A High-Resolution Global Map of Giant Kelp (Macrocystis pyrifera) Forests and Intertidal Green Algae (Ulvophyceae) with Sentinel-2 Imagery
Abstract
1. Introduction
- (a)
- New sensors onboard the recently launched European Space Agency’s (ESA) Sentinel satellites offer an alternative to Landsat in the remote detection of giant kelp forests. Although individual kelp blades can show different photo-acclimation responses to variable conditions of light, the concentration of pigment chlorophylls a and c and fucoxanthin (Fuc) give the plants a conspicuous brownish colour, with a higher concentration with increasing depth [35]. These characteristics result in the highest spectral reflectance of kelp canopies being in the Near-Infrared (780–890nm) [19], the lowest being in the blue (400–500nm) and red (675nm) areas [35], with reflectance increasing strongly in the red-edge area [16]. The sensors onboard Sentinel-2, with four bands of 10 m and three additional red-edge bands of 20 m of spatial resolution, can strongly contribute to highlight this spectral area with a level of detail that other multispectral sensors do not possess, such as those in Landsat (30 m of spatial resolution).
- (b)
- Cloud-based platforms such as Google Earth Engine (GEE, [36]) enable access to petabytes of open-access satellite imagery paired with an interactive development environment. Previous research in GEE in similar environments includes the analysis of the capabilities of Landsat imagery to detect kelp forests in British Columbia [17] and the use of Sentinel-2 imagery to estimate satellite-derived bathymetry [37] and to map seagrass [34]. Using GEE helps in processing large amounts of data to detect spatially persistent areas of giant kelp forests, taking into consideration that persistent kelp areas tend to occur at the centres of the forests, whereas borders are more variable [38]. This persistence has been associated with abiotic factors, such as water depth, the presence of a rocky substrate, substrate topology, and connectivity between the forests [38]. In contrast, variability is associated with ocean dynamics, such as wave height and sea-surface temperature ([14], more than seasonality), tidal ranges, or zenith angles [18]. Therefore, the central areas of forests should provide optimal material to build a global map of giant kelp.
- (c)
- The use of Unmanned Aerial Vehicles (UAV) for coastal habitat mapping is a simple, cost-effective and reliable technology [39] that has been successfully used to map and validate intertidal biogenic reefs [40], saltmarsh biomass [41], and algal blooms [42]. Recent surveys to detect macroalgae in temperate coastlines have shown that RGB (additive primary colors—red, green, and blue—model) and multispectral cameras mounted on UAVs produce accurate imagery able to detect water turbidity and a range of taxonomical groups of algae in surface or shallow water, with the exception of spectrally similar species [18,43]. To our knowledge, there are yet no standardized protocols for marine or coastal mapping with UAVs [42].
2. Materials and Methods
2.1. Training Data
2.2. Kelp Filter Algorithm
- Band-based threshold. The multispectral profiles of Land Vegetation, Coast, and Foam are clearly distinctive (Figure 1). One hundred percent of all Coast and Land Vegetation observations in the training data were larger than or equal to B11 = 0.028, which corresponds to the minimum value of Coast ROI at B11 (1610 nm at central wavelength, Figure 2). Consequently, all observations with value B11 ≥ 0.028 were masked out, which resulted in 305 training data grid cells remaining. This eliminated 21 observations in the upper quartile of the original kelp sample (Figure 2). This was done to avoid misclassification with coastal features at the expense of identifying some kelp-occupied grid cells with higher-than average reflectance values in band B11. These were found to be marginal portions of the identified giant kelp forest ROIs—i.e., grid cells occupying the periphery of kelp stands.
- Kelp Difference (KD). Giant kelp grid cells exhibited a conspicuous large difference in reflectance between bands in the red edge area of the spectrum (Bands 5, 6 and 7) and the red band (B4). Selecting B6 (central wavelength = 740 nm) as the band with the largest difference with B4 (Figure 1), we defined a Kelp Difference (KD) as the difference between both band values. Step 2 was applied after the band-based masking (Step 1), although the order of these two steps would not alter the result:
- KD-based threshold. A second masking threshold was applied to the KD-converted training dataset. This enabled 100% of the grid cells not belonging to Giant Kelp or Green Algae to be removed (Table 2 and Appendix A). The reflectance values for Giant Kelp and Green Algae were found to be too similar to be efficiently discriminated. In order to compare the performance of the KD in relation to other indices used to remotely detect algae in the past, we separately implemented this step employing NDVI and FAI. This resulted in the production of three different kelp maps.
2.3. Validation at High Spatial Resolution
2.4. Kelp Filter Algorithm in Google Earth Engine
- (a)
- cloud-free tool of [47] over Sentinel-2 grid cells scaled at 10−4 from 26th June 2015 to 23rd June 2019;
- (b)
- kelp filter threshold;
- (c)
- masking of all grid cells with elevation above sea level > 0 m using two digital elevation models: Advanced Land Observing Satellite (ALOS) and Shuttle Radar Topography Mission (SRTM), both at 30 m of spatial resolution. This last procedure was done to avoid any misclassification of elements on land with a similar reflectance to giant kelp that were not included in our ROI training data set. To improve the readability of the index, digital numbers were rescaled to values from 0 to 255 in the maps.
