Tsuga canadensis |
| [34] | SK, Canada | Boreal | 400–1000 | IS | R, T | Picea mariana, Pinus banksiana |
| [29] | SK, Canada | Boreal | 400–1000 | IS | R, T | Picea glauca, Picea mariana, Pinus banksiana |
| [104] | MS & KS, USA | Temperate | 400–850 | IS | R, T | Pinus palustris |
| [21] | NH, USA | Temperate | 306–1138 | NC | R | Abies balsamea, Picea rubens |
| [17] | UK | Temperate | 400–2500 | BF | R | Picea sitchensis |
| [32] | AK, USA | Boreal | 306–1138 | NC | R | Picea glauca, Picea mariana |
| [26] | WA, USA | Temperate | 200–2350 | IS | R, T | Pseudotsuga menziesii, Thuja plicata, Tsuga heterophylla |
| [105] | ON, Canada | Boreal | 400–800 | IS | R, T | Pinus banksiana |
| [18] | Australia | Temperate | 350–1100 | BF | R | Pinus radiata |
| [106] | NM, USA | Temperate | 350–2500 | IS | R | Juniperus monosperma, Pinus edulis |
| [107] | Czech Republic | Temperate | 450–1000 | IS | R, T | Picea abies |
| [48] | Czech Republic | Temperate | 350–1000 | BF | R, T | Picea abies |
| [108] | Sweden | Boreal | 400–1000 | IS | R, T | Picea abies |
| [60] | ON, Canada | Boreal | 350–1050 | IS | R, T | Pinus banksiana |
| [109] | ON, Canada | Boreal | 350–2500 | IS | R, T | Picea mariana |
| [110] | CA, USA | Temperate | 400–800 | IS | R, T | Pinus jefferyi |
| [111] | Estonia | Temperate | 400–1050 | IS | R | Picea abies, Pinus sylvestris |
| [30] | NW, USA; MB, Canada | Temperate/boreal | 400–1000 4 | NC | R | Pinus banksiana, Pinus ponderosa, Pseudotsuga menziesii, Tsuga heterophylla |
| [22] | Spain | Mediterr. | 306–1138 | NC | R | Pinus sylvestris |
| [69] | Switzerland | Temperate | 400–1800 | IS | R, T | Pinus sylvestris |
| [112] | ON, Canada | Temperate | 400–900 4 | IS | R, T | Picea mariana, Pinus banksiana |
| [113] | Finland | Boreal | 400–900 | BF | R | Pinus sylvestris |
| [19] | Czech Republic | Temperate | 350–2500 | CB | R | Pinus sylvestris |
| [20] | Finland | Boreal | 325–1075 | CB | R | Pinus sylvestris |
| [31] | Slovenia | Temperate | 250–880 | IS | R | Abies alba, Picea abies, Picea omorika, Pinus sylvestris |
| [51] | AB, Canada | Boreal | 400–900 | NC | R | Picea glauca, Pinus contorta, Pinus ponderosa |
| [52] | AB, Canada | Boreal | 400–900 | NC | R | Pinus contorta 5 |
| [114] | Finland | Boreal | 325–1075 | IS/CB | R, T | Picea pungens, Pinus sylvestris |
| [115] | Czech Republic | Temperate | 350–2500 | IS/CB | R | Picea abies |
| [33] | CA, USA | Temperate/mediterr. | 350–2500 | IS | R | Abies concolor, Abies magnifica, Calocedrus decurrens, Pinus jeffreyi, Pinus lambertiana, Pinus ponderosa, Pinus sabiniana |
| [47] | Finland | Boreal | 400–900 4 | CB | R | Picea abies, Pinus sylvestris |
| [23] | Finland | Boreal | 400–1100 | NC | R | Pinus sylvestris |
Appendix B
Appendix B.1. Data Description
Appendix B.2. Site Description
Appendix B.3. Field Data on Leaf Optical and Biochemical Properties
Appendix B.4. Airborne Hyperspectral Data
Appendix B.5. Multispectral Satellite Data
References
- Farjon, A. A Hand-Book of the World’s Conifers; Brill: Aylesbury, UK, 2010; pp. 1–1111. ISBN 9789047430629. [Google Scholar]
- Rocky Mountain Tree-Ring Research. Available online: http://www.rmtrr.org/oldlist.htm (accessed on 22 November 2017).
- Preston, R. Tall for its age. The New Yorker, 9 October 2006; 4. [Google Scholar]
- Ciais, P.; Sabine, C.; Bala, G.; Bopp, L.; Brovkin, V.; Canadell, J.; Chhabra, A.; DeFries, R.; Galloway, J.; Heimann, M.; et al. Carbon and Other Biogeochemical Cycles. In Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar]
- United Nations Economic Commission for Europe/Food and Agriculture Organization of the United Nations (UNECE/FAO). Forest Products Annual Market Review 2016; UNECE: Geneva, Switzerland, 2016; Available online: https://www.unece.org/fileadmin/DAM/timber/publications/FPAMR2017AdvanceDraft.pdf (accessed on 30 November 2017).
- Forest Europe. State of Europe’s Forests 2015; Forest Europe: Zvolen, Slovakia, 2015; pp. 1–314. Available online: http://www.foresteurope.org/docs/fullsoef2015.pdf (accessed on 30 November 2017).
