Evaluating Different Non-Destructive Estimation Methods for Winter Wheat (Triticum aestivum L.) Nitrogen Status Based on Canopy Spectrum
Abstract
:1. Introduction
2. Materials and methods
2.1. Field Experiments
2.2. Aerial Photography of UAVMC and the Reference VIs
2.3. Taking Photos of Winter Wheat with a Smartphone and the Reference Color-Based VIs
2.4. Measurements of Nitrogen (N) Status of Winter Wheat
2.5. Analytical Methods
3. Results
3.1. Variation of CNS in the Fertilizer Level Experiment
3.2. Estimation Models for the Method of UAVMC
- Estimation model for 0–30 cm:
- Estimation model for 30–60 cm:
- Estimation model for 60–90 cm:
- Estimation model for 0–90 cm:
3.3. Estimation Models for the SPAD Method
- Estimation model for 0–30 cm:
- Estimation model for 60–30 cm:
- Estimation model for 60–90 cm:
- Estimation model for 0–90 cm:
3.4. Estimation Models for the PHONEP Method
- Estimation model for 0–30 cm:
- Estimation model for 30–60 cm:
- Estimation model for 60–90 cm:
- Estimation model for 0–90 cm:
3.5. Validation
4. Discussion
4.1. Comparison of the Three Estimation Methods
4.2. Effect of P Fertilizer Shortage on CNS Estimation
4.3. The Saturation Response of the Estimation Indices
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mogollón, J.; Lassaletta, L.; Beusen, A.; Van Grinsven, H.; Westhoek, H.; Bouwman, A. Assessing future reactive nitrogen inputs into global croplands based on the shared socioeconomic pathways. Environ. Res. Lett. 2018, 13. [Google Scholar] [CrossRef]
- Hawkesford, M.J. Reducing the reliance on nitrogen fertilizer for wheat production. J. Cereal Sci. 2014, 59, 276–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raun, W.R.; Johnson, G.V. Improving nitrogen use efficiency for cereal production. Agron. J. 1999, 91, 357–363. [Google Scholar] [CrossRef] [Green Version]
- Cameira, M.; Mota, M. Nitrogen related diffuse pollution from horticulture production—Mitigation practices and assessment strategies. Horticulturae 2017, 3, 25. [Google Scholar] [CrossRef] [Green Version]
- Feng, W.; Zhu, Y.; Yao, X.; Tian, Y.; Zhuang, S.; Cao, W. Monitoring plant nitrogen accumulation dynamics with hyperspectral remote sensing in wheat. Sci. Agric. Sin. 2008, 41, 1937–1946. [Google Scholar]
- Qi, Y.; Leng, Y.; Wang, M.; Hu, Y.; Bai, Y. Design of decision support system for soil testing and formula fertilization based on the intelligent agriculture. In Proceedings of the 2016 4th International Conference on Machinery, Materials and Information Technology Applications, Xi’an, China, 10–11 December 2016. [Google Scholar]
- Li, G.; Zhu, L.; Li, J. Present status of research and application of non-destructive measurement of nitrogen nutrition diagnosis. Heilongjiang Agric. Sci. 2008, 4, 127–129. [Google Scholar]
- Ali, M.; Al-Ani, A.; Eamus, D.; Tan, D.K. Leaf nitrogen determination using non-destructive techniques—A review. J. Plant Nutr. 2017, 40, 928–953. [Google Scholar] [CrossRef]
