Blood Glucose Level Monitoring Using an FMCW Millimeter-Wave Radar Sensor
Abstract
1. Introduction
2. Why mm-Wave Sensing?
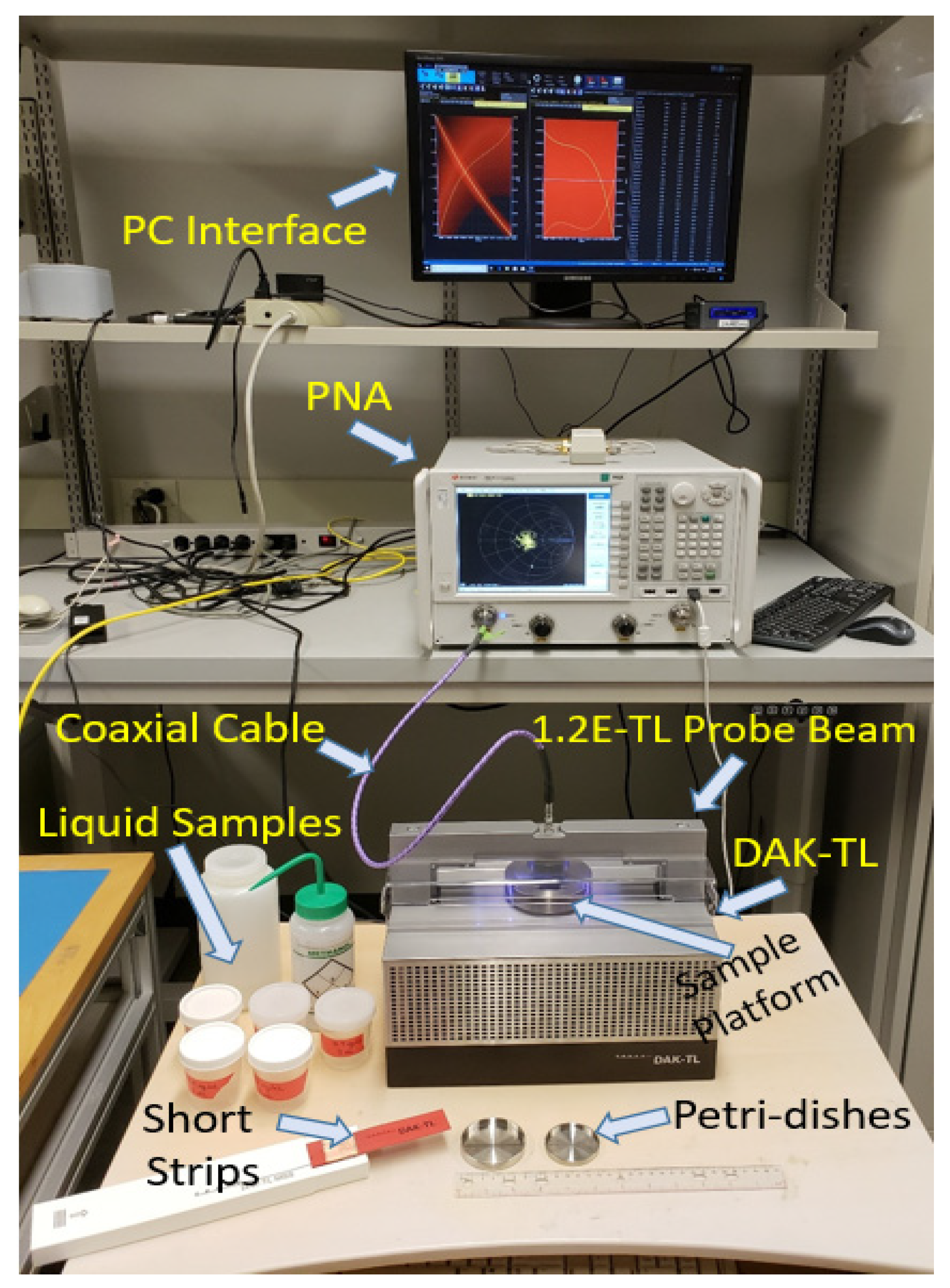
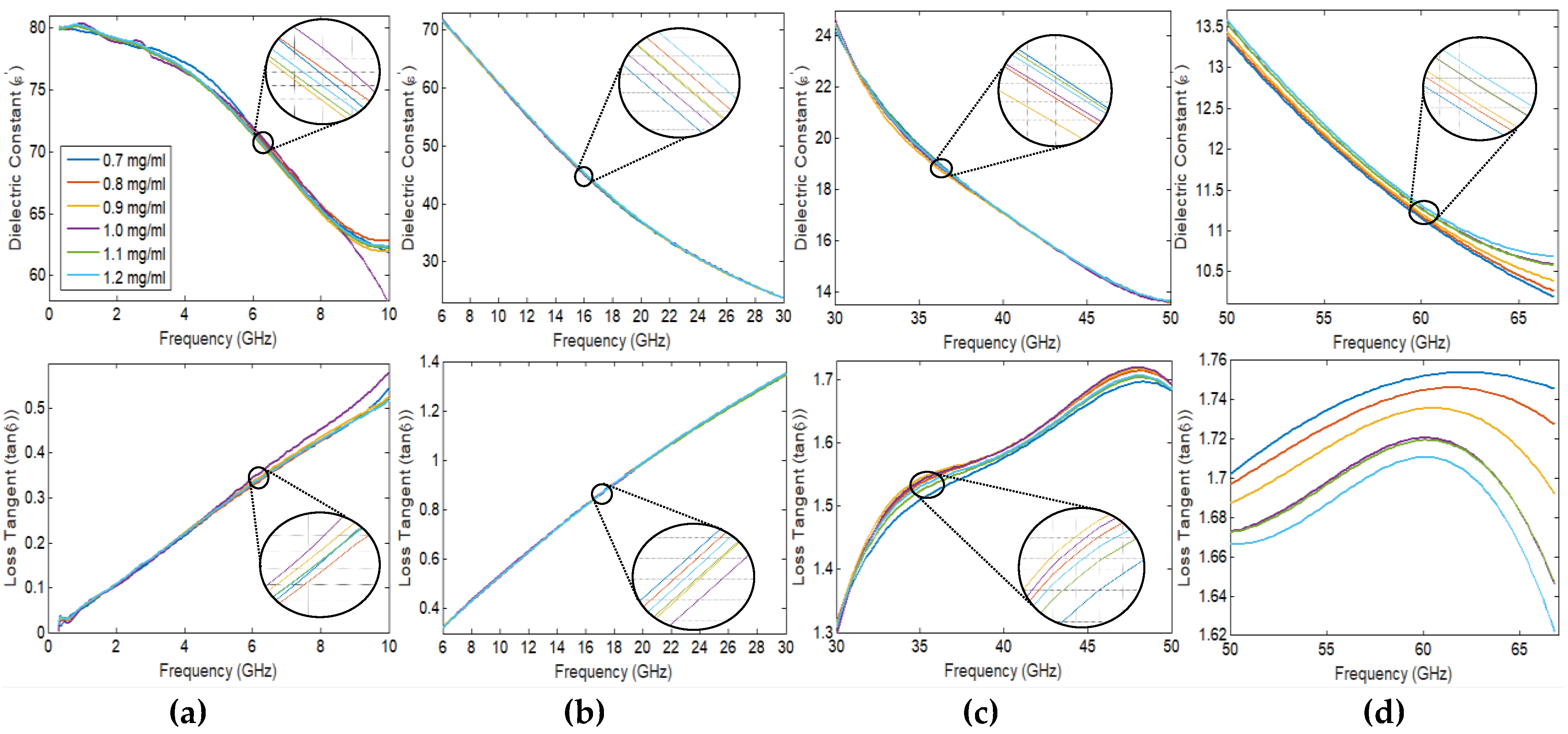
2.1. Spectroscopy Dielectric Measurements of Glucose Using DAK-TL
2.2. PNA Scattering Analysis for Clinical Blood Samples in the Frequency Range (50–67 GHz)
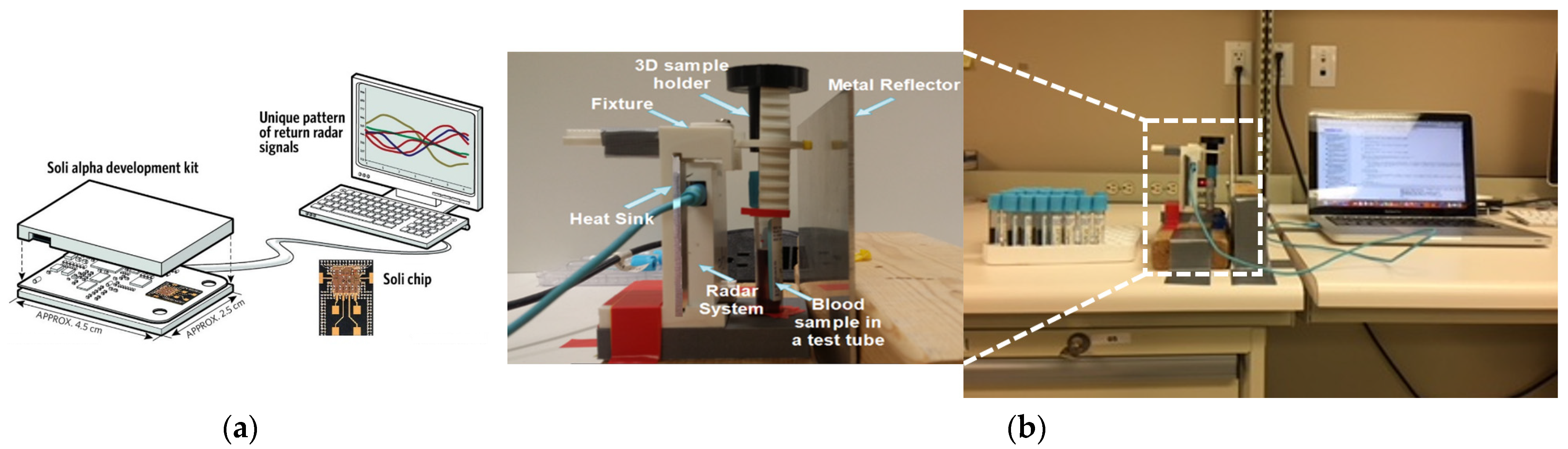
3. Proposed Low-Cost Sensor
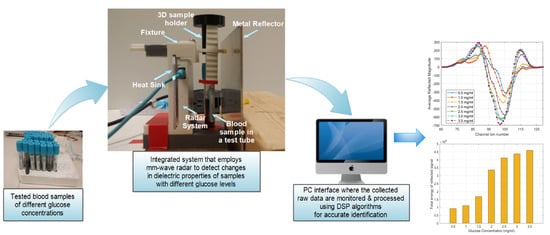
3.1. Working Principle of the Proposed Radar System
3.2. Radar Experimental Approach for Glucose Detection
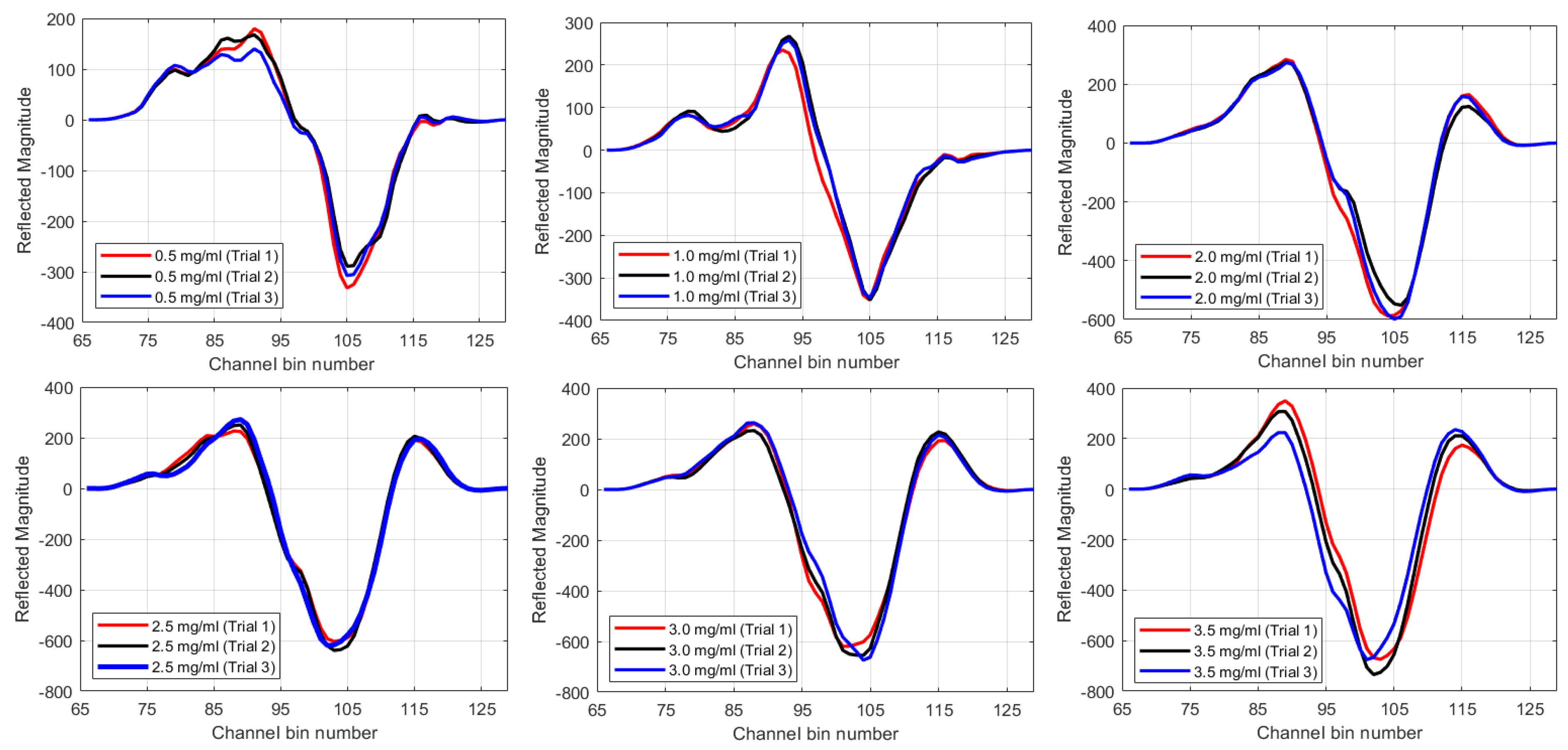
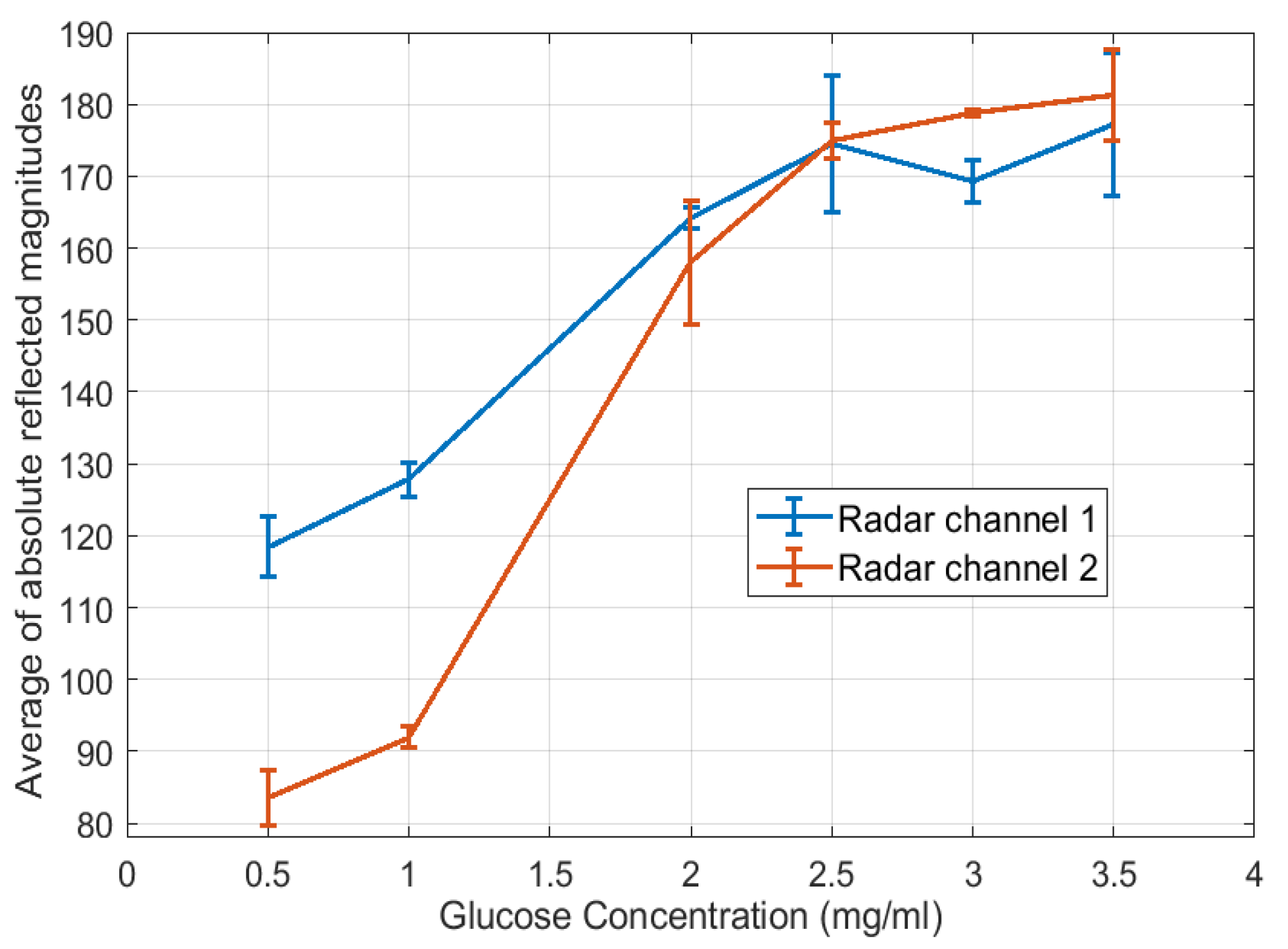
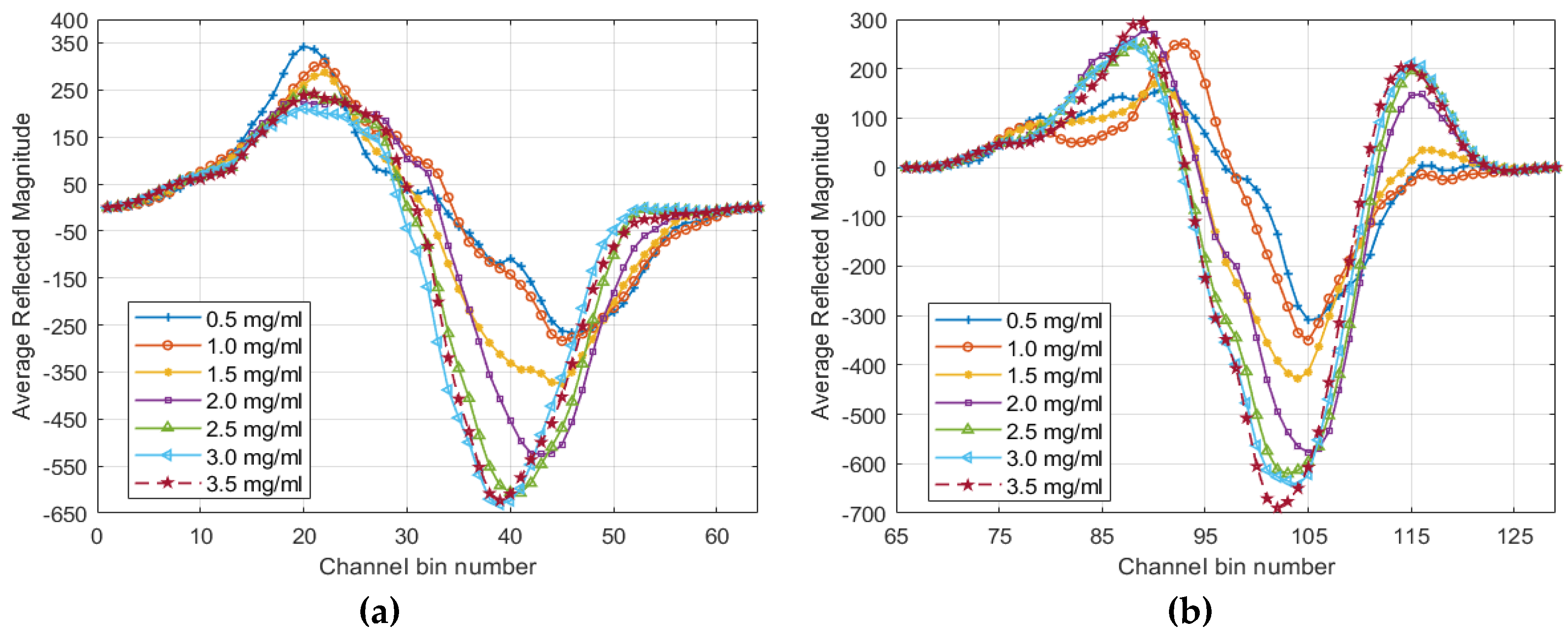
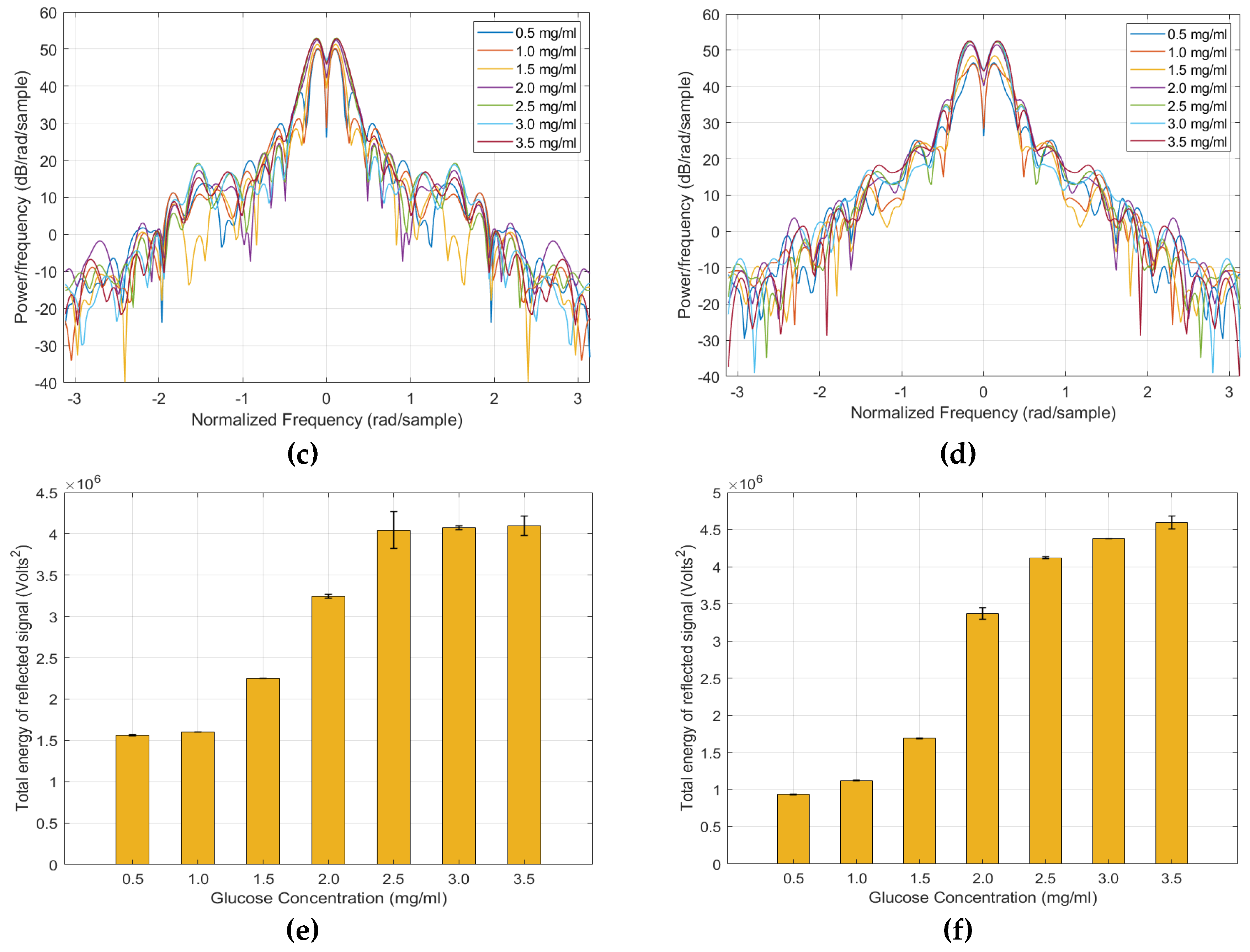
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Whiting, D.; Guariguata, L.; Weil, C.; Shaw, J.; IDF, J. Diabetes Atlas: Global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res. Clin. Pract. 2011, 94, 311–321. [Google Scholar] [CrossRef] [PubMed]
- WHO. World Health Day 2016: WHO calls for global action to halt rise in and improve care for people with diabetes. In Technical Report 2016; World Health Org.: Geneva, Switzerland, 2016. [Google Scholar]
- Cano-Garcia, H.; Kosmas, P.; Sotiriou, I.; Papadopoulos-Kelidis, I.; Parini, C.; Gouzouasis, I.; Palikaras, G.; Kallos, E. Detection of glucose variability in saline solutions from transmission and reflection measurements using V-band waveguides. Meas. Sci. Technol. 2015, 26, 125701–125710. [Google Scholar] [CrossRef]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2014, 37, S81–S90. [Google Scholar] [CrossRef] [PubMed]
- Holt, R.; Cockram, C.; Flyvbjerg, A.; Goldstein, B. Textbook of Diabetes; John Wiley & Sons: New York, NY, USA, 2011. [Google Scholar]