- Smolander, S.; Stenberg, P. Simple parameterizations of the radiation budget of uniform broadleaved and coniferous canopies. Remote Sens. Environ. 2005, 94, 355–363. [Google Scholar] [CrossRef]
- Rautiainen, M.; Stenberg, P. Application of photon recollision probability in coniferous canopy reflectance simulations. Remote Sens. Environ. 2005, 96, 98–107. [Google Scholar] [CrossRef]
- Yáñez-Rausell, L.; Schaepman, M.E.; Clevers, J.G.P.W.; Malenovský, Z. Minimizing measurement uncertainties of coniferous needle-leaf optical properties, part i: methodological review. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2014, 7, 399–405. [Google Scholar] [CrossRef]
- Yáñez-Rausell, L.; Malenovsky, Z.; Clevers, J.G.P.W.; Schaepman, M.E. Minimizing measurement uncertainties of coniferous needle-leaf optical properties, Part II: Experimental Setup and Error Analysis. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2014, 7, 406–420. [Google Scholar] [CrossRef]
- Hovi, A.; Raitio, P.; Rautiainen, M. Spectral analysis of 25 boreal tree species. Silva Fenn. 2017, 51, 1–16. [Google Scholar] [CrossRef]
- Rautiainen, M.; Lukeš, P. Spectral contribution of understory to forest reflectance in a boreal site: An analysis of EO-1 Hyperion data. Remote Sens. Environ. 2015, 171, 98–104. [Google Scholar] [CrossRef]
- Eriksson, H.; Eklundh, L.; Kuusk, A.; Nilson, T. Impact of understory vegetation on forest canopy reflectance and remotely sensed LAI estimates. Remote Sens. Environ. 2006, 103, 408–418. [Google Scholar] [CrossRef]
- Gates, D.M.; Keegan, H.J.; Schleter, J.C.; Weidner, V.R. Spectral properties of plants. Appl. Opt. 1965, 4, 11–20. [Google Scholar] [CrossRef]
- Daughtry, C.S.T.; Biehl, L.L. Changes in Spectral Properties of Detached Leaves; LARS Technical Report 061584; Purdue University Laboratory for Applications of Remote Sensing: West Lafayette, IN, USA, 1984; p. 25.
- Hosgood, B.; Jacquemoud, S.; Andreoli, G.; Verdebout, J.; Pedrini, A.; Schmuck, G. Leaf Optical Properties EXperiment 93 (LOPEX93); Report EUR 16095 EN (Revised 2005); Joint Research Centre/Institute for Remote Sensing Applications: Ispra, Italy, 2005; Available online: http://ies-webarchive.jrc.ec.europa.eu/ies/uploads/fileadmin/H03/LOPEX_Jan09.zip (accessed on 8 November 2017).
- O’Neill, A.L.; Kupiec, J.A.; Curran, P.J. Biochemical and reflectance variation throughout a Sitka spruce canopy. Remote Sens. Environ. 2002, 80, 134–142. [Google Scholar] [CrossRef]
- Coops, N.C.; Stone, C. A comparison of field-based and modelled reflectance spectra from damaged Pinus radiata foliage. Aust. J. Bot. 2005, 53, 417–429. [Google Scholar] [CrossRef]
- Lhotáková, Z.; Brodský, L.; Kupková, L.; Kopačková, V.; Potůčková, M.; Mišurec, J.; Klement, A.; Kovářová, M.; Albrechtová, J. Detection of multiple stresses in Scots pine growing at post-mining sites using visible to near-infrared spectroscopy. Environ. Sci. Process. Impacts 2013, 15, 2004–2015. [Google Scholar] [CrossRef]
- Olascoaga, B.; Juurola, E.; Lukeš, P.; Nikinmaa, E.; Bäck, J.; Porcar-Castell, A.; Juurola, E.; Pinho, P.; Halonen, L. Seasonal variation in the reflectance of photosynthetically active radiation from epicuticular waxes of Scots pine (Pinus sylvestris) needles. Boreal Environ. Res. 2014, 19, 132–141. [Google Scholar]
- Richardson, A.D.; Berlyn, G.P.; Gregoire, T.G. Spectral reflectance of Picea rubens (Pinaceae) and Abies balsamea (Pinaceae) needles along an elevational gradient, Mt. Moosilauke, New Hampshire, USA. Am. J. Bot. 2001, 88, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Clemente, R.; Navarro-Cerrillo, R.M.; Zarco-Tejada, P.J. Carotenoid content estimation in a heterogeneous conifer forest using narrow-band indices and PROSPECT+DART simulations. Remote Sens. Environ. 2012, 127, 298–315. [Google Scholar] [CrossRef]
- Mõttus, M.; Hernández-Clemente, R.; Perheentupa, V.; Markiet, V. In situ measurement of Scots pine needle PRI. Plant Methods 2017, 13, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.L. A comparison of spectral reflectance properties at the needle, branch, and canopy levels for selected conifer species. Remote Sens. Environ. 1991, 35, 79–93. [Google Scholar] [CrossRef]
- Hall, F.G.; Huemmrich, K.F.; Strebel, D.E.; Goetz, S.J.; Nickeson, J.E.; Woods, K.E. Biophysical, Morphological, Canopy Optical Property, and Productivity Data from the Superior National Forest; NASA Technical Memorandum 104568; NASA Goddard Space Flight Center: Greenbelt, MD, USA, 1992; p. 150.
