Remote Sensing to Detect Nests of the Leaf-Cutting Ant Atta sexdens (Hymenoptera: Formicidae) in Teak Plantations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Acquisition and Pre-processing of Satellite Images
2.3. Spectral Characterization of Leaf-cutting Ant Nests
2.4. Prediction and Mapping of the Leaf-cutting Ant Nests
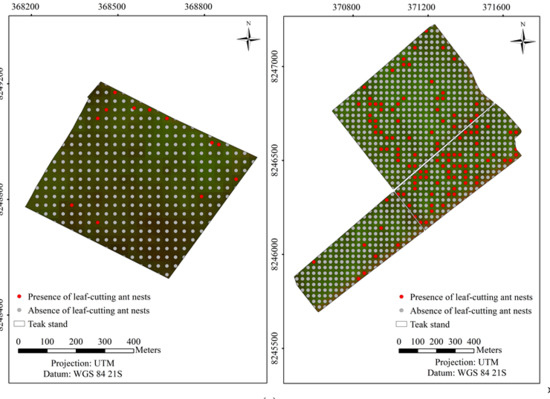
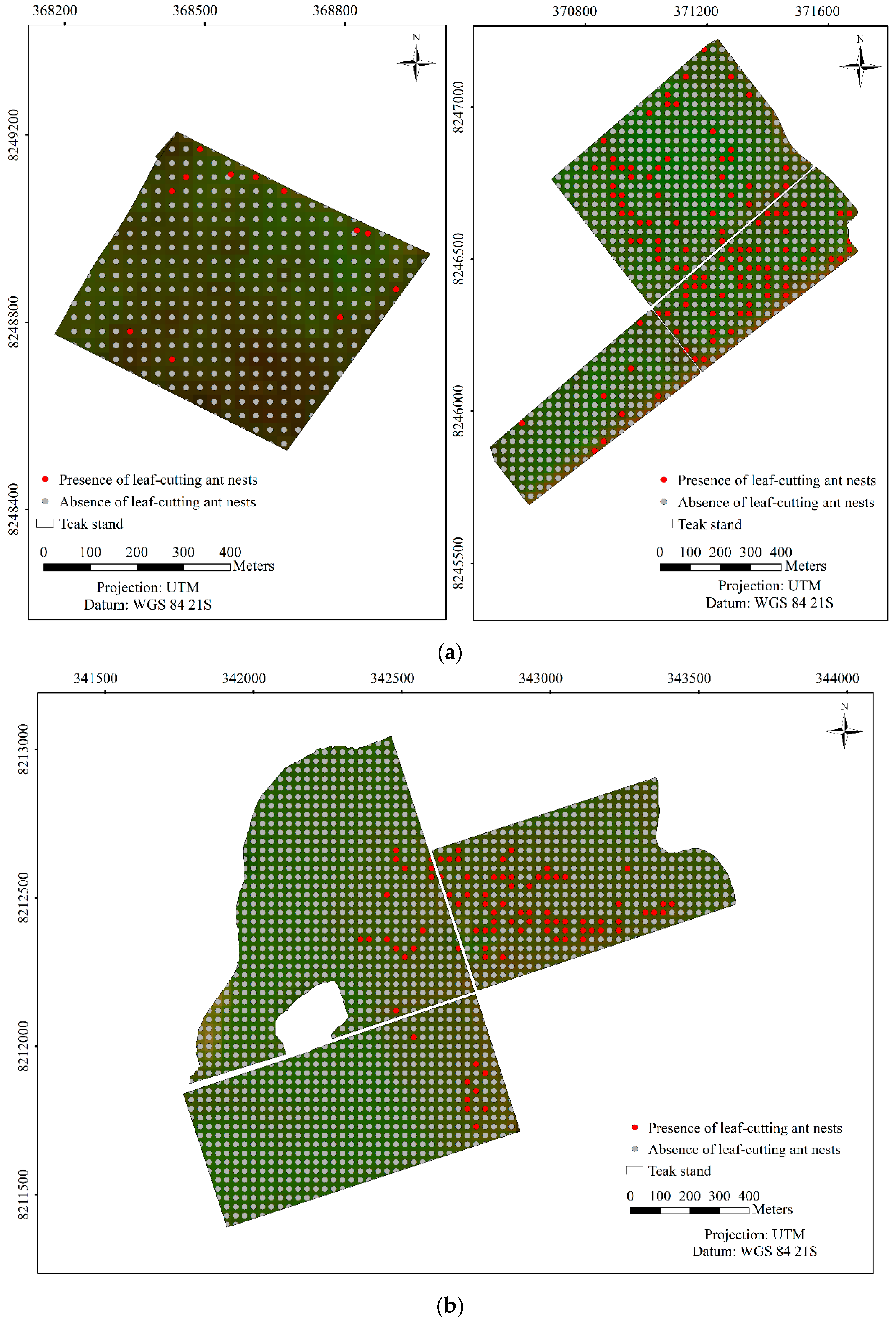
3. Results
3.1. Spectral Caracterization of the Leaf-cutting Ant Nests
3.2. Predicting and Mapping Leaf-cutting Ant Nests
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Deb, J.C.; Phinn, S.; Butt, N.; McAlpine, C.A. Climatic-induced shifts in the distribution of teak (Tectona grandis) in Tropical Asia: Implications for forest management and planning. Environ. Manag. 2017, 60, 422–435. [Google Scholar] [CrossRef]
- Brocco, V.F.; Paes, J.B.; da Costa, L.G.; Brazolin, S.; Arantes, M.D.C. Potential of teak heartwood extracts as a natural wood preservative. J. Clean. Prod. 2017, 142, 2093–2099. [Google Scholar] [CrossRef]
- Hansen, O.K.; Changtragoon, S.; Ponoy, B.; Kjær, E.D.; Minn, Y.; Finkeldey, R.; Nielsen, K.B.; Graudal, L. Genetic resources of teak (Tectona grandis Linn. f.)—Strong genetic structure among natural populations. Tree Genet. Genomes 2015, 11, 802–818. [Google Scholar] [CrossRef]
- Produce Conserve Include. Available online: http://www.pcimonitor.org/ (accessed on 19 April 2018).
- Zanetti, R.; Zanuncio, J.C.; Vilela, E.F.; Leite, H.G.; Jaffé, K.; Oliveira, A.C. Level of economic damage for leaf-cutting ants (Hymenoptera: Formicidae) in Eucalyptus plantations in Brazil. Sociobiology 2003, 42, 433–442. [Google Scholar]
- Souza, A.; Zanetti, R.; Calegario, N. Nível de dano econômico para formigas-cortadeiras em função do índice de produtividade florestal de eucaliptais em uma região de Mata Atlântica. Neotrop. Entomol. 2011, 40, 483–488. [Google Scholar]
- Hölldobler, B.; Wilson, E.O. The Leafcutter Ants: Civilization by Instinct; W.W. Norton & Company: New York, NY, USA, 2011. [Google Scholar]
- Arenas, A.; Roces, F. Avoidance of plants unsuitable for the symbiotic fungus in leaf-cutting ants: Learning can take place entirely at the colony dump. PLoS ONE 2017, 12, e0171388. [Google Scholar] [CrossRef]
