Detecting Frost Stress in Wheat: A Controlled Environment Hyperspectral Study on Wheat Plant Components and Implications for Multispectral Field Sensing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Glasshouse Setup
2.2. Controlled Environment Room (CER) Setup and Deployment
2.3. Hyperspectral Image Acquisition of Wheat Plant Components
2.4. Data extraction and Analysis
3. Experimental Results
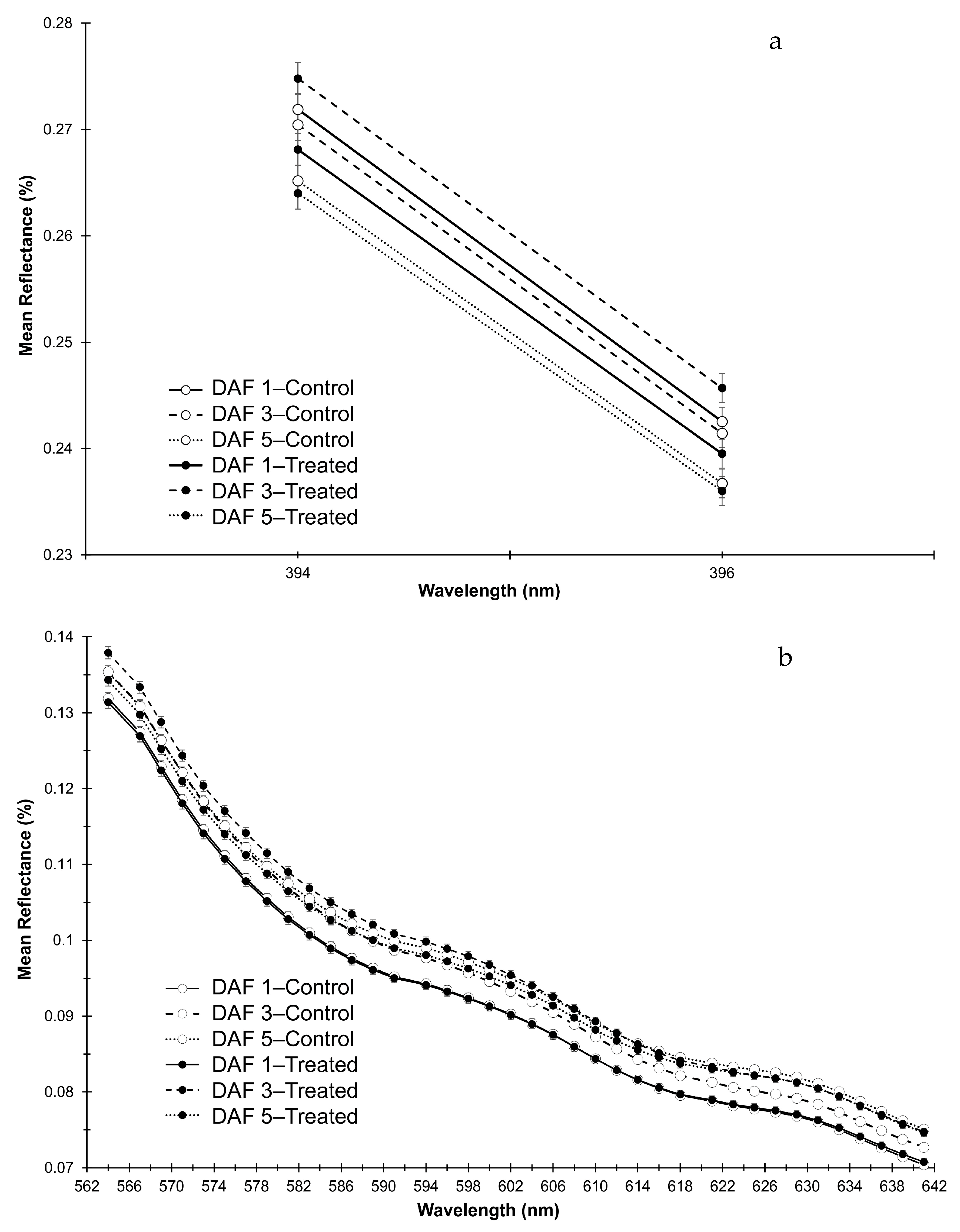
3.1. Effect of the Frost Treatment on Reflectance
3.2. Effect of DAF on Reflectance
3.3. Interaction of DAF and Treatment on Reflectance
3.4. Selecting an Appropriate Agri-sensor for Frost-stress Detection
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mittler, R. Abiotic stress, the field environment and stress combination. Trends Plant Sci. 2006, 11, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Kennelly, M.; O’Mara, J.; Rivard, C.; Miller, G.L.; Smith, D. Introduction to abiotic disorders in plants. Plant Health Instr. 2012. [Google Scholar] [CrossRef]
- Boyer, J.S. Plant productivity and environment. Science 1982, 218, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M. Crop plants and abiotic stresses. J. Biomol. Res. Ther. 2013, 3. [Google Scholar] [CrossRef] [Green Version]
- Das, B.; Mahajan, G.R.; Singh, R. Hyperspectral Remote Sensing: Use in Detecting Abiotic Stresses in Agriculture. In Advances in Crop Environment Interaction; Springer: Singapore, 2018; pp. 317–335. [Google Scholar]
- Chaerle, L.; Hagenbeek, D.; Vanrobaeys, X.; Van Der Straeten, D. Early detection of nutrient and biotic stress in Phaseolus vulgaris. Int. J. Remote Sens. 2007, 28, 3479–3492. [Google Scholar] [CrossRef]
- Zheng, B.; Chapman, S.C.; Christopher, J.T.; Frederiks, T.M.; Chenu, K. Frost trends and their estimated impact on yield in the Australian wheatbelt. J. Exp. Bot. 2015, 66, 3611–3623. [Google Scholar] [CrossRef] [Green Version]
- Loss, S.P. Factors affecting frost damage to wheat in Western Australia. In Department of Agriculture and Food, Western Australia, Perth. Report 6; 1987. Available online: https://researchlibrary.agric.wa.gov.au/cgi/viewcontent.cgi?article=1003&context=fc_technicalrpts (accessed on 26 November 2019).
- Whaley, J.M.; Kirby, E.J.; Spink, J.H.; Foulkes, M.J.; Sparkes, D.L. Frost damage to winter wheat in the UK: The effect of plant population density. Eur. J. Agron. 2004, 21, 105–115. [Google Scholar] [CrossRef]
- Frederiks, T.M.; Christopher, J.T.; Sutherland, M.W.; Borrell, A.K. Post-head-emergence frost in wheat and barley: Defining the problem, assessing the damage, and identifying resistance. J. Exp. Bot. 2015, 66, 3487–3498. [Google Scholar] [CrossRef] [Green Version]
- Barlow, K.M.; Christy, B.P.; O’Leary, G.J.; Riffkin, P.A.; Nuttall, J.G. Simulating the impact of extreme heat and frost events on wheat crop production: A review. Field Crops Res. 2015, 171, 109–119. [Google Scholar] [CrossRef] [Green Version]
