An Evaluation of Citizen Science Smartphone Apps for Inland Water Quality Assessment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Measurement Campaign
2.2. Water Sampling
2.3. Remote Sensing Reflectance
2.4. The HC Application
3. Results
3.1. Water Quality Conditions
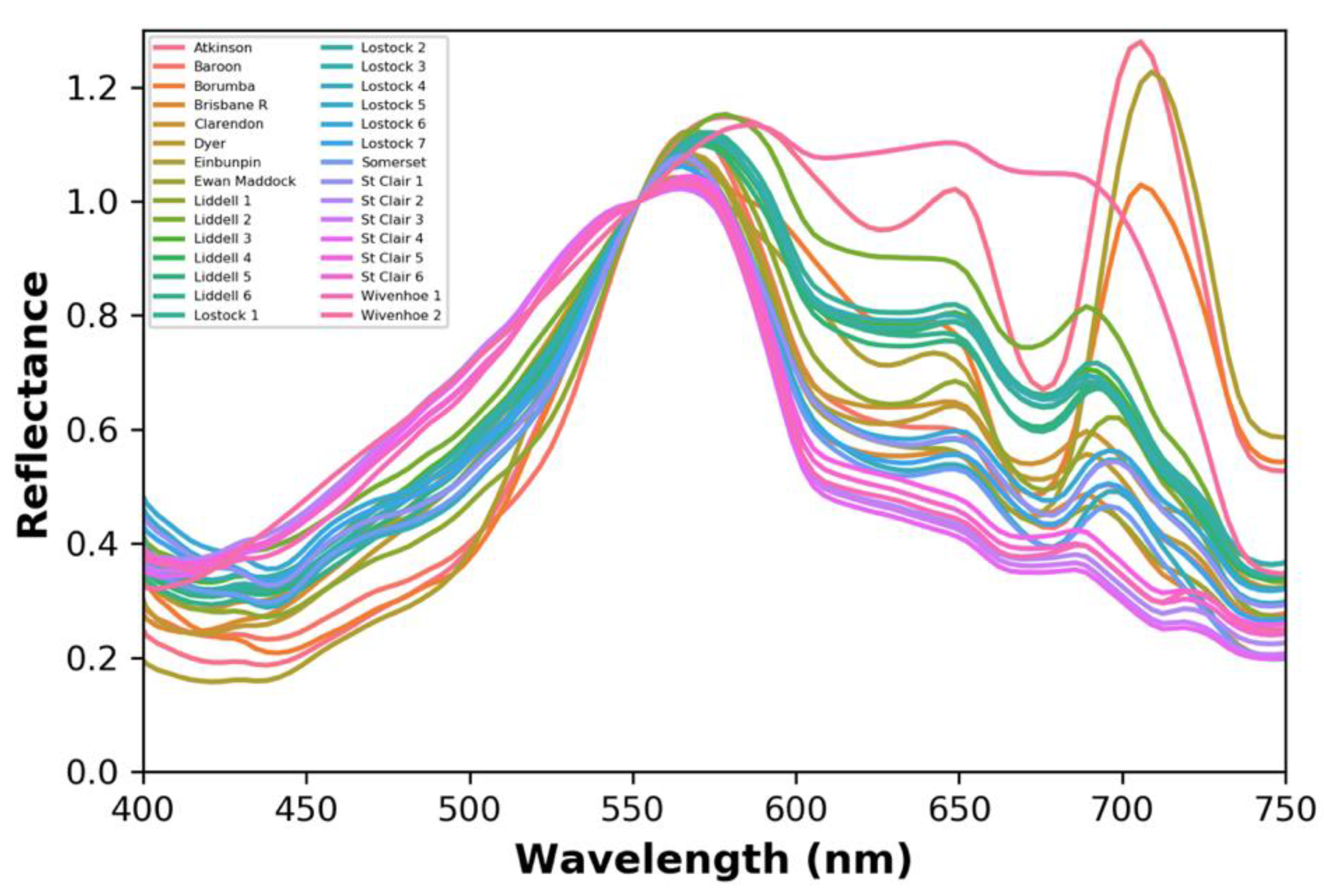
3.2. Remote Sensing Reflectance
3.3. EoW Performance
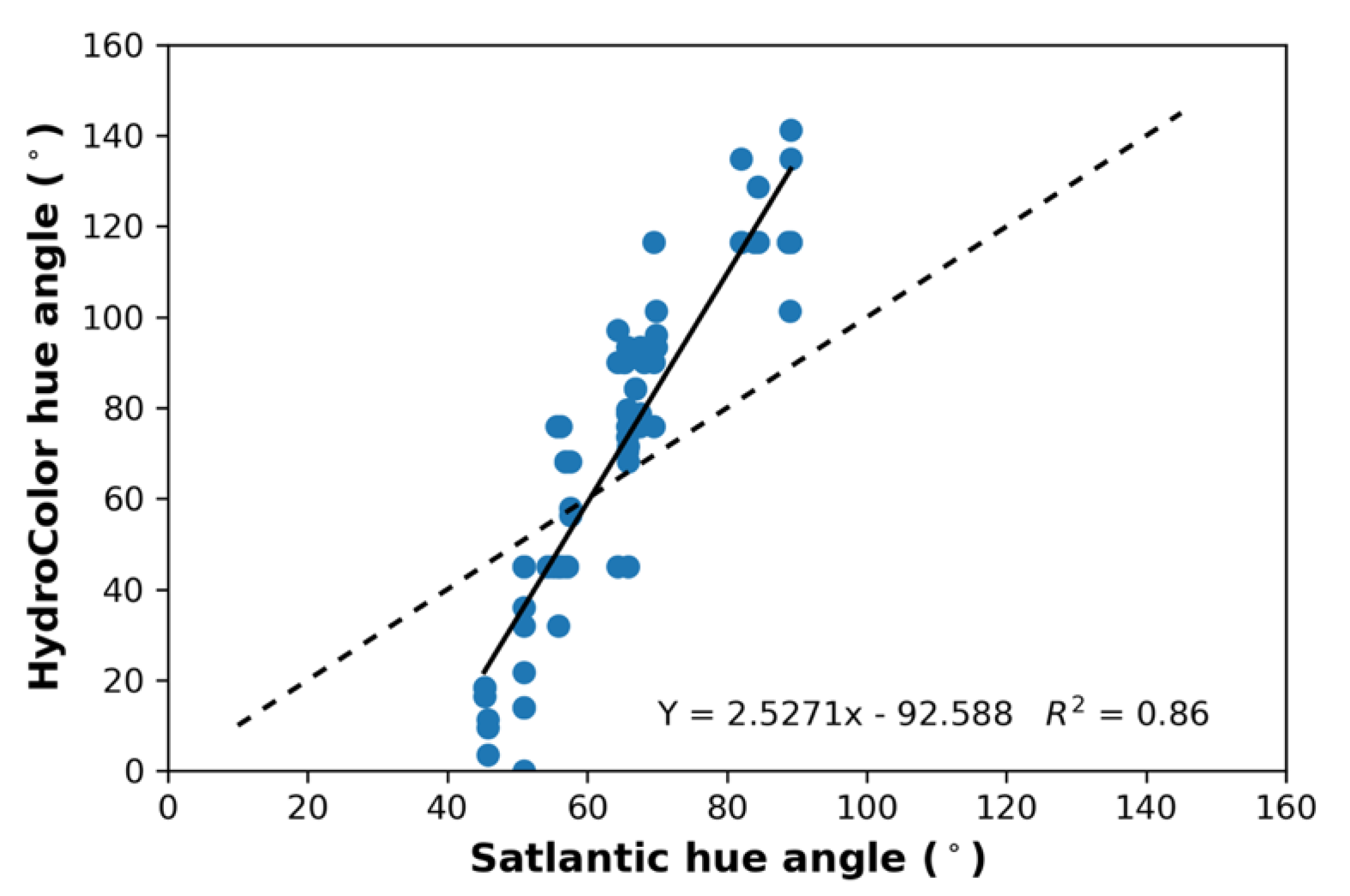
3.4. HC Performance
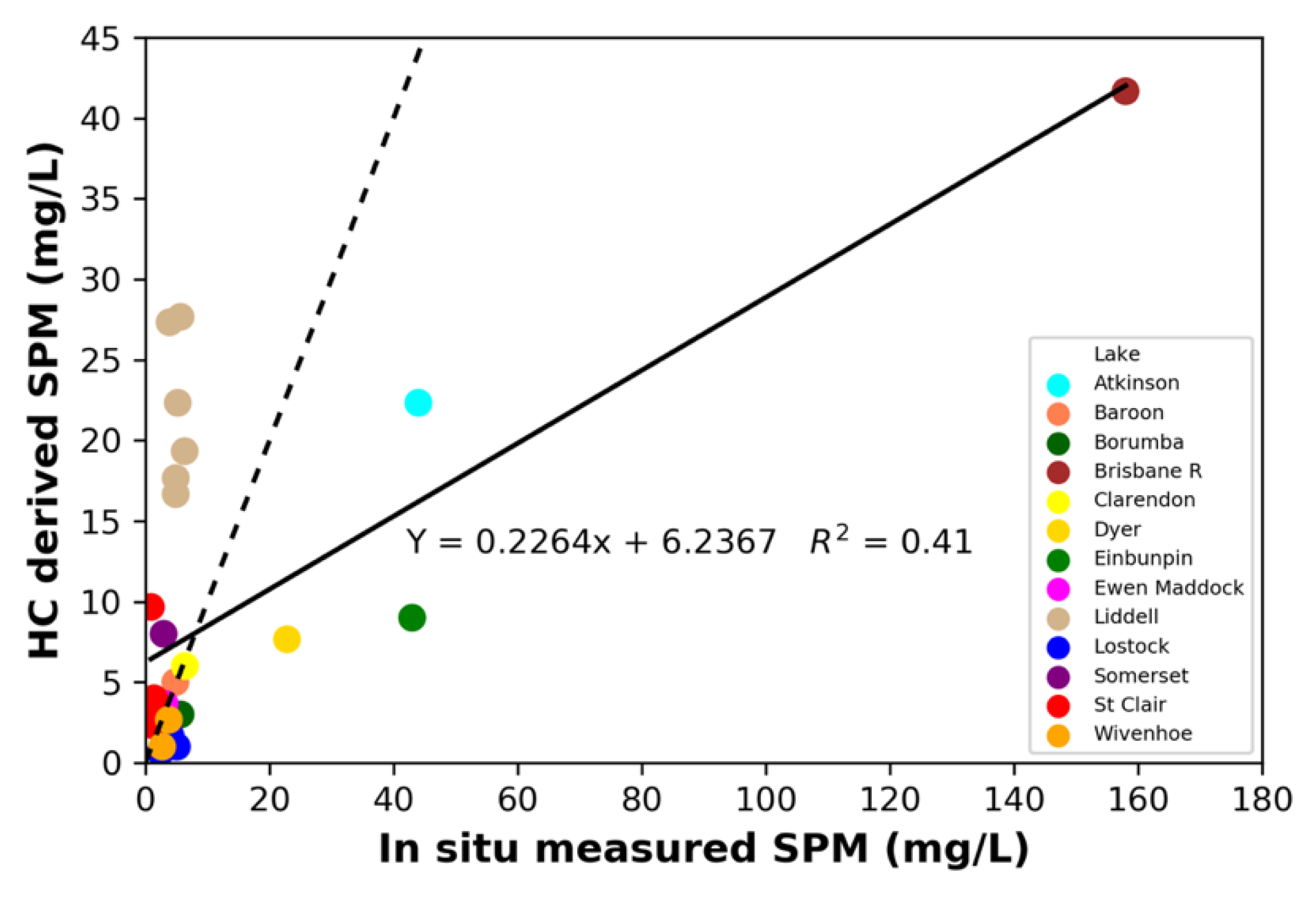
3.5. HC and EoW Information on Water Composition
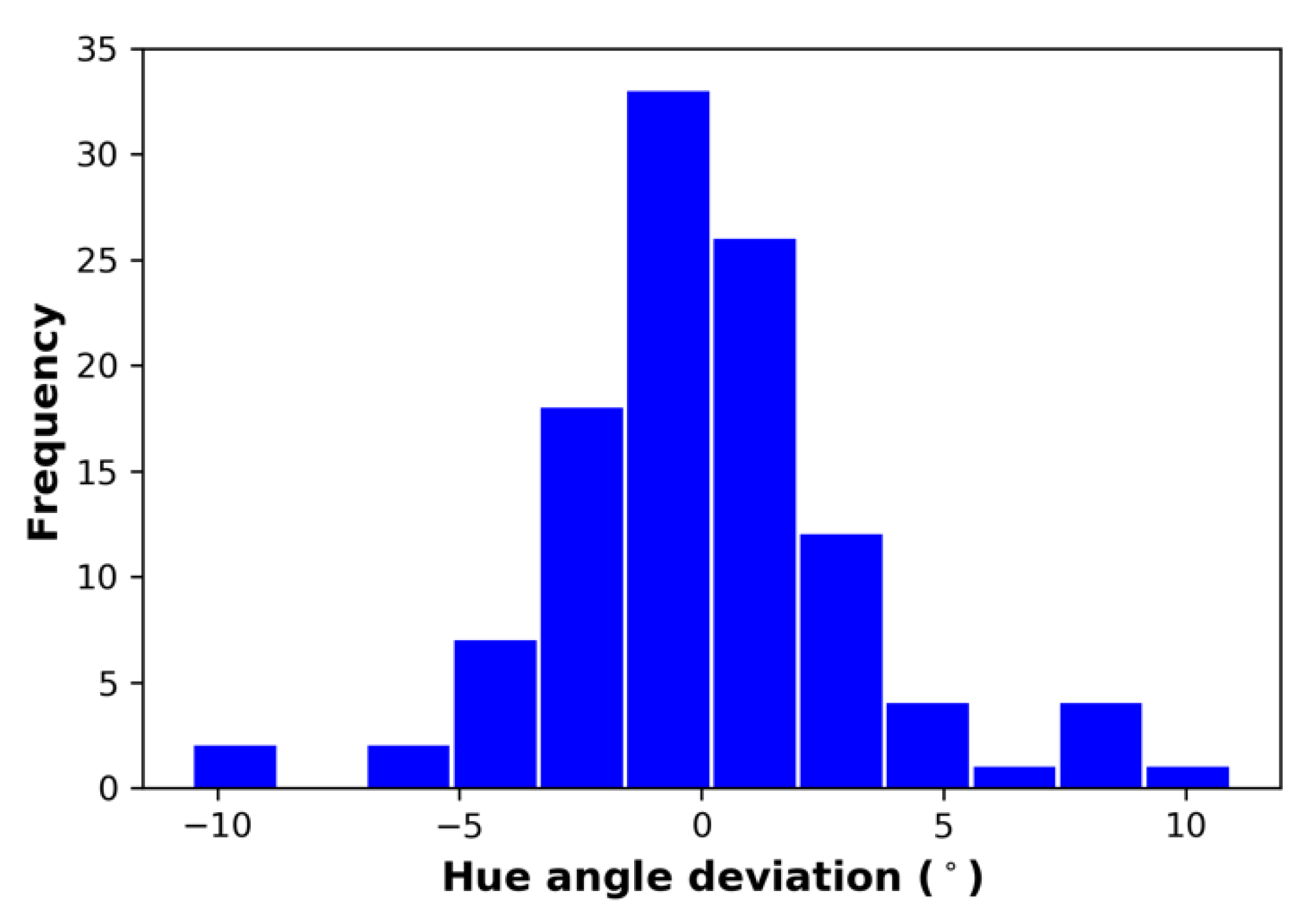
3.6. Spectral Integrity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khalil, B.; Ouarda, T.B.M.J.; St-Hilaire, A.; Chebana, F. A statistical approach for the rationalization of water quality indicators in surface water quality monitoring networks. 2010. J. Hydrol. 2010, 386, 173–185. [Google Scholar] [CrossRef]
- Paerl, H.W.; Huisman, J. Climate change: A catalyst for global expansion of harmful cyanobacterial blooms. Environ. Microbiol. Rep. 2009, 1, 27–37. [Google Scholar] [CrossRef]
- Fritz, S.; See, L.; Carlson, T.; Hakley, M.; Oliver, J.L.; Fraisl, D.; Mondardini, R.; Brocklehurst, M.; Shanley, L.A.; Schade, S.; et al. Citizen Science and the United Nations Sustainable Development Goals. Nat. Sustain. 2019, 2, 922–930. [Google Scholar] [CrossRef]
- Dekker, A.G. Detection of Optical Water Quality Parameters for Eutrophic Waters by High Resolution Remote Sensing. Ph.D. Thesis, Vrije University, Amsterdam, The Netherland, 1993. [Google Scholar]