- Roberts, D.A.; Ustin, S.L.; Ogunjemiyo, S.; Greenberg, J.; Dobrowski, S.Z.; Chen, J.; Hinckley, T.M. Spectral and Structural Measures of Northwest Forest Vegetation at Leaf to Landscape Scales. Ecosystems 2004, 7, 545–562. [Google Scholar] [CrossRef]
- Lukeš, P.; Stenberg, P.; Rautiainen, M.; Mõttus, M.; Vanhatalo, K. Optical properties of leaves and needles for boreal tree species in Europe. Remote Sens. Lett. 2013, 4, 667–676. [Google Scholar] [CrossRef]
- Rock, B.N.; Williams, D.L.; Moss, D.M.; Lauten, G.N.; Kim, M. High-spectral resolution field and laboratory optical reflectance measurements of red spruce and eastern hemlock needles and branches. Remote Sens. Environ. 1994, 47, 176–189. [Google Scholar] [CrossRef]
- Middleton, E.M.; Sullivan, J.H.; Bovard, B.D.; Deluca, A.J.; Chan, S.S.; Cannon, T.A. Seasonal variability in foliar characteristics and physiology for boreal forest species at the five Saskatchewan tower sites during the 1994 Boreal Ecosystem-Atmosphere Study. J. Geophys. Res. 1997, 102, 28831–28844. [Google Scholar] [CrossRef]
- Gamon, J.A.; Berry, J.A. Facultative and constitutive pigment effects on the Photochemical Reflectance Index (PRI) in sun and shade conifer needles. Isr. J. Plant Sci. 2012, 60, 85–95. [Google Scholar] [CrossRef]
- De Tomás Marín, S.; Novák, M.; Klančnik, K.; Gaberščik, A. Spectral signatures of conifer needles mainly depend on their physical traits. Pol. J. Ecol. 2016, 64, 1–13. [Google Scholar] [CrossRef]
- Richardson, A.D.; Berlyn, G.P.; Duigan, S.P. Reflectance of Alaskan black spruce and white spruce foliage in relation to elevation and latitude. Tree Physiol. 2003, 23, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Roth, K.L.; Casas, A.; Huesca, M.; Ustin, S.L.; Alsina, M.M.; Mathews, S.A.; Whiting, M.L. Leaf spectral clusters as potential optical leaf functional types within California ecosystems. Remote Sens. Environ. 2016, 184, 229–246. [Google Scholar] [CrossRef]
- Middleton, E.M.; Chan, S.S.; Rusin, R.J.; Mitchell, S.K. Optical properties of black spruce and jack pine needles at BOREAS sites in SK, Canada. Can. J. Remote Sens. 1997, 23, 108–119. [Google Scholar] [CrossRef]
- Hall, F.G.; Huemmrich, K.F.; Strebel, D.E.; Goetz, S.J.; Nickeson, J.E.; Woods, K.D. SNF Leaf Optical Properties: Cary-14; Superior National Forest Leaf Optical Properties: Cary-14; Data Set; Oak Ridge National Laboratory Distributed Active Archive Center: Oak Ridge, TN, USA, 1996. Available online: http://daac.ornl.gov (accessed on 8 November 2017).
- Middleton, E.; Sullivan, J. BOREAS TE-10 Leaf Optical Properties for SSA Species; Data Set; Oak Ridge National Laboratory Distributed Active Archive Center: Oak Ridge, TN, USA, 2000. Available online: http://www.daac.ornl.gov (accessed on 8 November 2017).
- Hueni, A.; Nieke, J.; Schopfer, J.; Kneubühler, M.; Itten, K. The spectral database SPECCHIO for improved long term usability and data sharing. Comput. Geosci. 2009, 35, 557–565. [Google Scholar] [CrossRef] [Green Version]
- SPECCHIO Spectral Information System. Available online: http://www.specchio.ch/ (accessed on 8 November 2017).
- Noda, H.M.; Motohka, T.; Murakami, K.; Muraoka, H.; Nasahara, K.N. Reflectance and transmittance spectra of leaves and shoots of 22 vascular plant species and reflectance spectra of trunks and branches of 12 tree species in Japan. Ecol. Res. 2014, 29, 111. [Google Scholar] [CrossRef]
- Noda, H. Reflectance and Transmittance Spectra of Leaves and Shoots of 22 Vascular Plant Species and Reflectance Spectra of Trunks and Branches of 12 Tree Species in Japan. ERDP-2013-02.1.1. Available online: http://db.cger.nies.go.jp/JaLTER/metacat/metacat/ERDP-2013-02.1.1/default (accessed on 8 November 2017).
- Serbin, S. Fresh Leaf Spectra to Estimate Leaf Morphology and Biochemistry for Northern Temperate Forests. Data Set. Ecological Spectral Information System (EcoSIS, USA), 2014. Available online: http://ecosis.org (accessed on 8 November 2017).
- EcoSIS Spectral Library. Available online: https://ecosis.org/ (accessed on 8 November 2017).
- Qi, Y.; Dennison, P.E.; Jolly, W.M.; Kropp, R.C.; Brewer, S.C. Spectroscopic analysis of seasonal changes in live fuel moisture content and leaf dry mass. Remote Sens. Environ. 2014, 150, 198–206. [Google Scholar] [CrossRef]
- Kokaly, R.F.; Clark, R.N.; Swayze, G.A.; Livo, K.E.; Hoefen, T.M.; Pearson, N.C.; Wise, R.A.; Benzel, W.M.; Lowers, H.A.; Driscoll, R.L.; et al. USGS Spectral Library Version 7; U.S. Geological Survey Data Series 1035; United States Geological Survey: Reston, VA, USA, 2017; p. 61. [CrossRef]
- USGS Spectral Library Version 7. Available online: https://speclab.cr.usgs.gov/spectral-lib.html (accessed on 8 November 2017).