- March, T.; Knights, S.; Biddulph, B.; Ogbonnaya, F.; Maccallum, R.; Belford, R. The GRDC National Frost Initiative. In Proceedings of the GRDC Updates, Adelaide, Australia, 10 February 2015. [Google Scholar]
- Sutka, J. Genetic studies of frost resistance in wheat. Theor. Appl. Genet. 1981, 59, 145–152. [Google Scholar] [CrossRef]
- Sutka, J. Genetic control of frost tolerance in wheat (Triticum aestivum L.). Euphytica 1994, 77, 277–282. [Google Scholar] [CrossRef]
- Sutka, J. Genes for frost resistance in wheat. Euphytica 2001, 119, 169–177. [Google Scholar] [CrossRef]
- Fowler, D.; Limin, A.; Ritchie, J. Low-temperature tolerance in cereals: Model and genetic interpretation. Crop Sci. 1999, 39, 626–633. [Google Scholar] [CrossRef]
- Thomashow, M.F. Plant cold acclimation: Freezing tolerance genes and regulatory mechanisms. Annu. Rev. Plant Biol. 1999, 50, 571–599. [Google Scholar] [CrossRef] [Green Version]
- Båga, M.; Chodaparambil, S.V.; Limin, A.E.; Pecar, M.; Fowler, D.B.; Chibbar, R.N. Identification of quantitative trait loci and associated candidate genes for low-temperature tolerance in cold-hardy winter wheat. Funct. Integr. Genom. 2007, 7, 53–68. [Google Scholar] [CrossRef]
- Eagles, H.A.; Wilson, J.; Cane, K.; Vallance, N.; Eastwood, R.F.; Kuchel, H.; Martin, P.J.; Trevaskis, B. Frost-tolerance genes Fr-A2 and Fr-B2 in Australian wheat and their effects on days to heading and grain yield in lower rainfall environments in southern Australia. Crop Pasture Sci. 2016, 67, 119–127. [Google Scholar] [CrossRef]
- Frederiks, T.M.; Christopher, J.T.; Harvey, G.L.; Sutherland, M.W.; Borrell, A.K. Current and emerging screening methods to identify post-head-emergence frost adaptation in wheat and barley. J. Exp. Bot. 2012, 63, 5405–5416. [Google Scholar] [CrossRef] [Green Version]
- Woodruff, D.R. ’WHEATMAN’ a decision support system for wheat management in subtropical Australia. Aust. J. Agr. Res. 1992, 43, 1483–1499. [Google Scholar] [CrossRef]
- Snape, J.W.; Sarma, R.; Quarrie, S.A.; Fish, L.; Galiba, G.; Sutka, J. Mapping genes for flowering time and frost tolerance in cereals using precise genetic stocks. Euphytica 2001, 120, 309–315. [Google Scholar] [CrossRef]
- Nuttall, J.G.; Perry, E.M.; Delahunty, A.J.; O’Leary, G.J.; Barlow, K.M.; Wallace, A.J. Frost response in wheat and early detection using proximal sensors. J. Agron. Crop Sci. 2019, 205, 220–234. [Google Scholar] [CrossRef] [Green Version]
- Jenkinson, R.; Biddulph, B. Role of stubble management on the severity and duration of frost and its impact on grain yield. In Proceedings of the Agribusines Crop Updates, Perth, Australia, 24 February 2014. [Google Scholar]
- Smith, R.; Minkey, D.; Butcher, T.; Hyde, S.; Jackson, S.; Reeves, K.; Biddulph, B. Stubble Management Recommendations And Limitations For Frost Prone Landscapes. In Proceedings of the GRDC Updates, Perth, Australia, 27 February 2017. [Google Scholar]