- Alma, A.M.; Farji-Brener, A.G.; Elizalde, L. Gone with the wind: Short and long-term responses of leaf-cutting ants to the negative effect of wind on their foraging activity. Behav. Ecol. 2016, 27, 1017–1024. [Google Scholar] [CrossRef]
- Jaffé, K. El Mundo De Las Hormigas; Equinocio: Caracas, Venezuela, 2004. [Google Scholar]
- Costa, N.A.; Vasconcelos, L.H.; Vieira-Neto, H.M.E.; Bruna, M.E. Do herbivores exert top-down effects in Neotropical savannas? Estimates of biomass consumption by leaf-cutter ants. J. Veg. Sci. 2008, 19, 849–854. [Google Scholar] [CrossRef]
- Zanuncio, J.C.; Torres, J.B.; Gasperazzo, W.L.; Zanuncio, T.V. Aferição de dosagens de iscas granuladas para controle de Atta laevigata (F. Smith) pelo número de olheiros ativos. Rev. Árvore 1996, 20, 241–246. [Google Scholar]
- Zanetti, R.; Zanuncio, J.C.; Santos, J.C.; Da Silva, W.L.P.; Ribeiro, G.T.; Lemes, P.G. An overview of integrated management of leaf-cutting ants (Hymenoptera: Formicidae) in Brazilian forest plantations. Forests 2014, 5, 439–454. [Google Scholar] [CrossRef]
- Reis, M.A.; Zanetti, R.; Scolforo, J.R.S.; Ferreira, M.Z. Sampling plans for leaf-cutting ant nests (Hymenoptera: Formicidae) by the methods of strip transects and line transects on eucalyptus plantations. Rev. Árvore 2010, 34, 1101–1108. [Google Scholar] [CrossRef]
- Zanetti, R.; Zanuncio, J.C.; Vilela, E.F.; Leite, H.G.; Della Lucia, T.M.C.; Couto, L. Efeito da espécie de eucalipto e da vegetação nativa circundante sobre o custo de combate a sauveiros em eucaliptais. Rev. Árvore 1999, 23, 321–325. [Google Scholar]
- Zanetti, R.; Reis, M.A.; Mendonça, L.A. Métodos de amostragem de formigas-cortadeiras em florestas cultivadas. In Insetos Sociais: Da Biologia à Aplicação, 1st ed.; Della Lucia, T.M.C., Ed.; Editora UFV: Viçosa, Brazil, 2008; pp. 397–412. [Google Scholar]
- Moshou, D.; Bravo, C.; Oberti, R.; West, J.; Bodria, L.; McCartney, A.; Ramon, H. Plant disease detection based on data fusion of hyper-spectral and multi-spectral fluorescence imaging using Kohonen maps. Real-Time Imaging 2005, 11, 75–83. [Google Scholar] [CrossRef]
