Developing an Atlas of Harmful Algal Blooms in the Red Sea: Linkages to Local Aquaculture
Abstract
:1. Introduction
2. Materials and Methods
2.1. Satellite Datasets
2.2. Aquaculture Production Dataset
2.3. Approach
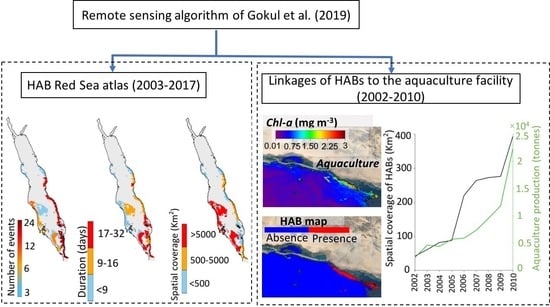
3. Results
3.1. Atlas of HABs in the Red Sea
3.1.1. North-Central Red Sea
3.1.2. South-Central Red Sea
3.1.3. Southern Red Sea
3.2. Interactions of HABs with Aquaculture
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Anderson, D.M.; Glibert, P.M.; Burkholder, J.M. Harmful algal blooms and eutrophication: Nutrient sources, composition, and consequences. Estuaries 2002, 25, 562–584. [Google Scholar] [CrossRef]
- Anderson, D.M. Approaches to monitoring, control and management of harmful algal blooms (HABs). Ocean Coast Manag. 2009, 52, 342–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gokul, E.A.; Shanmugam, P. An optical system for detecting and describing major algal blooms in coastal and oceanic waters around India. J. Geophys. Res. Oceans 2016, 121, 4097–4127. [Google Scholar] [CrossRef] [Green Version]
- Dale, B.; Edwards, M.; Reid, P.C. Climate change and harmful algal blooms. Ecol. Harmful Algae 2006, 367–378. [Google Scholar]
- Moore, S.K.; Trainer, V.L.; Mantua, N.J.; Parker, M.S.; Laws, E.A.; Backer, L.C.; Fleming, L.E. Impacts of climate variability and future climate change on harmful algal blooms and human health. Environ. Health 2008, 7, S4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glibert, P.M.; Allen, J.I.; Bouwman, A.F.; Brown, C.W.; Flynn, K.J.; Lewitus, A.J.; Madden, C.J. Modeling of HABs and eutrophication: Status, advances, challenges. J. Marine Syst. 2010, 83, 262–275. [Google Scholar] [CrossRef]
- Paerl, H.W.; Hall, N.S.; Calandrino, E.S. Controlling harmful cyanobacterial blooms in a world experiencing anthropogenic and climatic-induced change. Sci. Total Environ. 2011, 409, 1739–1745. [Google Scholar] [CrossRef]
- Mohamed, Z.A.; Mesaad, I. First report on Noctiluca scintillans blooms in the Red Sea off the coasts of Saudi Arabia: Consequences of eutrophication. Oceanologia 2007, 49, 337–351. [Google Scholar]
- Alkershi, A.; Menon, N. Phytoplankton in polluted waters of the Red Sea coast of Yemen. J. Mar. Biol. Ass. India 2011, 53, 161–166. [Google Scholar] [CrossRef]
- Mohamed, Z.A.; Al-Shehri, A.M. Occurrence and germination of dinoflagellate cysts in surface sediments from the Red Sea off the coasts of Saudi Arabia. Oceanologia 2011, 53, 121–136. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, Z.A.; Al-Shehri, A.M. The link between shrimp farm runoff and blooms of toxic Heterosigma akashiwo in Red Sea coastal waters. Oceanologia 2012, 54, 287–309. [Google Scholar] [CrossRef] [Green Version]
- Alkawri, A. Seasonal variation in composition and abundance of harmful dinoflagellates in Yemeni waters, southern Red Sea. Mar. Pollut. Bull. 2016, 112, 225–234. [Google Scholar] [CrossRef]