- Olmanson, L.G.; Brezonik, P.L.; Bauer, M.E. Airborne hyperspectral remote sensing to assess spatial distribution of water quality characteristics in large rivers: The Mississippi River and its tributaries in Minnesota. Remote Sens. Environ. 2013, 130, 254–265. [Google Scholar] [CrossRef]
- Hestir, E.L.; Brando, V.E.; Bresciani, M.; Giardino, C.; Matta, E.; Villa, P.; Dekker, A.G. Measuring freshwater aquatic ecosystems: The need for a hyperspectral global mapping satellite mission. Remote Sens. Environ. 2015, 167, 181–195. [Google Scholar] [CrossRef] [Green Version]
- Busch, J.A.; Zielinski, O.; Cembella, A.D. Subsea Optics and Imaging. In Subsea Optics and Imaging; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar] [CrossRef]
- Gons, H.J.; Rijkeboer, M.; Bagheri, S.; Ruddick, K.G. Optical teledetection of chlorophyll a in estuarine and coastal waters. Environ. Sci. Technol. 2000, 34, 5189–5192. [Google Scholar] [CrossRef]
- Gitelson, A.; Gurlin, D.; Moses, W.J.; Barrow, T. A bio-optical algorithm for the remote estimation of the chlorophyll- a concentration in case 2 waters. Environ. Res. Lett. 2009, 4, 45003. [Google Scholar] [CrossRef]
- Matthews, M.W. A current review of empirical procedures of remote sensing in inland and near-coastal transitional waters. Int. J. Remote Sens. 2011, 32, 6855–6899. [Google Scholar] [CrossRef]
- Dekker, A.G.; Vos, R.J.; Peters, S.W.M. Analytical algorithms for lake water TSM estimation for retrospective analyses of TM and SPOT sensor data. Int. J. Remote Sens. 2002, 23, 15–35. [Google Scholar] [CrossRef]