- Niinemets, Ü. A review of light interception in plant stands from leaf to canopy in different plant functional types and in species with varying shade tolerance. Ecol. Res. 2010, 25, 693–714. [Google Scholar] [CrossRef]
- Atherton, J.; Olascoaga, B.; Alonso, L.; Porcar-Castell, A. Spatial Variation of Leaf Optical Properties in a Boreal Forest Is Influenced by Species and Light Environment. Front. Plant Sci. 2017, 8, 309. [Google Scholar] [CrossRef] [PubMed]
- Lhotáková, Z.; Albrechtová, J.; Malenovský, Z.; Rock, B.N.; Polák, T.; Cudlín, P. Does the azimuth orientation of Norway spruce (Picea abies L. Karst.) branches within sunlit crown part influence the heterogeneity of biochemical, structural and spectral characteristics of needles? Environ. Exp. Bot. 2007, 59, 283–292. [Google Scholar] [CrossRef] [Green Version]
- Gebauer, R.; Čermák, J.; Plichta, R.; Špinlerová, Z.; Uban, J.; Volařík, D.; Ceulemans, R. Within-canopy variation in needle morphology and anatomy of vascular tissues in a sparse Scots pine forest. Trees 2015, 29, 1447–1457. [Google Scholar] [CrossRef]
- Miller, J.R.; Wu, J.; Boyter, M.G.; Belanger, M.; Hare, E.W. Seasonal patterns in leaf reflectance red-edge characteristics. Int. J. Remote Sens. 1991, 12, 1509–1523. [Google Scholar] [CrossRef]
- Wong, C.Y.S.; Gamon, J.A. The photochemical reflectance index provides an optical indicator of spring photosynthetic activation in evergreen conifers. New Phytol. 2015, 206, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Gamon, J.A.; Huemmrich, K.F.; Wong, C.Y.S.; Ensminger, I.; Garrity, S.; Hollinger, D.Y.; Noormets, A.; Peñuelas, J. A remotely sensed pigment index reveals photosynthetic phenology in evergreen conifers. Proc. Natl. Acad. Sci. USA 2016, 113, 13087–13092. [Google Scholar] [CrossRef] [PubMed]
- Peñuelas, J.; Filella, I.; Gamon, J.A. Assessment of photosynthetic radiation-use efficiency with spectral reflectance. New Phytol. 1995, 131, 291–296. [Google Scholar] [CrossRef]
- Mõttus, M.; Sulev, M.; Hallik, L. Seasonal course of the spectral properties of alder and birch leaves. IEEE J. Sel. Top. Appl. 2013, 7, 2496–2505. [Google Scholar] [CrossRef]
- Schaepman-Strub, G.; Schaepman, M.E.; Painter, T.H.; Dangel, S.; Martonchik, J.V. Reflectance quantities in optical remote sensing-definitions and case studies. Remote Sens. Environ. 2006, 103, 27–42. [Google Scholar] [CrossRef]
- Markiet, V.; Hernández-Clemente, R.; Mõttus, M. Spectral Similarity and PRI Variations for a Boreal Forest Stand Using Multi-angular Airborne Imagery. Remote Sens. 2017, 9, 1005. [Google Scholar] [CrossRef]
- Kokaly, R.F.; Asner, G.P.; Ollinger, S.V.; Martin, M.E.; Wessman, C.A. Characterizing canopy biochemistry from imaging spectroscopy and its application to ecosystem studies. Remote Sens. Environ. 2009, 113, S78–S91. [Google Scholar] [CrossRef]
- Ustin, S.L.; Roberts, D.A.; Gamon, J.A.; Asner, G.P.; Green, R.O. Using imaging spectroscopy to study ecosystem processes and properties. BioScience 2004, 54, 523–534. [Google Scholar] [CrossRef]
- Bond, B.J.; Farnsworth, B.T.; Coulombe, R.A.; Winner, W.E. Foliage physiology and biochemistry in response to light gradients in conifers with varying shade tolerance. Oecologia 1999, 120, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Moorthy, I.; Miller, J.R.; Noland, T.L. Estimating chlorophyll concentration in conifer needles with hyperspectral data: An assessment at the needle and canopy level. Remote Sens. Environ. 2008, 112, 2824–2838. [Google Scholar] [CrossRef]
- Homolová, L.; Lukeš, P.; Malenovský, Z.; Lhotáková, Z.; Kaplan, V.; Hanuš, J. Measurement methods and variability assessment of the Norway spruce total leaf area: Implications for remote sensing. Trees 2013, 27, 111–121. [Google Scholar] [CrossRef]
- Grant, L. Diffuse and specular characteristics of leaf reflectance. Remote Sens. Environ. 1987, 22, 309–322. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Lukjanova, A.; Turnbull, M.H.; Sparrow, A.D. Plasticity in mesophyll volume fractions modulates light-acclimation in needle photosynthesis in two pines. Tree Physiol. 2007, 12, 1137–1151. [Google Scholar] [CrossRef]