- Townsend, P.A.; Singh, A.; Foster, J.R.; Rehberg, N.J.; Kingdon, C.C.; Eshleman, K.N.; Seagle, S.W. A general Landsat model to predict canopy defoliation in broadleaf deciduous forests. Remote Sens. Environ. 2012, 119, 255–265. [Google Scholar] [CrossRef]
- Santos, A.; Oumar, Z.; Arnhold, A.; Silva, N.; Silva, C.O.; Zanetti, R. Multispectral characterization, prediction and mapping of Thaumastocoris peregrinus (Hemiptera: Thaumascoridae) attack in Eucalyptus plantations using remote sensing. J. Spat. Sci. 2016, 8596, 1–11. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Hornero, A.; Hernández-Clemente, R.; Beck, P.S.A. Understanding the temporal dimension of the red-edge spectral region for forest decline detection using high-resolution hyperspectral and Sentinel-2A imagery. ISPRS J. Photogramm. Remote Sens. 2018, 137, 134–148. [Google Scholar] [CrossRef] [PubMed]
- Peña, M.A.; Altmann, S.H. Use of satellite-derived hyperspectral indices to identify stress symptoms in an Austrocedrus chilensis forest infested by the aphid Cinara cupressi. Int. J. Pest. Manag. 2009, 55, 197–206. [Google Scholar] [CrossRef]
- Spruce, J.P.; Sader, S.; Ryan, R.E.; Smoot, J.; Kuper, P.; Ross, K.; Prados, D.; Russell, J.; Gasser, G.; McKellip, R.; et al. Assessment of MODIS NDVI time series data products for detecting forest defoliation by gypsy moth outbreaks. Remote Sens. Environ. 2011, 115, 427–437. [Google Scholar] [CrossRef]
- Sangüesa-Barreda, G.; Camarero, J.J.; García-Martín, A.; Hernández, R.; De la Riva, J. Remote-sensing and tree-ring based characterization of forest defoliation and growth loss due to the Mediterranean pine processionary moth. For. Ecol. Manag. 2014, 320, 171–181. [Google Scholar] [CrossRef] [Green Version]
- Çoban, H.O.; Özçelik, R.; Avci, M. Monitoring of damage from cedar shoot moth Dichelia cedricola Diakonoff (Lep: Tortricidae) by multi-temporal Landsat imagery. IForest 2014, 7, 126–131. [Google Scholar] [CrossRef]
- Isering, R.; Neumeister, L. Insecticides for Control of Pest Insects in FSC Certified Forests in Brazil: Recommendations by Technical Advisors; FSC Pesticides Committee: Bonn, Germany, 2010; Available online: http://www.ipef.br/pccf/arquivos/TA_Rec_alpha-Cypermethrin-Deltamethrin-Fenitrothion-Fipronil-Sulfluramid_BR_2010.pdf (accessed on 10 April 2018).