- Shou, L.; Jia, L.; Cui, Z.; Chen, X.; Zhang, F. Using high-resolution satellite imaging to evaluate nitrogen status of winter wheat. J. Plant Nutr. 2017, 30, 1669–1680. [Google Scholar] [CrossRef]
- Wright, D.L.; Rasmussen, V.P.; Ramsey, R.D.; Baker, D.J.; Ellsworth, J.W. Canopy reflectance estimation of wheat nitrogen content for grain protein management. GISci. Remote Sens. 2004, 41, 287–300. [Google Scholar] [CrossRef]
- Eitel, J.; Long, D.; Gessler, P.; Smith, A. Using in-situ measurements to evaluate the new RapidEye™ satellite series for prediction of wheat nitrogen status. Int. J. Remote Sens. 2007, 28, 4183–4190. [Google Scholar] [CrossRef]
- Jia, Y.; Su, Z.; Shen, W.; Yuan, J.; Xu, Z. UAV remote sensing image mosaic and its application in agriculture. Int. J. Smart Home 2016, 10, 159–170. [Google Scholar] [CrossRef]
- Dash, J.; Pearse, G.; Watt, M. UAV multispectral imagery can complement satellite data for monitoring forest health. Remote Sens. 2018, 10, 1216. [Google Scholar] [CrossRef] [Green Version]
- Hassan, M.A.; Yang, M.; Rasheed, A.; Yang, G.; Reynolds, M.; Xia, X.; Xiao, Y.; He, Z. A rapid monitoring of NDVI across the wheat growth cycle for grain yield prediction using a multi-spectral UAV platform. Plant Sci. 2019, 282, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, Z.; Chen, Z.; Zhou, L.; Yue, X.; Miao, Y. Nitrogen monitoring of winter wheat based on unmanned aerial vehicle remote sensing image. Trans. Chin. Soc. Agric. Mach. 2018, 49, 207–214. [Google Scholar]
- Li, H.; Li, J.; Lei, Y.; Zhang, Y. Diagnosis of nitrogen nutrition of winter wheat and summer corn using images from digital camera equipped on unmanned aerial vehicle. Chin. J. Eco Agric. 2017, 25, 1832–1841. [Google Scholar]
- Monostori, I.; Árendás, T.; Hoffman, B.; Galiba, G.; Gierczik, K.; Szira, F.; Vágújfalvi, A. Relationship between SPAD value and grain yield can be affected by cultivar, environment and soil nitrogen content in wheat. Euphytica 2016, 211, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Röll, G.; Hartung, J.; Graeff-Hönninger, S. Determination of plant nitrogen content in wheat plants via spectral reflectance measurements: Impact of leaf number and leaf position. Remote Sens. 2019, 11, 2794. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Jin, X.; Yang, G.; Drummond, J.; Yang, H.; Clark, B.; Li, Z.; Zhao, C. Remote sensing of leaf and canopy nitrogen status in winter wheat (Triticum aestivum L.) based on N-PROSAIL model. Remote Sens. 2018, 10, 1463. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Fang, Z.; Chen, Z.; Zhou, L.; Yue, X.; Wang, Z.; Wang, C.; Miao, Y. Nitrogen nutrition diagnosis of winter wheat based on ASD Field Spec3. Trans. Chin. Soc. Agric. Eng. 2018, 34, 162–169. [Google Scholar]