2.5. Validation at a Low Spatial Resolution
3. Results
3.1. Kelp Filter Algorithm
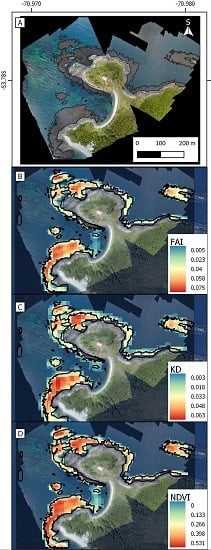
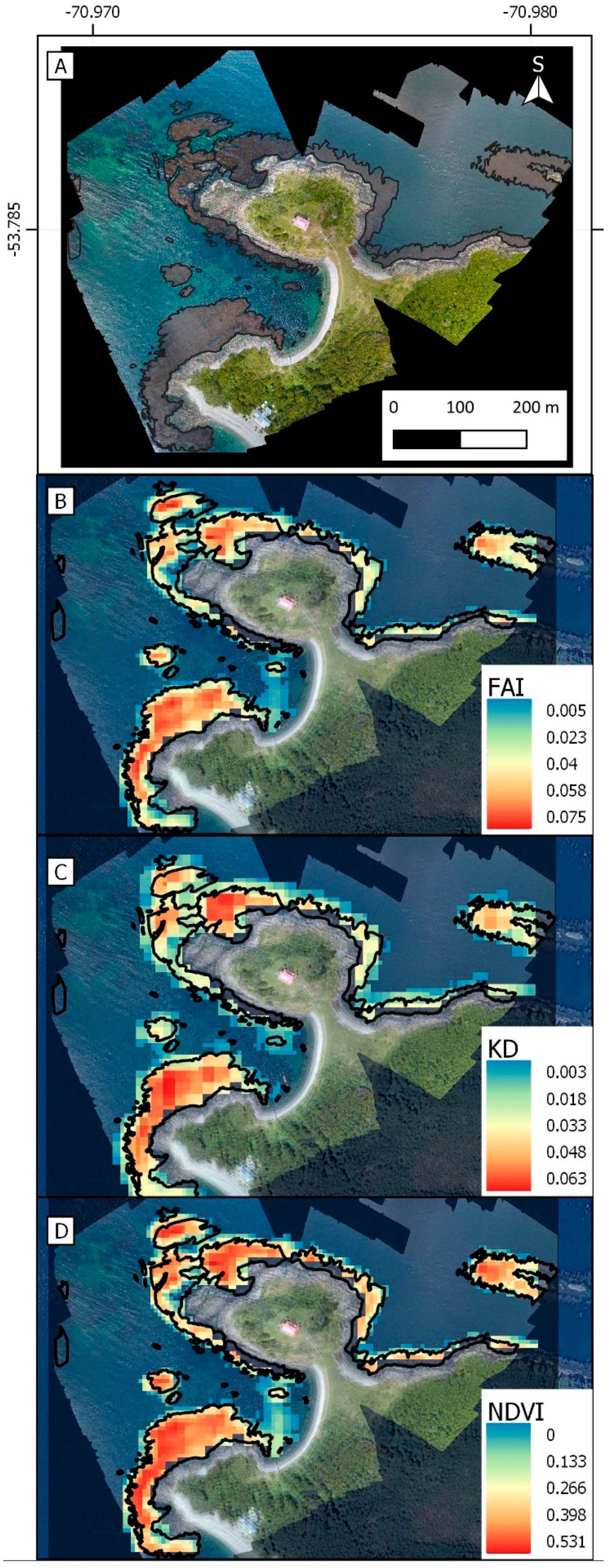
3.2. Validation at High Resolution
3.3. Validation at Low Resolution
4. Discussion
4.1. Kelp Detection and Mapping
4.2. Spatial and Biophysical Patterns
4.3. False Negatives
4.4. False Positives
4.5. Notes on UAV Surveying
4.6. Additional Considerations about the Global Map
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Miller, R.J.; Lafferty, K.D.; Lamy, T.; Kui, L.; Rassweiler, A.; Reed, D.C. Giant kelp, Macrocystis pyrifera, increases faunal diversity through physical engineering. Proc. R. Soc. B Boil. Sci. 2018, 285, 20172571. [Google Scholar] [CrossRef]
- Teagle, H.; Hawkins, S.J.; Moore, P.; Smale, D. The role of kelp species as biogenic habitat formers in coastal marine ecosystems. J. Exp. Mar. Boil. Ecol. 2017, 492, 81–98. [Google Scholar] [CrossRef]
- Fraser, C. Is bull-kelp kelp? The role of common names in science. New Zealand J. Mar. Freshw. Res. 2012, 46, 279–284. [Google Scholar] [CrossRef]
- Mann, K.H. Seaweeds: Their Productivity and Strategy for Growth: The role of large marine algae in coastal productivity is far more important than has been suspected. Science 1973, 182, 975–981. [Google Scholar] [CrossRef] [PubMed]
- Reed, D.C.; Rassweiler, A.; Arkema, K.K. BIOMASS RATHER THAN GROWTH RATE DETERMINES VARIATION IN NET PRIMARY PRODUCTION BY GIANT KELP. Ecology 2008, 89, 2493–2505. [Google Scholar] [CrossRef]
- Smale, D. Impacts of ocean warming on kelp forest ecosystems. New Phytol. 2019, 225, 1447–1454. [Google Scholar] [CrossRef]
- Dayton, P.K. Ecology of Kelp Communities. Annu. Rev. Ecol. Syst. 1985, 16, 215–245. [Google Scholar] [CrossRef]
- Graham, M.H. Effects of Local Deforestation on the Diversity and Structure of Southern California Giant Kelp Forest Food Webs. Ecosystems 2004, 7, 341–357. [Google Scholar] [CrossRef]
- Graham, M.H.; Vasquez, J.A.; Buschmann, A.H. Global ecology of the giant kelp Macrocystis: From ecotypes to ecosystems. Oceanogr. Marine Biol. 2007, 45, 39. [Google Scholar]
- Krumhansl, K.A.; Okamoto, D.K.; Rassweiler, A.; Novak, M.; Bolton, J.; Cavanaugh, K.C.; Connell, S.D.; Johnson, C.R.; Konar, B.; Ling, S.D.; et al. Global patterns of kelp forest change over the past half-century. Proc. Natl. Acad. Sci. USA 2016, 113, 13785–13790. [Google Scholar] [CrossRef]
- Schiel, D.R.; Foster, M.S. The Biology and Ecology of Giant Kelp Forests; University of California Press: Oakland, California, 2015; pp. 1–395. [Google Scholar]
- Division for Sustainable Development Goals, U.N. Transforming Our World: The 2030 Agenda for Sustainable Development. Available online: https://sustainabledevelopment.un.org/post2015/transformingourworld (accessed on 8 November 2019).