- Zanuncio, J.C.; Lemes, P.G.; Antunes, L.R.; Maia, J.L.S.; Mendes, J.E.P.; Tanganelli, K.M.; Salvador, J.F.; Serrão, J.E. The impact of the Forest Stewardship Council (FSC) pesticide policy on the management of leaf-cutting ants and termites in certified forests in Brazil. Ann. For. Sci. 2016, 73, 205–214. [Google Scholar] [CrossRef] [Green Version]
- FSC. Pesticides Policy Guidance Addendum: List of Approved Derogations for Use of ‘Highly Hazardous’ Pesticides; Forest Stewardship Council: Bonn, Germany, 2018; Available online: https://ic.fsc.org/file-download. list-of-approved-derogations-for-use-of-highly-hazardous-pesticides.a-2651.pdf (accessed on 10 April 2018).
- Lemes, P.G.; Zanuncio, J.C.; Serrão, J.E.; Lawson, S.A. Forest stewardship council (FSC) pesticide policy and integrated pest management in certified tropical plantations. Environ. Sci. Pollut. R. 2017, 24, 1283–1295. [Google Scholar] [CrossRef] [PubMed]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; Gonçalves, J.L.M.; Sparovek, G. Köppen’s climate classification map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef]
- Bolton, B. A New General Catalogue of the Ants of the World; Harvard University Press: Cambridge, MA, USA; London, UK, 1995; p. 504. [Google Scholar]
- QGIS Development Team. QGIS Geographic Information System. Open Source Geospatial Foundation Project. 2018. Available online: http://www.qgis.org/ (accessed on 5 February 2018).
- Lopes, J.F.S.; Brugger, M.S.; Menezes, R.B.; Camargo, R.S.; Forti, L.C.; Fourcassié, V. Spatio-temporal dynamics of foraging networks in the grass-cutting ant Atta bisphaerica Forel, 1908 (Formicidae, Attini). PLoS ONE 2016, 11, e0146613. [Google Scholar] [CrossRef] [PubMed]
- USGS. Landsat 8 (L8) Data Users Handbook, Sioux Falls: Department of the Interior: U.S. Geological Survey. 2016. Available online: https://www.usgs.gov/media/files/landsat-8-data-users-handbook (accessed on 3 April 2018).
- Teillet, P.M.; Guidon, B.; Goodenough, D.G. On the slope-aspect correction of multi-spectral scanner data. Can. J. Remote Sens. 1982, 8, 84–106. [Google Scholar] [CrossRef]
- Oumar, Z.; Mutanga, O. Using WorldView-2 bands and indices to predict bronze bug (Thaumastocoris peregrinus) damage in plantation forests. Int. J. Remote Sens. 2013, 34, 2236–2249. [Google Scholar] [CrossRef]
- Li, P.; Jiang, L.; Feng, Z. Cross-comparison of vegetation indices derived from Landsat-7 Enhanced Thematic Mapper Plus (ETM+) and Landsat-8 Operational Land Imager (OLI) sensors. Remote Sens. 2014, 6, 310–329. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017; Available online: https://www.r-project.org/ (accessed on 5 March 2018).
- Hijmans, R.J. Raster: Geographic Data Analysis and Modeling, R Package Version 2.8-19. 2019. Available online: https://CRAN.R-project.org/package=raster (accessed on 2 April 2019).
- Bivand, R.; Keitt, T.; Rowlingson, B. Rgdal: Bindings for the ‘Geospatial’ Data Abstraction Library, R Package Version 1.3-6. 2018. Available online: https://CRAN.R-project.org/package=rgdal (accessed on 2 April 2019).
- Rowlingson, B.; Diggle, P. Splancs: Spatial and Space-Time Point Pattern Analysis, R Package Version 2.01-40. 2017. Available online: https://CRAN.R-project.org/package=splancs (accessed on 2 April 2019).