- Warrick, B.E.; Miller, T.D. Freeze injury on wheat. In Texas Agricultural Extension Service; The Texas A and M University System: San Angelo, TX, USA, 1999. [Google Scholar]
- Shroyer, J.P.; Mikesell, M.E.; Paulsen, G.M. Spring freeze injury to Kansas wheat. In Agricultural Experiment Station and Cooperative Extension Service; Kansas State University: Manhattan, KS, USA, March 1995. [Google Scholar]
- White, C. Cereals-Frost Identification: The Back Pocket Guide; Bulletin 4375; grains Research and Development Corporation: Canberra, Australia; Government of Western Australia Dept. Of Agriculture: Kensington, Australia, 2000. [Google Scholar]
- The Western Australian Agricultural Authority. Frost Identification Guide for Cereals; Grains Research and Development Corporation; Department of Primary Industries and Regional Development: Western Australia, Australia, 2017. [Google Scholar]
- Marcellos, H. Wheat frost injury—freezing stress and photosynthesis. Aust. J. Agr. Res. 1977, 28, 557–564. [Google Scholar] [CrossRef]
- Rodriguez, D.; Fitzgerald, G.J.; Belford, R.; Christensen, L.K. Detection of nitrogen deficiency in wheat from spectral reflectance indices and basic crop eco-physiological concepts. Aust. J. Agr. Res. 2006, 57, 781–789. [Google Scholar] [CrossRef] [Green Version]
- Tilling, A.K.; O’Leary, G.J.; Ferwerda, J.G.; Jones, S.D.; Fitzgerald, G.J.; Rodriguez, D.; Belford, R. Remote sensing of nitrogen and water stress in wheat. Field Crops Res. 2007, 104, 77–85. [Google Scholar] [CrossRef]
- Fitzgerald, G.; Rodriguez, D.; O’Leary, G. Measuring and predicting canopy nitrogen nutrition in wheat using a spectral index—The canopy chlorophyll content index (CCCI). Field Crop Res. 2010, 116, 318–324. [Google Scholar] [CrossRef]
- Thorp, K.R.; Wang, G.; Bronson, K.F.; Badaruddin, M.; Mon, J. Hyperspectral data mining to identify relevant canopy spectral features for estimating durum wheat growth, nitrogen status, and grain yield. Comput. Electron. Agr. 2017, 136, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Das, B.; Sahoo, R.N.; Pargal, S.; Krishna, G.; Verma, R.; Chinnusamy, V.; Sehgal, V.K.; Gupta, V.K. Comparison of different uni- and multi-variate techniques for monitoring leaf water status as an indicator of water-deficit stress in wheat through spectroscopy. Biosyst. Eng. 2017, 160, 69–83. [Google Scholar] [CrossRef]
- Feng, W.; Qi, S.; Heng, Y.; Zhou, Y.; Wu, Y.; Liu, W.; He, L.; Li, X. Canopy Vegetation Indices from In situ Hyperspectral Data to Assess Plant Water Status of Winter Wheat under Powdery Mildew Stress. Front. Plant Sci. 2017, 8, 1219. [Google Scholar] [CrossRef]
- Mzid, N.; Todorovic, M.; Albrizio, R.; Cantore, V. Remote sensing based monitoring of durum wheat under water stress treatments. Spatial Analysis of GEOmatics. 2017. Available online: https://hal.archives-ouvertes.fr/hal-01643477 (accessed on 6 November 2019).