- Cavanaugh, K.C.; Siegel, D.A.; Kinlan, B.P.; Reed, D.C. Scaling giant kelp field measurements to regional scales using satellite observations. Mar. Ecol. Prog. Ser. 2010, 403, 13–27. [Google Scholar] [CrossRef]
- Cavanaugh, K.C.; Siegel, D.A.; Reed, D.C.; Dennison, P.E. Environmental controls of giant-kelp biomass in the Santa Barbara Channel, California. Mar. Ecol. Prog. Ser. 2011, 429, 1–17. [Google Scholar] [CrossRef]
- Pfister, C.A.; Berry, H.D.; Mumford, T. The dynamics of Kelp Forests in the Northeast Pacific Ocean and the relationship with environmental drivers. J. Ecol. 2018, 106, 1520–1533. [Google Scholar] [CrossRef]
- Jensen, J.R.; Cavanaugh, K.C.; Siegel, D.A.; Reed, D.C.; Dennison, P.E. Remote sensing techniques for kelp surveys. Photogramm. Eng. Remote Sens. 1980, 46, 743–755. [Google Scholar]
- Nijland, W.; Reshitnyk, L.; Rubidge, E. Satellite remote sensing of canopy-forming kelp on a complex coastline: A novel procedure using the Landsat image archive. Remote Sens. Environ. 2019, 220, 41–50. [Google Scholar] [CrossRef]
- Huovinen, P.; Ramírez, J.; Palacios, M.; Gómez, I. Satellite-derived mapping of kelp distribution and water optics in the glacier impacted Yendegaia Fjord (Beagle Channel, Southern Chilean Patagonia). Sci. Total Environ. 2019, 703, 135531. [Google Scholar] [CrossRef]
- Augenstein, E.; Stow, D.; Hope, A. Evaluation of SPOT HRV-XS data for kelp resource inventories. Photogramm. Eng. Remote Sens. 1991, 57, 501–509. [Google Scholar]
- Deysher, L. Evaluation of remote sensing techniques for monitoring giant kelp populations. Hydrobiologia 1993, 261, 307–312. [Google Scholar] [CrossRef]
- Tucker, C.J. Red and photographic infrared linear combinations for monitoring vegetation. Remote Sens. Environ. 1979, 8, 12–150. [Google Scholar] [CrossRef]
- Stekoll, M.; Deysher, L.; Hess, M. A remote sensing approach to estimating harvestable kelp biomass. In Eighteenth International Seaweed Symposium; Springer: Dordrecht, The Netherlands, 2006; Volume 1, pp. 97–108. [Google Scholar]
- Adams, J.B.; Smith, M.O.; Johnson, P.E. Spectral mixture modeling: A new analysis of rock and soil types at the Viking Lander 1 site. J. Geophys. Res. Solid Earth 1986, 91, 8098–8112. [Google Scholar] [CrossRef]
- Roberts, D.A.; Gardner, M.; Church, R.; Ustin, S.; Scheer, G.; Green, R.O. Mapping chaparral in the Santa Monica Mountains using multiple endmember spectral mixture models. Remote Sens. Environ. 1998, 65, 267–279. [Google Scholar] [CrossRef]
- Johansson, M.L.; Alberto, F.; Reed, D.C.; Raimondi, P.T.; Coelho, N.C.; Young, M.A.; Drake, P.T.; Edwards, C.A.; Cavanaugh, K.; Jorge, A. Seascape drivers of Macrocystis pyrifera population genetic structure in the northeast Pacific. Mol. Ecol. 2015, 24, 4866–4885. [Google Scholar] [CrossRef] [PubMed]
- Bell, T.W.; Allen, J.G.; Cavanaugh, K.C.; Siegel, D.A. Three decades of variability in California’s giant kelp forests from the Landsat satellites. Remote Sens. Environ. 2018, 238, 110811. [Google Scholar] [CrossRef]
- Lamy, T.; Reed, D.C.; Rassweiler, A.; Siegel, D.A.; Kui, L.; Bell, T.W.; Simons, R.D.; Miller, R.J. Scale-specific drivers of kelp forest communities. Oecologia 2018, 186, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Koweek, D.A.; Nickols, K.J.; Leary, P.R.; Litvin, S.Y.; Bell, T.W.; Luthin, T.; Lummis, S.; Mucciarone, D.A.; Dunbar, R.B. A year in the life of a central California kelp forest: physical and biological insights into biogeochemical variability. Biogeosciences 2017, 14, 31–44. [Google Scholar] [CrossRef]
- Friedlander, A.M.; Ballesteros, E.; Bell, T.W.; Giddens, J.; Henning, B.; Hune, M.; Munoz, A.; Salinas-de-León, P.; Sala, E. Marine biodiversity at the end of the world: Cape Horn and Diego Ramírez islands. PLoS ONE 2018, 13, e0189930. [Google Scholar] [CrossRef]