- Canadian Diabetes Association: Diabetes Self-Care. Available online: https://www.diabetes.ca/advocacy---policies/our-policy-positions/diabetes-self-care-in-public-places (accessed on 12 January 2019).
- Cunningham, D.; Stenken, J. In Vivo Glucose Sensing; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Sieg, A.; Guy, R.H.; Delgado-Charro, M.B. Noninvasive and minimally invasive methods for transdermal glucose monitoring. Diabetes Technol. Ther. 2005, 7, 174–197. [Google Scholar] [CrossRef] [PubMed]
- Weiss, R.; Yegorchikov, Y.; Shusterman, A.; Raz, I. Noninvasive continuous glucose monitoring using photoacoustic technology—Results from the first 62 subjects. Diabetes Technol. Ther. 2007, 9, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.; Zisser, H.; Schwartz, S.; Bailey, T.; Kaplan, R.; Ellis, S.; Jovanovic, L. Improvement in glycemic excursions with a transcutaneous, real-time continuous glucose sensor: A randomized controlled trial. Diabetes Care 2006, 29, 44–50. [Google Scholar] [CrossRef]
- Amir, O.; Weinstein, D.; Zilberman, S.; Less, M.; Perl-Treves, D.; Primack, H.; Weinstein, A.; Gabis, E.; Fikhte, B.; Karasik, A. Continuous Noninvasive Glucose Monitoring Technology Based on “Occlusion Spectroscopy”. J. Diabetes Sci. Technol. 2007, 1, 463–469. [Google Scholar] [CrossRef]
- Hotmartua, R.; Pangestu, P.W.; Zakaria, H.; Irawan, Y.S. Noninvasive Blood Glucose Detection Using Near Infrared Sensor. In Proceedings of the International Conference on Electrical Engineering and Informatics (ICEEI), Denpasar, Indonesia, 10–11 August 2015; pp. 687–692. [Google Scholar]
- Yadav, J.; Rani, A.; Singh, V.; Murari, B.M. Near-Infrared LED Based Non-Invasive Blood Glucose Sensor. In Proceedings of the International Conference on Signal Processing and Integrated Networks (SPIN), Noida, India, 20–21 February 2014; pp. 591–594. [Google Scholar]