- Lhotáková, Z.; Urban, O.; Dubánková, M.; Cvikrová, M.; Tomášková, I.; Kubínová, L.; Zvára, K.; Marek, M.V.; Albrechtová, J. The impact of long-term CO2 enrichment on sun and shade needles of Norway spruce (Picea abies): Photosynthetic performance, needle anatomy and phenolics accumulation. Plant Sci. 2012, 188–189, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Radovanović, B.; Šinžar-Sekulić, J.; Rakić, T.; Živković, I.; Lakušić, D. Variation in needle anatomy of Picea omorika (Pinaceae) plants belonging to different gene pools in natural populations on Tara Mt. in Serbia. Bot. Serbica 2014, 38, 237–246. [Google Scholar]
- Ross, J.; Meinander, O.; Sulev, M. Spectral scattering properties of Scots pine shoots. In Proceedings of the Surface and Atmospheric Remote Sensing: Technologies, Data Analysis and Interpretation, Pasadena, CA, USA, 8–12 August 1994; Volume 1–4, pp. 1451–1454. [Google Scholar]
- Nilson, T.; Ross, J. The use of remote sensing in the modeling of forest productivity. In Modeling Radiative Transfer through Forest Canopies: Implications for Canopy Photosynthesis and Remote Sensing; Gholz, H.L., Nakane, K., Shimoda, H., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1997; pp. 23–60. [Google Scholar]
- Mõttus, M.; Rautiainen, M.; Schaepman, M.E. Shoot scattering phase function for Scots pine and its effect on canopy reflectance. Agric. For. Meteorol. 2012, 154–155, 67–74. [Google Scholar] [CrossRef]
- Rautiainen, M.; Mõttus, M.; Yáñez-Rausell, L.; Homolová, L.; Malenovský, Z.; Schaepman, M.E. A note on upscaling coniferous needle spectra to shoot spectral albedo. Remote Sens. Environ. 2012, 117, 469–474. [Google Scholar] [CrossRef]
- Carter, G.A.; Smith, W.K. Influence of shoot structure on light interception and photosynthesis in conifers. Plant Physiol. 1985, 79, 1038–1043. [Google Scholar] [CrossRef] [PubMed]
- Woolley, J.T. Reflectance and Transmittance of Light by Leaves. Plant Physiol. 1971, 47, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Mõttus, M.; Rautiainen, M. Scaling PRI between coniferous canopy structures. IEEE J. Sel. Top. Appl. 2013, 6, 708–714. [Google Scholar] [CrossRef]
- Lang, M.; Kuusk, A.; Nilson, T.; Lükk, T.; Pehk, M.; Alm, G. Reflectance Spectra of Ground Vegetation in Sub-Boreal Forests. 2002. Available online: http://www.aai.ee/bgf/ger2600/ (accessed on 8 November 2017).
- Cole, E.; Newton, M.; Bailey, J. Understory vegetation dynamics 15 years post-thinning in 50-year-old Douglas-fir and Douglas-fir/western hemlock stands in western Oregon, USA. For. Ecol. Manag. 2017, 384, 358–370. [Google Scholar] [CrossRef]
- Iiames, J.S.; Congalton, R.G.; Pilant, A.N.; Lewis, T.E. Leaf Area Index (LAI) Change Detection Analysis on Loblolly Pine (Pinus taeda) Following Complete Understory Removal. Photogramm. Eng. Remote Sens. 2008, 11, 1389–1400. [Google Scholar] [CrossRef]
- Kuusinen, N.; Stenberg, P.; Tomppo, E.; Bernier, P.; Berninger, F. Variation in understory and canopy reflectance during stand development in Finnish coniferous forests. Can. J. For. Res. 2015, 45, 1077–1085. [Google Scholar] [CrossRef]
- Spanner, M.; Pierce, L.; Peterson, D.; Running, S. Remote sensing of temperate coniferous leaf area index: The influence of canopy closure, understory vegetation, and background reflectance. Int. J. Remote Sens. 1990, 11, 95–111. [Google Scholar] [CrossRef]
- Deering, D.W.; Eck, T.F.; Banerjee, B. Characterization of the reflectance anisotropy of three boreal forest canopies in spring–summer. Remote Sens. Environ. 1999, 67, 205–229. [Google Scholar] [CrossRef]
- Suzuki, R.; Kobayashi, H.; Delbart, N.; Asanuma, J.; Hiyama, T. NDVI responses to the forest canopy and floor from spring to summer observed by airborne spectrometer in eastern Siberia. Remote Sens. Environ. 2011, 115, 3615–3624. [Google Scholar] [CrossRef]
- Canisius, F.; Chen, J.M. Retrieving forest background reflectance in a boreal region from Multi-angle Imaging SpectroRadiometer (MISR) data. Remote Sens. Environ. 2007, 107, 312–321. [Google Scholar] [CrossRef]
- Chopping, M.; Nolin, A.W.; Moisen, G.G.; Martonchik, J.V.; Bull, M. Forest canopy height from the Multiangle Imaging Spectro-Radiometer (MISR) assessed with high resolution discrete return lidar. Remote Sens. Environ. 2009, 113, 2172–2185. [Google Scholar] [CrossRef]
- Pisek, J.; Chen, J.M.; Miller, J.; Freemantle, J.; Peltoniemi, J.; Simic, A. Mapping forest background in a boreal region using multiangle Compact Airborne Spectrographic Imager data. IEEE Trans. Geosci. Remote Sens. 2010, 48, 499–510. [Google Scholar] [CrossRef]
- Yang, W.; Kobayashi, H.; Suzuki, R.; Nasahara, K. A simple method for retrieving understory NDVI in sparse needleleaf forests in Alaska using MODIS BRDF data. Remote Sens. 2014, 6, 11936–11955. [Google Scholar] [CrossRef]