- Jia, L.; Chen, X.; Zhang, F.; Buerkert, A.; Roemheld, V. Optimum nitrogen fertilization of winter wheat based on color digital camera images. Commun. Soil Sci. Plant Anal. 2007, 38, 1385–1394. [Google Scholar] [CrossRef]
- Xia, S.; Zhang, C.; Li, H.; Zhang, Y.; Hu, C. Study on nitrogen diagnosis and fertilization recommendation of winter wheat using canopy digital images from cellphone camera. Chin. J. Eco Agric. 2018, 26, 538–546. [Google Scholar]
- Kaur, N.; Singh, D. Android based mobile application to estimate nitrogen content in rice crop. Int. J. Comput. Trends Technol. IJCTT 2016, 38, 87–91. [Google Scholar] [CrossRef]
- Intaravanne, Y.; Sumriddetchkajorn, S. Android-based rice leaf color analyzer for estimating the needed amount of nitrogen fertilizer. Comput. Electron. Agric. 2015, 116, 228–233. [Google Scholar] [CrossRef]
- Padilla, F.M.; de Souza, R.; Peña-Fleitas, M.T.; Gallardo, M.; Giménez, C.; Thompson, R.B. Different responses of various chlorophyll meters to increasing nitrogen supply in sweet pepper. Front. Plant Sci. 2018, 9, 1752. [Google Scholar] [CrossRef] [Green Version]
- Daughtry, C.S.T.; Walthall, C.L.; Kim, M.S.; Brown De Colstoun, E.; McMurtrey, J.E., III. Estimating corn leaf chlorophyll concentration from leaf and canopy reflectance. Remote Sens. Environ. 2000, 74, 229–239. [Google Scholar] [CrossRef]
- Navarro, G.; Caballero, I.; Silva, G.; Parra, P.C.; Vázquez, Á.; Caldeira, R. Evaluation of forest fire on Madeira Island using Sentinel-2A MSI imagery. Int. J. Appl. Earth Obs. Geoinf. 2017, 58, 97–106. [Google Scholar] [CrossRef] [Green Version]
- Gong, P.; Pu, R.; Biging, G.S.; Larrieu, M.R. Estimation of forest leaf area index using vegetation indices derived from Hyperion hyperspectral data. IEEE Trans. Geosci. Remote Sens. 2003, 41, 1355–1362. [Google Scholar] [CrossRef] [Green Version]
- Qi, J.; Chehbouni, A.; Huete, A.; Kerr, Y.; Sorooshian, S. A modified soil adjusted vegetation index. Remote Sens. Environ. 1994, 48, 119–126. [Google Scholar] [CrossRef]
- Chen, J. Evaluation of vegetation indices and a modified simple ratio for boreal applications. Can. J. Remote Sens. 1996, 22, 229–242. [Google Scholar] [CrossRef]
- Fieuzal, R.; Sicre, C.M.; Baup, F. Estimation of corn yield using multi-temporal optical and radar satellite data and artificial neural networks. Int. J. Appl. Earth Obs. Geoinf. 2017, 57, 14–23. [Google Scholar] [CrossRef]
- Li, X.; Xu, X.; Bao, Y.; Huang, W.; Luo, J.; Dong, Y.; Song, X.; Wang, J. Retrieving LAI of winter wheat based on sensitive vegetation index by the segmentation method. Sci. Agric. Sin. 2012, 45, 3486–3496. [Google Scholar]
- Rondeaux, G.; Steven, M.; Baret, F. Optimization of soil-adjusted vegetation indices. Remote Sens. Environ. 1996, 55, 95–107. [Google Scholar] [CrossRef]
- Roujean, J.L.; Breon, F.M. Estimating PAR absorbed by vegetation from bidirectional reflectance measurements. Remote Sens. Environ. 1995, 51, 375–384. [Google Scholar] [CrossRef]