- Nechad, B.; Ruddick, K.G.; Park, Y. Calibration and validation of a generic multisensor algorithm for mapping of total suspended matter in turbid waters. Remote Sens. Environ. 2010, 114, 854–866. [Google Scholar] [CrossRef]
- Al-Kharusi, E.S.; Tenenbaum, D.E.; Abdi, A.M.; Kutser, T.; Karlsson, J.; Bergström, A.-K.; Berggren, M. Large-Scale Retrieval of Coloured Dissolved Organic Matter in Northern Lakes Using Sentinel-2 Data. Remote Sens. 2020, 12, 157. [Google Scholar] [CrossRef] [Green Version]
- Dekker, A.; Hestir, E. Evaluating the Feasibility of Systematic Inland Water Quality Monitoring with Satellite Remote Sensing; CSIRO: Canberra, Australia, 2012.
- Hommersom, A.; Kratzer, S.; Laanen, M.; Ansko, I.; Ligi, M.; Bresciani, M.; Giardino, C.; Beltrán-Abaunza, J.M.; Moore, G.; Wernand, M.; et al. Intercomparison in the field between the new WISP-3 and other radiometers (TriOS Ramses, ASD FieldSpec, and TACCS). J. Appl. Remote Sens. 2012, 6, 63615. [Google Scholar] [CrossRef] [Green Version]
- Hunter, P.D.; Tyler, A.N.; Carvalho, L.; Codd, G.A.; Maberly, S.C. Hyperspectral remote sensing of cyanobacterial pigments as indicators for cell populations and toxins in eutrophic lakes. Remote Sens. Environ. 2010, 114, 2705–2718. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Sengpiel, R.E.; Pascual, D.L.; Tedesco, L.P.; Wilson, J.S.; Soyeux, E. Using hyperspectral remote sensing to estimate chlorophyll- a and phycocyanin in a mesotrophic reservoir. Int. J. Remote Sens. 2010, 31, 4147–4162. [Google Scholar] [CrossRef]