- Rosenthal, I.S.; Byrnes, J.E.; Cavanaugh, K.C.; Bell, T.W.; Harder, B.; Haupt, A.J.; Rassweiler, A.T.W.; Pérez-Matus, A.; Assis, J.; Swanson, A.; et al. Floating Forests: Quantitative validation of citizen science data generated from consensus classifications. arXiv 2018, arXiv:1801.08522. [Google Scholar]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Mishra, S.; Datta-Gupta, A. Applied Statistical Modeling and Data Analytics: A Practical Guide for the Petroleum Geosciences; Elsevier: Amsterdam, The Netherlands, 2017; pp. 225–231. [Google Scholar]
- Borsuk, M.E. Statistical Prediction. In Encyclopedia of Ecology; Academic Press: Cambridge, MA, USA, 2008; pp. 3362–3373. [Google Scholar] [CrossRef]
- Traganos, D.; Aggarwal, B.; Poursanidis, D.; Topouzelis, K.; Chrysoulakis, N.; Reinartz, P. Towards global-scale seagrass mapping and monitoring using Sentinel-2 on Google Earth Engine: The case study of the aegean and ionian seas. Remote Sens. 2018, 10, 1227. [Google Scholar] [CrossRef]
- Colombo-Pallotta, M.F.; Garcia-mendoza, E.; Ladah, L. Photosynthetic performance, light absorption, and pigment composition of Macrocystis pyrifera (Laminariales, phaeophyceae) blades from different depths. J. Phycol. 2006, 42, 1225–1234. [Google Scholar] [CrossRef]
- Gorelick, N.; Hancher, M.; Dixon, M.; Ilyushchenko, S.; Thau, D.; Moore, R. Google Earth Engine: Planetary-scale geospatial analysis for everyone. Remote Sens. Environ. 2017, 202, 18–27. [Google Scholar] [CrossRef]
- Traganos, D.; Poursanidis, D.; Aggarwal, B.; Chrysoulakis, N.; Reinartz, P. Estimating satellite-derived bathymetry (SDB) with the google earth engine and sentinel-2. Remote Sens. 2018, 10, 859. [Google Scholar] [CrossRef]
- Young, M.; Cavanaugh, K.; Bell, T.; Raimondi, P.; Edwards, C.A.; Drake, P.T.; Erikson, L.; Storlazzi, C. Environmental controls on spatial patterns in the long-term persistence of giant kelp in central California. Ecol. Monogr. 2016, 86, 45–60. [Google Scholar] [CrossRef]
- Turner, I.L.; Harley, M.D.; Drummond, C.D. UAVs for coastal surveying. Coast. Eng. 2016, 114, 19–24. [Google Scholar] [CrossRef]
- Collin, A.; Dubois, S.; James, D.; Houet, T. Improving Intertidal Reef Mapping Using UAV Surface, Red Edge, and Near-Infrared Data. Drones 2019, 3, 67. [Google Scholar] [CrossRef]
- Doughty, C.L.; Cavanaugh, K.C. Mapping Coastal Wetland Biomass from High Resolution Unmanned Aerial Vehicle (UAV) Imagery. Remote Sens. 2019, 11, 540. [Google Scholar] [CrossRef]
- Kislik, C.; Dronova, I.; Kelly, M. UAVs in support of algal bloom research: a review of current applications and future opportunities. Drones 2018, 2, 35. [Google Scholar] [CrossRef]
- Tait, L.; Bind, J.; Charan-Dixon, H.; Hawes, I.; Pirker, J.; Schiel, D. Unmanned Aerial Vehicles (UAVs) for Monitoring Macroalgal Biodiversity: Comparison of RGB and Multispectral Imaging Sensors for Biodiversity Assessments. Remote Sens. 2019, 11, 2332. [Google Scholar] [CrossRef]
- Hu, C. A novel ocean color index to detect floating algae in the global oceans. Remote Sens. Environ. 2009, 113, 2118–2129. [Google Scholar] [CrossRef]
- Baweja, P.; Sahoo, D. Classification of Algae. In The Algae World; Springer: Dordrecht, The Netherlands, 2015; pp. 31–55. [Google Scholar]
- Sentinel, E. User Handbook; European Space Agency Standard Document: Paris, France, 2013; pp. 1–64. [Google Scholar]
- Simonetti, D.; Simonetti, E.; Szantoi, Z.; Lupi, A.; Eva, H.D. First results from the phenology-based synthesis classifier using Landsat 8 imagery. IEEE Geosci. Remote. Sens. Lett. 2015, 12, 1496–1500. [Google Scholar] [CrossRef]
- Macaya, E.C.; Zuccarello, G.C. DNA Barcoding and genetic divergence in the Giant Kelp Macrocystis (Laminariales). J. Phycol. 2010, 46, 736–742. [Google Scholar] [CrossRef]
- Macaya, E.C. (Department of Oceanography, Faculty of Natural and Oceanographic Sciences, Universidad de Concepción, Concepción, CHILE). Personal communication, 2018.