- Moghimi, A.; Yang, C.; Miller, M.E.; Kianian, S.; Marchetto, P. A Novel Approach to Assess Salt Stress Tolerance in Wheat Using Hyperspectral Imaging. Front. Plant Sci. 2018, 9, 1182. [Google Scholar] [CrossRef]
- Lobell, D.B.; Ortiz-Monasterio, J.I. Impacts of day versus night temperatures on spring wheat yields. Agron. J. 2007, 99, 469–477. [Google Scholar] [CrossRef] [Green Version]
- Duncan, J.M.; Dash, J.; Atkinson, P.M. Elucidating the impact of temperature variability and extremes on cereal croplands through remote sensing. Glob. Change Biol. 2015, 21, 1541–1551. [Google Scholar] [CrossRef] [Green Version]
- Kyratzis, A.C.; Skarlatos, D.P.; Menexes, G.C.; Vamvakousis, V.F.; Katsiotis, A. Assessment of vegetation indices derived by UAV imagery for durum wheat phenotyping under a water limited and heat stressed mediterranean environment. Front. Plant Sci. 2017, 8, 1114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lelong, C.C.D.; Pinet, P.C.; Poilvé, H. Hyperspectral imaging and stress mapping in agriculture: A case study on wheat in Beauce (France). Remote Sens. Environ. 1998, 66, 179–191. [Google Scholar] [CrossRef]
- Moshou, D.; Pantazi, X.E.; Kateris, D.; Gravalos, I. Water stress detection based on optical multisensor fusion with a least squares support vector machine classifier. Biosyst. Eng. 2014, 117, 15–22. [Google Scholar] [CrossRef]
- Blackburn, G.A. Hyperspectral remote sensing of plant pigments. J. Exp. Bot. 2006, 58, 855–867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, D.; Reddy, K.R.; Kakani, V.G.; Reddy, V.R. Nitrogen deficiency effects on plant growth, leaf photosynthesis, and hyperspectral reflectance properties of sorghum. Eur. J. Agron. 2005, 22, 391–403. [Google Scholar] [CrossRef]
- Römer, C.; Wahabzada, M.; Ballvora, A.; Pinto, F.; Rossini, M.; Panigada, C.; Behmann, J.; Léon, J.; Thurau, C.; Bauckhage, C.; et al. Early drought stress detection in cereals: Simplex volume maximisation for hyperspectral image analysis. Funct. Plant Biol. 2012, 39, 878–890. [Google Scholar] [CrossRef]
- Fitzgerald, G.J.; Perry, E.M.; Flower, K.C.; Callow, J.N.; Boruff, B.; Delahunty, A.; Wallace, A.; Nuttall, J. Frost Damage Assessment in Wheat Using Spectral Mixture Analysis. Remote Sens-Basel. 2019, 11, 2476. [Google Scholar] [CrossRef] [Green Version]
- Biddulph, B.; Laws, M.; Eckermann, P.; Maccallam, R.; Leske, B.; March, T.; Eglinton, J. Preliminary ratings of wheat varieties for susceptibility to reproductive frost damage; Grains Research Development Corporation: Adelaide, Australia, 2015. [Google Scholar]
- Single, W. Studies on frost injury to wheat. II. Ice formation within the plant. Aust. J. Agr. Res. 1964, 15, 869–875. [Google Scholar] [CrossRef]
- Single, W.V.; Marcellos, H. Studies on frost injury to wheat. IV. Freezing of ears after emergence from the leaf sheath. Aust. J. Agr. Res. 1974, 25, 679–686. [Google Scholar] [CrossRef]
- Livingston, D.P.; Tuong, T.D.; Murphy, J.P.; Gusta, L.V.; Willick, I.; Wisniewski, M.E. High-definition infrared thermography of ice nucleation and propagation in wheat under natural frost conditions and controlled freezing. Planta 2018, 247, 791–806. [Google Scholar] [CrossRef] [Green Version]