- Le, C.; Li, Y.; Zha, Y.; Sun, D.; Huang, C.; Zhang, H. Remote estimation of chlorophyll a in optically complex waters based on optical classification. Remote Sens. Environ. 2011, 115, 725–737. [Google Scholar] [CrossRef]
- O’Donnell, D.M.; Effler, S.W.; Strait, C.M.; Peng, F.; Perkins, M. Remote sensing reflectance in the Great Lakes: In situ measurements, closure analyses, and a forward model. J. Great Lakes Res. 2013, 39, 137–150. [Google Scholar] [CrossRef]
- Kudela, R.M.; Palacios, S.L.; Austerberry, D.C.; Accorsi, E.K.; Guild, L.S.; Torres-Perez, J. Application of hyperspectral remote sensing to cyanobacterial blooms in inland waters. Remote Sens. Environ. 2015, 167, 196–205. [Google Scholar] [CrossRef] [Green Version]
- Busch, J.A.; Badají, R.; Ceccaroni, L.; Friedrichs, A.; Piera, J.; Simon, C.; Thijsse, P.; Wernand, M.; Van der Woerd, H.J.; Zielinski, O. Citizen bio-optical observations from coast- and ocean and their compatibility with ocean colour satellite measurements. Remote Sens. 2016, 8, 879. [Google Scholar] [CrossRef] [Green Version]
- Brando, V.E.; Lovell, J.L.; King, E.A.; Boadle, D.; Scott, R.; Schroeder, T. The Potential of Autonomous Ship-Borne Hyperspectral Radiometers for the Validation of Ocean Color Radiometry Data. Remote Sens. 2016, 150. [Google Scholar] [CrossRef] [Green Version]