- Belsher, T.; Mouchot, M.-C. Evaluation, par télédétection satellitaire, des stocks de Macrocystis pyrifera dans le golfe du Morbihan (archipel de Kerguelen). Oceanol. Acta. 1992, 15, 297–307. [Google Scholar]
- Wells, E.; Brewin, P.; Brickle, P. Intertidal and subtidal benthic seaweed diversity of South Georgia. South Georgia Heritage Trust, Joint Nature Conservation Committee Survey. 2011, pp. 1–20. Available online: https://pdfs.semanticscholar.org/d76f/ff633afbca1465e72741e929cc1c77a665cc.pdf (accessed on 3 February 2020).
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World map of the Köppen-Geiger climate classification updated. Meteorol. Z. 2006, 15, 259–263. [Google Scholar] [CrossRef]
- Cohen, J. A coefficient of agreement for nominal scales. Educ. Psychol. Meas. 1960, 20, 37–46. [Google Scholar] [CrossRef]
- Tadono, T.; Nagai, H.; Ishida, H.; Oda, F.; Naito, S.; Minakawa, K.; Iwamoto, H. Generation of the 30 m-mesh global digital surface model by ALOS prism. Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2016, XLI-B4, 157–162. [Google Scholar] [CrossRef]
- Spalding, M.D.; Fox, H.E.; Allen, G.R.; Davidson, N.; Ferdaña, Z.A.; Finlayson, M.; Halpern, B.S.; Jorge, M.A.; Lombana, A.; Lourie, S.A.; et al. Marine ecoregions of the world: A bioregionalization of coastal and shelf areas. BioScience 2007, 57, 573–583. [Google Scholar] [CrossRef]
- Cavanaugh, K.C.; Siegel, D.A.; Raimondi, P.T.; Alberto, F. Patch definition in metapopulation analysis: a graph theory approach to solve the mega-patch problem. Ecology 2014, 95, 316–328. [Google Scholar] [CrossRef]
- Bunting, P.; Rosenqvist, A.; Lucas, R.M.; Rebelo, R.-M.; Hilarides, L.; Thomas, N.; Hardy, A.; Itoh, T.; Shimada, M.; Finlayson, C.M. The global mangrove watch—A new 2010 global baseline of mangrove extent. Remote Sens. 2018, 10, 1669. [Google Scholar] [CrossRef]
- Gandhi, S.; Jones, T.G. Identifying Mangrove Deforestation Hotspots in South Asia, Southeast Asia and Asia-Pacific. Remote Sens. 2019, 11, 728. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, M.Y.; Wang, L.; Shimazaki, H.; Tamura, M. A new index for mapping lichen-dominated biological soil crusts in desert areas. Remote Sens. Environ. 2005, 96, 165–175. [Google Scholar] [CrossRef]
- Karnieli, A. Development and implementation of spectral crust index over dune sands. Int. J. Remote Sens. 1997, 18, 1207–1220. [Google Scholar] [CrossRef]
- Cavanaugh, K.C.; Kendall, B.E.; Siegel, D.A.; Reed, D.C.; Alberto, F.; Assis, J. Synchrony in dynamics of giant kelp forests is driven by both local recruitment and regional environmental controls. Ecology 2013, 94, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Bell, T.W.; Cavanaugh, K.C.; Reed, D.C.; Siegel, D.A. Geographical variability in the controls of giant kelp biomass dynamics. J. Biogeogr. 2015, 42, 2010–2021. [Google Scholar] [CrossRef]
- Masselink, G.; Hughes, M.G. An Introduction to Coastal Processes and Geomorphology; Routledge: Abingdon-on-Thames, UK, 2014. [Google Scholar] [CrossRef]