- Marcellos, H. A plant freezing chamber with radiative and convective energy exchange. J. Agr. Eng. Res. 1981, 26, 403–408. [Google Scholar] [CrossRef]
- Fuller, M.P.; Grice, P.L. A chamber for the simulation of radiation freezing of plants. Ann. App. Biol. 1998, 133, 111–121. [Google Scholar] [CrossRef]
- Nansen, C.; Zhang, X.; Aryamanesh, N.; Yan, G. Use of variogram analysis to classify field peas with and without internal defects caused by weevil infestation. J. Food Eng. 2014, 123, 17–22. [Google Scholar] [CrossRef] [Green Version]
- Tucker, C.J. Red and photographic infrared linear combinations for monitoring vegetation. Remote Sens. Environ. 1979, 8, 127–150. [Google Scholar] [CrossRef] [Green Version]
- ESRI. ArcMap, 10.5.1.; Environmental Systems Research Institute: Redlands, CA, USA, 2018. [Google Scholar]
- IBM Corp. IBM SPSS Statistics for Windows, 25.0; IBM Corp.: Armonk, NY, USA, 2017. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing, 3.5.1.; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.R-project.org/ (accessed on 3 February 2020).
- Patterson, H.D.; Thompson, R. Recovery of inter-block information when block sizes are unequal. Biometrika 1971, 58, 545–554. [Google Scholar] [CrossRef]
- VSN International Genstat for Windows, 19th ed.; VSN International: Hempel Hempstead, UK, 2017; Available online: https://www.vsni.co.uk/ (accessed on 3 February 2020).
- Flower, K.; Boruff, B.; Nansen, C.; Jones, H.; Thompson, S.; Lacoste, C.; Murphy, M. Proof of Concept: Remote Sensing Frost-Induced Stress in Wheat Paddocks; Grains Research and Development Corporation: Canberra, Australia, 2014. [Google Scholar]
- Puri, V.; Nayyar, A.; Raja, L. Agriculture drones: A modern breakthrough in precision agriculture. J. Stat. Manag. Syst. 2017, 20, 507–518. [Google Scholar] [CrossRef]
- King, A. Technology: The Future of Agriculture. Nature 2017, 544, S21–S23. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.; Zhu, D.; Wang, C.; Ma, Z.; Wang, J. Diagnosis of freezing stress in wheat seedlings using hyperspectral imaging. Biosyst. Eng. 2012, 112, 253–260. [Google Scholar] [CrossRef]
- Carter, G.A. Respones of leaf spectral reflectance to plant stress. Am. J. Bot. 1993, 80, 239–243. [Google Scholar] [CrossRef]
- Xue, L.; Yang, L. Deriving leaf chlorophyll content of green-leafy vegetables from hyperspectral reflectance. ISPRS J. Photogramm. 2009, 64, 97–106. [Google Scholar] [CrossRef]
- Horler, D.N.; Dockray, M.; Barber, J.; Barringer, A.R. Red edge measurements for remotely sensing plant chlorophyll content. Adv. Space Res. 1983, 3, 273–277. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Vegetation stress: An introduction to the stress concept in plants. J. Plant Physiol. 1996, 148, 4–14. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Merzlyak, M.N. Remote estimation of chlorophyll content in higher plant leaves. Int. J. Remote Sens. 1997, 18, 2691–2697. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Gitelson, A.A.; Schepers, J.S.; Walthall, C.L. Application of spectral remote sensing for agronomic decisions. Agron. J. 2008, 100, S-117. [Google Scholar] [CrossRef] [Green Version]
- Peñuelas, J.; Baret, F.; Filella, I. Semi-empirical indices to assess carotenoids/chlorophyll a ratio from leaf spectral reflectance. Photosynthetica 1995, 31, 221–230. [Google Scholar]