- Lymburner, L.; Botha, B.; Hestir, E.; Anstee, J.; Sagar, S.; Dekker, A.; Malthus, T. Landsat 8: Providing continuity and increased precision for measuring multi-decadal time series of total suspended matter. Remote Sens. Environ. 2016, 185, 108–118. [Google Scholar] [CrossRef]
- Malthus, T.J.; Lehmann, E.; Ho, X.; Botha, E.; Anstee, J. Implementation of a satellite based inland water algal bloom alerting system using analysis ready data. Remote Sens. 2019, 11, 2954. [Google Scholar] [CrossRef] [Green Version]
- Wernand, M.R.; Hommersom, A.; Van der Woerd, H.J. MERIS-based ocean colour classification with the discrete Forel-Ule scale. Ocean Sci. 2013, 9, 477–487. [Google Scholar] [CrossRef] [Green Version]
- Van der Woerd, H.J.; Wernand, M.R. True colour classification of natural waters with medium-spectral resolution satellites: SeaWiFS, MODIS, MERIS and OLCI. Sensors 2015, 15, 25663–25680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Woerd, H.J.; Wernand, M.R. Hue angle product for low to medium spectral resolution optical satellite sensors. Remote Sens. 2018, 10, 180. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Junsheng, L.; Zhang, B.; Spyrakos, E.; Tyler, A.N.; Shen, Q.; Zhang, F.; Kutser, T.; Lehmann, M.K.; Wu, Y.; et al. Trophic state assessment of global inland waters using a MODIS-derived Forel-Ule index. Remote Sens. Environ. 2018, 217, 444–460. [Google Scholar] [CrossRef] [Green Version]