- Edwards, M.S. Estimating scale-dependency in disturbance impacts: El Niños and giant kelp forests in the northeast Pacific. Oecologia 2004, 138, 436–447. [Google Scholar] [CrossRef] [PubMed]
- Buschmann, A.H.; Pereda, S.V.; Varela, D.A.; Rodríguez-Maulén, J.; López, A.; González-Carvajal, L.; Schilling, M.; Henríquez-Tejo, E.A.; Hernández-González, M.C. Ecophysiological plasticity of annual populations of giant kelp (Macrocystis pyrifera) in a seasonally variable coastal environment in the Northern Patagonian Inner Seas of Southern Chile. J. Appl. Phycol. 2014, 26, 837–847. [Google Scholar] [CrossRef]
- Vásquez, J.A. Production, use and fate of Chilean brown seaweeds: Re-sources for a sustainable fishery. J. Appl. Phycol. 2008, 20, 457–467. [Google Scholar] [CrossRef]
- Wernberg, T.; Bennett, S.; Babcock, R.C.; De Bettignies, T.; Cure, K.; Depczynski, M.; Dufois, F.; Fromont, J.; Fulton, C.J.; Hovey, R.K.; et al. Climate-driven regime shift of a temperate marine ecosystem. Science 2016, 353, 169–172. [Google Scholar] [CrossRef]
- Hinojosa, I.A.; Rivadeneira, M.M.; Thiel, M. Temporal and spatial distribution of floating objects in coastal waters of central–southern Chile and Patagonian fjords. Cont. Shelf. Res. 2011, 31, 172–186. [Google Scholar] [CrossRef]
- Hinojosa, I.A.; Pizarro, M.; Ramos, M.; Thiel, M. Spatial and temporal distribution of floating kelp in the channels and fjords of southern Chile. Estuar. Coast. Shelf Sci. 2010, 87, 367–377. [Google Scholar] [CrossRef]
- Bell, T.W.; Cavanaugh, K.C.; Siegel, D. Remote monitoring of giant kelp biomass and physiological condition: An evaluation of the potential for the Hyperspectral Infrared Imager (HyspIRI) mission. Remote Sens. Environ. 2015, 167, 218–228. [Google Scholar] [CrossRef]




| Class (ROI) | Site | Long, Lat | Images Processed | Reference |
|---|---|---|---|---|
| Kelp, Coast, Ocean | S. Australia—Warrnambool | 142.46, −38.4 | 163 | [48] |
| S. Africa—Oudekraal | 18.35, −33.98 | 310 | [48] | |
| Falkland Islands (Malvinas) | −57.75, −51.61 | 73 | [48] | |
| W. Canada—Nuchatlitz Islands | −126.53, 49.6 | 258 | [48] | |
| USA—Carmel Bay | −121.93, 36.55 | 76 | [48] | |
| C. Chile—Punta Parra | −72.97, −36.66 | 171 | [49] | |
| S. Chile—Grevy Island− Cape Horn | −67.61, −55.52 | 93 | [29] | |
| France—Kerguelen Islands | 69.68, −49.20 | 98 | [50] | |
| South Georgia & the South Sandwich Islands | −36.71, −54.11 | 129 | [51] | |
| Foam | S. Chile—Queule | −73.21, −39.35 | 77 | This study |
| River Grass | S. Chile—Queule | −73.21, −39.35 | 77 | This study |
| Land vegetation | S. Chile—Queule | −73.21, −39.35 | 77 | This study |
| Green algae (Ulvophyceae) | S. Chile—Puyuhuapi Channel S. Argentina − Puerto Deseado | −72.76, −44.71 −65.86, −47.85 | 78 147 | This study |
| Organic water | USA—Santa Barbara Channel S. Chile—Puyuhuapi Channel New Zealand—Kaimaumau | −119.95, 34.03 −72.71, −44.73 173.31, −34.96 | 282 78 136 | This study |
| Index | Masking Threshold | Defined by |