- Hoque, E.; Hutzler, P.J. Spectral blue-shift of red edge minitors damage class of beech trees. Remote Sens. Environ. 1992, 39, 81–84. [Google Scholar] [CrossRef]
- Filella, I.; Peñuelas, J. The red edge position and shape as indicators of plant chlorophyll content, biomass and hydric status. Int. J. Remote Sens. 1994, 15, 1459–1470. [Google Scholar] [CrossRef]
- Rock, B.N.; Hoshizaki, T.; Miller, J.R. Comparison of in situ and airborne spectral measurements of the blue shift associated with forest decline. Remote Sens. Environ. 1988, 24, 109–127. [Google Scholar] [CrossRef]
- Datt, B. Remote sensing of chlorophyll a, chlorophyll b, chlorophyll a+ b, and total carotenoid content in eucalyptus leaves. Remote Sens. Environ. 1998, 66, 111–121. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Merzlyak, M.N.; Lichtenthaler, H.K. Detection of red edge position and chlorophyll content by reflectance measurements near 700 nm. J. Plant Physiol. 1996, 148, 501–508. [Google Scholar] [CrossRef]
- Basso, B.; Cammarano, D.; De Vita, P. Remotely sensed vegetation indices: Theory and applications for crop management. Ital. J. Agrometeorol. 2004, 1, 36–53. [Google Scholar]
- Jones, H.G. Remote Sensing of Vegetation: Principles, Techniques, and Applications; Oxford University Press: Oxford, UK, 2010. [Google Scholar]
- Verrelst, J.; Camps-Valls, G.; Muñoz-Marí, J.; Rivera, J.P.; Veroustraete, F.; Clevers, J.G.; Moreno, J. Optical remote sensing and the retrieval of terrestrial vegetation bio-geophysical properties–A review. ISPRS J. Photogramm. 2015, 108, 273–290. [Google Scholar] [CrossRef]
- Bannari, A.; Morin, D.; Bonn, F.; Huete, A.R. A review of vegetation indices. Remote Sens. Rev. 1995, 13, 95–120. [Google Scholar] [CrossRef]
- Perry, E.M.; Nuttall, J.G.; Wallace, A.J.; Fitzgerald, G.J. In-field methods for rapid detection of frost damage in Australian dryland wheat during the reproductive and grain-filling phase. Crop Pasture Sci. 2017, 68, 516–526. [Google Scholar] [CrossRef] [Green Version]
- Daughtry, C.S.; Walthall, C.L.; Kim, M.S.; De Colstoun, E.B.; McMurtrey, J.E., III. Estimating corn leaf chlorophyll concentration from leaf and canopy reflectance. Remote Sens. Environ. 2000, 74, 229–239. [Google Scholar] [CrossRef]
- Gamon, J.A.; Peñuelas, J.; Field, C.B. A narrow-waveband spectral index that tracks diurnal changes in photosynthetic efficiency. Remote Sens. Environ. 1992, 41, 35–44. [Google Scholar] [CrossRef]
- Guendouz, A.; Guessoum, S.; Maamari, K.; Hafsi, M. Predicting the efficiency of using the RGB (Red, Green and Blue) reflectance for estimating leaf chlorophyll content of Durum wheat (Triticum durum Desf.) genotypes under semi arid conditions. Am.-Eurasian J. Sustain. Agric. 2012, 6, 102–107. [Google Scholar]
- Peñuelas, J.; Gamon, J.A.; Fredeen, A.L.; Merino, J.; Field, C.B. Reflectance indices associated with physiological changes in nitrogen- and water-limited sunflower leaves. Remote Sens. Environ. 1994, 48, 135–146. [Google Scholar] [CrossRef]
- Burke, M.J.; Gusta, L.V.; Quamme, H.A.; Weiser, C.J.; Li, P.H. Freezing and Injury in Plants. Annu. Rev. Plant Physiol. 1976, 27, 507–528. [Google Scholar] [CrossRef]
- Pearce, R.S. Extracellular ice and cell shape in frost-stressed cereal leaves: A low-temperature scanning-electron-microscopy study. Planta 1988, 175, 313–324. [Google Scholar] [CrossRef]