- Knyazikhin, Y.; Martonchik, J.; Myneni, R.; Diner, D.; Running, S. Synergistic algorithm for estimating vegetation canopy leaf area index and fraction of absorbed photosynthetically active radiation from MODIS and MISR data. J. Geophys. Res. 1998, D103, 32257–32276. [Google Scholar] [CrossRef]
- Rautiainen, M.; Mõttus, M.; Heiskanen, J.; Akujärvi, A.; Majasalmi, T.; Stenberg, P. Seasonal reflectance dynamics of common understory types in a northern European boreal forest. Remote Sens. Environ. 2011, 115, 3020–3028. [Google Scholar] [CrossRef]
- Rautiainen, M.; Suomalainen, J.; Mottus, M.; Stenberg, P.; Voipio, P.; Peltoniemi, J.; Manninen, T. Coupling forest canopy and understory reflectance in the Arctic latitudes of Finland. Remote Sens. Environ. 2007, 110, 332–343. [Google Scholar] [CrossRef]
- Goward, S.N.; Huemmrich, K.F.; Waring, R.H. Visible-near infrared spectral reflectance of landscape components in western Oregon. Remote Sens. Environ. 1994, 47, 190–203. [Google Scholar] [CrossRef]
- Miller, J.; White, P.; Chen, J.M.; Peddle, D.; McDemid, G.; Fournier, R.; Shepherd, P.; Rubinstein, I.; Freemantle, J.; Soffer, R.; et al. Seasonal change in the understory reflectance of boreal forests and influence on canopy vegetation indices. J. Geophys. Res. 1997, 102, 29475–29482. [Google Scholar] [CrossRef]
- Kuusk, A.; Lang, M.; Nilson, T. Simulation of the reflectance of ground vegetation in sub-boreal forests. Agric. For. Meteorol. 2004, 126, 33–46. [Google Scholar] [CrossRef]
- Nikopensius, M.; Pisek, J.; Raabe, K. Spectral reflectance patterns and seasonal dynamics of common understory types in three mature hemi-boreal forests. Int. J. Appl. Earth Obs. Geoinf. 2015, 43, 84–91. [Google Scholar] [CrossRef]
- Kobayashi, H.; Suzuki, R.; Kobayashi, S. Reflectance seasonality and its relation to the canopy leaf area index in an eastern Siberian larch forest: Multi-satellite data and radiative transfer analyses. Remote Sens. Environ. 2007, 106, 238–252. [Google Scholar] [CrossRef]
- Peterson, U. Seasonal Reflectance Profiles for Forest Clearcut Communities at Early Stages of Secondary Succession; Section of Physics and Astronomy; Preprint A-5; Academy of Sciences of the Estonian SSR: Tartu, Estonia, 1989. [Google Scholar]
- Lohila, A.; Minkkinen, K.; Aurela, M.; Tuovinen, J.-P.; Pentilla, T.; Ojanen, P.; Laurila, T. Greenhouse gas flux measurements in a forestry-drained peatland indicate a large carbon sink. Biogeosciences 2011, 8, 3203–3218. [Google Scholar] [CrossRef] [Green Version]
- Chopping, M.; Schaaf, C.B.; Zhao, F.; Wang, Z.; Nolin, A.W.; Moisen, G.G.; Martonchik, J.V.; Bull, M. Forest structure and aboveground biomass in the southwestern United States from MODIS and MISR. Remote Sens. Environ. 2011, 115, 2943–2953. [Google Scholar] [CrossRef]
- Peltoniemi, J.; Kaasalainen, S.; Näränen, J.; Rautiainen, M.; Stenberg, P.; Smolander, H.; Smolander, S.; Voipio, P. BRDF measurement of understory vegetation in pine forests: Dwarf shrubs, lichen and moss. Remote Sens. Environ. 2005, 94, 343–354. [Google Scholar] [CrossRef]
- Hallik, L.; Kull, O.; Nilson, T.; Penuelas, J. Spectral reflectance of multispecies herbaceous and moss canopies in the boreal forest understory and open field. Can. J. Remote Sens. 2009, 35, 474–485. [Google Scholar] [CrossRef]
- Schaepman-Strub, G.; Limpens, J.; Menken, M.; Bartholomeus, H.; Schaepman, M. Towards spatial assessment of carbon sequestration in peatlands: Spectroscopy based estimation of fractional cover of three plant functional types. Biogeosciences 2009, 6, 275–284. [Google Scholar] [CrossRef] [Green Version]
- Stenberg, P.; Mõttus, M.; Rautiainen, M. Photon recollision probability in modelling the radiation regime of canopies—A review. Remote Sens. Environ. 2016, 183, 98–108. [Google Scholar] [CrossRef]
- Rautiainen, M.; Lang, M.; Mõttus, M.; Kuusk, A.; Nilson, T.; Kuusk, J.; Lükk, T. Multi-angular reflectance properties of a hemiboreal forest: An analysis using CHRIS PROBA data. Remote Sens. Environ. 2008, 112, 2627–2642. [Google Scholar] [CrossRef]
- Verrelst, J.; Schaepman, M.E.; Koetz, B.; Kneubühler, M. Angular sensitivity analysis of vegetation indices derived from CHRIS/PROBA data. Remote Sens. Environ. 2008, 112, 2341–2353. [Google Scholar] [CrossRef]