- Goslee, S.C. Analyzing remote sensing data in R: The Landsat package. J. Stat. Softw. 2011, 43, 1–17. [Google Scholar] [CrossRef]
- Santos, A. Landsat8: Landsat 8 Imagery Rescaled to Reflectance, Radiance and/or Temperature, R Package Version 0.1-10. 2017. Available online: https://CRAN.R-project.org/package=landsat8 (accessed on 2 April 2019).
- Stabler, B. Shapefiles: Read and Write ESRI Shapefiles, R Package Version 0.7. 2013. Available online: https://CRAN.R-project.org/package=shapefiles (accessed on 2 April 2019).
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral. Ecol. 2001, 26, 32–46. [Google Scholar] [CrossRef]
- Perumal, K.; Bhaskaran, R. Supervised classification performance of multispectral images. J. Comput. 2010, 2, 124–129. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Peter, R.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; et al. Vegan Community Ecology R Package Version 2.5-3. 2018. Available online: https://CRAN.R-project.org/package=vegan (accessed on 16 July 2018).
- Pérez-Enciso, M.; Tenenhaus, M. Prediction of clinical outcome with microarray data: A partial least squares discriminant analysis (PLS-DA) approach. Hum. Genet. 2003, 112, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Mevik, B.H.; Wehrens, R.; Liland, K.L. Pls: Partial Least Squares and Principal Component Regression, R Package Version 2.7-0. 2018. Available online: https://CRAN.R-project.org/package=pls (accessed on 2 April 2019).
- Geladi, P.; Kowalski, B.R. Partial least-squares regression: A tutorial. Anal. Chim. Acta 1986, 185, 1–17. [Google Scholar] [CrossRef]
- Tenenhaus, M. La régression PLS: Théorie et pratique; Editions Thecnip: Paris, France, 1998. [Google Scholar]
- Rohart, F.; Gautier, B.; Singh, A.; Le Cao, K.A. Mixomics: An R package for ‘omics feature selection and multiple data integration. PLoS Comput. Biol. 2017, 13, e1005752. [Google Scholar] [CrossRef] [PubMed]
- Pastore, T.C.M.; Braga, J.W.B.; Coradin, V.T.R.; Magalhães, W.L.E.; Okino, E.Y.A.; Camargos, J.A.A.; Muñiz, G.I.B.; Bressan, O.A.; Davrieux, F. Near infrared spectroscopy (NIRS) as a potential tool for monitoring trade of similar woods: Discrimination of true mahogany, cedar, andiroba, and curupixá. Holzforschung 2011, 65, 73–80. [Google Scholar] [CrossRef]
- Seasholtz, M.B.; Kowalski, B. The parsimony principle applied to multivariate calibration. Anal. Chim. Acta 1993, 277, 165–177. [Google Scholar] [CrossRef]
- Oumar, M.S.; Peerbhay, K.; Germishuizen, I.; Mutanga, O.; Oumar, Z. Detecting canopy damage caused by Uromycladium acaciae on South African Black Wattle forest compartments using moderate resolution satellite imagery. S. Afr. J. Geomat. 2019, 8, 69–83. [Google Scholar] [CrossRef]
- Christian, B.; Krishnayya, N.S.R. Spectral signatures of teak (Tectona grandis L.) using hyperspectral (EO-1) data. Curr. Sci. 2007, 93, 1291–1296. [Google Scholar]
- Lobell, D.B.; Asner, G.P. Moisture effects on soil reflectance. Soil Sci. Soc. Am. J. 2002, 66, 722–727. [Google Scholar] [CrossRef]
- Xu, H. Modification of normalized difference water index (NDWI) to enhance open water features in remotely sensed imagery. Int. J. Remote Sens. 2006, 27, 3025–3033. [Google Scholar] [CrossRef]