- Vashist, S.K. Non-invasive glucose monitoring technology in diabetes management: A review. Anal. Chim. Acta 2012, 750, 16–27. [Google Scholar] [CrossRef]
- Glennon, T.; O’Quigley, C.; McCaul, M.; Matzeu, G.; Beirne, S.; Wallace, G.G.; Stroiescu, F.; O’Mahoney, N.; White, P.; Diamond, D. ‘SWEATCH’: A wearable platform for harvesting and analyzing sweat sodium content. Electroanalysis 2016, 28, 1283–1289. [Google Scholar] [CrossRef]
- Heikenfeld, J. Non-invasive analyte access and sensing through eccrine sweat: Challenges and outlook circa. Electroanalysis 2016, 28, 1242–1249. [Google Scholar] [CrossRef]
- Guo, D.; Zhang, D.; Zhang, L.; Lu, G. Non-invasive blood glucose monitoring for diabetics by means of breath signal analysis. Sens. Actuators B Chem. 2012, 173, 106–113. [Google Scholar] [CrossRef]
- Malik, S.; Gupta, S.; Khadgawat, R.; Anand, S. A Novel Non-Invasive Blood Glucose Monitoring Approach Using Saliva. In Proceedings of the IEEE International Conference on Signal Processing, Informatics, Communication and Energy Systems (SPICES), Kozhikode, India, 19–21 February 2015. [Google Scholar]
- Gao, W.; Emaminejad, S.; Nyein, H.Y.; Challa, S.; Chen, K.; Peck, A.; Javey, A. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 2016, 529, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hodge, W.; Hutnick, C.; Wang, X. Noninvasive Diagnostic Devices for Diabetes through Measuring Tear Glucose. J. Diabetes Sci. Technol. 2011, 5, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Naylon, J.; Luzio, S.; Beutler, J.; Birchall, J.; Martin, C.; Porch, A. Design and In Vitro Interference Test of Microwave Noninvasive Blood Glucose Monitoring Sensor. IEEE Trans. Microw. Theory Tech. 2015, 63, 3016–3025. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Hovsepyan, A.; Babajanyan, A.; Lee, K.; Friedman, B. Microwave dielectric resonator biosensor for aqueous glucose solution. Rev. Sci. Instrum. 2008, 79, 086107–086109. [Google Scholar] [CrossRef] [PubMed]