- Lehmann, M.K.; Nguyen, U.; Allan, M.; Van der Woerd, H.J. Colour classification of 1486 lakes across a wide range of optical water types. Remote Sens. 2018, 10, 1273. [Google Scholar] [CrossRef] [Green Version]
- Giardino, C.; Koks, K.; Bolpagni, R.; Luciani, G.; Candiani, G.; Lehmann, M.K.; Van der Woerd, H.J.; Bresciani, M. The color of water from space: A case study for Italian lakes from Sentinel-2. In Earth Observation and Geospatial Analysis; Intech Open: London, UK, 2019. [Google Scholar] [CrossRef] [Green Version]
- Novoa, S.; Wernand, M.R.; Van der Woerd, H.J. WACODI: A generic algorithm to derive the intrinsic color of natural waters from digital images. Limnol. Oceanogr. Methods 2015, 13, 697–711. [Google Scholar] [CrossRef] [Green Version]
- Leeuw, T.; Boss, E. The HydroColor App: Above water measurements of remote sensing reflectance and turbidity using a smartphone camera. Sensors 2018, 18, 256. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Cowen, L.L.E.; Costa, M. Is ocean reflectance acquired by citizen scientists robust for science applications? Remote Sens. 2018, 10, 835. [Google Scholar] [CrossRef] [Green Version]
- Ouma, Y.O.; Waga, J.; Okech, O.; Lavisa, O.; Mbuthia, D. Estimation of reservoir bio-optical water quality parameters using smartphone sensor apps and Landsat ETM+: Review and comparative experimental results. J. Sens. 2018, 3490757. [Google Scholar] [CrossRef]
- Fargoin, G.S.; Mueller, J.L. Ocean Optics Protocols for Satellite Ocean Color Sensor Validation, 3rd ed.; NASA Goddard Space Flight Center: Greenbelt, MD, USA, 2002.