- Alkawri, A.; Al-Areeki, M.; Alsharaby, K. The first recorded bloom of Protoperidinium quinquecorne and its link to a massive fish kill in Yemeni coastal waters, Southern Red Sea. Plankton Benthos Res. 2016, 11, 75–78. [Google Scholar] [CrossRef] [Green Version]
- Alkawri, A.; Abker, M.; Qutaei, E.; Alhag, M.; Qutaei, N.; Mahdy, S. The first recorded bloom of Pyrodinium bahamense var bahamense plate in Yemeni coastal waters off Red Sea, near Al Hodeida City. Turk. J. Fish. Aquat. Sci. 2016, 16, 275–282. [Google Scholar] [CrossRef]
- Banguera-Hinestroza, E.; Eikrem, W.; Mansour, H.; Solberg, I.; Curdia, J.; Holtermann, K.; Edvardsen, B.; Kaartvedt, S. Seasonality and toxin production of Pyrodinium bahamense in a Red Sea lagoon. Harmful Algzae 2016, 55, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Catania, D.; Richlen, M.L.; Mak, Y.L.; Morton, S.L.; Laban, E.H.; Xu, Y.; Anderson, D.M.; Chan, L.L.; Berumen, M.L. The prevalence of benthic dinoflagellates associated with ciguatera fish poisoning in the central Red Sea. Harmful Algae 2017, 68, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Gokul, E.A.; Raitsos, D.E.; Gittings, J.A.; Alkawri, A.; Hoteit, I. Remotely sensing harmful algal blooms in the Red Sea. PLoS ONE 2019, 14, e0215463. [Google Scholar] [CrossRef]
- Mohamed, Z.A. Potentially harmful microalgae and algal blooms in the Red Sea: Current knowledge and research needs. Mar. Environ. Res. 2018, 140, 234–242. [Google Scholar] [CrossRef]
- Brewin, J.R.W.; Morán, X.A.J.; Raitsos, D.E.; Gittings, J.A.; Calleja, M.L.; Viegas, M.; Ansari, M.I.; Al-Otaibi, N.; Huete-Stauffer, T.M.; Hoteit, I. Factors regulating the relationship between total and size-fractionated chlorophyll-a in coastal waters of the Red Sea. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Kürten, B.; Khomayis, H.S.; Devassy, R.; Audritz, S.; Sommer, U.; Struck, U.; El-Sherbiny, M.M.; Al-Aidaroos, A.M. Ecohydrographic constraints on biodiversity and distribution of phytoplankton and zooplankton in coral reefs of the Red Sea, Saudi Arabia. Mar. Ecol. 2015, 36, 1195–1214. [Google Scholar] [CrossRef]
- Hozumi, A.; Hong, P.Y.; Kaartvedt, S.; Røstad, A.; Jones, B.H. Water quality, seasonality, and trajectory of an aquaculture-wastewater plume in the Red Sea. Aquac. Environ. Interacact. 2018, 10, 61–77. [Google Scholar] [CrossRef] [Green Version]
- Gittings, J.A.; Brewin, R.J.W.; Raitsos, D.E.; Kheireddine, M.; Ouhssain, M.; Jones, B.; Hoteit, I. Remotely sensing phytoplankton size structure in the Red Sea. Remote Sens. Environ. 2019, 234. [Google Scholar] [CrossRef]
- Brewin, R.J.; Raitsos, D.E.; Dall’Olmo, G.; Zarokanellos, N.; Jackson, T.; Racault, M.F.; Boss, E.S.; Sathyendranath, S.; Jones, B.H.; Hoteit, I. Regional ocean- color Chl-a algorithms for the Red Sea. Remote Sens. Environ. 2015, 165, 64–85. [Google Scholar] [CrossRef] [Green Version]
- Racault, M.F.; Raitsos, D.E.; Berumen, M.L.; Brewin, R.J.; Platt, T.; Sathyendranath, S.; Hoteit, I. Phytoplankton phenology indices in coral reef ecosystems: Application to ocean-color observations in the Red Sea. Remote Sens. Environ. 2015, 160, 222–234. [Google Scholar] [CrossRef] [Green Version]