- Cano-Garcia, H.; Gouzouasis, I.; Sotiriou, I.; Saha, S.; Palikaras, G.; Kosmas, P.; Kallos, E. Reflection and Transmission Measurements Using 60 GHz Patch Antennas in the Presence of Animal Tissue for Non-Invasive Glucose Sensing. In Proceedings of the 10th European Conference on Antennas and Propagation (EuCAP), Davos, Switzerland, 10–15 April 2016; pp. 1–3. [Google Scholar]
- Siegel, P.H.; Lee, Y.; Pikov, V. Millimeter-Wave Non-Invasive Monitoring of Glucose in Anesthetized Rats. In Proceedings of the International Conference on Infrared, Millimeter, and Terahertz Waves (IRMMW-THz), Tucson, AZ, USA, 14–19 September 2014; pp. 1–2. [Google Scholar]
- Hofmann, M.; Bloss, M.; Weigel, R.; Fischer, G.; Kissinger, D. Non-Invasive Glucose Monitoring Using Open Electromagnetic Waveguides. In Proceedings of the 42nd European Microwave Conference, Amsterdam, The Netherlands, 29 October–1 November 2012; pp. 546–549. [Google Scholar]
- Baker-Jarvis, J.; Janezic, M.D.; Domich, P.D.; Geyer, R. Analysis of an Open-Ended Coaxial Probe with Lift-Off for Nondestructive Testing. IEEE Trans. Instrum. Meas. 1994, 43, 711–718. [Google Scholar] [CrossRef]
- Bakhtiari, S.; Ganchev, S.I.; Zoughi, R. Analysis of radiation from an open-ended coaxial line into stratified dielectrics. IEEE Trans. Microw. Theory Tech. 1994, 42, 1261–1267. [Google Scholar] [CrossRef]
- Meaney, P.M.; Gregory, A.P.; Seppälä, J.; Lahtinen, T. Open-Ended Coaxial Dielectric Probe Effective Penetration Depth Determination. IEEE Trans. Microw. Theory Tech. 2016, 64, 915–923. [Google Scholar] [CrossRef]
- Omer, A.E.; Shaker, G.; Safavi-Naeini, S.; Shubair, R.M. EM Measurements of Glucose-Aqueous Solutions. In Proceedings of the 2019 IEEE International Symposium on Antennas and Propagation and USNC-URSI Radio Science Meeting, Atlanta, GA, USA, 7–12 July 2019; pp. 103–104. [Google Scholar]