- Diner, D.J.; Beckert, J.C.; Reilly, T.H.; Bruegge, C.J.; Conel, J.E.; Kahn, R.A.; Martonchik, J.V.; Ackerman, T.P.; Davies, R.; Gerstl, S.A.W.; et al. Multi-angle Imaging SpectroRadiometer (MISR) instrument description and experiment overview. IEEE Trans. Geosci. Remote Sens. 1998, 36, 1072–1087. [Google Scholar] [CrossRef]
- Barnsley, M.J.; Settle, J.J.; Cutter, M.A.; Lobb, D.R.; Teston, F. The PROBA/CHRIS mission: A low-cost smallsat for hyperspectral multiangle observations of the Earth surface and atmosphere. IEEE Trans. Geosci. Remote Sens. 2004, 42, 1512–1520. [Google Scholar] [CrossRef]
- Hernández-Clemente, R.; Kolari, P.; Porcar-Castell, A.; Korhonen, L.; Mõttus, M. Tracking the seasonal dynamics of boreal forest photosynthesis using EO-1 Hyperion reflectance: sensitivity to structural and illumination effects. IEEE Trans. Geosci. Remote Sens. 2016, 54, 5105–5116. [Google Scholar] [CrossRef]
- Knapp, A.K.; Carter, G.A. Variability in leaf optical properties among 26 species from a broad range of habitats. Am. J. Bot. 1998, 85, 940–946. [Google Scholar] [CrossRef] [PubMed]
- Zarco-Tejada, P.J.; Miller, J.R.; Harron, J.; Hu, B.; Noland, T.L.; Goel, N.; Mohammed, G.H.; Sampson, P. Needle chlorophyll content estimation through model inversion using hyperspectral data from boreal conifer forest canopies. Remote Sens. Environ. 2004, 89, 189–199. [Google Scholar] [CrossRef]
- Stimson, H.C.; Breshears, D.D.; Ustin, S.L.; Kefauver, S.C. Spectral sensing of foliar water conditions in two co-occurring conifer species: Pinus edulis and Juniperus monosperma. Remote Sens. Environ. 2005, 96, 108–118. [Google Scholar] [CrossRef]
- Malenovský, Z.; Albrechtová, J.; Lhotáková, Z.; Zurita-Milla, R.; Clevers, J.G.P.W.; Schaepman, M.E.; Cudlín, P. Applicability of the PROSPECT model for Norway spruce needles. Int. J. Remote Sens. 2006, 27, 5315–5340. [Google Scholar] [CrossRef]
- Huang, D.; Knyazikhin, Y.; Dickinson, R.E.; Rautiainen, M.; Stenberg, P.; Disney, M.; Lewis, P.; Cescatti, A.; Tian, Y.; Verhoef, W.; et al. Canopy spectral invariants for remote sensing and model applications. Remote Sens. Environ. 2007, 106, 106–122. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, J.M.; Miller, J.R.; Noland, T.L. Retrieving chlorophyll content in conifer needles from hyperspectral measurements. Can. J. Remote Sens. 2008, 34, 296–310. [Google Scholar] [CrossRef]
- Di Vittorio, A.V.; Biging, G.S. Spectral identification of ozone-damaged pine needles. Int. J. Remote Sens. 2009, 30, 3041–3073. [Google Scholar] [CrossRef]
- Kuusk, A.; Kuusk, J.; Lang, M. A dataset for the validation of reflectance models. Remote Sens. Environ. 2009, 113, 889–892. [Google Scholar] [CrossRef]
- Croft, H.; Chen, J.M.; Zhang, Y.; Simic, A. Modelling leaf chlorophyll content in broadleaf and needle leaf canopies from ground, CASI, Landsat TM 5 and MERIS reflectance data. Remote Sens. Environ. 2013, 133, 128–140. [Google Scholar] [CrossRef]
- Dengel, S.; Grace, J.; Aakala, T.; Hari, P.; Newberry, S.L.; Mizunuma, T. Spectral characteristics of pine needles at the limit of tree growth in subarctic Finland. Plant Ecol. Divers. 2013, 6, 31–44. [Google Scholar] [CrossRef]
- Olascoaga, B.; Mac Arthur, A.; Atherton, J.; Porcar-Castell, A. A comparison of methods to estimate photosynthetic light absorption in leaves with contrasting morphology. Tree Physiol. 2016, 36, 368–379. [Google Scholar] [CrossRef] [PubMed]
- Potůčková, M.; Červená, L.; Kupková, L.; Lhotáková, Z.; Lukeš, P.; Hanuš, J.; Novotný, J.; Albrechtová, J. Comparison of reflectance measurements acquired with a contact probe and an integration sphere: Implications for the spectral properties of vegetation at a needle-level. Sensors 2016, 16, 1801. [Google Scholar] [CrossRef] [PubMed]
- Homolová, L.; Janoutová, R.; Lukeš, P.; Hanuš, J.; Novotný, J.; Brovkina, O.; Loyaza Fernandez, R.R. In situ data collection supporting remote sensing estimation of spruce forest parameters at the ecosystem station Bílý Kříž. Beskydy 2017. under review. [Google Scholar]
- Porra, R.J.; Thompson, W.A.; Kriedemann, P.E. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: Verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochem. Biophys. Acta 1989, 975, 384–394. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic membranes. Method Enzymol. 1987, 148, 350–382. [Google Scholar]
- Hanuš, J.; Fabiánek, T.; Fajmon, L. Potential of airborne imaging spectroscopy at CzechGlobe. Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2016, XLI-B1, 15–17. [Google Scholar] [CrossRef]