- Jordan, C.F. Derivation of leaf-area index from quality of light on the forest floor. Ecology 1969, 50, 663–666. [Google Scholar] [CrossRef]
- Huete, A.R. A soil-adjusted vegetation index (SAVI). Remote Sens. Environ. 1988, 25, 295–309. [Google Scholar] [CrossRef]
- Baresel, J.P.; Rischbeck, P.; Hu, Y.; Kipp, S.; Barmeier, G.; Mistele, B.; Schmidhalter, U. Use of a digital camera as alternative method for non-destructive detection of the leaf chlorophyll content and the nitrogen nutrition status in wheat. Comput. Electron. Agric. 2017, 140, 25–33. [Google Scholar] [CrossRef]
- Pagola, M.; Ortiz, R.; Irigoyen, I.; Bustince, H.; Barrenechea, E.; Aparicio-Tejo, P.; Lamsfus, C.; Lasa, B. New method to assess barley nitrogen nutrition status based on image colour analysis: Comparison with SPAD-502. Comput. Electron. Agric. 2009, 65, 213–218. [Google Scholar] [CrossRef]
- Karcher, D.E.; Richardson, M.D. Quantifying turfgrass color using digital image analysis. Crop Sci. 2003, 43, 943–951. [Google Scholar] [CrossRef]
- Guerrero, J.M.; Pajares, G.; Montalvo, M.; Romeo, J.; Guijarro, M. Support vector machines for crop/weeds identification in maize fields. Expert Syst. Appl. 2012, 39, 11149–11155. [Google Scholar] [CrossRef]
- Tucker, C.J. Red and photographic infrared linear combinations for monitoring vegetation. Remote Sens. Environ. 1979, 8, 127–150. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wang, D.; Zhang, G.; Wang, C. Digital camera-based image segmentation of rice canopy and diagnosis of nitrogen nutrition. Trans. Chin. Soc. Agric. Eng. 2012, 28, 131–136. [Google Scholar]
- Beniaich, A.; Naves Silva, M.L.; Avalos, F.A.P.; Menezes, M.D.; Candido, B.M. Determination of vegetation cover index under different soil management systems of cover plants by using an unmanned aerial vehicle with an onboard digital photographic camera. Semin. Cienc. Agrar. 2019, 40, 49–66. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wang, D.; Zhang, G.; Wang, J. Estimating nitrogen status of rice using the image segmentation of GR thresholding method. Field Crops Res. 2013, 149, 33–39. [Google Scholar] [CrossRef]
- Li, Y.; Chen, D.; Walker, C.N.; Angus, J.F. Estimating the nitrogen status of crops using a digital camera. Field Crops Res. 2010, 118, 221–227. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Viña, A.; Arkebauer, T.J.; Rundquist, D.C.; Keydan, G.; Leavitt, B. Remote estimation of leaf area index and green leaf biomass in maize canopies. Geophys. Res. Lett. 2003, 30. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X. Crop Roots and Utilization of Soil Water; China Meteorological Press: Beijing, China, 1999. [Google Scholar]
- Gao, L.; Yang, G.; Li, H.; Li, Z.; Feng, H.; Wang, L.; Dong, J.; He, P. Winter wheat LAI estimation using unmanned aerial vehicle RGB-imaging. Chin. J. Eco Agric. 2016, 24, 1254–1264. [Google Scholar]