- Shimin, D. A New Method for Measuring Dielectric Constant Using the Resonant Frequency of a Patch Antenna. IEEE Trans. Microw. Theory Tech. 1986, 34, 923–931. [Google Scholar] [CrossRef]
- Bernard, P.A.; Gautray, J.M. Measurement of dielectric constant using a microstrip ring resonator. IEEE Trans. Microw. Theory Tech. 1991, 39, 592–595. [Google Scholar] [CrossRef]
- Noviosense. Available online: http://noviosense.com/ (accessed on 22 September 2019).
- Novartis. Available online: http://www.novartis.com/ (accessed on 22 September 2019).
- QuickLLC. Available online: http://www.iquickitsalivaanalyzer.com/ (accessed on 22 September 2019).
- Cnoga Medical. Available online: http://cnogacare.co/portfolioitem/combo-glucometer/ (accessed on 22 September 2019).
- Glucosense Diagnostic Ltd. Available online: http://www.glucosense.net/ (accessed on 22 September 2019).
- Grove Instruments. Available online: http://www.groveinstruments.com/ (accessed on 22 September 2019).
- Harman-Boehm, I.; Gal, A.; Raykhman, A.M.; Naidis, E.; Mayzel, Y. Noninvasive glucose monitoring: Increasing accuracy by combination of multi-technology and multi-sensors. J. Diabetes Sci. Technol. 2010, 4, 583–595. [Google Scholar] [CrossRef] [PubMed]
- Gouzouasis, I.; Cano-Garcia, H.; Sotiriou, I.; Saha, S.C.; Palikaras, G.; Kosmas, P.; Kallos, E. Detection of Varying Glucose Concentrations in Water Solutions Using a Prototype Biomedical Device for Millimeter-Wave Non-Invasive Glucose Sensing. In Proceedings of the 10th European Conference on Antennas and Propagation (EuCAP), Davos, Switzerland, 10–15 April 2016; pp. 1–4. [Google Scholar]
- Glucowise. Available online: http://www.gluco-wise.com/ (accessed on 22 September 2019).