- Richter, R.; Schläpfer, D. Geo-atmospheric processing of airborne imaging spectrometry data. Part 2: Atmospheric/topographic correction. Int. J. Remote Sens. 2002, 23, 2631–2649. [Google Scholar] [CrossRef]








| Dataset | Region | nm | Geom 1 | R or T 2 | Species |
|---|---|---|---|---|---|
| [16] | Italy (Temperate) | 400–2500 | IS | R | Picea abies, Pinus contorta, Pinus wallichiana, Pseudotsuga menziesii |
| [25,35] | MN, USA (Boreal) | 350–2100 | NA | R, T | Abies balsamea, Larix laricina, Picea mariana, Pinus banksiana, Pinus resinosa |
| [36] | SK, Canada (Boreal) | 400–1000 | IS | R, T | Picea glauca, Picea mariana, Pinus banksiana |
| [27,37,38] | Finland (Boreal) | 350–2500 | IS | R, T | Picea abies, Pinus sylvestris |
| [39,40] | Honshu, Japan (Temperate) | 350–2500 | IS | R, T | Chamaecyparis obtusa, Cryptomeria japonica, Cunninghamia lanceolata, Larix kaempferi, Picea abies, Pinus densiflora |
| [41,42] | North Central and NE USA (Temperate) | 350–2500 | NC/IS 4 | R, T | Abies balsamea, Juniperus virginiana, Larix laricina, Picea mariana, Picea rubens, Pinus banksiana, Pinus resinosa, Pinus strobus, Pinus sylvestris, Thuja occidentalis, Tsuga canadensis |
| [42,43] | MT, USA (Temperate) | 350–2500 | IS | R | Pinus contorta |
| [11,37,38] | Finland (Boreal) | 350–2500 | IS | R, T | Abies balsamea, Abies sibirica, Larix gmelinii, Larix laricina, Larix sibirica, Picea abies, Picea glauca, Picea mariana, Pinus banksiana, Pinus contorta, Pinus sylvestris, Pseudotsuga menziesii |
| [44,45] | USA (Temperate) | 350–2500 3 | BF/NA 5 | R | Picea engelmannii, Picea pungens, Pinus edulis, Pinus sabiniana |
| Species (n) | Chl a+b (mg/g) | Car (mg/g) | Nitrogen (%) | Cellulose (%) | Lignin (%) | Starch (%) |
|---|---|---|---|---|---|---|
| Picea abies (5) | 7.36 | 1.53 | 7.08 | 27.23 | 13.43 | 0.59 |
| Pseudotsuga menziesii (2) | 6.67 | 1.43 | 8.58 | 25.17 | 14.92 | n.a. |
| Pinus contorta (2) | 10.44 | 2.35 | 8.93 | 34.17 | 12.83 | 0.82 |
| Stand Age | Canopy Position | Needle Age | SLAh (cm2/g) | LAT CF | Cab Per Dry Matter (mg/g) | Car Per Dry Matter (mg/g) | Cab Per Needle Area (µg/cm2) | Car Per Needle Area (µg/cm2) | Cm Per Needle Area (g/cm2) | Cw Per Needle Area (g/cm2) |
|---|---|---|---|---|---|---|---|---|---|---|
| Immature (~40 years) | Exposed | current | 64.73 | 3.46 | 0.75 | 0.14 | 29.27 | 5.68 | 0.016 | 0.024 |
| older | 53.74 | 3.48 | 1.03 | 0.23 | 42.17 | 9.24 | 0.019 | 0.022 | ||
| Shaded | current | 104.26 | 2.88 | 0.80 | 0.16 | 22.05 | 4.39 | 0.010 | 0.017 | |
| older | 69.34 | 2.88 | 1.25 | 0.26 | 44.12 | 9.12 | 0.014 | 0.020 | ||
| Mature (~80 years) | Exposed | current | 79.57 | 3.51 | 0.72 | 0.14 | 22.51 | 4.45 | 0.013 | 0.018 |
| older | 58.44 | 3.36 | 1.09 | 0.23 | 41.86 | 8.78 | 0.017 | 0.020 | ||
| Shaded | current | 102.70 | 2.78 | 0.96 | 0.19 | 23.65 | 4.77 | 0.010 | 0.014 | |
| older | 92.38 | 2.76 | 1.21 | 0.24 | 30.29 | 6.08 | 0.011 | 0.014 |
| Dataset | Region | Biome | Wavelengths | Species |
|---|---|---|---|---|
| [25,35] | MN, USA | Boreal | 350–2100 nm | Abies balsamea, Larix laricina, Picea mariana |
| [73] | Estonia | (Hemi) boreal | 400–2460 nm | Picea abies, Pinus sylvestris |
| [39,40] | Honshu, Japan | Temperate | 350–2500 nm | Cryptomeria japonica, Larix kaempferi |
| Strengths | Weaknesses |
|
|
| Opportunities | Threats |
|
|
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rautiainen, M.; Lukeš, P.; Homolová, L.; Hovi, A.; Pisek, J.; Mõttus, M. Spectral Properties of Coniferous Forests: A Review of In Situ and Laboratory Measurements. Remote Sens. 2018, 10, 207. https://doi.org/10.3390/rs10020207
Rautiainen M, Lukeš P, Homolová L, Hovi A, Pisek J, Mõttus M. Spectral Properties of Coniferous Forests: A Review of In Situ and Laboratory Measurements. Remote Sensing. 2018; 10(2):207. https://doi.org/10.3390/rs10020207
Chicago/Turabian StyleRautiainen, Miina, Petr Lukeš, Lucie Homolová, Aarne Hovi, Jan Pisek, and Matti Mõttus. 2018. "Spectral Properties of Coniferous Forests: A Review of In Situ and Laboratory Measurements" Remote Sensing 10, no. 2: 207. https://doi.org/10.3390/rs10020207
APA StyleRautiainen, M., Lukeš, P., Homolová, L., Hovi, A., Pisek, J., & Mõttus, M. (2018). Spectral Properties of Coniferous Forests: A Review of In Situ and Laboratory Measurements. Remote Sensing, 10(2), 207. https://doi.org/10.3390/rs10020207