- Dreano, D.; Raitsos, D.E.; Gittings, J.; Krokos, G.; Hoteit, I. The Gulf of Aden intermediate water intrusion regulates the southern Red Sea summer phytoplankton blooms. PLoS ONE 2016, 11. [Google Scholar] [CrossRef] [Green Version]
- Gittings, J.A.; Raitsos, D.E.; Kheireddine, M.; Racault, M.F.; Claustre, H.; Hoteit, I. Evaluating tropical phytoplankton phenology metrics using contemporary tools. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Pauly, D. The Sea Around Us Project: Documenting and communicating global fisheries impacts on marine ecosystems. AMBIO J. Hum. Environ. 2007, 36, 290–295. [Google Scholar] [CrossRef]
- Garibaldi, L. The FAO global capture production database: A six-decade effort to catch the trend. Mar. Policy 2012, 36, 760–768. [Google Scholar] [CrossRef]
- Campbell, B.; Pauly, D. Mariculture: A global analysis of production trends since 1950. Mar. Policy 2013, 39, 94–100. [Google Scholar] [CrossRef]
- Raitsos, D.E.; Pradhan, Y.; Brewin, R.J.; Stenchikov, G.; Hoteit, I. Remote sensing the phytoplankton seasonal succession of the Red Sea. PLoS ONE 2013, 8, e64909. [Google Scholar] [CrossRef] [Green Version]
- Patzert, W.C. Wind-induced reversal in Red Sea circulation. Deep Sea Res. Oceanogr. Abstr. 1974, 21, 109–121. [Google Scholar] [CrossRef]
- Murray, S.P.; Johns, W. Direct observations of seasonal exchange through the Bab el Mandab Strait. Oceanogr. Lit. Rev. 1998, 3, 438. [Google Scholar] [CrossRef]
- Sofianos, S.S.; Johns, W.E. An oceanic general circulation model (OGCM) investigation of the Red Sea circulation, 1. Exchange between the Red Sea and the Indian Ocean. J. Geophys. Res. Oceans 2002, 107, 1–11. [Google Scholar] [CrossRef]
- Aiki, H.; Takahashi, K.; Yamagata, T. The Red Sea outflow regulated by the Indian monsoon. Cont. Shelf Res. 2006, 26, 1448–1468. [Google Scholar] [CrossRef]
- Churchill, J.H.; Bower, A.S.; McCorkle, D.C.; Abualnaja, Y. The transport of nutrient-rich Indian Ocean water through the Red Sea and into coastal reef systems. J. Mar. Res. 2014, 72, 165–181. [Google Scholar] [CrossRef]
- Yao, F.; Hoteit, I.; Pratt, L.J.; Bower, A.S.; Zhai, P.; Köhl, A.; Gopalakrishnan, G. Seasonal overturning circulation in the Red Sea: 1. Model validation and summer circulation. J. Geophys. Res. Oceans 2014, 119, 2238–2262. [Google Scholar] [CrossRef] [Green Version]
- Gittings, J.A.; Raitsos, D.E.; Racault, M.F.; Brewin, R.J.; Pradhan, Y.; Sathyendranath, S.; Platt, T. Seasonal phytoplankton blooms in the Gulf of Aden revealed by remote sensing. Remote Sens. Environ. 2017, 189, 56–66. [Google Scholar] [CrossRef] [Green Version]
- Raitsos, D.E.; Brewin, R.J.; Zhan, P.; Dreano, D.; Pradhan, Y.; Nanninga, G.B.; Hoteit, I. Sensing coral reef connectivity pathways from space. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Raitsos, D.E.; Krokos, G.; Gittings, J.A.; Zhan, P.; Hoteit, I. Physical connectivity simulations reveal dynamic linkages between coral reefs in the southern Red Sea and the Indian Ocean. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef]