- Amrane, S.; Azami, N.; Elboulqe, Y. Optimized Algorithm of Dermis Detection for Glucose Blood Monitoring Based on Optical Coherence Tomography. In Proceedings of the 10th International Conference on Intelligent Systems: Theories and Applications (SITA), Rabat, Morocco, 20–21 October 2015; pp. 1–5. [Google Scholar]
- Wadamori, N. Behavior of Long-Period Measurements Using a Small-Sized Photoacoustic Cell for Aqueous Glucose Monitoring. In Proceedings of the 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; pp. 1267–1270. [Google Scholar]
- Ramasahayam, S.; Arora, L.; Chowdhury, S.R.; Anumukonda, M. FPGA Based System for Blood Glucose Sensing Using Photoplethysmography and Online Motion Artifact Correction Using Adaline. In Proceedings of the 2015 9th International Conference on Sensing Technology (ICST), Auckland, New Zealand, 8–10 December 2015; pp. 22–27. [Google Scholar]
- Asaduzzaman, A.; Samadarsinee, S.; Chidella, K.K. Simulating Multi Sensor Noninvasive Blood Glucose Monitoring Systems. In Proceedings of the SoutheastCon 2016, Norfolk, VA, USA, 30 March–3 April 2016; pp. 1–7. [Google Scholar]
- Pai, P.P.; Sanki, P.K.; De, A.; Banerjee, S. Nir Photoacoustic Spectroscopy for Non-Invasive Glucose Measurement. In Proceedings of the 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; pp. 7978–7981. [Google Scholar]
- Li, X.; Li, C. Research on Non-Invasive Glucose Concentration Measurement by NIR Transmission. In Proceedings of the IEEE International Conference on Computer and Communications (ICCC), Chengdu, China, 10–11 October 2015; pp. 223–228. [Google Scholar]
- Ling, S.H.; San, P.P.; Nguyen, H.T. Non-invasive hypoglycemia monitoring system using extreme learning machine for type 1 diabetes. ISA Trans. 2016, 64, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Yang, F.; Xia, F.; Zhang, Q.; Chen, Y. A Novel Miniature Spiral Sensor for Non-Invasive Blood Glucose Monitoring. In Proceedings of the 10th European Conference on Antennas and Propagation (EuCAP), Davos, Switzerland, 10–15 April 2016; pp. 1–2. [Google Scholar]
- Adhyapak, A.; Sidley, M.; Venkataraman, J. Analytical Model for Real Time, Noninvasive Estimation of Blood Glucose Level. In Proceedings of the 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014; pp. 5020–5023. [Google Scholar]
- Baghbani, R.; Rad, M.A.; Pourziad, A. Microwave sensor for noninvasive glucose measurements design and implementation of a novel linear. IET Wirel. Sens. Syst. 2015, 5, 51–57. [Google Scholar] [CrossRef]
- Yilmaz, T.; Foster, R.; Hao, Y. Broadband tissue mimicking phantoms and a patch resonator for evaluating noninvasive monitoring of blood glucose levels. IEEE Trans. Antennas Propag. 2014, 62, 3064–3075. [Google Scholar] [CrossRef]
- Deshmukh, V.V.; Ghongade, R.B. Measurement of Dielectric Properties of Aqueous Glucose Using Planar Ring Resonator. In Proceedings of the International Conference on Microelectronics, Computing and Communications (MicroCom), Durgapur, India, 23–25 January 2016; pp. 1–5. [Google Scholar]
- XeThru: Single-Chip Radar Sensors with Sub-Mm Resolution. Available online: https://www.xethru.com/ (accessed on 23 September 2019).
- Project Soli—Google ATAP. Available online: https://atap.google.com/soli/ (accessed on 23 September 2019).
- Walabot Sensor Radio Frequency Technology for Advanced Detection. Available online: https://walabot.com/ (accessed on 23 September 2019).
- Yeo, H.-S.; Quigley, A. Radar Sensing in Human-computer Interaction. Interactions 2017, 25, 70–73. [Google Scholar] [CrossRef]
- Lien, J.; Gillian, N.; Karagozler, M.E.; Amihood, P.; Schwesig, C.; Olson, E.; Raja, H.; Poupyrev, I. Soli: Ubiquitous Gesture Sensing with Millimeter Wave Radar. ACM Trans. Graph. 2016, 35, 142–161. [Google Scholar] [CrossRef]
- Wang, S.; Song, J.; Lien, J.; Poupyrev, I.; Hilliges, O. Interacting with Soli: Exploring Fine-Grained Dynamic Gesture Recognition in the Radio-Frequency Spectrum. In Proceedings of the 29th Annual Symposium on User Interface Software and Technology (UIST ’16), Tokyo, Japan, 16–19 October 2016; pp. 851–860. [Google Scholar]
- Ens, B.; Quigley, A.; Yeo, H.-S.; Irani, P.; Piumsomboon, T.; Billinghurst, M. Exploring Mixed-Scale Gesture Interaction. In Proceedings of the SIGGRAPH Asia 2017 Posters (SA ’17), Bangkok, Thailand, 27–30 November 2017. [Google Scholar]
- Avrahami, D.; Patel, M.; Yamaura, Y.; Kratz, S. Below the Surface: Unobtrusive Activity Recognition for Work Surfaces Using RF-radar Sensing. In Proceedings of the 23rd International Conference on Intelligent User Interfaces (IUI ’18), Tokyo, Japan, 7–11 March 2018; pp. 439–451. [Google Scholar]
- Dhekne, A.; Gowda, M.; Zhao, Y.; Hassanieh, H.; Choudhury, R.R. LiquID: A Wireless Liquid IDentifier. In Proceedings of the 16th Annual International Conference on Mobile Systems, Applications, and Services (MobiSys ’18), Munich, Germany, 10–15 June 2018; pp. 442–454. [Google Scholar]
- Yeo, H.-S.; Flamich, G.; Schrempf, P.; Harris-Birtill, D.; Quigley, A. RadarCat: Radar Categorization for Input & Interaction. In Proceedings of the 29th Annual Symposium on User Interface Software and Technology (UIST ’16), Tokyo, Japan, 16–19 October 2016; pp. 833–841. [Google Scholar]
- Rahman, T.; Adams, A.; Ravichandran, R.V.; Zhang, M.; Patel, S.N.; Kientz, J.A.; Choudhury, T. DoppleSleep: A Contactless Unobtrusive Sleep Sensing System Using Short-Range Doppler Radar. In Proceedings of the 2015 ACM International Joint Conference on Pervasive and Ubiquitous Computing (UbiComp ’15), Osaka, Japan, 7–11 September 2015; pp. 39–50. [Google Scholar]
- Adib, F.; Mao, H.; Kabelac, Z.; Katabi, D.; Miller, R.C. Smart Homes That Monitor Breathing and Heart Rate. In Proceedings of the 33rd Annual ACM Conference on Human Factors in Computing Systems (CHI ’15), Seoul, Republic of Korea, 18–23 April 2015; pp. 837–846. [Google Scholar]
- Zhao, M.; Li, T.; Abu Alsheikh, M.; Tian, Y.; Zhao, H.; Torralba, A.; Katabi, D. Through-Wall Human Pose Estimation Using Radio Signals. In Proceedings of the 2018 IEEE/CVF Conference on Computer Vision and Pattern Recognition (CVPR), Salt Lake City, UT, USA, 18–22 June 2018; pp. 7356–7365. [Google Scholar]
- Liu, S.; Shaker, G.; Safavi-Naeini, S.; Chong, J.M. Low-cost Gas Sensors Utilizing Mm-Wave Radars. In Proceedings of the 2017 IEEE International Symposium on Antennas and Propagation & USNC/URSI National Radio Science Meeting, San Diego, CA, USA, 9–15 July 2017; pp. 1853–1854. [Google Scholar]
- Shaker, G.; Smith, K.; Omer, A.E.; Liu, S.; Csech, C.; Wadhwa, U.; Safavi-Naeini, S.; Hughson, R. Non-Invasive Monitoring of Glucose Level Changes Utilizing a mm-Wave Radar System. Int. J. Mob. Hum. Comput. Interact. 2018, 10, 10–29. [Google Scholar] [CrossRef]
- Scientific American, “Fake Blood Made Scientific”. Available online: https://www.scientificamerican.com/article/fake-blood-made-scientific (accessed on 19 January 2018).