|---|---|---|
| NDVI | ≥−0.003411 | River grass max value |
| FAI | ≥0.005352 | Organic water max value |
| KD | ≥0.003216 | River grass max value |
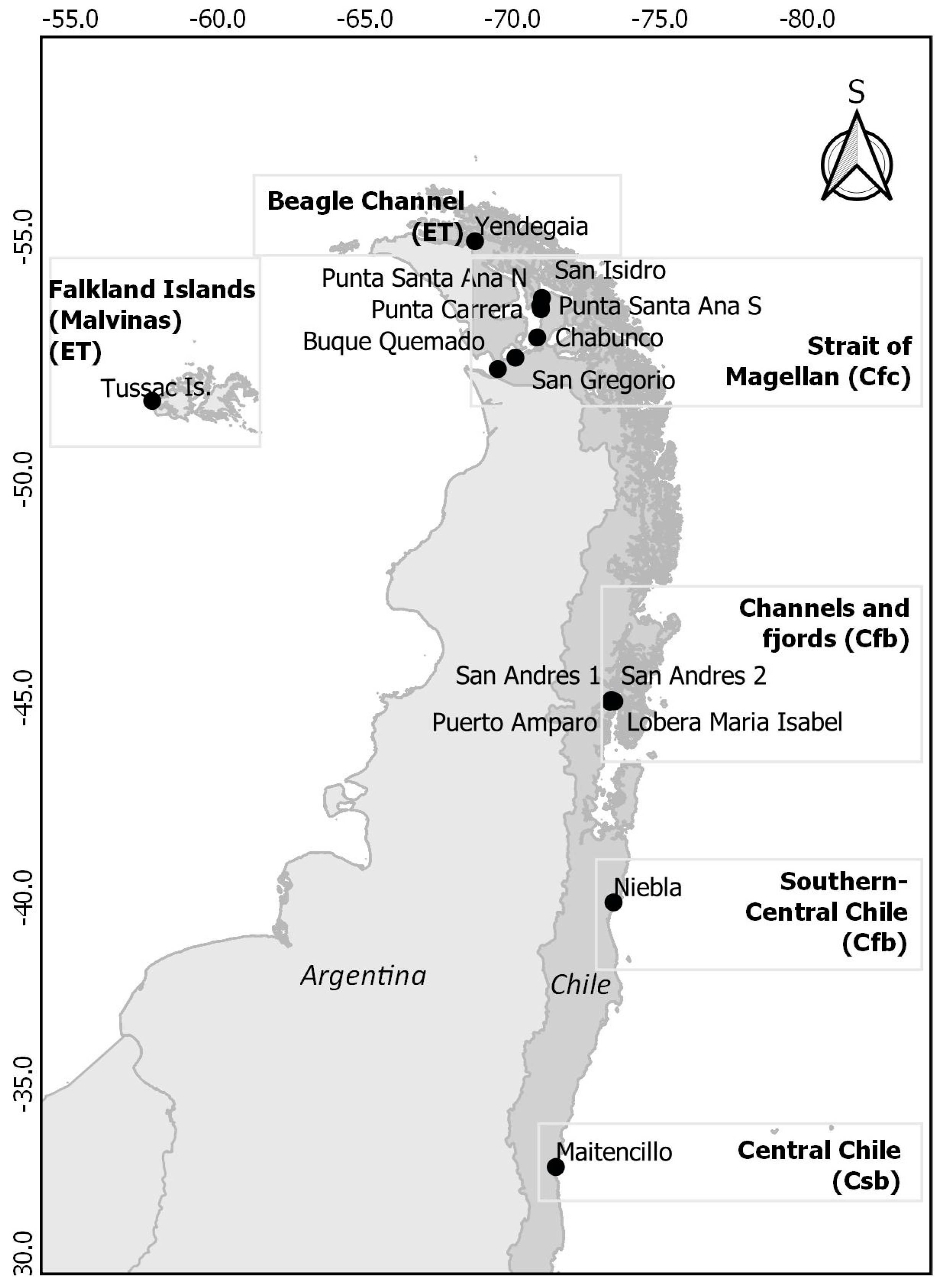
| Main Area | Site | Long, Lat | Area (ha) | Köppen−Geiger Climate Classification |
|---|---|---|---|---|
| C. Chile | Maitencillo | −71.44199, −32.64762 | 0.5 | Csb |
| S. C. Chile | Niebla | −73.40054, −39.87498 | 2.1 | Cfb |
| Channels and Fjords | Lobera María Isabel | −73.42381, −44.90923 | 0.3 | Cfb |
| Channels and Fjords | San Andrés 1 and 2 | −73.32865, −44.9348 | 0.3 1 | Cfb |
| Channels and Fjords | Puerto Amparo | −73.28257, −44.89874 | 0.3 | Cfb |
| Strait of Magellan | San Isidro | −70.97483, −53.78515 | 5.0 | Cfc |
| Strait of Magellan | Santa Ana Sur | −70.92467, −53.63006 | 0.4 | Cfc |
| Strait of Magellan | Santa Ana Norte | −70.91918, −53.62731 | 1.1 | Cfc |
| Strait of Magellan | Punta Carrera | −70.93902, −53.55859 | 5.2 | Cfc |
| Strait of Magellan | Chabunco | −70.81101, −52.98648 | 1.4 | Cfc |
| Strait of Magellan | San Gregorio | −70.07255, −52.57044 | 0.8 | Cfc |
| Strait of Magellan | Buque Quemado | −69.47702, −52.33489 | 1.2 | Cfc |
| Beagle Channel | Yendegaia | −68.70262, −54.9045 | 1.5 | ET |
| Falkland Islands (Malvinas) | Tussac Islands: Kelly Rocks, Bottom, Top | −57.74472, −51.67233 | 11.5 16.3 11.8 | ET |
| Index | No. of Observations Total | No. of Observations Kelp | No. of Observations Green Algae |
|---|---|---|---|
| NDVI | 102 | 50 | 52 |
| FAI | 103 | 50 | 53 |
| KD | 114 | 61 | 53 |
| FAI | NDVI | KD | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PA | UA | OA | Kappa | PA | UA | OA | Kappa | PA | UA | OA | Kappa | |
| Punta Santa Ana Norte | 59.3 | 84.3 | 92.3 | 0.65 | 61.2 | 80.0 | 91.9 | 0.65 | 62.5 | 75.0 | 91.3 | 0.63 |
| San Isidro | 69.2 | 76.7 | 92.9 | 0.69 | 71.0 | 74.4 | 92.7 | 0.68 | 75.1 | 67.4 | 91.6 | 0.66 |
| Chabunco | 69.2 | 76.8 | 92.7 | 0.69 | 72.0 | 73.9 | 92.5 | 0.69 | 82.7 | 64.5 | 91.2 | 0.67 |
| Punta Carrera | 76.0 | 94.6 | 91.9 | 0.79 | 78.7 | 93.7 | 92.4 | 0.80 | 84.2 | 92.9 | 93.7 | 0.84 |
| Niebla | 62.6 | 79.7 | 80.9 | 0.56 | 66.5 | 77.0 | 80.9 | 0.57 | 67.9 | 69.8 | 78.0 | 0.52 |
| Tussac Kelly | 66.2 | 89.5 | 86.2 | 0.67 | 61.0 | 90.9 | 85.0 | 0.63 | 72.8 | 87.4 | 87.5 | 0.71 |
| Tussac Bottom | 60.8 | 88.4 | 89.4 | 0.66 | 57.6 | 88.4 | 88.8 | 0.63 | 71.4 | 85.5 | 90.8 | 0.72 |
| Tussac Top | 69.1 | 74.6 | 80.8 | 0.57 | 60.8 | 76.4 | 79.6 | 0.53 | 77.6 | 73.6 | 82.3 | 0.62 |
| Yendegaia | 56.1 | 80.8 | 89.3 | 0.60 | 51.2 | 82.1 | 88.8 | 0.57 | 63.2 | 71.2 | 88.4 | 0.60 |
| Total average | 65.4 | 82.8 | 88.5 | 0.65 | 64.4 | 81.9 | 88.1 | 0.64 | 73.1 | 76.4 | 88.3 | 0.66 |
| Range (m) | N | % | Cumulative% |
|---|---|---|---|
| 50 | 42 | 26.8 | 26.8 |
| 100 | 17 | 10.8 | 37.6 |
| 200 | 27 | 17.2 | 54.8 |
| 300 | 17 | 10.8 | 65.6 |
| >300, undetected | 54 | 34.4 | 100.0 |
| Total | 157 | 100 |
| Province | Ecoregion | km2 | Detected/Georeferenced |
|---|---|---|---|
| Agulhas | Agulhas Bank | 136.2 | |