- Yu, C.; Qin, J.; Xu, J.; Nie, H.; Luo, Z.; Cen, K. Straw combustion in circulating fluidized bed at low-temperature: Transformation and distribution of potassium. Can. J. Chem. Eng. 2010, 88, 874–880. [Google Scholar] [CrossRef]
- Gu, Y.; Wylie, B.K.; Howard, D.M.; Phuyal, K.P.; Ji, L. NDVI saturation adjustment: A new approach for improving cropland performance estimates in the Greater Platte River Basin, USA. Ecol. Indic. 2013, 30, 1–6. [Google Scholar] [CrossRef]
- De Souza, R.; Peña-Fleitas, M.T.; Thompson, R.B.; Gallardo, M.; Grasso, R.; Padilla, F.M. The use of chlorophyll meters to assess crop N status and derivation of sufficiency values for sweet pepper. Sensors 2019, 19, 2949. [Google Scholar] [CrossRef] [Green Version]
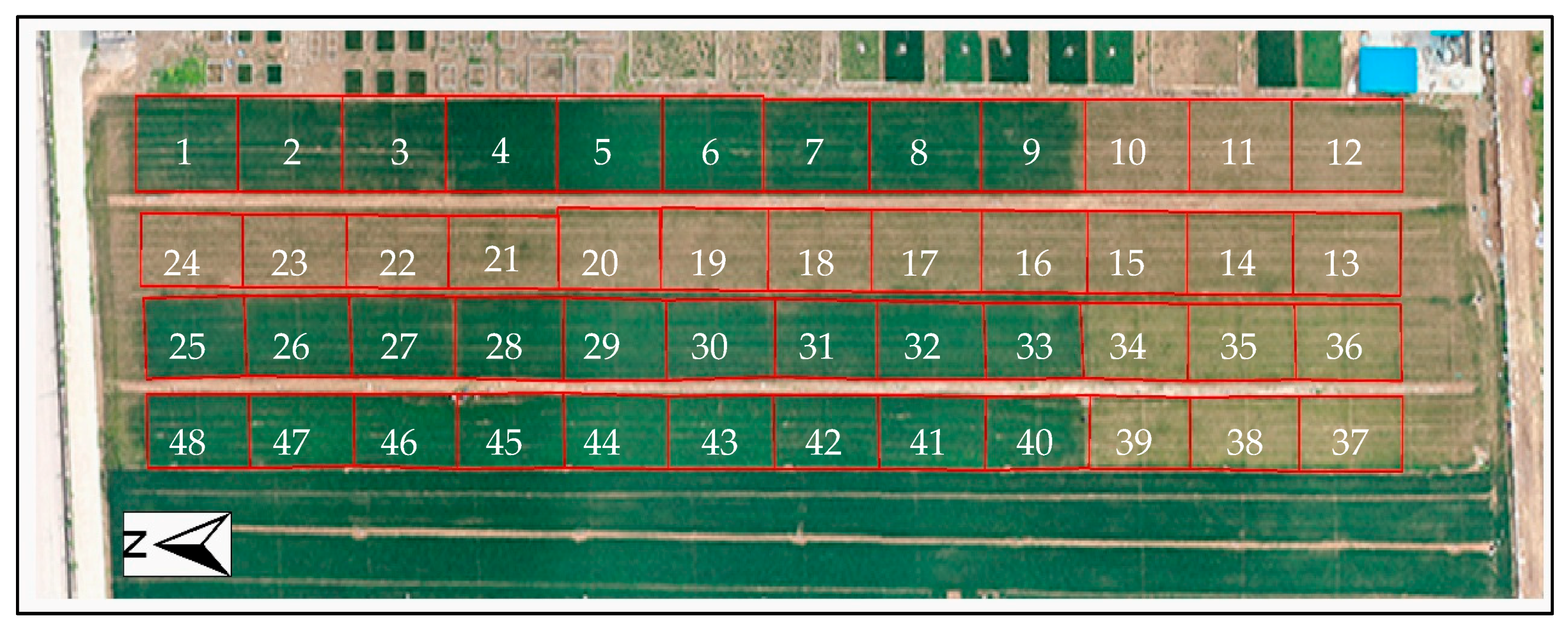





| Treatment | Plot Number in Figure 1 | Treatment | Plot Number in Figure 1 | Treatment | Plot Number in Figure 1 | Treatment | Plot Number in Figure 1 |
|---|---|---|---|---|---|---|---|
| N3P1K1 | 1, 2, 3 | N2P2K1 | 4, 5, 6 | N1P1K1 | 7, 8, 9 | N0P0K1 | 10, 11, 12 |
| N3P0K0 | 22, 23, 24 | N2P0K0 | 19, 20, 21 | N1P0K0 | 16, 17, 18 | N0P0K0 | 13, 14, 15 |
| N3P1K0 | 25, 26, 27 | N2P1K0 | 28, 29, 30 | N1P1K0 | 31, 32, 33 | N0P1K0 | 34, 35, 36 |
| N3P2K0 | 46, 47, 48 | N2P2K0 | 43, 44, 45 | N1P2K0 | 40, 41, 42 | N0P2K0 | 37, 38, 39 |
| Band | Band Width (nm) | Wave Width (nm) | Image Resolution | Field of View H° × V° |
|---|---|---|---|---|
| Green | 40 | 550 | 1280 × 960 | 62.2 × 48.7 |
| Red | 40 | 660 | 1280 × 960 | 62.2 × 48.7 |
| Red edge | 40 | 735 | 1280 × 960 | 62.2 × 48.7 |
| Near Infrared | 40 | 790 | 1280 × 960 | 62.2 × 48.7 |
| Name of VI | Abbreviation | Equation | Reference |
|---|---|---|---|
| Difference vegetation index | DVI | [26] | |
| Green normalized difference vegetation index | GNDVI | [27] | |
| Modified non-linear vegetation index | MNLI | [28] | |
| The second modified soil-adjusted vegetation index | MSAVI2 | [29] | |
| Modified simple ratio | MSR | [30] | |
| Normalized vegetation index | NDVI | [31] | |
| Non-linear vegetation index | NLI | [32] | |
| Optimized soil-adjusted vegetation index | OSAVI | [33] | |
| Renormalized difference vegetation index | RDVI | [34] | |
| Ratio vegetation index | RVI | [35] | |
| Soil-adjusted vegetation index | SAVI | [36] |
| Name of VI | Abbreviation | Equation | Reference |
|---|---|---|---|
| The dark green color index | DGCI | [39] | |
| Excess green index | EXG | [40] | |
| Green leaf index | GLI | [41] | |
| The difference between green and red | GMR | [42,43] | |
| Green-red vegetation index | GRVI | [41] | |
| Normalized blueness intensity | NBI | [44] | |
| Normalized greenness intensity | NGI | [44] | |
| Normalized redness intensity | NRI | [44] | |
| SAVI green | SAVIGreen | [45] | |
| Visible atmospherically resistant index | VARI | [46] | |