- Stylinski, C.D.; Gamon, J.; Oechel, W.C. Seasonal patterns of reflectance indices, carotenoid pigments and photosynthesis of evergreen chaparral species. Oecologia 2002, 131, 366–374. [Google Scholar] [CrossRef]
- Gamon, J.A.; Surfus, J.S. Assessing leaf pigment content with a reflectometer. New Phytol. 1999, 143, 105–117. [Google Scholar] [CrossRef]
- Hao, P.; Chen, Z.; Tang, H.; Li, D.; Li, H. New workflow of plastic-mulched farmland mapping using multi-temporal Sentinel-2 data. Remote Sens. 2019, 11, 1353. [Google Scholar] [CrossRef]
- Cantarelli, E.B.; Costa, E.C.; Zanetti, R.; Pezzutti, R. Acromyrmex spp. (Hymenoptera: Formicidae) sampling plan in Pinus spp. pre-planting áreas. Cienc. Rural 2006, 36, 385–390. [Google Scholar] [CrossRef]
- Reis, M.A.; Zanetti, R.; Scolforo, J.R.S.; Ferreira, M.Z.; Zanuncio, J.C. Sampling of leaf-cutting ant nests (Hymenoptera: Formicidae) in Eucalyptus plantations using quadrant and Prodan methods. Sociobiology 2008, 51, 21–29. [Google Scholar]


| Landsat OLI Bands | Spectral Resolution (Micrometers) |
|---|---|
| Band 2—Blue | 0.452–0.512 |
| Band 3—Green | 0.533–0.590 |
| Band 4—Red | 0.636–0.673 |
| Band 5—Near Infrared (NIR) | 0.851–0.879 |
| Band 6—Shortwave Infrared SWIR 1 | 1.566–1.651 |
| Band 7—Shortwave Infrared SWIR 2 | 2.107–2.294 |
| Vegetation Indexes (VI) | Reflectance |
|---|---|
| Atmospherically resistant vegetation index (ARVI) | (Near infrared − 2Red − Blue)/(Near infrared + 2Red − Blue) |
| Land surface water index (LSWI) | (Near infrared − Shortwave infrared 1)/(Near infrared + Shortwave infrared 1) |
| Modified normalized difference water index (MNDWI) | (Green − Shortwave infrared 1)/(Green + Shortwave infrared 1) |
| Normalized burn ratio (NBR) | (Near infrared − Shortwave infrared 2)/(Near infrared + Shortwave infrared 2) |
| Normalized difference vegetation index (NDVI) | (Near infrared − Red)/(Near infrared + Red) |
| Photochemical reflectance index (PRI) | (Blue − Green)/(Blue + Green) |
| Red green ratio index (RGI) | Red/Green |
| Simple ratio (SR) | Near infrared/Red |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, I.C.d.L.; Santos, A.d.; Oumar, Z.; Soares, M.A.; Silva, J.C.C.; Zanetti, R.; Zanuncio, J.C. Remote Sensing to Detect Nests of the Leaf-Cutting Ant Atta sexdens (Hymenoptera: Formicidae) in Teak Plantations. Remote Sens. 2019, 11, 1641. https://doi.org/10.3390/rs11141641
Santos ICdL, Santos Ad, Oumar Z, Soares MA, Silva JCC, Zanetti R, Zanuncio JC. Remote Sensing to Detect Nests of the Leaf-Cutting Ant Atta sexdens (Hymenoptera: Formicidae) in Teak Plantations. Remote Sensing. 2019; 11(14):1641. https://doi.org/10.3390/rs11141641
Chicago/Turabian StyleSantos, Isabel Carolina de Lima, Alexandre dos Santos, Zakariyyaa Oumar, Marcus Alvarenga Soares, Július César Cerqueira Silva, Ronald Zanetti, and José Cola Zanuncio. 2019. "Remote Sensing to Detect Nests of the Leaf-Cutting Ant Atta sexdens (Hymenoptera: Formicidae) in Teak Plantations" Remote Sensing 11, no. 14: 1641. https://doi.org/10.3390/rs11141641
APA StyleSantos, I. C. d. L., Santos, A. d., Oumar, Z., Soares, M. A., Silva, J. C. C., Zanetti, R., & Zanuncio, J. C. (2019). Remote Sensing to Detect Nests of the Leaf-Cutting Ant Atta sexdens (Hymenoptera: Formicidae) in Teak Plantations. Remote Sensing, 11(14), 1641. https://doi.org/10.3390/rs11141641