- Tilstone, G.H.; Moore, G.F.; Doerffer, R.; Røttgers, R.; Ruddick, K.G.; Pasterkamp, R.; Jørgensen, P.V. Regional Validation of MERIS Chlorophyll products in North Sea REVAMP Protocols. In Proceedings of the Working Meeting on MERIS and AATSR Calibration and Geophysical Validation (ENVISAT MAVT-2003), Frascati, Rome, Italy, 20–24 October 2003; ESA Special Publication WPP-233: Noordwijk, The Netherlands, 2002; pp. 1–77. [Google Scholar]
- Clementson, L.A.; Parslow, J.S.; Turnbull, A.R.; Mckenzie, D.C.; Rathbone, C.E. Optical properties of waters in the Australasian sector of the Southern Ocean. J. Geophys. Res. Oceans 2001, 106, 31611–31625. [Google Scholar] [CrossRef]
- Zibordi, G.; Talone, M. On the equivalence of near-surface methods to determine the water-leaving radiance. Opt. Express 2020, 28, 3200. [Google Scholar] [CrossRef] [PubMed]
- Ruddick, K.G.; Vos, K.; Boss, E.; Castagna, A.; Frouin, R.; Gilerson, A.; Hieronymi, M.; Johnson, B.C.; Kuusk, J.; Lee, Z.; et al. A Review of Protocols for Fiducial Reference Measurements of Water-Leaving Radiance for Validation of Satellite Remote-Sensing Data over Water. Remote Sens. 2019, 11, 2198. [Google Scholar] [CrossRef] [Green Version]
- Simis, S.G.H.; Peters, S.W.M.; Gons, H.J. Remote sensing of the cyanobacterial pigment phycocyanin in turbid inland water. Limnol. Oceanogr. 2005, 50, 237–245. [Google Scholar] [CrossRef]
- Ruiz-Verdu, A.; Simis, S.G.H.; De Hoyos, C.; Gons, H.J.; Peña-Martínez, R. An evaluation of algorithms for the remote sensing of cyanobacterial biomass. Remote Sens. Environ. 2008, 112, 3996–4008. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Dall’Olmo, G.; Moses, W.; Rundquist, D.C.; Barrow, T.; Fisher, T.R.; Gurlin, D.; Holz, J. A simple semi-analytical model for remote estimation of chlorophyll-a in turbid waters-Validation. Remote Sens. Environ. 2008, 112, 3582–3593. [Google Scholar] [CrossRef]
- Li, L.; Li, L.; Song, K. Remote sensing of freshwater cyanobacteria: An extended IOP Inversion Model of Inland Waters (IIMIW) for partitioning absorption coefficient and estimating phycocyanin. Remote Sens. Environ. 2015, 157, 9–23. [Google Scholar] [CrossRef]
- Liu, G.; Lin, L.; Song, K.; Li, Y.; Lyu, H.; Wen, Z.; Fang, C.; Bi, S.; Sun, X.; Wang, Z.; et al. An OLCI-based algorithm for semi-empirically partitioning absorption coefficient and estimating chlorophyll a concentration in various turbid case-2 waters. Remote Sens. Environ. 2020, 239, 111648. [Google Scholar] [CrossRef]
- Ogashawara, I.; Mishra, D.R.; Mishra, S.; Curtarelli, M.P.; Stech, J.L. A Performance Review of Reflectance Based Algorithms for Predicting Phycocyanin Concentrations in Inland Waters. Remote Sens. 2013, 5, 4774–4798. [Google Scholar] [CrossRef] [Green Version]
- Spyrakos, E.; O’Donnell, R.; Hunter, P.D.; Miller, C.; Scott, M.; Simis, S.G.H.; Niel, C.; Barbosa, C.; Binding, C.E.; Bradt, S.; et al. Optical types of inland and coastal waters. Limnol. Oceanogr. 2017, 63, 846–870. [Google Scholar] [CrossRef] [Green Version]
- Kirk, J.T. Light and Photosynthesis in Aquatic Ecosystems, 3rd ed.; Cambridge University Press: Cambridge, NY, USA, 2011. [Google Scholar]
- CIE. Commission Internationale de l’Éclairage Proceedings, 1931; Cambridge University Press: Cambridge, UK, 1932; pp. 19–29. [Google Scholar]
- Novoa, S.; Wernand, M.R.; Van der Woerd, H.J. The Forel-Ule scale revisited spectrally: Preparation protocols, transmission measurements and chromaticity. J. Eur. Opt. Soc. RP 2013, 8, 13057. [Google Scholar] [CrossRef] [Green Version]
- Leeuw, T. Crowdsourcing Water Quality Data Using the iPhone Camera; Paper 2118; University of Maine: Orono, ME, USA, 2014; Available online: http://digitalcommons.library.umaine.edu/etd/2118 (accessed on 24 January 2020).