| The dark green color index | DGCI | [39] |
| Spectral VIs | With TN of Plants | With Soil Nitrate Nitrogen Content | |||
|---|---|---|---|---|---|
| 0–30 cm | 30–60 cm | 60–90 cm | 0–90 cm | ||
| DVI | 0.88 ** | 0.49 ** | 0.46 * | 0.38 * | 0.50 ** |
| GNDVI | 0.90 ** | 0.52 ** | 0.48 ** | 0.42 ** | 0.52 ** |
| MNLI | 0.87 ** | 0.51 ** | 0.47 ** | 0.38 * | 0.51 ** |
| MSAVI2 | 0.87 ** | 0.50 ** | 0.46 ** | 0.37 * | 0.51 ** |
| MSR | 0.89 ** | 0.51 ** | 0.48 ** | 0.39 ** | 0.52 ** |
| NDVI | 0.88 ** | 0.47 ** | 0.43 ** | 0.37 * | 0.48 ** |
| NLI | 0.89 ** | 0.44 ** | 0.39 * | 0.34 * | 0.44 ** |
| OSAVI | 0.89 ** | 0.45 ** | 0.39 * | 0.34 * | 0.45 ** |
| RDVI | 0.89 ** | 0.51 ** | 0.43 ** | 0.37 * | 0.50 ** |
| RVI | 0.83 ** | 0.50 ** | 0.38 * | 0.26 * | 0.46 ** |
| SAVI | 0.88 ** | 0.45 ** | 0.40 ** | 0.35 * | 0.45 ** |
| With TN of Plants | With Soil Nitrate Nitrogen Content | ||||
|---|---|---|---|---|---|
| 0–30 cm | 30–60 m | 60–90 cm | 0–90 cm | ||
| SPAD | 0.85 ** | 0.57 ** | 0.50 ** | 0.43 ** | 0.55 ** |
| Color-Based VIs | With TN of Plants | With Soil Nitrate Nitrogen Content | |||
|---|---|---|---|---|---|
| 0–30 cm | 30–60 cm | 60–90 cm | 0–90 cm | ||
| DGCI | 0.70 ** | 0.64 ** | 0.63 ** | 0.62 ** | 0.66 ** |
| EXG | −0.71 ** | −0.65 ** | −0.64 ** | −0.59 ** | −0.64 ** |
| GLI | −0.54 ** | −0.64 ** | −0.61 ** | −0.56 ** | −0.61 ** |
| GMR | 0.68 ** | 0.45 ** | 0.33 * | 0.21 * | 0.40 ** |
| GRVI | 0.83 ** | 0.55 ** | 0.55 ** | 0.53 ** | 0.57 ** |
| NBI | 0.65 ** | 0.67 ** | 0.60 ** | 0.60 ** | 0.64 ** |
| NGI | −0.49 ** | −0.57 ** | −0.54 ** | −0.50 ** | 0.54 ** |
| NRI | −0.77 ** | −0.64 ** | −0.62 ** | −0.54 ** | 0.65 ** |
| SAVIGreen | 0.68 ** | 0.54 ** | 0.48 ** | 0.38 * | 0.52 ** |
| VARI | 0.91 ** | 0.72 ** | 0.67 ** | 0.60 ** | 0.72 ** |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Zhang, Y.; Lei, Y.; Antoniuk, V.; Hu, C. Evaluating Different Non-Destructive Estimation Methods for Winter Wheat (Triticum aestivum L.) Nitrogen Status Based on Canopy Spectrum. Remote Sens. 2020, 12, 95. https://doi.org/10.3390/rs12010095
Li H, Zhang Y, Lei Y, Antoniuk V, Hu C. Evaluating Different Non-Destructive Estimation Methods for Winter Wheat (Triticum aestivum L.) Nitrogen Status Based on Canopy Spectrum. Remote Sensing. 2020; 12(1):95. https://doi.org/10.3390/rs12010095
Chicago/Turabian StyleLi, Hongjun, Yuming Zhang, Yuping Lei, Vita Antoniuk, and Chunsheng Hu. 2020. "Evaluating Different Non-Destructive Estimation Methods for Winter Wheat (Triticum aestivum L.) Nitrogen Status Based on Canopy Spectrum" Remote Sensing 12, no. 1: 95. https://doi.org/10.3390/rs12010095
APA StyleLi, H., Zhang, Y., Lei, Y., Antoniuk, V., & Hu, C. (2020). Evaluating Different Non-Destructive Estimation Methods for Winter Wheat (Triticum aestivum L.) Nitrogen Status Based on Canopy Spectrum. Remote Sensing, 12(1), 95. https://doi.org/10.3390/rs12010095