- Pearce, R.S.; Ashworth, E.N. Cell shape and localisation of ice in leaves of overwintering wheat during frost stress in the field. Planta 1992, 188, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Cromey, M.G.; Wright, D.S.; Boddington, H.J. Effects of frost during grain filling on wheat yield and grain structure. New-Zeal J. Crop Hort. Sci. 1998, 26, 279–290. [Google Scholar] [CrossRef]
- Seelig, H.; Hoehn, A.; Stodieck, L.S.; Klaus, D.M.; Adams III, W.W.; Emery, W.J. The assessment of leaf water content using leaf reflectance ratios in the visible, near-, and short-wave-infrared. Int. J. Remote Sens. 2008, 29, 3701–3713. [Google Scholar] [CrossRef]
- Suplick-Ploense, M.R.; Alshammary, S.F.; Qian, Y.L. Spectral Reflectance Response of Three Turfgrasses to Leaf Dehydration. Asian J. Plant Sci. 2011, 10, 67. [Google Scholar] [CrossRef]







| Main Effect Treatment | Central Band | Predicted Mean Control (%) | Predicted Mean Treated (%) | LSD (p ≤ 0.05) | |
| Heads | |||||
| R419–512 | R465 | 0.1182 | 0.1218 | 0.0018 | |
| R610–675 | R641 | 0.1468 | 0.1496 | 0.0023 | |
| R749–754 | R752 | 0.7157 | 0.7223 | 0.0060 | |
| Leaves | |||||
| R415–494 | R454 | 0.0763 | 0.7780 | 0.0015 | |
| R670–687 | R679 | 0.0753 | 0.7670 | 0.0014 | |
| R727–889 | R808 | 0.8082 | 0.8172 | 0.0090 | |
| Main Effect DAF | Central Band | Predicted Mean DAF 1 (%) | Predicted Mean DAF 3 (%) | Predicted Mean DAF 5 (%) | LSD (p ≤ 0.05) |
| Heads | |||||
| R392–398 | R394 | 0.2998 | 0.3060 | 0.3016 | 0.0029 |
| R400–498 | R450 | 0.1237 | 0.1273 | 0.1341 | 0.0019 |
| R500–542 | R521 | 0.1818 | 0.1878 | 0.1926 | 0.0023 |
| R576–598 | R589 | 0.1750 | 0.1829 | 0.1849 | 0.0023 |
| R600–697 | R641 | 0.1434 | 0.1475 | 0.1536 | 0.0021 |
| R700–754 | R729 | 0.5527 | 0.5675 | 0.5716 | 0.0047 |
| Leaves | |||||
| R398–498 | R448 | 0.0789 | 0.0813 | 0.0816 | 0.0010 |
| R500–562 | R531 | 0.1360 | 0.1397 | 0.1398 | 0.0016 |
| R642–697 | R670 | 0.0670 | 0.0702 | 0.0719 | 0.0010 |
| R700–889 | R795 | 0.8017 | 0.8147 | 0.8194 | 0.0065 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murphy, M.E.; Boruff, B.; Callow, J.N.; Flower, K.C. Detecting Frost Stress in Wheat: A Controlled Environment Hyperspectral Study on Wheat Plant Components and Implications for Multispectral Field Sensing. Remote Sens. 2020, 12, 477. https://doi.org/10.3390/rs12030477
Murphy ME, Boruff B, Callow JN, Flower KC. Detecting Frost Stress in Wheat: A Controlled Environment Hyperspectral Study on Wheat Plant Components and Implications for Multispectral Field Sensing. Remote Sensing. 2020; 12(3):477. https://doi.org/10.3390/rs12030477
Chicago/Turabian StyleMurphy, Mary E., Bryan Boruff, J. Nikolaus Callow, and Ken C. Flower. 2020. "Detecting Frost Stress in Wheat: A Controlled Environment Hyperspectral Study on Wheat Plant Components and Implications for Multispectral Field Sensing" Remote Sensing 12, no. 3: 477. https://doi.org/10.3390/rs12030477
APA StyleMurphy, M. E., Boruff, B., Callow, J. N., & Flower, K. C. (2020). Detecting Frost Stress in Wheat: A Controlled Environment Hyperspectral Study on Wheat Plant Components and Implications for Multispectral Field Sensing. Remote Sensing, 12(3), 477. https://doi.org/10.3390/rs12030477