| Natal | 1.7 | ||
| Benguela | Namaqua | 96.5 | 1/1 |
| Cold Temperate Northeast Pacific | Gulf of Alaska | 483.9 | |
| North American Pacific Fjordland | 2074.2 | ||
| Northern California | 193.7 | 1/1 | |
| Oregon, Washington, Vancouver Coast and Shelf | 333.6 | ||
| Puget Trough/Georgia Basin | 118.2 | ||
| Magellanic | Channels and Fjords of Southern Chile | 4840.7 | 23/32 |
| Chiloense | 687.0 | 17/22 | |
| Falkland Islands (Malvinas) | 3081.1 | 1/1 | |
| Patagonian Shelf | 144.5 | ||
| Northern New Zealand | Northeastern New Zealand | 76.6 | |
| Three Kings–North Cape | 0.6 | ||
| Scotia Sea | South Georgia & the South Sandwich Islands | 145.9 | 2/2 |
| Southeast Australian Shelf | Bassian | 389.3 | 10/13 |
| Cape Howe | 128.4 | ||
| Western Bassian | 42.4 | 1/3 | |
| Southern New Zealand | Central New Zealand | 75.7 | 5/11 |
| Chatham Island | 23.3 | 1/1 | |
| Snares Island | ND | ||
| South New Zealand | 148.8 | 7/8 | |
| Subantarctic Islands | Crozet Islands | 73.6 | |
| Heard and McDonald Islands | 0.5 | ||
| Kerguelen Islands | 3397.6 | ||
| Macquarie Island | 17.2 | ||
| Prince Edward Islands | 46.4 | 1/1 | |
| Subantarctic New Zealand | Auckland Island | 29.1 | 1/1 |
| Bounty and Antipodes Islands | 0.6 | 1/2 | |
| Campbell Island | 1.5 | 1/1 | |
| Tristan Gough | Tristan Gough | 6.0 | |
| Southern California Bight | 222.1 | ||
| Warm Temperate Southeastern Pacific | Araucanian | 54.9 | 12/18 |
| Central Chile | 11.7 | 9/10 | |
| Humboldtian | 4.7 | 9/29 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mora-Soto, A.; Palacios, M.; Macaya, E.C.; Gómez, I.; Huovinen, P.; Pérez-Matus, A.; Young, M.; Golding, N.; Toro, M.; Yaqub, M.; et al. A High-Resolution Global Map of Giant Kelp (Macrocystis pyrifera) Forests and Intertidal Green Algae (Ulvophyceae) with Sentinel-2 Imagery. Remote Sens. 2020, 12, 694. https://doi.org/10.3390/rs12040694
Mora-Soto A, Palacios M, Macaya EC, Gómez I, Huovinen P, Pérez-Matus A, Young M, Golding N, Toro M, Yaqub M, et al. A High-Resolution Global Map of Giant Kelp (Macrocystis pyrifera) Forests and Intertidal Green Algae (Ulvophyceae) with Sentinel-2 Imagery. Remote Sensing. 2020; 12(4):694. https://doi.org/10.3390/rs12040694
Chicago/Turabian StyleMora-Soto, Alejandra, Mauricio Palacios, Erasmo C. Macaya, Iván Gómez, Pirjo Huovinen, Alejandro Pérez-Matus, Mary Young, Neil Golding, Martin Toro, Mohammad Yaqub, and et al. 2020. "A High-Resolution Global Map of Giant Kelp (Macrocystis pyrifera) Forests and Intertidal Green Algae (Ulvophyceae) with Sentinel-2 Imagery" Remote Sensing 12, no. 4: 694. https://doi.org/10.3390/rs12040694
APA StyleMora-Soto, A., Palacios, M., Macaya, E. C., Gómez, I., Huovinen, P., Pérez-Matus, A., Young, M., Golding, N., Toro, M., Yaqub, M., & Macias-Fauria, M. (2020). A High-Resolution Global Map of Giant Kelp (Macrocystis pyrifera) Forests and Intertidal Green Algae (Ulvophyceae) with Sentinel-2 Imagery. Remote Sensing, 12(4), 694. https://doi.org/10.3390/rs12040694