- Yaws, K.; Mixon, D.; Roach, W. Electromagnetic Properties of Tissue in the Optical Region. In Proceedings of the Biomedical Optics (BiOS), San Jose, CA, USA, 28 May 2004. [Google Scholar]
- Hofmann, M.; Fischer, G.; Weigel, R.; Kissinger, D. Microwave-based noninvasive concentration measurements for biomedical applications. IEEE Trans. Microw. Theory Tech. 2013, 61, 2195–2204. [Google Scholar] [CrossRef]
- Meriakri, V.V.; Chigrai, E.E.; Nikitin, I.P.; Parkhomenko, M.P. Dielectric Properties of Water Solutions with Small Content of Glucose in the Millimeter-Wave Band and the Determination of Glucose in Blood. In Proceedings of the International Kharkov Symposium Physics and Engrg. of Millimeter and Sub-Millimeter Waves (MSMW), Kharkov, Ukraine, 25–30 June 2007; pp. 873–875. [Google Scholar]
- Meriakri, V.V.; Chigrai, E.E.; Kim, D.; Nikitin, I.P.; Pangonis, L.I.; Parkhomenko, M.P.; Won, J.H. Dielectric properties of glucose solutions in the millimetre-wave range and control of glucose content in blood. Meas. Sci. Technol. 2007, 18, 977–982. [Google Scholar] [CrossRef]
- Saha, S.; Cano-Garcia, H.; Sotiriou, I.; Lipscombe, O.; Gouzouasis, I.; Koutsoupidou, M.; Palikaras, G.; MacKenzie, R.; Reeve, T.; Kosmas, P.; et al. A Glucose Sensing System Based on Transmission Measurements at Millimetre Waves using Micro strip Patch Antennas. Sci. Rep. 2017, 7, 6855–6865. [Google Scholar] [CrossRef]
- Brady, A.; McCabe, C.; McCann, M. Fundamentals of Medical Surgical Nursing; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Ekoé, J.; Punthakee, Z.; Ransom, T.; Prebtani, A.; Goldenberg, R. Screening for Type1 and Type2 Diabetes. Can. J. Diabetes 2013, 37, S12–S15. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nasr, I.; Jungmaier, R.; Baheti, A.; Noppeney, D.; Bal, J.S.; Wojnowski, M.; Karagozler, E.; Raja, H.; Lien, J.; Poupyrev, I.; et al. A Highly Integrated 60 GHz 6-Channel Transceiver with Antenna in Package for Smart Sensing and Short-Range Communications. IEEE J. Solid State Circuits 2016, 51, 2066–2076. [Google Scholar] [CrossRef]
- Heinzel, G.; Rüdiger, A.; Schilling, R. Spectrum and Spectral Density Estimation by the Discrete Fourier transform (DFT), Including a Comprehensive List of Window Functions and Some New at-Top Windows; Technical Report; Albert-Einstein-Institut: Hannover, Germany, 2002. [Google Scholar]
- Omer, A.E.; Shaker, G.; Safavi-Naeini, S.; Murray, K.; Hughson, R. Glucose Levels Detection Using mm-Wave Radar. IEEE Sens. Lett. 2018, 2, 1–4. [Google Scholar] [CrossRef]
- Zhadobov, M.; Augustine, R.; Sauleau, R.; Alekseev, S.; Di Paola, A.; Le Quément, C.; Mahamoud, Y.S.; Le Dréan, Y. Complex permittivity of representative biological solutions in the 2–67 GHz range. Bioelectromagnetics 2012, 33, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Nikawa, Y.; Someya, D. Application of Millimeter Waves to Measure Blood Sugar Level. In Proceedings of the 2001 Asia-Pacific Microwave Conference (Cat. No.01TH8577), Taipei, Taiwan, 3–6 December 2001; pp. 1303–1306. [Google Scholar]
- Turgul, V.; Kale, I. On the Accuracy of Complex Permittivity Model of Glucose/Water Solutions for Non-Invasive Microwave Blood Glucose Sensing. In Proceedings of the 2015 E-Health and Bioengineering Conference (EHB), Iasi, Romania, 19–21 November 2015; pp. 1–4. [Google Scholar]
- Karacolak, T.; Moreland, E.C.; Topsakal, E. Cole-Cole model for glucose-dependent dielectric properties of blood plasma for continuous glucose monitoring. Microw. Opt. Technol. Lett. 2013, 55, 1160–1164. [Google Scholar] [CrossRef]
- Hofmann, M.; Trenz, F.; Weigel, R.; Fischer, G.; Kissinger, D. A Microwave Sensing System for Aqueous Concentration Measurements Based on a Microwave Reflectometer. In Proceedings of the 2012 IEEE/MTT-S International Microwave Symposium Digest, Montreal, QC, Canada, 17–22 June 2012; pp. 1–3. [Google Scholar]
- Diederichs, K.; Qiu, A.; Shaker, G. Wireless Biometric Individual Identification Utilizing Millimeter Waves. IEEE Sens. Lett. 2017, 1, 1–4. [Google Scholar] [CrossRef]
- Yeo, H.-S.; Minami, R.; Rodriguez, K.; Shaker, G.; Quigley, A. Exploring Tangible Interactions with Radar Sensing. Proc. ACM Interact. Mob. Wearable Ubiquitous Technol. 2018, 2, 1–25. [Google Scholar] [CrossRef]

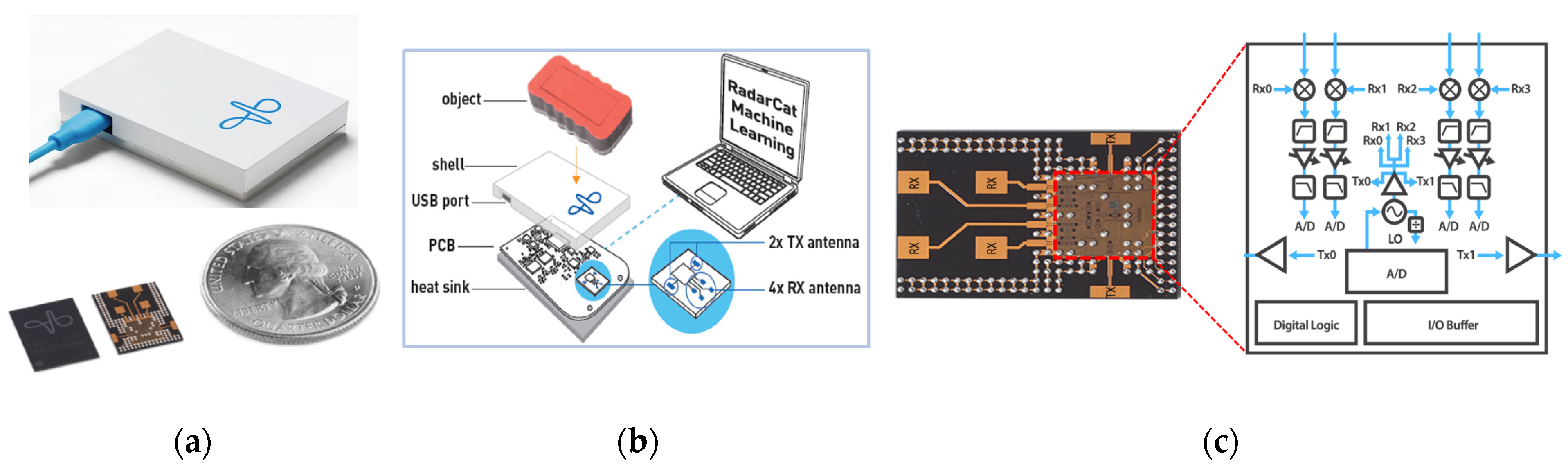


| Device/Company | Technique | Placement | Needs | Attributes |
|---|---|---|---|---|
| NovioSense (Noviosense BV) [32] | Electrochemical enzymatic-based tear analysis | Lower eye lid (inferior conjunctival fornix) | Continuous monitoring provided a sample | Compact, painless, flexible, wireless power, smartphone connected for data analysis |
| Smart Contact Lens (Novartis & Google) [33] | Electrochemical enzymatic-based tear analysis | Eye | Continuous monitoring provided a sample | Painless, power efficient, portable, low relief, hazardous when overheated, withdrawn from market! |
| iQuicklt Saliva Analyzer (Quick LLC) [34] | Saliva analysis | Saliva of the mouth | Intermittent monitoring provided a sample | Portable, convenient to use, accurate, time efficient (real-time readings), under development and clinical trials |
| TensorTip Combo Glucometer (Cnoga Medical) [35] | Photometric and photography-based techniques | Fingertip | Intermittent monitoring without a sample | Convenient to use, accurate when calibrated on individual-basis, smartphone compatible, battery operated (rechargeable), cost-effective, certified! |