- Johns, W.E.; Sofianos, S.S. Atmospherically-forced exchange through the Bab el Mandeb. J. Phys. Oceanogr. 2012, 42, 1143–1157. [Google Scholar] [CrossRef]
- Yao, F.; Hoteit, I.; Pratt, L.J.; Bower, A.S.; Köhl, A.; Gopalakrishnan, G.; Rivas, D. Seasonal overturning circulation in the Red Sea: 2. Winter circulation. J. Geophys. Res. Oceans 2014, 119, 2263–2289. [Google Scholar] [CrossRef] [Green Version]
- Triantafyllou, G.; Yao, F.; Petihakis, G.; Tsiaras, K.P.; Raitsos, D.E.; Hoteit, I. Exploring the Red Sea seasonal ecosystem functioning using a three-dimensional biophysical model. J. Geophys. Res. Oceans 2014, 119, 1791–1811. [Google Scholar] [CrossRef] [Green Version]
- Post, A.F.; Dedej, Z.; Gottlieb, R.; Li, H.; Thomas, D.N.; El-Absawi, M.; El-Naggar, A.; El-Gharabawi, M.; Sommer, U. Spatial and temporal distribution of Trichodesmium spp. in the stratified Gulf of Aqaba, Red Sea. Mar. Ecol. Prog. Ser. 2002, 239, 241–250. [Google Scholar] [CrossRef] [Green Version]
- Madkour, F.; El-Sherbiny, M.; Aamer, M. Phytoplankton population along certain Egyptian coastal regions of the Red Sea. Egypt. J. Aquat. Biol. Fish. 2010, 14, 95–109. [Google Scholar] [CrossRef] [Green Version]
- Nassar, M.Z.; El-Din, N.G.; Gharib, S.M. Phytoplankton variability in relation to some environmental factors in the eastern coast of Suez Gulf, Egypt. Environ. Monit. Assess. 2015, 648, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Touliabah, H.E.; Abu El-Kheir, W.S.; Kuchari, M.G. Phytoplankton composition at Jeddah coast-Red Sea, Saudi Arabia in relation to some ecological factors. J. King Abdulaziz Univ. Sci. 2010, 148, 1–34. [Google Scholar] [CrossRef] [Green Version]
- Elhag, A.G.; Nasr, D.H. Studies in planktonic populations in Port Sudan coastal area. Sudan J. Sci. 1989, 4, 12–26. [Google Scholar]
- Ali, M.H.M. Ecological Study on the Phytoplankton in the Sudanese Red Sea Coast (Port Sudan Area). Master’s Thesis, University of Khartoum, Khartoum, Sudan, 2015. [Google Scholar]
- Beyer, J.; Staalstrøm, A.; Wathne, B.M.; Omer, R.K.; Ahmed, S.E. Marine Ecological Baselines and Environmental Impact Assessment Studies in the Sudanese Coastal Zone. A Review; Norsk Institutt for Vannforskning: Oslo, Norway, 2015. [Google Scholar]
- Ali, E.B.; Churchill, J.H.; Barthel, K.; Skjelvan, I.; Omar, A.M.; de Lange, T.E.; Eltaib, E.B. Seasonal variations of hydrographic parameters off the Sudanese coast of the Red Sea, 2009–2015. Reg. Stud. Mar. Sci. 2018, 18, 1–10. [Google Scholar] [CrossRef]
- Klaus, R. Coral reefs and communities of the central and southern Red Sea (Sudan, Eritrea, Djibouti, and Yemen). In the Red Sea; Springer: Berlin/Heidelberg, Germany, 2015; pp. 409–451. [Google Scholar]
- Steinmetz, F.; Deschamps, P.Y.; Ramon, D. Atmospheric correction in presence of sun glint: Application to MERIS. Opt. Express 2011, 19, 9783–9800. [Google Scholar] [CrossRef]