- Garaba, S.; Voss, D.; Wollschlager, J.; Zielinski, O. Modern approaches to shipborne ocean color remote sensing. Appl. Opt. 2015, 54, 3602–3612. [Google Scholar]
- Mueller, J.L.; Fargion, G.S.; Mcclain, C.R.; Mueller, J.L.; Morel, A.; Frouin, R.; Davis, C.; Arnone, R.; Carder, K.; Steword, R.G.; et al. NASA/TM-2003-Ocean Optics Protocols for Satellite Ocean Color Sensor Validation, Revision 4, Volume III: Radiometric Measurements and Data Analysis Protocols; NASA: Washington DC, USA, 2003.
- Mobley, C.D. Estimation of the remote-sensing reflectance from above-surface measurements. Appl. Opt. 1999, 38, 7442–7455. [Google Scholar] [CrossRef] [PubMed]
- Pascale, D. A review of RGB color spaces... from xyY to R’G’B’. 2003. Babel Color. pp. 1–35. Available online: www.babelcolor.com (accessed on 24 January 2020).
- Lindell, L.T.; Pierson, D.; Premazzi, G.; Zillioli, E. Manual for Monitoring European Lakes Using Remote Sensing Techniques; Joint Research Centre European Commission: Ispra, Italy, 1999; ISBN 978-9282853900. [Google Scholar]
- Van der Woerd, H.J.; Pasterkamp, R. Mapping of the North Sea turbid coastal waters using SeaWiFS data. Can. J. Remote Sens. 2004, 30, 44–53. [Google Scholar] [CrossRef]
- Pitarch, J.; Van der Woerd, H.J.; Brewin, R.J.W.; Zielinsky, O. Optical properties of Forel-Ule water types deduced from 15 years of global satellite ocean color observations. Remote Sens. Environ. 2018, 231, 111249. [Google Scholar] [CrossRef]
- Malthus, T.J.; Lehmann, E.; Ho, X.; Gensemer, S.; Anstee, J.; Botha, E.; Brayan, J.; Bowling, L.; Shaikh, M.; Ohmsen, R.; et al. An algal bloom early warning system for NSW using satellite and near-surface observations. In Proceedings of the OzWater17 Conference, 16–18 May 2017; Australian Water Association: Municipality, Australia. Available online: https://awa.sharefile.com/share?#/view/s69fcd4a5d344354b (accessed on 23 February 2020).
- Bowling, L.; Shaikh, M.; Brayan, J.; Malthus, T. An evaluation of a handheld spectroradiometer for the near real-time measurement of cyanobacteria for bloom management purposes. Environ. Monit. Assess. 2017, 189, 495. [Google Scholar] [CrossRef]
- Flanagin, A.J.; Metzger, M.J. The credibility of volunteered geographic information. GeoJournal 2008, 72, 137–148. [Google Scholar] [CrossRef]
- Wernand, M.R.; Van der Woerd, H.J.; Gieskes, W.W.C. Trends in Ocean Colour and Chlorophyll Concentration from 1889 to 2000, Worldwide. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Burggraaff, O.; Schmidt, N.; Zamorano, J.; Puly, K.; Pascual, S.; Tapia, C.; Spyrakos, E.; Snik, F. Standardized spectral and radiometric calibration of consumer cameras. Opt. Express. 2019, 19075. [Google Scholar] [CrossRef] [Green Version]











© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malthus, T.J.; Ohmsen, R.; Woerd, H.J.v.d. An Evaluation of Citizen Science Smartphone Apps for Inland Water Quality Assessment. Remote Sens. 2020, 12, 1578. https://doi.org/10.3390/rs12101578
Malthus TJ, Ohmsen R, Woerd HJvd. An Evaluation of Citizen Science Smartphone Apps for Inland Water Quality Assessment. Remote Sensing. 2020; 12(10):1578. https://doi.org/10.3390/rs12101578
Chicago/Turabian StyleMalthus, Tim J., Renee Ohmsen, and Hendrik J. van der Woerd. 2020. "An Evaluation of Citizen Science Smartphone Apps for Inland Water Quality Assessment" Remote Sensing 12, no. 10: 1578. https://doi.org/10.3390/rs12101578
APA StyleMalthus, T. J., Ohmsen, R., & Woerd, H. J. v. d. (2020). An Evaluation of Citizen Science Smartphone Apps for Inland Water Quality Assessment. Remote Sensing, 12(10), 1578. https://doi.org/10.3390/rs12101578