| Glucosense (Glucosense Diagnostic Ltd.) [36] | Low-powered laser sensors that use photonic technology (infrared light) | Fingertip | Intermittent monitoring without a sample | Convenient to use, portable, affordable, power-efficient, time efficient (30 s), under development! |
| Groves’s Device (Groves Instrument Inc) [37] | NIR spectroscopy | Fingertip or earlobe | Intermittent monitoring without a sample | Fast processed readings, time-efficient (20 s), compact, portable, uses capillary-level blood, less accurate due lacking subjective calibration |
| GlucoTrack (Integrity Applications) [38] | Thermal, ultrasonic, and microwave EM technology | Earlobe | Intermittent monitoring without a sample | Affordable, convenient to use, high accuracy due earlobe placement, unit-connected for results processing/display, complex processing, FDA approved! |
| GlucoWise (MediWise) [39,40] | RF/Microwave | Amid fingers (thumb and forefinger) | Intermittent monitoring without a sample | Convenient to use, affordable, accurate, Bluetooth-based data transmission, compact, integrable with insulin pumps, uses capillary-level blood, time-efficient, fast readings (10 s), hurtful due localized energy usage, under development! |
| Ref | Technique | Main Items | Sensing Parameter | Pros | Cons |
|---|---|---|---|---|---|
| [41] | OCT | Light source + coupler + interferometer | OCT slope | - Good resolution images with high SNR | - Affected by pressure and temperature - Compromise between resolution and penetration depth -Time consuming for glucose estimation |
| [42] | Optical/thermal spectroscopy + photoacoustic | Microphone + laser diode | Change in optical absorption coefficient and pressure | - Compact size - Improved sensitivity | - Affected by pressure and temperature |
| [43] | NIR + PPG + ANN | Photo diode + LED | Difference in optical density | - Reduced cost - Fast FPGA implementation -Good accuracy | - PPG is affected by noise, motion, fatty tissues - Increased complex processing -Time consuming -Filtering overhead |
| [44] | Photoacoustic + NIR + MEMS | IR + ultrasonic micro-electro-micro mechanical (MEMS) sensors | Acoustic pressure and absorption | - Compact size - Efficient power - Accurate and sensitive - Deep penetration in ear lobe area | - Affected by pressure, temperature, and humidity - Performance dependency on surface |
| [45] | NIR + photoacoustic | Piezoelectric sensor + pulsed laser diode (PLD) | Change in pressure (Photoacoustic amplitude) | - Good sensitivity - Limited scattering - User friendly | - Nonuniform absorption Affected by pressure and temperature -Time consuming -Power inefficient |
| [46] | NIR | Thermal/laser sensors + spectroscope | Change in power output of the transmitted light beam | - Not expensive - Harmless | - Data limitation - Less accurate |
| [47] | ECG + ML | Compumedics ECG leads | Heart rate and QT interval | - Fast processing - Converged computation - Detecting hypoglycemia and hyperglycemia | - ECG signal parameters possibly affected by abnormal heart condition (e.g., arrhythmia) |
| [48] | RF/Microwave | Microstrip patch antenna (spiral) | Shift in resonance frequency | - Compact size - Affordable cost - High Q-factor | - Affected by noises in atmosphere - Not validated for glucose sensing using real measurements - Loss parameters not considered in simulation |
| [49] | RF/Microwave | PNA + antenna + Cole-Cole model | Shift in resonant frequency | - Good correlation with glucometer when calibrated | - Affected by environmental changes (sweat, temperature, pressure, etc.) - Subject-based calibration |
| [50] | RF/Microwave | Microstrip patch antenna (triangular) | Shift in resonance frequency | - Cost effective - Miniaturized size - Linear | - Not demonstrated for different glucose levels detection |
| [51] | RF/Microwave | Patch resonator | Changes in input impedance | - Miniaturized size - Good sensitivity - Tested on wideband tissue mimicking phantom | - Large error margin - Phantom simulated with non-dispersive dielectric properties |
| [39] | Millimeter-wave Antenna | Two patch antennas (Glucowise) | Changes in transmission coefficient S21 | - Portable - Less susceptible by interference | - Possible harmful effects due localized energy |
| [52] | RF/Microwave | Planar ring resonator | Shift in resonant frequency | - Compact size - Accurate (Resonant-based) | - Affected by environment status - Low sensitivity - Tested on impractical glucose ranges |
| Targeted concentration for the prepared sample (mg/mL) | 0.5 | 1.0 | 1.5 | 2.0 | 2.5 | 3.0 | 3.5 |
| Measured concentration using glucometer (mg/mL) | 0.5 | 1.06 | 1.63 | 2.16 | 2.79 | 3.15 | 3.32 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omer, A.E.; Safavi-Naeini, S.; Hughson, R.; Shaker, G. Blood Glucose Level Monitoring Using an FMCW Millimeter-Wave Radar Sensor. Remote Sens. 2020, 12, 385. https://doi.org/10.3390/rs12030385
Omer AE, Safavi-Naeini S, Hughson R, Shaker G. Blood Glucose Level Monitoring Using an FMCW Millimeter-Wave Radar Sensor. Remote Sensing. 2020; 12(3):385. https://doi.org/10.3390/rs12030385
Chicago/Turabian StyleOmer, Ala Eldin, Safieddin Safavi-Naeini, Richard Hughson, and George Shaker. 2020. "Blood Glucose Level Monitoring Using an FMCW Millimeter-Wave Radar Sensor" Remote Sensing 12, no. 3: 385. https://doi.org/10.3390/rs12030385
APA StyleOmer, A. E., Safavi-Naeini, S., Hughson, R., & Shaker, G. (2020). Blood Glucose Level Monitoring Using an FMCW Millimeter-Wave Radar Sensor. Remote Sensing, 12(3), 385. https://doi.org/10.3390/rs12030385