- Raitsos, D.E.; Yi, X.; Platt, T.; Racault, M.F.; Brewin, R.J.; Pradhan, Y.; Papadopoulos, V.P.; Sathyendranath, S.; Hoteit, I. Monsoon oscillations regulate fertility of the Red Sea. Geophys. Res. Lett. 2015, 42, 855–862. [Google Scholar] [CrossRef] [Green Version]
- Yap, W.G. Shrimp culture: A global overview. SEAFDEC Asian Aquac. 1999, 21, 18–21. [Google Scholar]
- Grindle, A.K.; Siddiqi, A.; Anadon, L.D. Food security amidst water scarcity: Insights on sustainable food production from Saudi Arabia. Sustain. Prod. Consum. 2015, 2, 67–78. [Google Scholar] [CrossRef]
- Becker, C.; Hughen, K.; Mincer, T.J.; Ossolinski, J.; Weber, L.; Apprill, A. Impact of prawn farming effluent on coral reef water nutrients and microorganisms. Aquac. Environ. Interact. 2017, 9, 331–346. [Google Scholar] [CrossRef] [Green Version]
- Alday-Sanz, V.; Brock, J.; Flegel, T.W.; McIntosh, R.; Bondad-Reantaso, M.G.; Salazar, M.; Subasinghe, R. Facts, truths and myths about SPF shrimp in Aquaculture. Rev. Aquac. 2020, 12, 76–84. [Google Scholar] [CrossRef]
- DeVantier, L.; Turak, E.; Al-Shaikh, K.; De ath, G. Coral communities of the central-northern Saudi Arabian Red Sea. Fauna Arabia. 2000, 18, 23–66. [Google Scholar]
- Genevier, L.G.; Jamil, T.; Raitsos, D.E.; Krokos, G.; Hoteit, I. Marine heatwaves reveal coral reef zones susceptible to bleaching in the Red Sea. Glob. Chang. Biol. 2019, 25, 2338–2351. [Google Scholar] [CrossRef] [Green Version]
- Hozumi, A.; Kaartvedt, S.; Røstad, A.; Berumen, M.L.; Cochran, J.E.; Jones, B.H. Acoustic backscatter at a Red Sea whale shark aggregation site. Reg. Stud. Mar. Sci. 2018, 20, 23–33. [Google Scholar] [CrossRef] [Green Version]
- Cochran, J.E.; Braun, C.D.; Cagua, E.F.; Campbell, M.F., Jr.; Hardenstine, R.S.; Kattan, A.; Priest, M.A.; Sinclair-Taylor, T.H.; Skomal, G.B.; Sultan, S.; et al. Multi-method assessment of whale shark (Rhincodon typus) residency, distribution, and dispersal behavior at an aggregation site in the Red Sea. PLoS ONE 2019, 14, e0222285. [Google Scholar] [CrossRef]
- Acker, J.; Leptoukh, G.; Shen, S.; Zhu, T.; Kempler, S. Remotely-sensed chlorophyll a observations of the northern Red Sea indicate seasonal variability and influence of coastal reefs. J. Mar. Syst. 2008, 69, 191–204. [Google Scholar] [CrossRef]
- Alkawri, A.; Gamoyo, M. Remote sensing of phytoplankton distribution in the Red Sea and Gulf of Aden. Acta Oceanol. Sin. 2014, 33, 93–99. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gokul, E.A.; Raitsos, D.E.; Gittings, J.A.; Hoteit, I. Developing an Atlas of Harmful Algal Blooms in the Red Sea: Linkages to Local Aquaculture. Remote Sens. 2020, 12, 3695. https://doi.org/10.3390/rs12223695
Gokul EA, Raitsos DE, Gittings JA, Hoteit I. Developing an Atlas of Harmful Algal Blooms in the Red Sea: Linkages to Local Aquaculture. Remote Sensing. 2020; 12(22):3695. https://doi.org/10.3390/rs12223695
Chicago/Turabian StyleGokul, Elamurugu Alias, Dionysios E. Raitsos, John A. Gittings, and Ibrahim Hoteit. 2020. "Developing an Atlas of Harmful Algal Blooms in the Red Sea: Linkages to Local Aquaculture" Remote Sensing 12, no. 22: 3695. https://doi.org/10.3390/rs12223695